Abstract
The comorbidity of major depressive disorder (MDD) and cardiovascular disease (CVD) is among the 10th leading cause of morbidity and mortality worldwide. Thus, understanding the co-occurrence of these disorders will have major public health significance. MDD is associated with an abnormal stress response, manifested in brain circuitry deficits, gonadal dysfunction, and autonomic nervous system (ANS) dysregulation. Contribution of the relationships between these systems to the pathophysiology of MDD is not well understood. The objective of this preliminary study was to investigate, in parallel, relationships between HPG-axis functioning, stress response circuitry activation, and parasympathetic reactivity in healthy controls and women with MDD. Using fMRI with pulse oximetry [from which we calculated the high frequency (HF) component of R-R interval variability (HF-RRV), a measure of parasympathetic modulation] and hormone data, we studied eight women with recurrent MDD in remission and six controls during a stress response paradigm. We demonstrated that hypoactivations of hypothalamus, amygdala, hippocampus, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and subgenual ACC were associated with lower parasympathetic cardiac modulation in MDD women. Estradiol and progesterone attenuated group differences in the effect of HF-RRV on hypoactivation in the amygdala, hippocampus, ACC, and OFC in MDD women. Findings have implications for understanding the relationship between mood, arousal, heart regulation, and gonadal hormones, and may provide insights into MDD and CVD risk comorbidity.
INTRODUCTION
The comorbidity of major depressive disorder (MDD) and cardiovascular disease (CVD) will be the leading cause of disability world wide by 2020 [28], and is significantly higher in women [8, 30]. Literature on the pathophysiology of sex differences in MDD provides evidence of disruption of several circuits involved in the response to stress [13, 18], including hypothalamic-pituitary-gonadal (HPG) axis, the network of brain regions associated with arousal, and the parasympathetic component of the autonomic nervous system (ANS) [5].
The approach of linking ANS activity to metabolic abnormalities has gained popularity in recent attempts to investigate mechanisms underlying brain activity deficits in affective disorders [20]. Fourier or autoregressive analysis of cyclical oscillations in the R-R interval (R-R variability; RRV) produces power spectra, portions of which reflect autonomic influences on heart rate and blood pressure. Research suggests that RRV dysregulation, characterized by increased sympathetic and decreased parasympathetic activity in response to stress, may represent a unique window into understanding underlying biological mechanisms involved in affective disorders [21] and the comorbidity with cardiovascular disease [10]. In particular, MDD has been associated with parasympathetic cardiac dysregulation [10]. We recently demonstrated, in a large population-level cohort study, that fetal risk factors have a significant impact on the comorbidity between MDD and low parasympathetic reactivity in adulthood, a finding specific to women [8], suggesting that MDD-CVD comorbidity in women has its origins during fetal development. With the current pilot study, we take the next step in this research, extending the focus to examine the pathophysiology of this sex-specific comorbidity in two additional key systems, stress response circuitry in the brain and the HPG-axis, in parallel with RRV.
At the neuroendocrine level, women with MDD display reduced estradiol [37] and increased progesterone [11], suggesting HPG axis dysfunction. These hormones have the potential to act at the receptor level in subcortical areas that show deficits in MDD (hypothalamus, hippocampus), given the density of estrogen and progesterone receptors in these highly sexually dimorphic regions [31]. Work by our group and others offers evidence of relationships between peripheral serum hormone levels and stress response region activation, with correlations between estradiol and reward-related activation of the amygdala-hippocampal complex in healthy controls [6] and estradiol and progesterone and hypoactivation in MDD compared with controls during stress [13]. Interest in the effect of hormones on cardiovascular function in post-menopausal women has prompted investigation of the relationship between peripheral endocrine markers and HF-RRV. Animal studies demonstrate significant links between HPG-axis hormones and ANS regulation, with data suggesting that endogenous estrogens increase HF-RRV [16]. Findings in healthy women indicate higher HF-RRV during elevated estradiol phases of the menstrual cycle [26], although others report no differences across cycle phase [19]. In general, however, compelling evidence suggests important relationships between HPG-axis functioning, stress response circuitry activation, and parasympathetic reactivity to stress paradigms in healthy controls and in the pathophysiology of MDD. However, studies have not explored relationships between these systems in parallel in MDD.
Several studies have investigated the brain circuitry correlates of ANS regulation during emotional tasks in healthy participants [4–5, 29, 34, 36]. These investigations report activation of hypothalamic nuclei, brainstem regions, amygdala, hippocampus, medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and insula. In a recent study, we demonstrated hypoactivity of the stress response circuitry in women with recurrent MDD in remission, including hypothalamus, amygdala, hippocampus, OFC, and ACC [13]. In the current preliminary study, we test the hypothesis that these brain activity deficits in women with MDD are significantly associated with low parasympathetic regulation of the heart, operationalized as the HF-RRV, and further predicted that estradiol and progesterone would account for significant variability in the relationship between stress response circuitry hypoactivation and HF-RRV dysfunction in women with MDD compared with controls.
MATERIAL AND METHODS
Participants were a community sample of eight women with a history of severe and persistent MDD, currently in remission, and six healthy controls (HC), recruited from the general community through flyers and online classified search engines. All participants were interviewed by an experienced Masters-level clinical interviewer, using the Structured Clinical Interview for DSM-IV Axis 1 Disorders (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition). Diagnoses were confirmed by two senior diagnostic experts. Full remission was defined as the absence of DSM-IV MDD symptoms for the past one month; 5/8 MDD women met criteria for sustained full remission for the past 1 to 5 years. MDD women had a mean age of symptom onset of 20.9±5.0 years, duration of illness of 13.3±8.5 years, and 2.9±1.9 episodes of MDD. Comorbid diagnoses in the MDD group: alcohol dependence, in full remission >3 years (1 subject); generalized anxiety disorder, current (1 subject). Four MDD women were taking selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors: citalopram (1), sertraline (1), venlafaxine (2). The HC group was made comparable to the MDD group on age (MDD: mean=34.2±4.4; HC: mean=34.4±4.5), ethnicity (all Caucasian), handedness (all right-handed), and verbal IQ (WAIS-III vocabulary subtest scaled score; MDD: mean=14.3±1.3; HC: mean=14.3±3.1). Subjects in the HC group had no history of any DSM-IV axis I disorder and were not taking any psychotropic medications. Two subjects were current light (average 1.5–2 cigarettes/day) smokers (1 MDD, 1 HC); neither had smoked within 17 or 36 hours (respectively) prior to the study visit.
Menstrual cycles of all study subjects were monitored for 3 months prior to fMRI scanning. On the day of the scan, study procedures were described and written informed consent was obtained. Women with MDD were assessed using the 21-item Hamilton Rating Scale for Depression (HAM-D). All subjects scored less than 8 (mean=3.5±2.5), indicating that MDD subjects were not clinically depressed at the time of scanning. The study protocol was approved by the local institutional review board through the Partners Human Research Committee. Participants received an honorarium for their time. Remaining study procedures are only briefly described here, as they are identical to those in our previous report [13].
A visual stimuli task paradigm was presented to evoke a stress response, adapted from the International Affective Picture System (IAPS) and validated in our previous publications [9], including with this sample [13], while participants underwent standard gradient echo planar imaging functional imaging on a 3-Tesla MRI scanner (Signa VH, GE Medical Systems, Wisconsin, USA). For additional experimental setup details see [13].
To measure heart rate (HR) and RRV, a photoplethysmography (PPG) sensor was placed on the left thumb and recorded at a rate of 100 samples/second (Hz). The PPG operates at a near infrared wavelength, and corresponding waveforms are synchronized with heartbeats. PPG provides valuable information about the cardiovascular system, including autonomic function in a non-invasive manner [1, 3]. Pulse oximetry recording was synchronized with the start of scanning, and recorded values saved in an ASCII file generated by scanner software. The peak of each pulse pressure wave was detected automatically using the ‘PhysioNet’ software toolbox (www.physionet.org) and the time interval between two successive pulse pressure waves (inverse of HR) was used to estimate RR-interval. Incorrect or missed pulse pressure waves were corrected manually via visual inspection.
To estimate RRV, frequency components of the pulse-pressure derived beat timeseries within a 20-second time window from the end of the corresponding TR were calculated using a Lomb periodogram, which allows the frequency components of unevenly spaced data to be obtained on the basis of a least-squares method [22]. This 20-second time window from the end of the TR to calculate the HF-RRV signal was shifted with every TR interval (i.e. 2-sec, the duration of each EPI volume acquisition) to pair with the EPI volumes of each run. The Lomb periodogram approach is crucial in calculating frequency components of heartbeat signals, since the RR-intervals are not evenly spaced in the time-domain, and Fourier transform approach is therefore unsuitable [17]. Using the Lomb periodogram of the RR-intervals, the average power of all frequency components between 0.15 Hz and 0.4 Hz was calculated within the 20-sec time frame window and adopted as the level of HF-RRV of the corresponding time-point of each EPI volume acquisition. Thus, resulting HF-RRV values with the same temporal resolution to the EPI volumes could be successfully adopted as the regressor of the 1st level general linear model (GLM) analysis of EPI data.
fMRI data were preprocessed usingSPM2 (www.fil.ion.ucl.ac.uk/spm) (see [13]). A GLM was applied to fMRI data of each subject, with anticipated hemodynamic responses to visual stimuli (negative, neutral) convolved with a canonical hemodynamic response function and subsequently used as regressors. In this study, HF-RRV from each scan run was used as the third regressor [29]. Six head-motion parameters (three translations, three rotations) estimated during the realignment were also used as regressors to adjust for spurious brain activations due to head-motion. From the least-squares estimation of the GLM, brain activations of each voxel in association with negative and neutral stimuli, ANS activity as measured via the HF-RRV signal, and six motion parameters were estimated from regressor coefficients (i.e., beta weights).
For the second-level group analysis, beta weights associated with HF-RRV regressors from each subject were transformed into t-scores. Using contrast images from HC and MDD subjects within the whole brain, voxel-wise two-sample t-tests were used to compare the group-dependent effect size of HF-RRV-associated brain activations evoked in response to negative versus neutral stimuli. The resulting level of activation from the t-test estimation was analyzed within anatomic regions-of-interest (ROIs) [hypothalamus, amygdala, hippocampus, OFC, ACC, and subgenual ACC (sgACC) (ACC and sgACC ROIs did not overlap), anatomic borders defined using a manually segmented MNI-152 brain] using the ROI-analysis toolbox as implemented through Wake Forest University (WFU) PickAtlas for SPM8. Spatial patterns of active voxels for each ROI were detected from clusters with a minimum of 5 voxels (p < 0.05 uncorrected) considering the marginal size of the hypothalamus and sgACC. False positives were corrected by adjusting effective number of voxels within the cluster using the spherical search volume with 3 mm radius (small volume correction) based on a family-wise error (FWE) correction for multiple comparisons.
Data from the current subjects indicated lower hormone levels in MDD (estradiol: MDD=125.8 pg/mL; HC=164.7 pg/mL; progesterone: MDD=1.0 ng/mL; HC=0.73 ng/mL). To assess the degree to which hormone level differences accounted for variability in the relationship between stress response circuitry hypoactivation and HF-RRV dysfunction in MDD, second-level group analyses described above were repeated with inclusion of estradiol and progesterone levels as additional covariates. The effect of adding these hormone covariates was quantified using t-test to compare between (1) t-scores without hormones and (2) t-scores with hormones for each ROI.
RESULTS
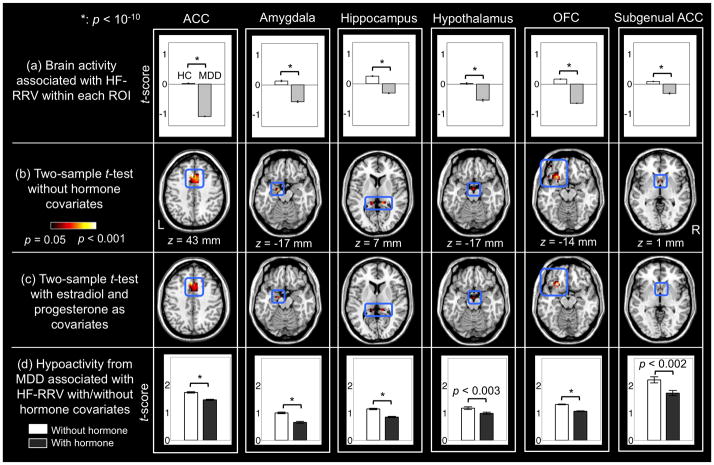
The MDD group showed a significantly lower level of HF-RRV variation than HC [standard deviation (STD) MDD and HC: 5.1 (±0.8) and 5.8 (±1.0), respectively; t-score=2.71, p=0.0098; inter quartile range (IQR): 6.8 (±1.4) and 8.5 (±1.7), respectively; t=3.36, p=0.0017]. HR variation did not differ between groups (STD: t=0.51, p=0.61; IQR: t=1.14, p=0.26). Similarly, R-R interval variations did not differ between groups (STD: t =−0.91, p=0.37; IQR: t =−0.18, p=0.86) (see Supplementary Figure). [Table 1 and Figure 1 here]
Table 1.
Brain Regions in the Stress Response Circuitry Significantly Associated with HF-RRV Comparing Healthy Women and Women with Depression1
| Contrast | Region of Interest | Side | Size | x | y | z | Z-score | PFWE-corr |
|---|---|---|---|---|---|---|---|---|
| HC > MDD | Hypothalamus | 7 | 0 | −7 | −17 | 2.24 | 0.039 | |
|
| ||||||||
| Amygdala | L | 19 | −15 | −7 | −17 | 2.76 | 0.012 | |
|
| ||||||||
| Hippocampus | R | 13 | 12 | −37 | 7 | 2.55 | 0.020 | |
| 32 | 33 | −19 | −20 | 2.42 | 0.026 | |||
| 42 | −19 | −14 | 2.18 | 0.044 | ||||
| L | 16 | −12 | −37 | 4 | 2.34 | 0.031 | ||
|
| ||||||||
| Orbitofrontal cortex (OFC) | L | 135 | −27 | 20 | −17 | 3.04 | 0.006 | |
| −27 | 32 | −23 | 2.15 | 0.047 | ||||
| −30 | 29 | −5 | 2.08 | 0.054 | ||||
| L | 33 | −48 | 17 | −5 | 2.43 | 0.026 | ||
| R | 10 | 54 | 38 | −5 | 2.29 | 0.035 | ||
| L | 12 | −42 | 47 | −17 | 2.22 | 0.041 | ||
| R | 17 | 42 | 53 | −8 | 2.12 | 0.050 | ||
|
| ||||||||
| Anterior cingulate cortex (ACC) | L | 555 | −3 | 20 | 43 | 3.22 | 0.003 | |
| R | 6 | 56 | 16 | 3.00 | 0.006 | |||
| 6 | 8 | 49 | 2.52 | 0.017 | ||||
|
| ||||||||
| Subgenual ACC | 7 | 0 | 11 | −2 | 2.44 | 0.025 | ||
PFWE-corr < 0.05 with a family-wise error (FWE) using a small volume correction (SVC) within a 3mm radius of sphere for clusters with p < 0.05 (uncorrected) with at least 5 voxels; Size of clusters is a number of voxels within a cluster; x,y,z MNI coordinates in mm; Z-score is the maximum value within the cluster
Figure 1.
(a) Bar graphs of average brain activations associated with HF-RRV within each ROI illustrated using group t-scores (error bars reflect STD). (b) Brain activations within the stress response circuitry in association with HF-RRV observed in HC compared with MDD (p<0.05; cluster minimum: 5 voxels). (c) Brain activations related to attenuation of HC>MDD differences associated with HF-RRV, following addition of estradiol and progesterone covariates. (d) Average level of hypoactivations in MDD compared to HC across voxels within each ROI shown as t-scores in bar graphs, in which the t-scores decreased following addition of hormone covariates.
Table 1 shows stress response circuitry activations that are significantly associated with HF-RRV. Greater activations associated with HF-RRV in HC compared with MDD (Figure 1a) were found in all hypothesized ROIs, i.e. hypothalamus, amygdala, hippocampus, OFC, ACC, and sgACC (p<0.05, FWE-corrected; see Figure 1b). There were no regions in which significantly greater brain activations were associated with greater HF-RRV in MDD compared to healthy women.
Addition of estradiol and progesterone as covariates significantly attenuated the hypoactivation in association with HF-RRV in the MDD group (Figure 1c). Quantification of this attenuation revealed significant changes in the ACC (p<10−10), amygdala (p<10−10), hippocampus (p<10−10), hypothalamus (p<0.003), OFC (p<10−10), and subgenual ACC (p<0.002) as evaluated from two-sample t-tests (Figure 1d).
DISCUSSION
This preliminary study demonstrated that hypoactivation of the hypothalamus, amygdala, hippocampus, OFC, ACC, and sgACC is associated with low parasympathetic control of the heart (HF-RRV) in MDD women. Additionally, our pilot data indicate that increased progesterone and lower estradiol in women with MDD are associated with parasympathetic dysregulation of the amygdala, hippocampus, ACC, and OFC, suggesting that activity in these regions is related to gonadal hormone dysfunction. Examining the confluence of these systems – regulation of mood in the brain, ANS control, and HPG-axis – in MDD is critical to defining the nature of the illness.
These findings are consistent with previous work investigating the neural control of the heart in emotional tasks [4–5, 29, 34, 36]. Prior studies have demonstrated similar activations in association with HF-RRV or heart rate reactivity in the hypothalamus [15], hippocampal formation [5], ACC [5, 36], and OFC [36]. Our findings implicating limbic brain regions (hippocampus, amygdala) in ANS regulation are not surprising given that they are critical in mood regulation. These regions also affect autonomic regulation through projections to the anterior hypothalamus and control of the hypothalamic-pituitary-adrenal (HPA-) and HPG-axis circuitries. Further, paralimbic regions, such as the OFC and ACC, also regulate autonomic tone, demonstrated in human [27] and animal [12] studies. The hippocampus is uniquely positioned to be involved in the convergence of dysfunction in these systems. With a high density of gonadal [31] and glucocorticoid [25] receptors, the hippocampus is a key region in the interaction between the HPG- and HPA-axes and response to stress. Similarly, both ACC and OFC provide inhibitory control of the HPA-axis during stress. Importantly, estradiol also has an inhibitory effect on the HPA-axis whereby lower estradiol, as in women with MDD, is associated with reduced ability to control the stress response.
The current preliminary findings are the first to localize specific brain regions associated with low parasympathetic control in women with MDD. Although our sample size was small, it is comparable to a number of other functional imaging studies on autonomic nervous system function [4, 29]. We also had a set of a priori ROIs and controlled for menstrual status (which significantly affects RRV [26]), thus increasing the power of our tests. Further, although using pulse oximetry to measure HF-RRV is not as sensitive or direct as acquisition via electrocardiogram, recent data support its use as a valid means of measuring HRV compared to ECG [24]. Also, our results are remarkably consistent with previous studies reporting activation in our ROIs, several of which are in the central autonomic network [23]. We were interested in group differences (MDD vs. HC) in HF-RRV effects and one would not expect a methods bias (i.e., PPG sensor) by group status. The reduced sensitivity introduced by a sampling rate of 100 Hz may have increased Type II error rate, which would result in a decreased estimation of the effect of HF-RRV on brain activity. Thus, the results likely represent an underestimation of the relationships of HF-RRV with our ROIs. Moreover, although four MDD women were taking antidepressants, exclusion of these subjects did not change the findings with the exception of loss of significance in the hypothalamus and sgACC (data not shown), suggesting medication status did not drive the associations between mood, stress, and ANS functioning in MDD.
It has been hypothesized that neurophysiological processes underlying stress-induced affective states will be reflected in static and dynamic characteristics of corresponding neuronal networks [32]. Functional connectivity analyses, involving investigation of the temporal correlation and causation of BOLD signals in these networks [7], would be of benefit in determining how brain activations interact across networks implicated in the stress response. Given that increased sympathetic activity and decreased vagal activity have been proposed as biomarkers of the severity of depression [14], clinical trials would be helpful in determining whether RRV control [33] may alleviate some depressive symptoms, and whether vagus nerve stimulation techniques might represent a potential adjunct to standard antidepressant therapy. Further, emerging treatment options in MDD, such as transcranial magnetic stimulation (TMS), have incorporated approaches which have effects on brain and heart [35], suggesting potential for effective treatment of co-occurring depressive and cardiovascular symptoms in individuals with MDD-CVD comorbidity. Interestingly, some studies report moderating effects of HPG-axis hormones on the efficacy of repetitive TMS for MDD [2]. Thus, findings may also facilitate the development of alternative non-psychopharmacological strategies for the treatment of symptoms of MDD.
This study provides preliminary evidence that hypoactivity of the stress response circuitry in MDD is significantly associated with dysregulation of the ANS, specifically parasympathetic cardiac control as measured by HF-RRV, and HPG-axis dysfunction. Although a larger study of MDD women and men is needed to replicate these findings, the results provide support for shared brain regions regulating mood, hormonal regulation, and cardiac control, suggesting possible pathways to understanding the comorbidity of MDD and CVD risk, for which women are at a significantly higher risk.
Supplementary Material
Highlights.
Decreased high-frequency RRV (HF-RRV) in women with major depression (MDD).
No differences between MDD and control women in heart rate or R-R intervals.
Stress response circuitry hypoactivity related to lower HF-RRV in women with MDD.
Gonadal hormones attenuated group differences in the HF-RRV effect on hypoactivity.
Abnormal brain-heart coupling may have implications for MDD-CVD comorbidity.
Acknowledgments
This work was supported by grants from the National Institute of Health to J.M.G.: ORWH-NIMH P50 MH082679 and pilot funds for fMRI scans from NIH NCRR-GCRC M01 RR02635 at Brigham and Women's Hospital's General Clinical Research Center. Additional funding for support of Dr. Lee’s effort is from the World Class University program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science and Technology (R31-10008) and Basic Science Research Program, NRF grant of Korea (2011-0004794). We thank Drs. Tamara Gersh, Seung-Schik Yoo, and Matthew Jerram for help in earlier phases of the study, Harlyn Aizley, M.Ed. for clinical interviewing, and Jo-Ann Donatelli, Ph.D. for her contributions to diagnostic review. We also appreciate the input of Stuart Tobet, Ph.D. and Robert Handa, Ph.D. (Co-PIs on ORWH-NIMH P50 MH082679) for their comments on earlier drafts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 2.Baeken C, De Raedt R, Vanderhasselt MA, Leyman L, Schiettecatte J, Poppe K, Anckaert E, Bossuyt A. A “hypersensitive” hypothalamic-pituitary-adrenal system could be indicative for a negative clinical high-frequency repetitive transcranial magnetic stimulation outcome in melancholic depressed patients. Brain Stimul. 2010;3:54–57. doi: 10.1016/j.brs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 5.Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friston K. Functional and effective connectivity inneuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- 8.Goldstein JM, Cherkerzian S, Buka SL, Fitzmaurice G, Hornig M, O'Toole S, Sloan RP. Sex-specific impact of maternal-fetal risk factors on depression and cardiovascular risk 40 years later. Journal of Developmental Origins of Health and Disease. 2011;2:353–364. doi: 10.1017/S2040174411000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 11.Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. 2006;26:379–384. doi: 10.1097/01.jcp.0000229483.52955.ec. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman BL, Rasmussen T. Stimulation studies of insular cortex of Macaca mulatta. J Neurophysiol. 1953;16:343–351. doi: 10.1152/jn.1953.16.4.343. [DOI] [PubMed] [Google Scholar]
- 13.Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131:379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Kuniecki M, Urbanik A, Sobiecka B, Kozub J, Binder M. Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiol Exp (Warsz) 2003;63:39–48. doi: 10.55782/ane-2003-1453. [DOI] [PubMed] [Google Scholar]
- 16.Kuo TB, Lai CT, Hsu FC, Tseng YJ, Li JY, Shieh KR, Tsai SC, Yang CC. Cardiac neural regulation oscillates with the estrous cycle in freely moving female rats: the role of endogenous estrogens. Endocrinology. 2010;151:2613–2621. doi: 10.1210/en.2009-1410. [DOI] [PubMed] [Google Scholar]
- 17.Laguna P, Moody GB, Mark RG. Power spectral density of unevenly sampled data by least-square analysis: performance and application to heart rate signals. IEEE Trans Biomed Eng. 1998;45:698–715. doi: 10.1109/10.678605. [DOI] [PubMed] [Google Scholar]
- 18.Lee BT, Seong Whi C, Hyung Soo K, Lee BC, Choi IG, Lyoo IK, Ham BJ. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuropsychopharmacol B ol Psychiatry. 2007;31:1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp Physiol. 2003;88:441–446. doi: 10.1113/eph8802535. [DOI] [PubMed] [Google Scholar]
- 20.Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- 21.Licht CM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ, DeRijk RH, Vogelzangs N, Zitman FG, de Geus EJ, Penninx BW. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 22.Lomb N. Least-squares frequency analysis of equally spaced data. Astrophys Space Sci Libr. 1976;39:447–462. [Google Scholar]
- 23.Lowey AD, Spyer KM. Central regulation of autonomic functions. Oxford University Press; New York: 1990. [Google Scholar]
- 24.Lu G, Yang F, Taylor JA, Stein JF. A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. J Med Eng Technol. 2009;33:634–641. doi: 10.3109/03091900903150998. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 26.McKinley PS, King AR, Shapiro PA, Slavov I, Fang Y, Chen IS, Jamner LD, Sloan RP. The impact of menstrual cycle phase on cardiac autonomic regulation. Psychophysiology. 2009;46:904–911. doi: 10.1111/j.1469-8986.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical ouput and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 28.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 29.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. NeuroImage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naqvi TZ, Naqvi SS, Merz CN. Gender differences in the link between depression and cardiovascular disease. Psychosom Med. 2005;67(Suppl 1):S15–18. doi: 10.1097/01.psy.0000164013.55453.05. [DOI] [PubMed] [Google Scholar]
- 31.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 33.Siepmann M, Aykac V, Unterdorfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback. 2008;33:195–201. doi: 10.1007/s10484-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 34.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Raju TR, Gangadhar BN. Modulation of cardiac autonomic functions in patients with major depression treated with repetitive transcranial magnetic stimulation. J Affect Disord. 2007;104:231–236. doi: 10.1016/j.jad.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.