Abstract
We show that fluorine NMR can be used to monitor the insertion and change in conformation of a 19F-labeled cell-penetrating peptide upon interacting with the cellular plasma membrane. α-Synuclein and a construct comprising a cell-penetrating peptide covalently attached to its N-terminus were studied. Important information about the interaction of the proteins with CHO-K1 cells was obtained by monitoring the diminution of 19F resonances of 3-fluoro-L-tyrosine labeled proteins. For α-synuclein, a decrease in the resonance from position 39 was observed indicating that only the N-terminal third region of the protein interacts with plasma membrane. However, when the fusion construct was incubated with the cells, a decrease in the resonance from the fusion peptide region was noted with no change in the resonances from α-synuclein region. Longer incubation, studied by using confocal fluorescence microscopy, revealed that the fusion construct translocates into the cells, but α-synuclein alone did not cross the membrane in significant amounts.
Keywords: cell-penetrating peptide, delivery system, eukaryotic cell, 19F NMR, α-synuclein
INTRODUCTION
Several groups have used nuclear magnetic resonance spectroscopy (NMR) to study the interaction of proteins and peptides with artificial membranes1 and lipid bilayers,2 but understanding the interaction, insertion, and conformational dynamics of proteins and peptides with native membranes remains challenging. The greatest barrier to delivery of proteins into cells is the plasma membrane. A series of delivery systems has been developed to overcome this problem. Cell-penetrating peptides cross the plasma membrane in an energy independent manner.3–5 These peptides can be covalently attached to the cargo,6 overexpressed as a fusion protein in bacteria,7, 8 or bound through noncovalent (charge-charge and/or hydrophobic) interactions.9 Several cell-penetrating peptides have been developed.7, 8 One of the most popular is the trans-acting activator of transcription (TAT) from the human immunodeficiency virus, which can deliver proteins,10 oligonucleotides,11, 12 liposomes,13 and nanoparticles.14 TAT targets the fused proteins to the nucleus.15 The N-terminal TAT-α-synuclein construct was delivered to astrocytes16 and PC1217 cells. Kim et al.7 designed a cytoplasmic transduction peptide (CTP) derived from TAT that has increased transduction potential and delivers biomolecules to the cytoplasm rather than the nucleus. Among the sequences tested, the eleven amino-acid version, YGR2AR6, proved most efficient in translocating β-galactosidase into several cell lines. After transduction, the peptide is cleaved by cytoplasmic enzymes, releasing the cargo.
Fluorine NMR (19F NMR) is highly sensitive to chemical environment. It is particularly useful for in-cell NMR because there is no background signal. The utility of 19F NMR for assessing biologically important interactions relies on the broadening of resonances from a small molecule when it is immobilized by binding to a large one. For instance, 19F resonances from a labeled protein like α-synuclein are broadened into the baseline when the protein, or part of it, is immobilized upon binding to cells, vesicles, or micelles.18–23 Under these conditions, the fraction bound can be easily quantified by measuring the decrease in area under each resonance. 19F NMR can also be used to determine which part of a protein interacts with the larger molecule because resonances from bound but internally mobile regions remain sharp.
19F NMR has been used to study protein-ligand interactions,18 fibril formation,19 protein interaction with lipid vesicles,20 and sodium dodecyl sulfate micelles.21 It has also been used to study the interaction of peptides with small unilamellar vesicles, bicelles22 and living cells23 as well as the physicochemical properties of antimicrobial peptides24 and the biological activity of different peptides.25, 26 Here, we used 19F NMR to study the interaction of wild-type α-synuclein and a CTP-α-synuclein fusion protein with the plasma membrane of Chinese hamster ovary (CHO-K1) cells.
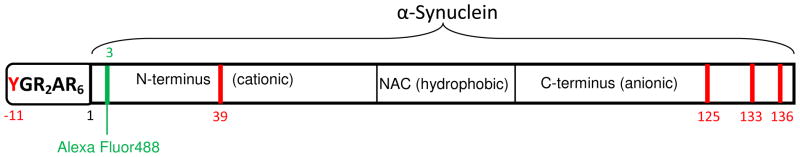
α-Synuclein is a 140-residue intrinsically-disordered protein comprising a positively-charged N-terminus, a hydrophobic middle region, and a negatively charged C-terminus (Figure 1). It is the main component of Lewy bodies found in the cytoplasm of neurons in the substantia nigra pars compacta of patients with Parkinson’s disease.27 The N-terminal region of α-synuclein forms an α-helix upon interaction with vesicles of different lipid composition.20
Figure 1.
Schematic representation of the cytoplasmic transduction peptide (CTP), YGR2AR6, covalently attached to the N-terminus of α-synuclein. Red indicates the positions of tyrosines. Green shows the position of the fluorescent label.
MATERIALS AND METHODS
Site-directed Mutagenesis
The CTP-α-synuclein construct was created in two steps with a Stratagene site-directed mutagenesis kit. First, YGR2A was inserted at the N-terminus of the wild-type α-synuclein gene by using the primers:
Forward 5′-3′: GCAGGAGATATACATATGTATGGCCGTCGTGCGGATGTATTCATGAAAGG
Reverse 5′-3′: CCTTTCATGAATACATCCGCACGACGGCCATACATATGTATATCTCCTGC
Second, the R6 fragment was inserted using the primers:
Forward 5′-3′: GTATGGCCGTCGTGCGCGTCGTCGTCGTCGTCGTGATGTATTCATGAAAGG
Reverse 5′-3′: CTTTCATGAATACATCACGACGACGACGACGACGCGCACGACGGCCATAC′
The insertions were confirmed with the sequencing primer, 5′-GGGAGACCACAACGGTTTCCCTCTAG-3′.
Expression and Purification of α-Synuclein Variants
V3C CTP-α-synuclein was expressed in Escherichia coli and purified as described by Ruf et al.28 The insertion of YGR2AR6 was confirmed by using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) of Lys C digested samples (Figure S1). 3-Fluoro-L-tyrosine labeled variants were expressed and purified as described by Li et al.19 Purified α-synuclein was lyophilized and stored at −80 °C.
Alexa Fluor Labeling
Twelve mg of V3C CTP-α-synuclein were dissolved in sterile degassed H2O to a final concentration of 2 mg/mL. Tris(2-carboxyethyl)phosphine and NaHCO3 were added in a ten fold molar excess over protein. The mixture was incubated at room temperature with shaking for 30 min. Next, Alexa Fluor 488 C5-maleimide (Invitrogen) was added in a ten fold molar excess over protein. The mixture was incubated at room temperature with shaking for 2 h. The labeled protein was purified by gel filtration chromatography on a Superdex 75 column with 20% acetonitrile in phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) as an eluent. The labeled protein was dialyzed against water, and the labeling efficiency was determined as described in the Alexa Fluor 488 C5-maleimide labeling protocol (Invitrogen). The absorbance at 494 nm along with the extinction coefficient of 71,000 M−1cm−1 for Alexa Fluor 488 was used to quantify the labeled α-synuclein. The Lowry method was used to quantify the total amount of protein (Lowry protein assay kit, Pierce). The labeling efficiency for V3C CTP-α-synuclein with the dye was 84%. Aliquots of 1 mg labeled protein were lyophilized and stored at −80 °C.
Cell Culture
CHO-K1 cells were obtained from the UNC Lineberger Cancer Center. The cells were seeded in 6-well glass plates (Corning Life Sciences) at a density of ~2 × 105 cells/well in F-12 media supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL) at 37 °C in 5% CO2.
Fluorine NMR
Spectra of 19F-labeled α-synuclein were acquired at 37 °C on a Varian Inova 600 MHz NMR spectrometer at a frequency of 564.5 MHz using a 5 mm triple-resonance probe (Varian 600 H-F(C,X)). Each spectrum comprised 2048 transients with a 1 s delay between transients. Each transient was acquired using a 60 kHz sweep width and an acquisition time of 1.9 s. The CHO-K1 cells were incubated with 19F-labeled CTP-α-synuclein in media at 37 °C. Aliquots were combined with equal volumes of Ficoll dissolved in cell media. The final sample contained 100 μM 19F CTP-α-synuclein and ~1.5 × 107 cells/mL in media with 20% (w/v) Ficoll and 10% D2O. The interaction of CHO-K1 cells with 19F-labeled wild-type and Y125F α-synucleins were studied under the same conditions. Cell viability was tested after each NMR experiment by using the trypan blue exclusion assay. The viability was always greater than 90%.
Fluorescence Image Acquisition
The cells were treated with fluorescently-labeled CTP-α-synuclein for 20 h. For imaging, four washing steps with phosphate buffered saline were performed after translocation. The cells were imaged in their complete media using a Zeiss confocal microscope equipped with LSM 5 software and a 40x oil objective. Mitochondria were stained with Mito Tracker Red CMX Ros (Invitrogen) immediately prior to imaging. The measurements were acquired with multichannel detection using 488 nm excitation (Ar laser) for Alexa Fluor 488 and 543 nm excitation (HeNe1 laser) for Mito Tracker Red. Untreated cells were tested under the same conditions and no autofluorescence was noted.
RESULTS AND DISCUSSION
CTP-α-Synuclein Expression, Purification and Labeling
The fused construct (Figure 1) was expressed in E. coli. The CTP-α-synuclein expression level is ~2/3 that of the wild-type protein under the same conditions. The purity was confirmed by SDS-PAGE, and the presence of the CTP was confirmed by matrix-assisted laser desorption/ionization mass spectroscopy analysis (Figure S1). V3C CTP-α-synuclein was labeled with Alexa Fluor 488 maleimide dye with labeling efficiencies of ~80%. The presence of a single fluorescent band at the appropriate molecular weight on the SDS-PAGE indicated purity.
Cell Suspensions for NMR Experiments
Studying the interaction of wild-type and CTP-α-synuclein with the plasma membrane by using NMR requires that the cells remain in suspension during data acquisition. CHO-K1 cells tend to settle quickly to the bottom of the NMR tube. This settling removes the bound α-synuclein from the NMR detection zone, degrading accuracy.
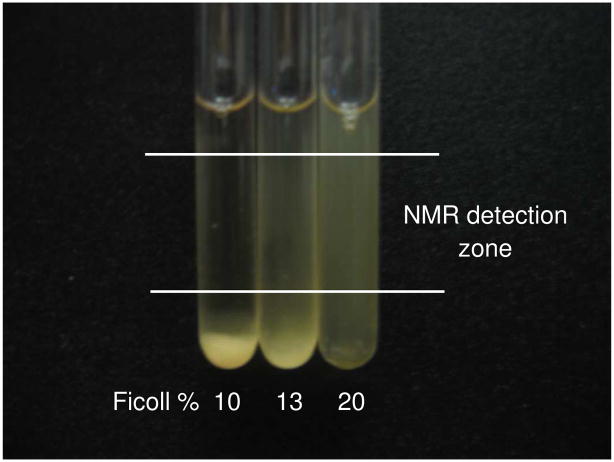
Several devices,29 gels,30 and natural biodegradable polymers31 have been used to facilitate suspension and prolong cells viability during NMR experiments. We overcame the settling problem by using a hydrophilic polysaccharide, Ficoll, and adjusting its concentration so as to keep the CHO-K1 cells suspended (Figure 2). After 3 h in the NMR tube, the cells settled in 10% (w/v) Ficoll and exhibited low viability. At 20% (w/v) Ficoll the cells remained suspended with a viability higher than 90%. Ficoll has also been used to prevent settling of Xenopus laevis oocytes during NMR data acquisition.32
Figure 2.
NMR tubes containing an initial uniform suspension of ~1.5 × 107 CHO-K1 cells/mL in F-12 media with 10% D2O and various (w/v) concentrations of Ficoll at 37 °C after 3 h. The cells viability was greater than 90% for 20% Ficoll.
Interaction of Wild-type and Y125F α-Synucleins with the Plasma Membrane
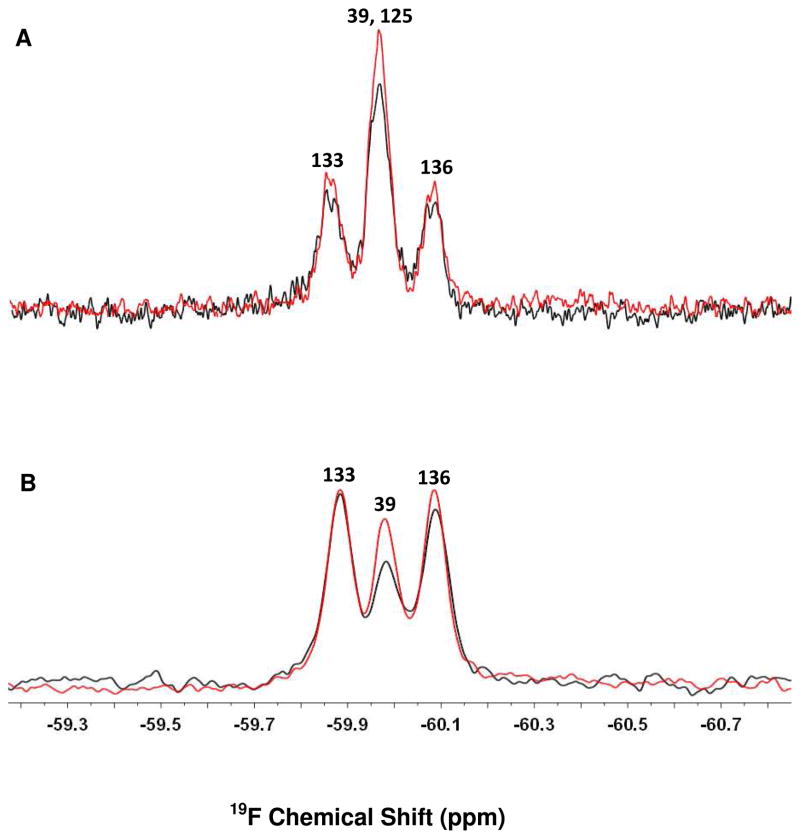
In the absence of cells, the middle peak in the spectrum of wild-type α-synuclein represents an overlap of the resonances from the 3-fluoro-tyrosines at positions 39 and 125.19 Upon incubating 19F-labeled wild-type α-synuclein with the cells a 35% decrease in the area under the middle peak was noted (Figure 3A), but the resonances from the 3-fluoro-tyrosines at positions 133 and 136 decreased by only 10% and 6%, respectively. The decrease could arise from increased heterogeneity, but several studies show that higher eukaryotic cells in the media do not dramatically interfere with the NMR spectra of intracellular proteins and extracellular peptides.8, 23, 33 Studies conducted in detergent micelles and synthetic lipid vesicles demonstrate that the decreases in peak intensities arise from binding, without interference from the heterogeneity of the environment.1, 20 Instead, we interpret the decrease in the middle peak as arising from the restricted motion experienced by that part of the protein interacting with the plasma membrane.
Figure 3.
19F-labeled α-synuclein spectra in the presence (black) and absence (red) of CHO-K1 cells. (A) wild-type α-synuclein (B) Y125F α-synuclein. The protein concentration was 100 μM. Residue assignments19 are shown above the resonances.
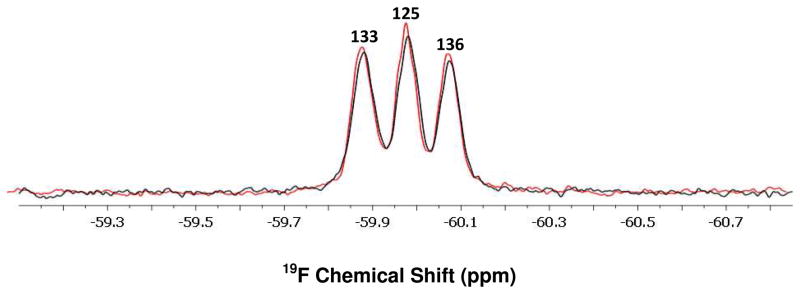
We used Y125F α-synuclein to determine which of the two resonances changed upon interaction with the cells. For this variant, the middle peak corresponds only to the resonance from position 39. A significant decrease in the resonance from position 39 was noted upon incubating Y125F α-synuclein with the cells, but the resonances from positions 133 and 136 remained almost unchanged (Figure 3B). Control experiments (Figure S2) show that the decrease observed in the resonance from position 39 is not caused by a change in pH or the presence of Ficoll. Furthermore, no significant change in peaks’ intensities was noted when Y39F α-synuclein was used (Figure 4). The slight decrease in the area under resonances from samples containing cells (Figures 3A and 4) arises from dilution by the cells themselves and by residual media remaining after collecting the cells. Based on our findings, it appears that the N-terminal region interacts with plasma membrane, while the C-terminal tail retains its motional freedom. Although high concentrations of polysaccharides (ca. 300–400 g/L) can induce protein aggregation34, this is not a concern for the work reported here because no changes in NMR spectra were observed for a control sample where the protein was stored for 12 h in 20% Ficoll (Figure S2).
Figure 4.
19F-labeled Y39F α-synuclein spectra in the presence (black) and absence (red) of CHO-K1 cells. Residue assignments19 are shown above the resonances.
These data suggest a model wherein at least the N-terminal third of α-synuclein, but not its C-terminal region, lie on or in the membrane surface. This observation is based on the fact that the Y39F resonance decreases, but the resonances from the C-terminal region remain nearly constant. Also, no significant translocation of the protein was noted when fluorescently-labeled V3C α-synuclein was used (data not shown) indicating that the bound protein does not cross the plasma membrane. The interaction of 19F-labeled α-synuclein with SDS micelles20 and LUVs35 has also been studied, and a similar binding pattern was noted. Our findings agree with NMR data on α-synuclein binding to small unilamellar vesicles where signal attenuation was noted for different segments of the protein with uniform, narrow widths for all resonances.1 Furthermore, changes in chemical shifts were absent and NOE experiments excluded contributions from fast exchange. Also, higher molecular weight biomolecules are not as prone to changes in chemical shift as are smaller ones, such as peptides.22
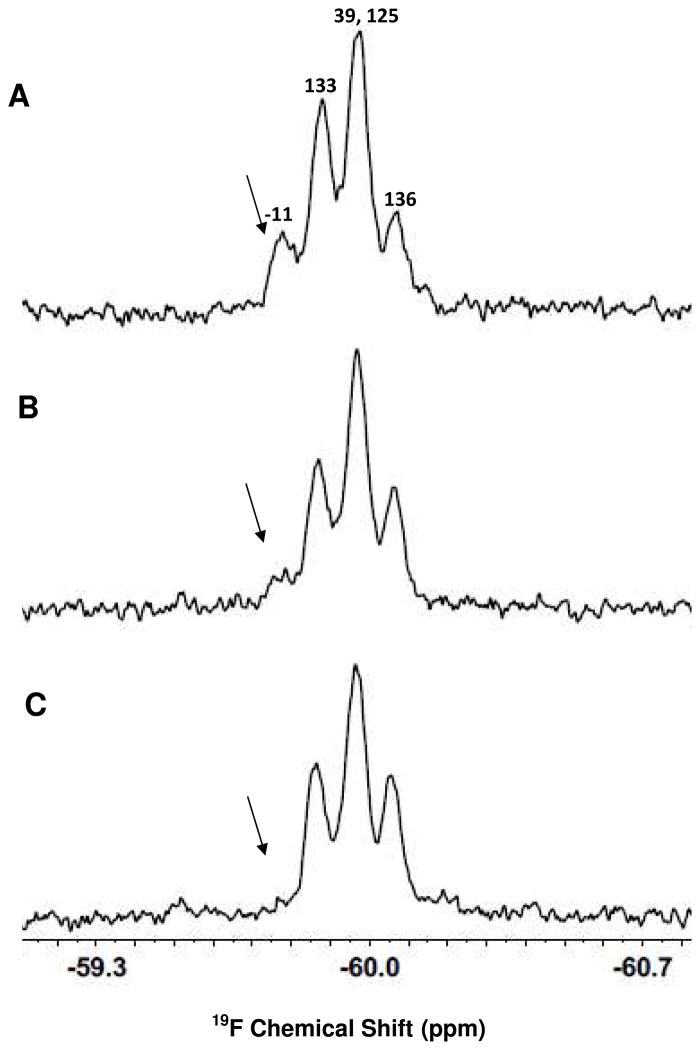
Interaction of CTP-α-Synuclein with Plasma Membrane
19F-labeled CTP-α-synuclein was used to study the early stage insertion of the CTP into the plasma membrane. CTP-α-synuclein has five tyrosines, one in the CTP at position -11, one at the position 39 in the N-terminal region, and three in the C-terminal region (Figure 1). Figure 5A shows the spectrum of the 19F-labeled fusion protein in cell media containing 20% (w/v) Ficoll and 10% D2O. A significant change was noted immediately after adding CHO-K1 cells (Figure 5B). After 1 h, the most downfield resonance, from the 3-fluoro-tyrosine at position -11, disappeared leaving a spectrum resembling that of wild-type α-synuclein (Figure 5C). The disappearance of this resonance is attributed to the interaction of the CTP in the fused construct with the plasma membrane. The resonance from 3-fluoro-tyrosine at position 133 partially overlaps the resonance from the 3-fluoro-tyrosine in the CTP. The CTP loses motional freedom upon interacting with the cell membrane, diminishing its intensity. The partial overlap makes it appear that the intensity of the resonance from 3-fluoro-tyrosine 133 also decreases. From these data we conclude that most of the fusion construct bound the cells in the first hour.
Figure 5.
Interaction of 19F-labeled CTP-α-synuclein with CHO-K1 cells. (A) CTP-α-synuclein in cells media containing 20% (w/v) Ficoll and 10% D2O (B) CTP-α-synuclein and CHO-K1 cells in the same media and (C) after 1 h incubation. The protein concentration was 100 μM. The arrow indicates the decrease in the resonance from Y-11.
The data for CTP-α-synuclein suggest a different type of binding compared with that of the wild-type α-synuclein. The CTP inserts into the lipid bilayer while the α-synuclein portion does not interact with plasma membrane after 1 h of incubation, as suggested by the observation that the resonance at position 39 is unaffected. To confirm that the fusion protein interacts with plasma membrane and to verify that CTP-α-synuclein can be translocating into the cells, we used a different approach.
Confocal Microscopy Studies of CTP-α-Synuclein Translocation
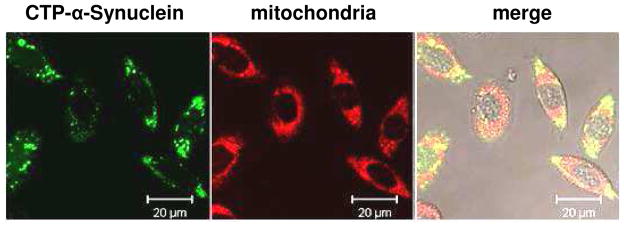
Translocation of CTP-α-synuclein into mammalian cells was assessed by incubating the fluorescently-labeled construct with CHO-K1 cells for 20 h (Figure 6). Mitochondrial staining confirmed localization of the protein to the cytoplasm with no fluorescence present in the nucleus. Some co-localization of α-synuclein and mitochondria was noted, in agreement with other studies.36–40 Control experiments using fluorescently-labeled α-synuclein under the same conditions showed no significant intracellular fluorescence. These observations indicate that α-synuclein does not cross the plasma membrane in significant amounts over this time period in the absence of CTP. These data support our models where, in the absence of CTP, the N-terminal region of α-synuclein is located on the plasma membrane, but CTP disrupts this structure as a result of its penetration into the lipid bilayer.
Figure 6.
CTP mediated delivery of fluorescently-labeled α-synuclein into CHO-K1 cells. From left to right: Alexa Fluor 488 fluorescence (green), Mito Tracker Red fluorescence (red), and merged images. No autofluorescence was noted.
CONCLUSIONS
Fluorine NMR, combined with fluorescence microscopy, provides information on the interaction of α-synuclein and CTP-α-synuclein with the plasma membrane of higher eukaryotic cells. This study also shows the utility of 19F NMR for investigating the interactions between proteins and the plasma membrane as well for monitoring the insertion of cell-penetrating peptides. The results advance our understanding of the interaction of proteins and peptides with the plasma membrane and indicate that 19F NMR will be a valuable tool for studying the translocation of other carriers.
Supplementary Material
Acknowledgments
We thank Dr. Marc ter Horst for NMR spectrometer maintenance, Dr. Michael Chua for assistance with confocal microscopy imaging, Alexander Krois for assistance with protein purification, and Elizabeth Pielak for critical evaluation of the manuscript. This work was supported by a NIH Director’s Pioneer Award (5DP1OD783) and a National Science Foundation grant (MCB-1051819) to G.J.P. and a Foundation for Aging Research GlaxoSmithKline Award to I.G.Z.
Footnotes
MALDI-MS mass spectra of CTP-α-synuclein and 19F spectra of Y125F α-synuclein at pH 6 and Y125F α-synuclein after 12 h of incubation in Ficoll. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bodner CR, Dobson CM, Bax A. Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J Mol Biol. 2009;390(4):775–90. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandasamy SK, Lee DK, Nanga RP, Xu J, Santos JS, Larson RG, Ramamoorthy A. Solid-state NMR and molecular dynamics simulations reveal the oligomeric ion-channels of TM2-GABA(A) stabilized by intermolecular hydrogen bonding. Biochim Biophys Acta. 2009;1788(3):686–95. doi: 10.1016/j.bbamem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Henriques ST, Quintas A, Bagatolli LA, Homble F, Castanho MA. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol Membr Biol. 2007;24(4):282–93. doi: 10.1080/09687860601142936. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. TAT peptide-modified liposomes for intracellular delivery of drugs and DNA. Cell Mol Biol Lett. 2002;7(2):265–7. [PubMed] [Google Scholar]
- 5.Lamaziere A, Wolf C, Lambert O, Chassaing G, Trugnan G, Ayala-Sanmartin J. The homeodomain derived peptide Penetratin induces curvature of fluid membrane domains. PLoS One. 2008;3(4):e1938. doi: 10.1371/journal.pone.0001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, Kahl U, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Hokfelt T, Bartfai T, Langel U. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998;16(9):857–61. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Jeon C, Kim JH, Kim MS, Yoon CH, Choi IS, Kim SH, Bae YS. Cytoplasmic transduction peptide (CTP): new approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res. 2006;312(8):1277–88. doi: 10.1016/j.yexcr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Inomata K, Ohno A, Tochio H, Isogai S, Tenno T, Nakase I, Takeuchi T, Futaki S, Ito Y, Hiroaki H, Shirakawa M. High-resolution multidimensional NMR spectroscopy of proteins in human cells. Nature. 2009;458(7234):106–9. doi: 10.1038/nature07839. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19(12):1173–6. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 10.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294(3):C842–55. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi A, Akuta T, Okuyama H, Senda T, Yokoi H, Inokuchi H, Fujita S, Hayakawa T, Takeda K, Hasegawa M, Nakanishi M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J Biol Chem. 2001;276(28):26204–10. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler A, Seelig J. High affinity of the cell-penetrating peptide HIV-1 Tat-PTD for DNA. Biochemistry. 2007;46(27):8138–45. doi: 10.1021/bi700416h. [DOI] [PubMed] [Google Scholar]
- 13.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA. 2001;98(15):8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry CC, de la Fuente JM, Mullin M, Chu SW, Curtis AS. Nuclear localization of HIV-1 TAT functionalized gold nanoparticles. IEEE Trans Nanobioscience. 2007;6(4):262–9. doi: 10.1109/tnb.2007.908973. [DOI] [PubMed] [Google Scholar]
- 15.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 16.Choi HS, Lee SH, Kim SY, An JJ, Hwang SI, Kim DW, Yoo KY, Won MH, Kang TC, Kwon HJ, Kang JH, Cho SW, Kwon OS, Choi JH, Park J, Eum WS, Choi SY. Transduced Tat-α-synuclein protects against oxidative stress in vitro and in vivo. J Biochem Mol Biol. 2006;39(3):253–62. doi: 10.5483/bmbrep.2006.39.3.253. [DOI] [PubMed] [Google Scholar]
- 17.Albani D, Peverelli E, Rametta R, Batelli S, Veschini L, Negro A, Forloni G. Protective effect of TAT-delivered α-synuclein: relevance of the C-terminal domain and involvement of HSP70. Faseb J. 2004;18(14):1713–5. doi: 10.1096/fj.04-1621fje. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Hajduk PJ, Mack J, Olejniczak ET. Structural studies of Bcl-xL/ligand complexes using 19F NMR. J Biomol NMR. 2006;34(4):221–7. doi: 10.1007/s10858-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Lutz EA, Slade KM, Ruf RA, Wang GF, Pielak GJ. 19F NMR studies of α-synuclein conformation and fibrillation. Biochemistry. 2009;48(36):8578–84. doi: 10.1021/bi900872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GF, Li C, Pielak GJ. 19F NMR studies of α-synuclein-membrane interactions. Protein Sci. 2010;19(9):1686–91. doi: 10.1002/pro.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GF, Li C, Pielak GJ. Probing the micelle-bound aggregation-prone state of α-synuclein with 19F NMR spectroscopy. Chembiochem. 2010;11(14):1993–6. doi: 10.1002/cbic.201000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buer BC, Chugh J, Al-Hashimi HM, Marsh EN. Using fluorine nuclear magnetic resonance to probe the interaction of membrane-active peptides with the lipid bilayer. Biochemistry. 2010;49(27):5760–5. doi: 10.1021/bi100605e. [DOI] [PubMed] [Google Scholar]
- 23.Okamura E, Ninomiya K, Futaki S, Nagai Y, Kimura T, Wakai C, Matubayasi N, Sugiura Y, Nakahara M. Real-time in-cell F-19 NMR study on uptake of fluorescent and nonfluorescent F-19-octaarginines into human Jurkat cells. Chemistry Letters. 2005;34(7):1064–1065. [Google Scholar]
- 24.Gottler LM, de la Salud Bea R, Shelburne CE, Ramamoorthy A, Marsh EN. Using fluorous amino acids to probe the effects of changing hydrophobicity on the physical and biological properties of the beta-hairpin antimicrobial peptide protegrin-1. Biochemistry. 2008;47(35):9243–50. doi: 10.1021/bi801045n. [DOI] [PubMed] [Google Scholar]
- 25.Gottler LM, Lee HY, Shelburne CE, Ramamoorthy A, Marsh EN. Using fluorous amino acids to modulate the biological activity of an antimicrobial peptide. Chembiochem. 2008;9(3):370–3. doi: 10.1002/cbic.200700643. [DOI] [PubMed] [Google Scholar]
- 26.Marsh EN, Buer BC, Ramamoorthy A. Fluorine--a new element in the design of membrane-active peptides. Mol Biosys. 2009;5(10):1143–7. doi: 10.1039/b909864j. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruf RA, Lutz EA, Zigoneanu IG, Pielak GJ. α-Synuclein conformation affects its tyrosine-dependent oxidative aggregation. Biochemistry. 2008;47(51):13604–9. doi: 10.1021/bi801884z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharaf NG, Barnes CO, Charlton LM, Young GB, Pielak GJ. A bioreactor for in-cell protein NMR. J Magn Reson. 2010;202(2):140–6. doi: 10.1016/j.jmr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu FY, Tsai SW, Wang FF, Wang YJ. The collagen-containing alginate/poly(L-lysine)/alginate microcapsules. Artif Cells Blood Substit Immobil Biotechnol. 2000;28(2):147–54. doi: 10.3109/10731190009118577. [DOI] [PubMed] [Google Scholar]
- 31.Ghidoni I, Chlapanidas T, Bucco M, Crovato F, Marazzi M, Vigo D, Torre ML, Faustini M. Alginate cell encapsulation: new advances in reproduction and cartilage regenerative medicine. Cytotechnology. 2008;58(1):49–56. doi: 10.1007/s10616-008-9161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodart JF, Wieruszeski JM, Amniai L, Leroy A, Landrieu I, Rousseau-Lescuyer A, Vilain JP, Lippens G. NMR observation of Tau in Xenopus oocytes. J Magn Reson. 2008;192(2):252–7. doi: 10.1016/j.jmr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Selenko P, Serber Z, Gadea B, Ruderman J, Wagner G. Quantitative NMR analysis of the protein G B1 domain in Xenopus laevis egg extracts and intact oocytes. Proc Natl Acad Sci USA. 2006;103(32):11904–9. doi: 10.1073/pnas.0604667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Berg B, Ellis RJ, Dobson CM. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 1999;18(24):6927–33. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zigoneanu IG, Yang YJ, Krois AS, Haque ME, Pielak GJ. Interaction of α-synuclein with vesicles that mimic mitochondrial membranes. Biochim Biophys Acta. 2012;1818(3):512–519. doi: 10.1016/j.bbamem.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol Cell Biol. 2005;25(22):10190–201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of α-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65(7–8):1272–84. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devi L, Anandatheerthavarada HK. Mitochondrial trafficking of APP and α-synuclein: Relevance to mitochondrial dysfunction in Alzheimer’s and Parkinson’s diseases. Biochim Biophys Acta. 2010;1802(1):11–9. doi: 10.1016/j.bbadis.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of α-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314(10):2076–89. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–33. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.