Abstract
The purpose of this work was to determine if and where Angiopoietin-like genes are expressed in the mouse uterus during the implantation period of pregnancy, and to determine if uterine expression of such genes is controlled by estrogen or progesterone. We found that all six known murine angiopoietin-like genes were expressed in the mouse uterus during implantation. The expression of four genes was controlled by either estrogen or progesterone. Only the levels of angiopoietin-like 4 (Angptl4) mRNA dramatically increased in implantation segments of the uterus during decidualization and was conceptus-independent. Due to this increased expression, and the fact that angiopoietin-like 4 protein plays a role in lipid metabolism and angiogenesis in other tissues, only the expression of Angptl4 was further examined in the uterus and developing placenta. Angptl4 mRNA was localized to subpopulations of the endometrial stromal fibroblast and endothelial cell populations during decidualization. It was also localized to the ectoplacental cone, trophoblast giant cells and parietal endoderm of the conceptus at this time. By mid-pregnancy, Angptl4 mRNA was localized mainly to the mesometrial lymphoid aggregate region plus mesometrial endothelial cells of the uterus as well as in various cell types of the conceptus. Additional work showed that Angptl4 expression increases in mouse endometrial stromal cells as they undergo decidualization in vitro. As in other cell types, the expression of Angptl4 in endometrial stromal cells was increased in response to an agonist of the peroxisome proliferator activated receptors. Taken together, the results of this work support the hypothesis that locally expressed Angptl4 might play a role in local uterine/placental lipid metabolism and vascular changes during implantation and thus provides a basis for future research.
Keywords: Uterus, Decidualization, Endometrium, Angiopoietin-like
INTRODUCTION
Implantation begins with the attachment of the embryo to the uterine wall and ends in the formation of the definitive placenta. In rodents and man, implantation involves the differentiation of the endometrial stroma into decidual tissue, a process called decidualization (Abrahamsohn and Zorn 1993, Dunn et al. 2003). This process involves the transdifferentiation of endometrial stromal fibroblast cells to decidual cells as well as other changes such as extracellular matrix remodeling, vascular remodeling and changes in immune cell populations (Abrahamsohn and Zorn 1993, Blois et al. 2011, Dunn et al. 2003, Gellersen et al. 2007, Herington and Bany 2009) Implantation also involves the development of the placenta from trophoblast cell lineages of the conceptus (Simmons and Cross 2005). In mice the mature functioning form of the placenta with normal blood flow is formed after mid-pregnancy while in humans this occurs approximately at the end of the first trimester. Therefore, prior to this time the uterus must provide a nutritive environment for the conceptus to survive and develop without the presence of significant nutritive exchange from a placenta.
Uterine natural killer (uNK) cells are the major immune cell type in the uterus during decidualization and is the main source of interferon γ (IFNG) found in the uterus during implantation (Croy et al. 2003). uNK cells and IFNG play a key role in the spiral artery modification of pregnancy and decidual integrity by just after mid-pregnancy as revealed by several knockout models where uteri lack uNK cells (Croy et al. 2003). Maternal expression of interleukin-15 (Il15) is required for uNK cells to appear in the uterus during decidualization (Barber and Pollard 2003) and is a useful model to study the effects of depleting uNK cells, and thus significantly reducing IFNG levels, in the uterus during decidualization on uterine gene expression.
Seven genes encoding proteins similar to those of the angiopoietins have been discovered in humans and are now commonly referred to as the angiopoietin-like (Angptl) genes. These 7 members are called angiopoietin-like 1 (Angptl1), 2 (Angptl2), 3 (Angptl3), 4 (Angptl4), 5 (Angptl5), 6 (Angptl6), and 7 (Angptl7). Notably, Angptl5 was originally discovered in humans and no mouse ortholog has been described (Zeng et al. 2003). However, the other six Angptl genes are represented in the mouse. In a similar fashion to the related angiopoietin proteins, the angiopoietin-like (ANGPTL) proteins contain C- and N-terminal fibrinogen-like and coiled-coil domains, respectively (Kadomatsu et al. 2011). However, the ANGPTL proteins are orphan ligands as none bind to endothelial-specific receptor tyrosine kinase (TEK), the receptor of angiopoietin-1 (ANGPT1) and -2 (ANGPT2), or the related receptor tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE1)(Katoh and Katoh 2006). Nonetheless, various functions of Angptl proteins have been described in developmental (Lai et al. 2007), physiological (Cazes et al. 2006, Ito et al. 2003, Le Jan et al. 2003, Mandard et al. 2006, Xu et al. 2005, Yang et al. 2008) and pathophysiological (Hu et al. 2011, Kikuchi et al. 2008, Zhu et al. 2011) processes. Uterine expression of Angpt1 and Angpt2 plus their receptor Tek during implantation in mice has been previously described (Bany and Cross 2006, Matsumoto et al. 2002). However, the expression and function of the related Angptl genes in the uterus during implantation remain to be explored. Therefore, the purpose of this study was to examine uterine Angptl gene expression in the mouse uterus in response to steroids and during embryo implantation.
MATERIALS AND METHODS
Animals
All animal work was approved by the Southern Illinois University IACUC committee. CD1 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Il15 knockout (Il15−/−) mice on a CD1 background were also used and have been described previously(Eckstrum and Bany 2011). Mice were housed under controlled light conditions (lights on 6 a.m. to 8 p.m.) with free access to food and water. Mice were ovariectomized and allowed to recover for 10–14 days. Some were then injected subcutaneously with vehicle, estradiol-17β (10 ng), progesterone (1 mg) or both progesterone plus estradiol-17β in 0.1 ml sesame oil and the uteri were collected 24 h later. Other ovariectomized mice were used to collect endometrial stromal cells as described below.
Pregnant animals were obtained by mating 10–12 week old females with mature males and Day 0.5 of pregnancy was assigned the morning a vaginal plug was discovered. Mice were sacrificed on Day 3.5 of pregnancy just before the onset of implantation as well as after the onset of implantation on Days 4.5 to 11.5 and uteri were collected. In some cases, females were mated with vasectomized males and Day 0.5 of pseudopregnancy was assigned the morning a vaginal plug was observed. As described in detail elsewhere (Herington and Bany 2007b), concanavalin A-coated agarose beads were transferred into lumen of the uteri of some of these mice on Day 2.5 of pseudopregnancy. These beads induce decidualization of the uterus in a similar fashion to the implanting blastocyst resulting in the formation of focal deciduomas and are thus useful when comparing gene expression to pregnant mice (Herington et al. 2009).
Tissue Collection and Processing
Both uterine horns of ovariectomized mice were dissected. Implantation (IS) and non-implantation (NIS) segments of the pregnant uteri were separated. Bead-induced deciduoma (BID) and non-bead segments of the uteri of pseudopregnant mice were separated. No attempt was made to separate myometrium from endometrium of the segments. However, for RNA isolation, conceptuses were dissected out of IS tissue. Tissues were either collected for RNA isolation or for in situ hybridization. Tissues collected for RNA isolation were homogenized in Trizol Reagent (InVitrogen) while those used for in situ hybridization were fixed in 4% paraformaldehyde in PBS for 24 h.
Quantitative-RT-PCR (qRT-PCR)
Total RNA was isolated from the tissue homogenized in Trizol Reagent as directed by the manufacturer (InVitrogen, Carlsbad, CA). The isolated RNA was then treated with RNAse-free DNAse (Promega, Madison, WI) and re-extracted using Trizol. Quantitative RT-PCR was validated and carried out essentially as previously described (McConaha, et al., 2011) using High Capacity RNA to cDNA Kits (Applied Biosystems, Carlsbad, CA) and BioRAD SYBR Green Mastermix (BioRAD, Hercules, CA). Primer sequences and annealing temperatures used are shown in Table 1. Relative mRNA levels were calculated essentially as previously described, using 18 S rRNA as the housekeeping gene (Herington and Bany 2007a). Briefly, for each of the samples, the ΔCt values (CtmRNA−Ct18SRNA) were calculated where CtmRNA and Ct18SrRNA are the Ct values for Angptl mRNAs and 18S rRNA respectively. Next, the average ΔCt for the values found for the tissue type and time point we wanted to normalize to a mean of 1 was subtracted from all individual ΔCt values. Finally, the normalized ΔCt values for each of the samples were then transformed to relative expression levels using the following: 2−(ΔCt).
Table 1.
Oligonucleotide primer sequences (5′–3′) used for qRT-PCR and annealing temperature (Temp, °C) utilized.
| Gene | Upstream Primer | Downstream Primer | Temp |
|---|---|---|---|
| 18 S rRNA | CGGCTACCACATCCAA | GCTGGAATTACCGCGGCT | 64 |
| Angptl1 | TCTTCTCTGCTGCCCAC | GTGAGCCTCTGCACAATC | 62 |
| Angptl2 | GGGACCTTAACTGTGC | GAATGGCTACAGGTACCA | 62 |
| Angptl3 | GATGGCTCTGTCAATT | CATCAATGTTTCCAAACCC | 62 |
| Anfptl4 | CCTCTTCAACTGAACG | TCTGTTATAAACGGCAGA | 62 |
| Angptl6 | TATCCACCGGCTCACC | TCTGCGTAGCGTGCATTG | 62 |
| Angptl7 | CACCGTGAGGCATGTG | GGTTGGTCTTTATGAACTG | 62 |
| Lpl | CCTACTTCAGCTGGCC | TGGGAGCAAATGATTCCT | 62 |
| Prl8a2 | GCTGCATCAATTCCTG | CCTCATCACGTCTATACAT | 62 |
In situ hybridization
Fixed tissue was processed into paraffin blocks using standard methods then sectioned (5 μm) using a rotary microtome. In situ hybridization was carried out exactly as previously described (Simmons et al. 2008) using digoxigenin (DIG)-labeled riboprobes and BCIP/NBT as the colorimetric substrate. The slides were counterstained with nuclear fast red prior to mounting coverslips. The mRNA signals were purple and nuclei stained red and photomicrographs were captured using a Leica Microscope (Leica Microsystems Inc., Buffalo Grove, IL) equipped with a Retiga camera and Qcapture pro software (QImaging, Burnaby, BC). Only linear adjustments were made in the color levels and brightness of the photomicrographs when preparing the figures in order to show what was seen visually with the microscope.
A PCR amplicon of Angptl4 was produced using the upstream 5′-AGATGACCCAGCTCATTGGCTTGA-3′ and downstream 5′-AGAGGCTCTTGGCACAGTTAAGGT-3′ primers that produced a 510 bp amplicon. After subjecting the PCR reaction to agarose gel electrophoresis the amplicon was isolated using a Gel Extraction Kit (Omega Bio-tek, Norcross, GA) then cloned into the pGEM-T Easy vector (Promega) as recommended by the manufacturers. The sequence and orientation of the clone was verified by DNA sequencing (Biotechnology Center, University of Illinois, Urbana-Champaign, IL). The cDNA was then used to prepare antisense and control sense riboprobes using methods described previously (McConaha et al. 2011). No signals were seen in all hybridizations where sense probes were used (data are not shown).
Measurement of Blood Triacylglyceride Levels
After removing food for 1 hour, at noon on Day 8.5 of pregnancy and pseudopregnancy, mice were sacrificed and blood was collected from the abdominal vein. Blood triglyceride levels (mg/dl) were measured using a CardioChek Meter and test strips (CardioChek, Indianapolis, IN) as recommended by the manufacturer.
Primary Mouse Endometrial Cell Culture
Ovariectomized mice were injected with estradiol and progesterone to sensitize their uteri for a deciduogenic stimulus as previously described (Bany et al. 2000). On the day of optimal sensitization mice were sacrificed and the uteri were dissected. Endometrial stromal cells were isolated and plated exactly as described previously (Bany et al. 2000). Cells were then cultured in DMEM:F12 containing 10% charcoal-stripped fetal calf serum, 0.1 U/ml penicillin, 0.1 mg/ml streptomycin, 1.25 μg/ml fungizone (InVitrogen, Carlesbad, CA), 10 nM estradiol-17β, 1 μM medroxyprogesterone (Sigma, Saint Louis, MO) and 8Br-cAMP (Santa Cruz Biotechnology, Santa Cruz, CA) the medium was changed every 24 h. Some cells were treated with carbaprostacyclin (10 μM)(Santa Cruz Biotechnology, Santa Cruz, CA) or vehicle (ethanol) in place of the 8Br-cAMP. The medium was replaced at 24 h of culture. Cells were scraped into Trizol Reagent for RNA isolation and qRT-PCR as described above.
Statistical Analysis
Data were analyzed using SigmaStat Software as directed by the manufacturer (Systat Software Inc., Chicago, IL). To deal with a significant heterogeneity of variance, data were logarithmically transforming before statistical analysis. A repeated measures analysis of variance (ANOVA) was used to determine the effect of tissue type (NIS and IS tissue) and Day of pregnancy (Day 4.5–8.5) on mRNA levels. When a significant (P<0.05) interaction was detected, Duncan’s multiple range test was used for group comparisons. Comparison of the mRNA levels between pregnant IS and pseudopregnant BID tissues and IS tissues between wild-type and Il15−/− mice on Days 3.5–8.5 were carried out using a 2-way ANOVA. A 1-way ANOVA was used to determine the effect of Day (6.5–10.5) on is tissue Angptl4 mRNA levels followed by Duncan’s multiple range test to determine differences between means. T-Tests were used to compare blood triglyceride levels and relative mRNA levels in culture cells. Finally, the effects of steroids on uterine mRNA levels were analyzed using a 2-way ANOVA.
RESULTS
Angiopoietin-like (Angpl) Gene mRNA Levels in the Pregnant Uterus
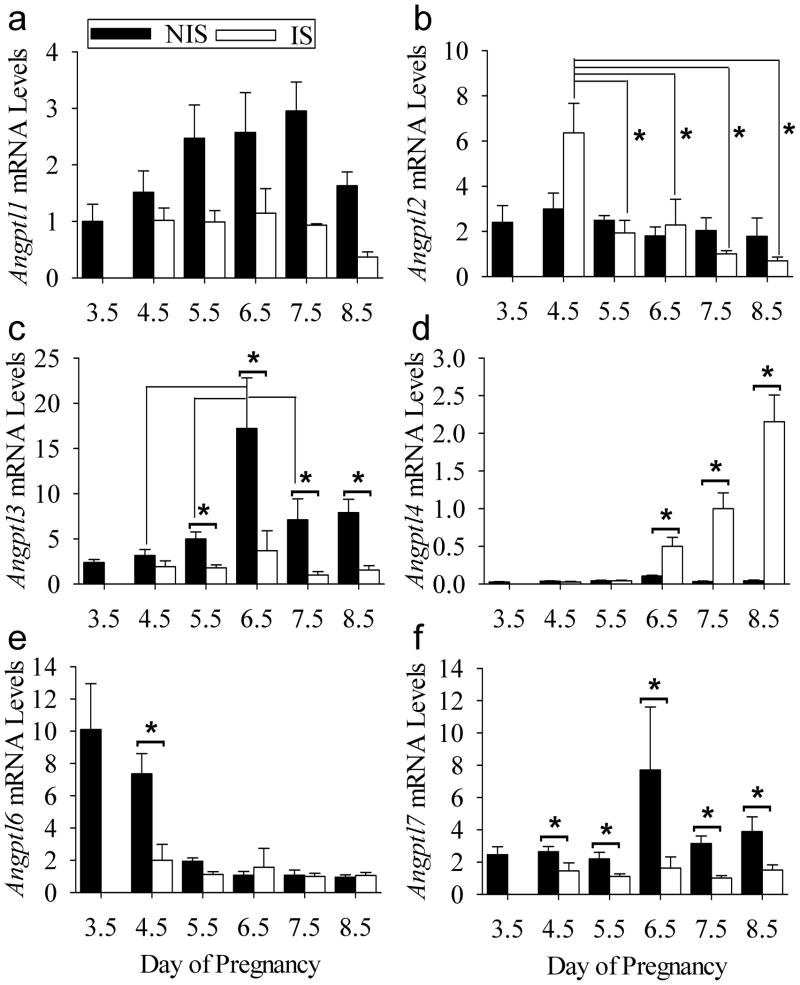
The levels of several Angpl mRNAs in the peri-implantation mouse uterus were determined by qRT-PCR. The mRNAs of Angptl1, Angptl2, Angptl3, Angptl4, Angptl6 and Angptl7 were detected in pregnant uterine tissues immediately before (day 3.5) and after the onset of implantation (days 4.5–8.5)(Fig. 1). There was an effect (P<0.001) of tissue type on Angptl1 mRNA levels (Fig. 1A). This was due to higher (P<0.05) mRNA levels in NIS compared to IS tissue on days 5.5, 6.5, 7.5 and 8.5. Angptl2 mRNA levels remained relatively constant in NIS tissue throughout the periimplantation period at a level not different (P>0.05) from that in IS tissues on Days 5.5–8.5 (Fig 1B). However, Angptl2 mRNA levels were greater (P<0.05) in IS tissue on days 4.5 compared to all other samples. Angptl3 mRNA levels remained relatively constant in IS tissue throughout the peri-implantation period (Fig 1C). However, mRNA levels were elevated (P<0.05) in NIS compared to IS tissue on Days 5.5–8.5 of pregnancy (Fig. 1C). Angptl3 mRNA levels were higher (P<0.05) in NIS tissues on Day 6.5 compared to those on Day 4.5, 5.5, and 7.5. Angptl4 mRNA levels increased (P<0.05) in IS compared to NIS tissue on Days 6.5–8.5 of pregnancy compared to NIS tissue by approximately 5-, 31- and 49-fold, respectively (Fig. 1D). Angpl6 mRNA was high on day 3.5 of pregnancy just before the onset of implantation and in NIS tissue on Day 4.5 (Fig 1E). However, after the onset of implantation, the mRNA level decreased (P<0.05) in IS compared to NIS tissue on Day 4.5. This decrease was to the same (P>0.05) levels seen NIS and IS tissue on Days 5.5–8.5. Finally, Angptl7 mRNA levels were greater (P<0.05) in NIS compared to IS tissues on Days 4.5–8.5 (Fig. 1F).
Figure 1.
qRT-PCR analysis of angiopoietin-like expression in the mouse uterus during implantation. Relative (A) Angptl1, (B) Angptl2, (C) Angptl3, (D) Angptl4, (E) Angptl6 and (F) Angptl7 mRNA levels in non-implantation (NIS) compared to implantation (IS) site tissue segments on Day 3.5–8.5 of pregnancy. Bars represent mean ± SEM (N=4). *P<0.05. The data were normalized to the mean relative mRNA level of Day 7.5 IS tissue.
Localization of Angptl4 Gene Expression in the Pregnant Uterus
Uteri collected from day 6.5 (Fig. 2A) and later (data are not shown) pregnant mice showed limited hybridization signals in NIS tissues. In IS tissues from Day 6.5 mice (Fig. 2B), hybridization signals were seen in both the embryo and ectoplacental cone cells of the conceptus (Fig. 2C). Strong hybridization signals were also seen in the vascular layer between the longitudinal and circular muscles of the myometrium (Fig. 2D) and in the endometrial stromal cells near the myometrium (Fig. 2D,E). In the mesometrial side of the Day 6.5 IS tissue, light Angptl4 hybridization signals were seen in the endothelial cells lining the sinusoids (Fig. 2F), endometrial stromal cells (Fig. 2B) and in the vessels in the mesometrial-myometrial area (Fig. 2G). In IS tissues from Day 7.5 mice (Fig. 2H), hybridization signals were seen in both the embryo (Fig. 3I) and ectoplacental cone cells (Fig. 2J) of the conceptus. In the antimesometrial side of the IS tissue, hybridization signals were seen in the vascular layer between the longitudinal and circular muscles of the myometrium and in the endometrial stromal cells adjacent to the myometrium (Fig. 2K,M). In the mesometrial side of the Day 7.5 IS tissue, Angptl4 hybridization signals were seen in the endothelial cells lining the sinusoids (Fig. 2L). In IS tissues from Day 8.5 mice (Fig. 2O), strong hybridization signals were seen in flattened ectoplacental cone cells and embryo (Fig. 2P), as well as the parietal endoderm cells and trophoblast giant cells (Fig. 2Q). In the antimesometrial side of the Day 8.5 IS tissue, hybridization signals were seen in the vascular layer between the longitudinal and circular muscles of the myometrium (Fig. 2R) and in endometrial stromal cells of the antimesometrial decidua (Fig. 2R). In the mesometrial side, strong Angptl4 hybridization signals were seen in the endothelial cells lining the sinusoids (Fig. 2S) as well as in the mesometrial decidua and the developing mesometrial lymphoid aggregate of pregnancy (MLap)(Fig. 2O,T).
Figure 2.
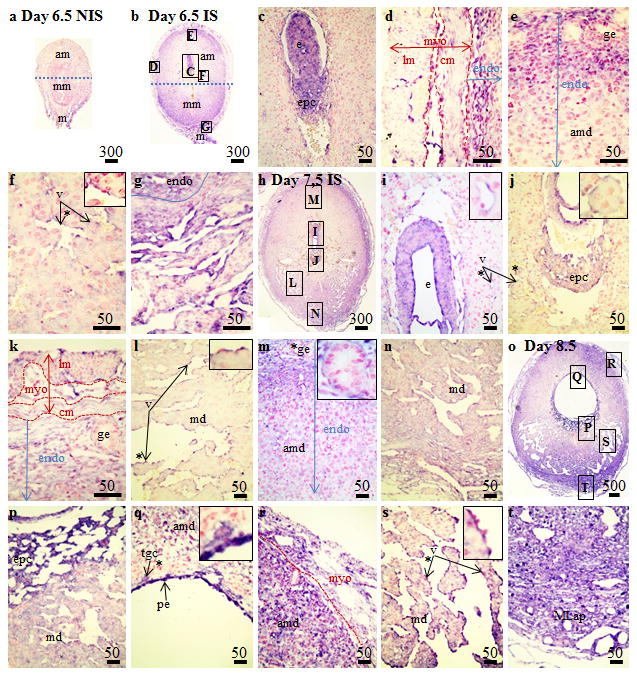
Localization of Angptl4 mRNA in non-implantation (NIS) and implantation (IS) tissue segments from Day 6.5–8.5 pregnant mice by in situ hybridization. (A) Day 6.5 NIS, (B–G) Day 6.5 IS, (H–N) Day 7.5 IS and (O–T) Day 8.5 IS. These are representative of at least 3 independent samples. Global linear adjustments of the brightness and color level were made on the photomicrographs to more accurately represent what was seen on the slides under the microscope. Numbers above scale bars are in microns. Abbrev: am, antimesometrial decidua; c, conceptus; cm, circular smooth muscle layer; endo, endometrium; epc, ectoplacental cone; ge, glandular epithelium; lm, longitudinal smooth muscle layer; m, mesometrium; MLap, mesometrial lymphoid aggregate of pregnancy; mm, mesometrial decidua; myo, myometrium; pe, parietal endoderm; plac, developing placenta; tgc, trophoblast giant cells; v, vascular endothelium.* Denotes area of higher magnification inset in the panel.
Figure 3.
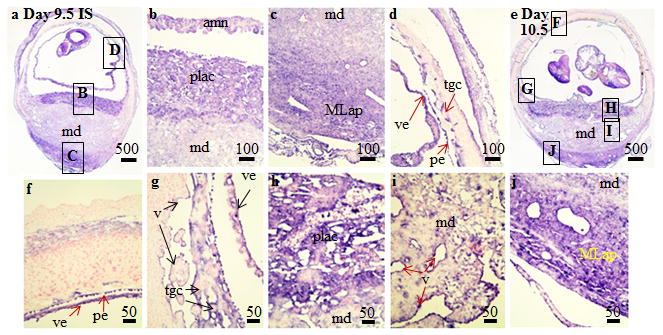
Localization of Angptl4 mRNA in implantation (IS) tissue segments from (A–D) Day 9.5 and (E–J) Day 10.5 pregnant mice using in situ hybridization. These are representative of at least 3 independent samples. Global linear adjustments of the brightness and color level were made on the photomicrographs to more accurately represent what was seen on the slides under the microscope. Numbers above scale bars are in microns. Abbrev: mm, mesometrial decidua; MLap, mesometrial lymphoid aggregate of pregnancy; pe, parietal endoderm; plac, developing placenta; tgc, trophoblast giant cells; v, vascular endothelium; ve, visceral endoderm.
On Day 9.5 of pregnancy, placental development is well underway. Strong hybridization signals in the IS tissue collected from Day 9.5 pregnant mice was seen in the in the fetus (Fig. 3A) as well as the amnion (Fig. 3B), MLap (Fig. 3C) and trophoblast giant cells and yolk sac (Fig. 3D). Strong hybridization signals in the IS tissue collected from Day 10.5 pregnant mice were seen in the fetus (Fig. 3E) plus trophoblast giant, amnion, and yolk sac cells (Fig. 3F,G). Cells in the developing labyrinth of the placenta exhibited strong hybridization signals (Fig. 3E,H). Finally, hybridization signals are seen in the endothelial cells of the mesometrial decidual region (Fig. 3I) and within mesometrial lymphocyte aggregate of pregnancy (Fig. 3J) in the Day 10.5 IS tissue.
Angptl4 Expression in Mouse Uterus During Artificially-Induced Decidualization
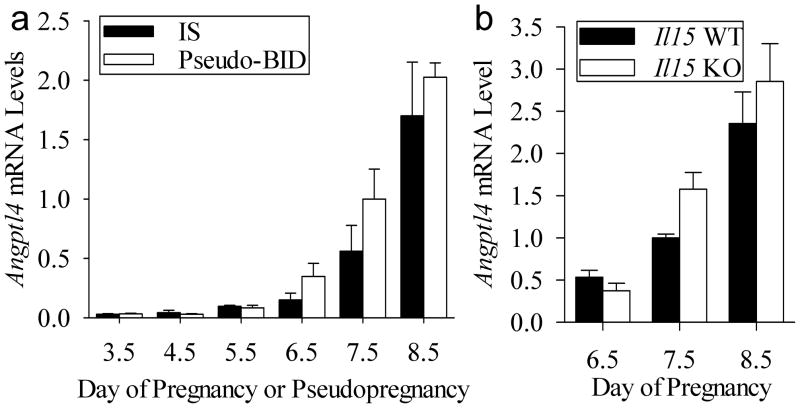
Next we investigated if the increase Angptl4 in the IS tissues (Figure 1D) requires the presence of a conceptus or can be mimicked using an artificial deciduogenic stimulus. To examine this we used an artificial model where the uterine transfer of blastocyst-sized beads are used as a deciduogenic stimulus in pseudopregnant mice. There was no effect (P>0.05) of tissue type (IS and BID tissues) and the lack of an interaction (P>0.05) between tissue type and day of pregnancy revealed mRNA levels did not differ between IS and BID tissue on each day examined (Fig. 4A).
Figure 4.
Effect of the conceptus and uNK cells on uterine Angptl4 expression. (A) qRT-PCR analysis of relative Angptl4 mRNA levels in IS tissue of pregnant mice to BID tissue of pseudopregnant mice on Days 3.5–8.5. (B) qRT-PCR analysis of relative Angptl4 mRNA levels in IS tissue segments of Il15+/+ compared to Il15−/− mice on Days 6.5–8.5 of pregnancy. Bars represent mean ± SEM (N=3–4). The data were normalized to the mean relative mRNA level of Day 7.5 IS tissue.
Angptl4 Expression in the uNK Cell Deficient Uteri
We compared Angptl4 mRNA levels in IS tissues from interleukin-15 wild-type (Il15+/+) to that of Il15−/− mice on Days 6.5–8.5 of pregnancy. A difference in Angptl4 mRNA levels in IS tissues from wild-type and Il15−/− mice was not detected on each day examined (Fig. 4B).
Expression of Lpl in Mouse Uterus During Implantation
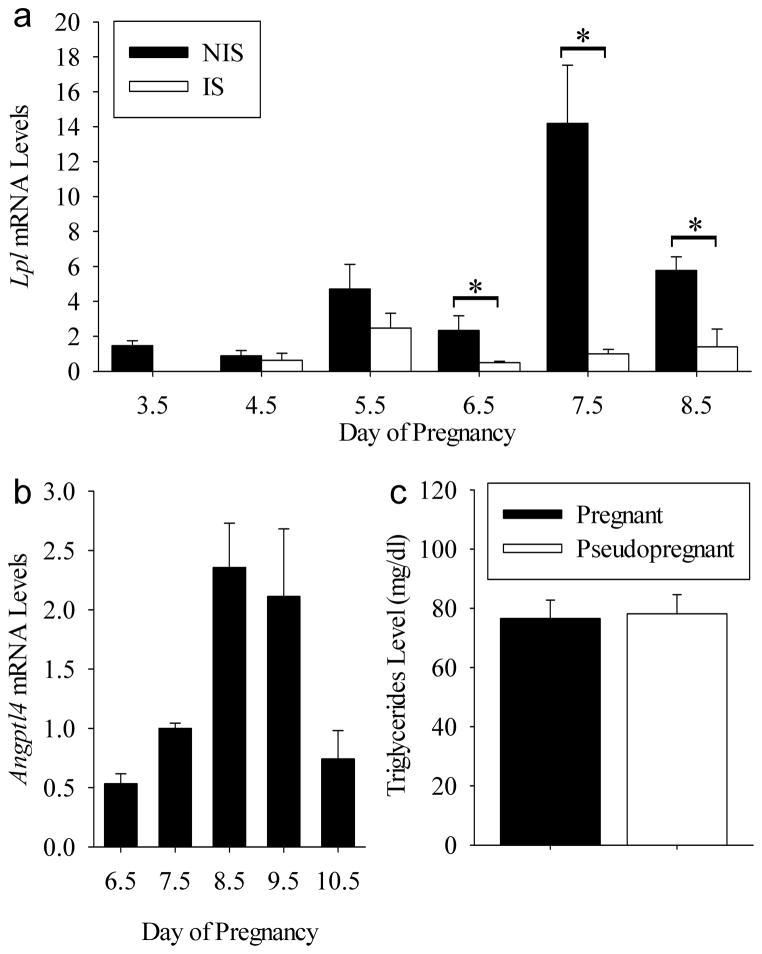
Next we determined if Lpl is expressed locally in the uterus during the peri-implantation period on days 3.5–8.5. As shown in Fig. 5A Lpl mRNA was detected throughout the uterus on Days 3.5 to 8.5 of pregnancy. There were increases (P<0.05) in Lpl mRNA levels in NIS compared to IS tissues on Days 6.5 to 8.5 of pregnancy.
Figure 5.
Potential function of uterine Angptl4 expression during implantation. (A) Lpl mRNA levels in the mouse uterus during implantation. *P<0.05. (B) Angptl4 mRNA levels in IS tissues form Days 6.5–10.5 pregnant mice. Bars with different letters are significantly (P<0.05 different. (C) Triglyceride concentrations in the blood of Day 9 pregnant compared to pseudopregnant mice. Bars represent mean ± SEM (N=3–4). For graphs A and B, the data were normalized to the mean relative mRNA level of Day 7.5 IS tissue.
Effect of Decidualization on Maternal Blood Triglyceride (TG) Levels in Pseudopregnant Mouse
To determine if uterine Angptl4 expression has an impact on maternal blood triglyceride levels, we first examined mRNA levels in IS tissue from day 6.5–10.5 of pregnancy (Fig. 5B). There was an effect (P<0.005) of day on mRNA levels. This effect was due to a peak in Angptl4 mRNA levels in the IS tissue on Days 8.5 and 9.5. Next we measured maternal blood triglyceride levels in Day 9 pregnant and pseudopregnant mice. Blood triglyceride levels did not differ (P>0.05) between these mice (Fig. 5C)
Expression of Angptl4 in Mouse Endometrial Stromal Cells During Decidualization In Vitro
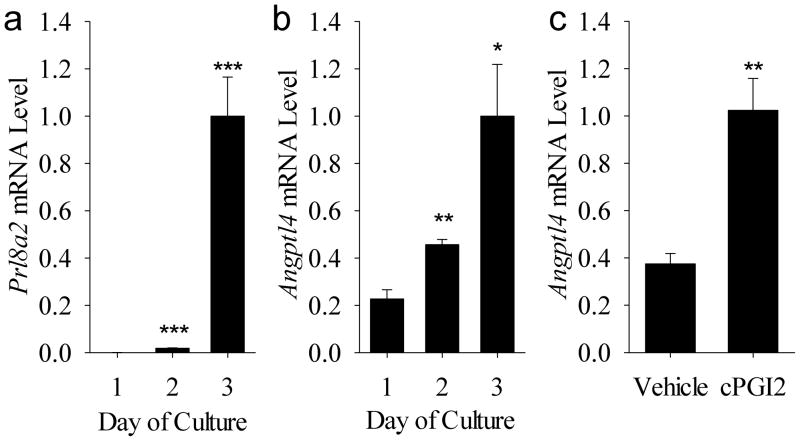
As shown in Fig. 6A, the mRNA levels for the decidualization marker Prl8a2 increased (P<0.005) on days 2 and 3 of culture compared to Day 1. This was accompanied by an increase in Angptl4 mRNA levels on Days 2 (P<0.05) and 3 (P<0.01) compared to Day 1 of culture (Fig. 6B). Incubation with cPGI2 for 72 h caused an increase (P<0.01) in Angptl4 mRNA levels in mouse endometrial stromal cells compared to those treated with vehicle for the same amount of time (Fig. 6C)
Figure 6.
Expression of Angptl4 in mouse endometrial stromal cells during decidualization in vitro. (A) Prl8a2 and (B) Angptl4 mRNA levels in endometrial stromal cells during days 1–3 of culture with medium that contains 8Br-cAMP which stimulates decidualization. (C) Effect of cPGI2 on Angptl4 mRNA levels in endometrial cells. Bars represent mean ± SEM (N=3–4). *P<0.05, **P<0.01 and ***P<0.005 compared to Day 1 or vehicle treatment. The data were normalized to the mean relative mRNA level on Day 3 of culture for graphs A plus B and for the cPGI2 treatment group for graph C.
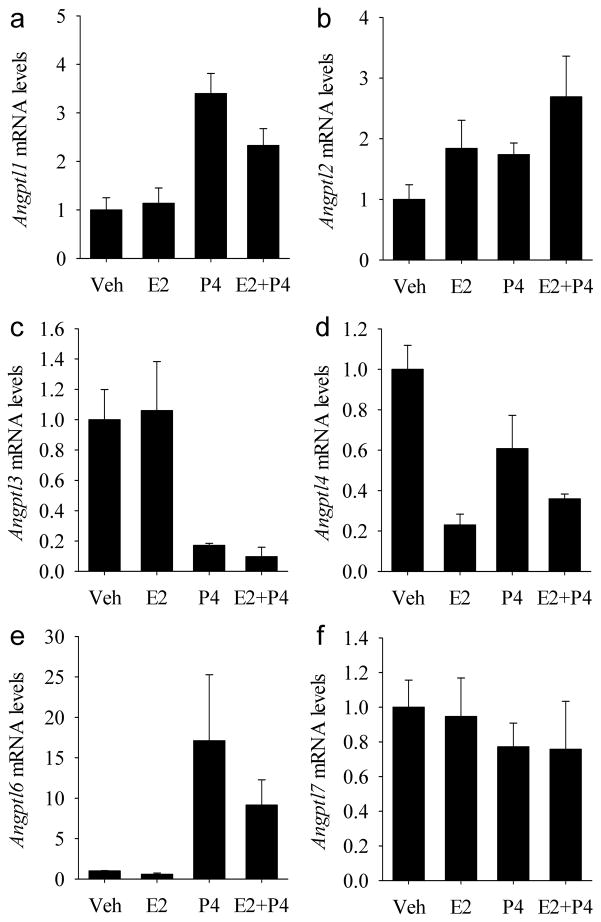
Effect of Estrogen and Progesterone on Angptl Gene Expression
The individual and combined effects of progesterone and estrogen on uterine Angpl gene expression in ovariectomized mice were determined using qRT-PCR. Angptl1 mRNA levels increased (P<0.001) to the same level in response to progesterone whether estrogen was present or not (Fig. 7A). There were no effects (P>0.05) of estrogen or progesterone on Angptl2 mRNA levels (Fig. 7B). Angptl3 mRNA levels decreased (P<0.001) to the same level in response to progesterone whether estrogen was present or absent (Fig. 7C). Estrogen, but not progesterone, caused a reduction (P<0.001) in Angptl4 mRNA levels (Fig. 7D). An interaction interaction (P<0.05) between the treatments revealed that the effect of estrogen in decreasing mRNA levels was slightly reduced in the presence of progesterone. Angptl6 mRNA levels increased (P<0.001) to the same level in response to progesterone alone and progesterone plus estrogen (Fig. 7E). There were no effects (P>0.05) of estrogen or progesterone on Angptl7 mRNA levels (Fig. 7F).
Figure 7.
The effects of steroids on angiopoietin-like gene expression in the uteri of ovariectomized mice. Quantitative RT-PCR analysis of relative (A) Angptl1, (B) Angptl2, (C) Angptl3, (D) Angptl4, (E) Angptl6 and (F) Angptl7 mRNA levels in response to vehicle (Veh), estradiol-17α (E2), progesterone (P4) or estradiol-17α plus progesterone (E2+P4). Bars represent mean ± SEM (N=4). The data were normalized to the mean relative mRNA level of vehicle-treated mice.
DISSCUSSION
Implantation is the process by which the embryo forms a functional nutritive attachment to the mother and ends in the development of the functional placenta. Previous work has shown that members of the angiopoietin family are expressed in the mouse uterus during implantation (Bany and Cross 2006, Matsumoto et al. 2002). The present study now shows that all members of the related Angptl family (Angptl1, Angptl2, Angptl3, Angpl4, Angptl6 and Angptl7) are expressed in both the NIS and IS tissue of the mouse uterus during implantation. Of these, the most abundant increase in expression was seen for Angptl4 in the IS tissue as mid-pregnancy approached, with levels peaking on Days 8.5 and 9.5. On the other hand, the most significant decrease in expression was seen for Angptl6 in both NIS and IS tissues of the uterus during implantation. This provides the first descriptive data showing that expression of all six mouse Angptl genes occurs in the uterus during implantation.
Lipoprotein lipase (LPL) plays a key role in fatty acid metabolism and ANGPLT4 inhibits its activity. Fatty acids are transported in the body mainly in the form of triglycerides which are composed of three fatty acid molecules joined to a glycerol molecule. Most triglycerides transported in the blood are in the form of lipoproteins such as very low density lipoprotien (VLDL) or chylomicrons from the liver and intestines, respectively (Nicoll and Lewis 1980). Notably, we detected Lpl expression in the uterus during implantation. LPL is an enzyme made in parenchymal tissue of most organs and is transported to the luminal surface of the capillary endothelium where members are anchored as active dimers by heparin sulfate proteoglycans(Goldberg and Merkel 2001). This endothelial cell-bound LPL hydrolyzes the triglycerides transported in the blood into free fatty acids which can then be taken up by the tissues for metabolism or storage. Recent work has shown that ANGPTL4 can inhibit LPL activity (Shan et al. 2009). Our findings that Angptl4 expression dramatically increases in the IS tissue during implantation in mice and is expressed in the developing placenta raised the question of its role in implantation. We hypothesized that if the ANGPTL4 produced in the uterus and conceptus may be released into the circulation and then modulate blood triglyceride levels. However, we found that serum triglyceride levels did not differ between pseudopregnant and pregnant mice indicating that ANGPTL4 produced in the uterus and placenta do not act as an endocrine regulator of maternal serum triglyceride levels. However, uterine ANGPTL4 might potentially play a role in regulating uterine and conceptus uptake of fatty acids during implantation. Notably, Angptl4 knockout mice do not have a reported implantation defect (Backhed et al. 2004). Although this lack of an overt implantation phenotype might be due to a functional redundancy of other related factors, it appears that maternal ANGPTL4 is not absolutely required for implantation.
Recent data indicates that a full length and glycosylated ANGPTL4 is secreted from cells which can then be degraded into C- and N-terminal fragments with differing functions. The secreted full-length form of ANGPTL4 binds to the glycosylaminoglycans of the extracellular matrix via its N-terminal coiled-coil domain (CCD)(Chomel et al. 2009) and is also found in the plasma(Mandard et al. 2004). In this form, the protein is a poor inhibitor of LPL and its CCD mediates its anti-angiogeneic properties(Zeng et al. 2003). Enzymes shown to cleave ANGPL4 are proprotein convertase subtilisin/kexin-type 5 (PCSK5), 6 (PCSK6) and 7 (PCSK7). Cleavage releases the soluble CCD domain which is a potent inhibitor of LPL(Lei et al. 2011). On the other hand, the extracellular matirx bound CCD domain and soluble C-terminal fibrinogen-like domain (FLD) are believed to inhibit and promote angiogenesis, respectively(Zeng et al. 2003). Therefore, regulation of ANGPTL4 function appears to be complex but involves the activities of protein convertase enzymes. Notably, Pcks5 is expressed in the uterus and placenta during implantation in mice (Nie et al. 2003, Wong et al. 2002). Further, blocking expression of this gene prevents implantation in mice (Nie et al. 2005) and normal decidual differentiation of human endometrial cells (Tang et al. 2005). Therefore, PCKS5 plays an important role in implantation and may be responsible for processing ANGPTL4 that is produced in the uterus.
Currently little is known about the regulation of ANGPTL4 expression in the uterus and placenta. However, Angptl4 expression is also controlled by peroxisome proliferator activated receptors (PPARs)(Belanger et al. 2002, Mandard et al. 2004, Staiger et al. 2009, Yoon et al. 2000), fatty acids, hypoxia (Gonzalez-Muniesa et al. 2011) and IFNG (Lu et al. 2010) in other tissues/cells. This is interesting because PPARs have been found to play a key role in placental development (Wieser et al. 2008) and are expressed in the uterus and developing placenta during implantation in rodents (Ding et al. 2003a, Ding et al. 2003b, Nishimura et al. 2011). The present study shows that Angptl4 expression in cultured mouse endometrial stromal cells increases during decidualization in vitro and incubation of these cells with a PPAR agonist can mimic this increase. This suggests that PPARs may regulate Angptl4 expression in the mouse endometrial stromal cells during decidualization. Other work has suggested that PPARγ is required for normal placental development and regulation of Angtpl4 expression in the placenta (Parast et al. 2009, Yoon et al. 2000). However, to the best of our knowledge, it is yet to be determined whether expression of Angptl4 is altered in Pparγ–knockout conceptuses during placental development. Specific fatty acids control increase the expression of ANGPTL4 in cultured human first trimester trophoblast cells (Johnsen et al. 2011). However, whether this fatty acid-regulated Angptl4 expression plays an important role in placental development or LPL activity remains to be determined. Finally, since its expression can be induced in other cell types by IFNG(Lu et al. 2010), we hypothesized the increased uterine expression of Angptl4 in the uterus during decidualization may be regulated by uNK cells. This is because uNK cells are the major source of this cytokine in the uterus during pregnancy in mice (Ashkar and Croy 1999). However, our results suggest that neither uNK cells themselves nor their source of IFNG are not required for the increased Angptl4 expression in IS tissues. Therefore, IFNG is likely not a regulator of uterine Angptl4 expression during implantation.
In addition to Angptl4, there are five other Angptl genes in the mouse. Angptl1 and Angptl3 genes encode proteins with anti-angiogenic and angiogenic properties, respectively (Camenisch et al. 2002, Dhanabal et al. 2002). Additionally, in a similar fashion to AGPTL4, ANGPTL3 can regulate LPL activity, albeit through a different mechanism (Shan et al. 2009). Angptl2 encodes a protein that has angiogenic properties and also regulates insulin sensitivity in fat tissues (Kitazawa et al. 2011). The Angplt6 gene encodes a protein mainly expressed in the liver that may modulate energy metabolism and adiposity (Oike et al. 2005). Finally Angptl7 encodes a protein that regulates the expression of extracellular matrix genes in the eye (Comes et al. 2011). The present study showed that all of these five Angptl genes are expressed in the mouse uterus during the peri-implantation period some of which show changes in expression.
Estrogen and progesterone are major regulators of uterine gene expression during the estrous cycle and pregnancy. The present study showed that all 6 Angptl genes are expressed in the uteri of ovariectomized mice. Although no effect was seen on Angptl2 and Angplt7 expression, steroids appeared to control the expression of the other four Angpl genes. Progesterone caused increased uterine Angptl1 and Angptl6 expression while that of Angptl3 was reduced. Finally, Angptl4 expression decreased in response to estrogen. This provides the first descriptive data showing that expression of four of the Angptl genes are regulated by either progesterone or estrogen and more work is required to determine the importance of this regulation in uterine biology. Notably, previous work showed that estrogen causes a significant increase in uterine LPL activity in rodents (Gray and Greenwood 1983). Since ANGPTL4 decreases the activity of LPL, it is tempting to speculate from our data that the mechanism by which estradiol decreases uterine LPL activity may be due at least in part to its effect on decreasing uterine Angptl4 expression.
In summary we have characterized Angpl gene expression in the mouse uterus in response to steroids and during implantation. All six of the mouse Angptl genes were expressed in the uterus with the notable observation that Angpl4 expression is dramatically increased in IS tissue of the uterus during decidualization. Further, we showed that Angptl4 was expressed by distinct cell populations of both the uterus and conceptus during implantation. Taken together with what has been previously published about ANGPTL4 function in other tissues or cells, the results of this work support the hypothesis that ANGPTL4 may play a role in lipid metabolism or vascular changes in the uterus and also in the developing placenta during implantation. This work provides a basis for future research on examining the function of Angtpl4 expression in the mouse uterus and developing placenta during implantation.
Acknowledgments
This work was supported in part by Southern Illinois University School of Medicine and a NIH - Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD049010) grant (to BB). Personnel support from Southern Illinois University was received in the form of Undergraduate Research Assistantships (to CS). The authors gratefully acknowledge Sheila Scillufo and Jennifer Herington for providing technical assistance in animal husbandry and qRT-PCR work in this study, respectively.
References
- Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266:603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294:445–456. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bany BM, Harvey MB, Schultz GA. Expression of matrix metalloproteinases 2 and 9 in the mouse uterus during implantation and oil-induced decidualization. J Reprod Fertil. 2000;120:125–134. doi: 10.1530/jrf.0.1200125. [DOI] [PubMed] [Google Scholar]
- Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. 2011;88:86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- Cazes A, Galaup A, Chomel C, Bignon M, Brechot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S, Monnot C. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99:1207–1215. doi: 10.1161/01.RES.0000250758.63358.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel C, Cazes A, Faye C, Bignon M, Gomez E, Ardidie-Robouant C, Barret A, Ricard-Blum S, Muller L, Germain S, Monnot C. Interaction of the coiled-coil domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. Faseb J. 2009;23:940–949. doi: 10.1096/fj.08-115170. [DOI] [PubMed] [Google Scholar]
- Comes N, Buie LK, Borras T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011;16:243–259. doi: 10.1111/j.1365-2443.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, He H, Esadeg S, Wei Q, McCartney D, Zhang J, Borzychowski A, Ashkar AA, Black GP, Evans SS, Chantakru S, van den Heuvel M, Paffaro VA, Jr, Yamada AT. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003;126:149–160. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanabal M, LaRochelle WJ, Jeffers M, Herrmann J, Rastelli L, McDonald WF, Chillakuru RA, Yang M, Boldog FL, Padigaru M, McQueeney KD, Wu F, Minskoff SA, Shimkets RA, Lichenstein HS. Angioarrestin: an antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 2002;62:3834–3841. [PubMed] [Google Scholar]
- Ding NZ, Ma XH, Diao HL, Xu LB, Yang ZM. Differential expression of peroxisome proliferator-activated receptor delta at implantation sites and in decidual cells of rat uterus. Reproduction. 2003a;125:817–825. doi: 10.1530/rep.0.1250817. [DOI] [PubMed] [Google Scholar]
- Ding NZ, Teng CB, Ma H, Ni H, Ma XH, Xu LB, Yang ZM. Peroxisome proliferator-activated receptor delta expression and regulation in mouse uterus during embryo implantation and decidualization. Mol Reprod Dev. 2003b;66:218–224. doi: 10.1002/mrd.10348. [DOI] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003;7:151–161. doi: 10.1016/s1472-6483(10)61745-2. [DOI] [PubMed] [Google Scholar]
- Eckstrum K, Bany BM. Tumor necrosis factor receptor subfamily 9 (Tnfrsf9) gene is expressed in distinct cell populations in mouse uterus and conceptus during implantation period of pregnancy. Cell Tissue Res. 2011;344:567–576. doi: 10.1007/s00441-011-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ, Merkel M. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci. 2001;6:D388–405. doi: 10.2741/goldberg. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Muniesa P, de Oliveira C, Perez de Heredia F, Thompson MP, Trayhurn P. Fatty Acids and Hypoxia Stimulate the Expression and Secretion of the Adipokine ANGPTL4 (Angiopoietin-Like Protein 4/Fasting-Induced Adipose Factor) by Human Adipocytes. J Nutrigenet Nutrigenomics. 2011;4:146–153. doi: 10.1159/000327774. [DOI] [PubMed] [Google Scholar]
- Gray JM, Greenwood MR. Uterine and adipose lipoprotein lipase activity in hormone-treated and pregnant rats. Am J Physiol. 1983;245:E132–137. doi: 10.1152/ajpendo.1983.245.2.E132. [DOI] [PubMed] [Google Scholar]
- Herington JL, Bany BM. The conceptus increases secreted phosphoprotein 1 gene expression in the mouse uterus during the progression of decidualization mainly due to its effects on uterine natural killer cells. Reproduction. 2007a;133:1213–1221. doi: 10.1530/REP-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. Effect of the conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod. 2007b;76:579–588. doi: 10.1095/biolreprod.106.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. Do molecular signals from the conceptus influence endometrium decidualization in rodents? J Exp Zool B Mol Dev Evol. 2009;312:797–816. doi: 10.1002/jez.b.21308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Underwood T, McConaha M, Bany BM. Paracrine signals from the mouse conceptus are not required for the normal progression of decidualization. Endocrinology. 2009;150:4404–4413. doi: 10.1210/en.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Jham BC, Ma T, Friedman ER, Ferreira L, Wright JM, Accurso B, Allen CM, Basile JR, Montaner S. Angiopoietin-like 4: a novel molecular hallmark in oral Kaposi’s sarcoma. Oral Oncol. 2011;47:371–375. doi: 10.1016/j.oraloncology.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y, Suda T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63:6651–6657. [PubMed] [Google Scholar]
- Johnsen GM, Basak S, Weedon-Fekjaer MS, Staff AC, Duttaroy AK. Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta. 2011;32:626–632. doi: 10.1016/j.placenta.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. Febs J. 2011;278:559–564. doi: 10.1111/j.1742-4658.2010.07979.x. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Comparative integromics on Angiopoietin family members. Int J Mol Med. 2006;17:1145–1149. [PubMed] [Google Scholar]
- Kikuchi R, Tsuda H, Kozaki K, Kanai Y, Kasamatsu T, Sengoku K, Hirohashi S, Inazawa J, Imoto I. Frequent inactivation of a putative tumor suppressor, angiopoietin-like protein 2, in ovarian cancer. Cancer Res. 2008;68:5067–5075. doi: 10.1158/0008-5472.CAN-08-0062. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Nagano M, Masumoto KH, Shigeyoshi Y, Natsume T, Hashimoto S. Angiopoietin-like 2, a circadian gene, improves type 2 diabetes through potentiation of insulin sensitivity in mice adipocytes. Endocrinology. 2011;152:2558–2567. doi: 10.1210/en.2010-1407. [DOI] [PubMed] [Google Scholar]
- Lai DM, Tu YK, Hsieh YH, Hsu WM, Lee CC, Cheng WC, Hsieh FJ, Li H. Angiopoietin-like protein 1 expression is related to intermuscular connective tissue and cartilage development. Dev Dyn. 2007;236:2643–2652. doi: 10.1002/dvdy.21262. [DOI] [PubMed] [Google Scholar]
- Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, Jin W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286:15747–15756. doi: 10.1074/jbc.M110.217638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–1741. doi: 10.1016/j.bbrc.2009.12.145. [DOI] [PubMed] [Google Scholar]
- Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, Muller M, Kersten S. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–34420. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M, Kersten S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002;277:29260–29267. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- McConaha ME, Eckstrum K, An J, Steinle JJ, Bany BM. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction. 2011;141:511–527. doi: 10.1530/REP-10-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A, Lewis B. Evaluation of the roles of lipoprotein lipase and hepatic lipase in lipoprotein metabolism: in vivo and in vitro studies in man. Eur J Clin Invest. 1980;10:487–495. doi: 10.1111/j.1365-2362.1980.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Nie G, Li Y, Wang M, Liu YX, Findlay JK, Salamonsen LA. Inhibiting uterine PC6 blocks embryo implantation: an obligatory role for a proprotein convertase in fertility. Biol Reprod. 2005;72:1029–1036. doi: 10.1095/biolreprod.104.036889. [DOI] [PubMed] [Google Scholar]
- Nie GY, Li Y, Minoura H, Findlay JK, Salamonsen LA. Specific and transient up-regulation of proprotein convertase 6 at the site of embryo implantation and identification of a unique transcript in mouse uterus during early pregnancy. Biol Reprod. 2003;68:439–447. doi: 10.1095/biolreprod.102.006676. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Yamauchi N, Chowdhury VS, Torii M, Hattori MA, Kaneto M. Expression of peroxisome proliferator-activated receptor isoforms in the rat uterus during early pregnancy. Cell Tissue Res. 2011;345:275–284. doi: 10.1007/s00441-011-1208-4. [DOI] [PubMed] [Google Scholar]
- Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, Urano T, Kimura Y, Kubota Y, Maekawa H, Miyamoto T, Miyata K, Matsumoto S, Sakai J, Nakagata N, Takeya M, Koseki H, Ogawa Y, Kadowaki T, Suda T. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. Plos One. 2009;4:e8055. doi: 10.1371/journal.pone.0008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Yu XC, Liu Z, Hu Y, Sturgis LT, Miranda ML, Liu Q. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J Biol Chem. 2009;284:1419–1424. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. doi: 10.1186/1471-2164-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Haring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Mikhailik A, Pauli I, Giudice LC, Fazelabas AT, Tulac S, Carson DD, Kaufman DG, Barbier C, Creemers JW, Tabibzadeh S. Decidual differentiation of stromal cells promotes Proprotein Convertase 5/6 expression and lefty processing. Endocrinology. 2005;146:5313–5320. doi: 10.1210/en.2005-0684. [DOI] [PubMed] [Google Scholar]
- Wieser F, Waite L, Depoix C, Taylor RN. PPAR Action in Human Placental Development and Pregnancy and Its Complications. PPAR Res. 2008;2008:527048. doi: 10.1155/2008/527048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BS, Liu S, Schultz GA, Rancourt DE. Subtilisin proprotein convertase-6 expression in the mouse uterus during implantation and artificially induced decidualization. Mol Reprod Dev. 2002;61:453–459. doi: 10.1002/mrd.10113. [DOI] [PubMed] [Google Scholar]
- Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci U S A. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–840. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Dai J, Ying K, Zhao E, Jin W, Ye Y, Xu J, Xie Y, Mao Y. Identification of a novel human angiopoietin-like gene expressed mainly in heart. J Hum Genet. 2003;48:159–162. doi: 10.1007/s100380300025. [DOI] [PubMed] [Google Scholar]
- Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, Kersten S, Li HY, Ding JL, Tan NS. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19:401–415. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]