Abstract
Background
Percutaneous transluminal angioplasty +/− stent (PTA/S) and surgical bypass are both accepted treatments for claudication due to superficial femoral artery (SFA) occlusive disease. However, long-term results comparing these modalities for primary intervention in patients who have had no prior intervention has not been reported. We report our results with three year follow-up.
Methods
We reviewed all lower extremity bypass procedures at Beth Israel Deaconess Medical Center from 2001–2009 and all PTA/S performed from 2005 through 2009 for claudication. We excluded all limb salvage procedures and included only those that were undergoing their first intervention for claudication due to SFA disease. We recorded patient demographics, comorbidities, perioperative medications, TASC classification, and runoff. Outcomes included complications, restenosis, symptom recurrence, reinterventions, major amputation, and mortality.
Results
We identified 113 bypass grafts and 105 PTA/S of femoral-popliteal lesions without prior interventions. Bypasses were above the knee in 62% (45% vein) and below the knee in 38% (100% vein). Mean age was 63 (bypass) vs. 69 (PTA/S) (P<.01). Mean length of stay (LOS) was 3.9 vs. 1.2 days (P<.01). Bypass grafts were used less for TASC A (17% vs. 40%, P<.01), and more for TASC C (36% vs. 11%, P<.01) and TASC D (13% vs. 3%, P<.01) lesions. There were no differences in perioperative (2% vs. 0%, NS) or 3 year mortality (9 vs. 8%, NS). Wound infection was higher with bypass (16% vs. 0%, P<.01). None involved grafts. Bypass showed improved freedom from restenosis (73% vs. 42% - 3 years, HR 0.4, 95% CI 0.23–0.71), symptom recurrence (70% and 36% at 3 years, HR 0.37, 95% CI 0.2–0.56), and freedom from symptoms at last follow-up (83% vs. 49%, (HR 0.18, 95% CI 0.08–0.40). There was no difference in freedom from reintervention (77% vs. 66% at 3 years, NS). Multivariable analysis of all patients showed that restenosis was predicted by PTA/S (HR 2.5, 95% CI 1.4–4.4) and TASC D (HR 3.7, 95% CI 3.5–9) lesions. Recurrence of symptoms was similarly predicted by PTA/S (HR 3.0, 95% CI 1.8–5) and TASC D lesions (HR 3.1, 95% CI 1.4–7). Statin use postoperatively was predictive of patency (HR 0.6 95% CI 0.35–0.97) and freedom from recurrent symptoms (HR 0.6 95% CI 0.36–0.93).
Conclusions
Surgical bypass for the primary treatment of claudication showed improved freedom from restenosis and symptom relief despite treatment of more extensive disease, but was associated with increased LOS and wound infection. Statins improved freedom from restenosis and symptom recurrence overall.
Introduction
Claudication, commonly the initial symptom of lower extremity chronic arterial occlusive disease, affects 5% of men and women between the ages of 55–74 and has an increasing prevalence with age affecting up to 20% at age 70.1–3 Many patients’ symptoms will improve or remain stable without intervention.1–4 Surgical bypass, either with native vein or prosthetic material, had been the standard intervention for refractory patients and had been done with relatively low morbidity and with good results.5, 6 However, as endovascular interventions for peripheral vascular disease have become more common, percutaneous transluminal angioplasty +/− stent (PTA/S) has become increasingly used as an intervention for claudicants.7–17 In fact, at many institutions, it now has become the first-line intervention.8, 16 Comparisons between bypass grafts and PTA/S show that PTA/S has good results in regard to primary patency and symptom relief, however endovascular interventions have a higher recurrence of symptoms.9 Initially it was thought that advanced, TASC C-D, lesions should be reserved for bypass.16, 17 Recently these have been shown to be successfully treated endovascularly. 18, 20, 21
The durability of PTA/S in comparison to surgical bypass has previously been evaluated. However, many of these analyses also include patients with rest pain and tissue loss, with only a percentage of the patients being claudicants.8, 14 Studies looking specifically at claudicants do not specifically exclude those who have had prior ipsilateral interventions, nor do they stratify the lesions by TASC classification, exclude distal interventions, or include the perioperative medication profile in the analyses.7, 9–13
Our institution is a large referral center and university hospital with a high volume of patients with lower extremity vascular disease. Our objective is to examine our experience in treating patients with claudication by surgical bypass or endovascular intervention for primary treatment of lesions in the femoral and popliteal region comparing restenosis, our primary outcome, secondary restenosis, symptom relief, reinterventions, limb loss, and death.
Materials and Methods
We performed a retrospective cohort review of all patients undergoing interventions by the Division of Vascular and Endovascular Surgery at Beth Israel Deaconess Medical Center (BIDMC) with femoral-popliteal disease and claudication. We individually reviewed the records of all surgical bypasses from 2001–2009 and all endovascular interventions from 2005–2009. Inclusion criteria included a diagnosis of claudication, first time ipsilateral intervention, lesions in the femoral-popliteal region, and procedures performed by vascular surgeons. Patients with more distal interventions, rest pain, or tissue loss were excluded. Demographics, perioperative medications, Trans-Atlantic Inter-Society Consensus (TASC) II classification, runoff, comorbidities, perioperative complications, thirty-day and late survival, recurrent symptoms, reinterventions, and limb loss were recorded. The choice of intervention was surgeon dependent at the time and varied with acquisition of endovascular skills over time. Prosthetic grafts were not used for below knee popliteal bypass. The choice of PTA/S intervention varied by surgeon preference, however, in general we use primary angioplasty with selective stenting for residual stenosis >30% or flow limiting dissection. Routine statin use was introduced over time. We generally treat with clopidogrel for one month and aspirin indefinitely. Technical success for PTA/S was defined as less than 30% residual stenosis and no flow limiting dissection. For bypass, a patent graft at completion of OR case and no significant defect in vein on continuous wave doppler interrogation and angioscopy were required for technical success.
Follow-up is variable, but in general patients are seen three to four times per year for two years and then one to two times per year. Restenosis was evaluated by ultrasound or angiography with 81% of patients receiving post-operative ultrasounds.22 Duplex criteria for restenosis was 75% or >3.5 fold increase in peak systolic velocity. Intervention was performed for symptomatic graft restenosis and asymptomatic grafts that are threatened (peak systolic velocity ratio >3.5 – 4, low graft velocities <30cm/second). In general, we do not reintervene for asymptomatic restenosis post PTA alone, however we are more likely to re-intervene for an asymptomatic in-stent restenosis with peak systolic velocity ratio of >3.5–4. We are less likely to re-intervene for a symptomatic restenosis if the disease was extensive and restenosis was rapid, as we feel this has a low likelihood of deriving a durable benefit. Symptom recurrence and development of rest pain were determined by the attending surgeon at follow-up with correlation to ankle-brachial index values. All angiographic images were reviewed with lesions graded according to the TASC II classification system with run-off vessels’ patency recorded according to the Society for Vascular Surgery’s reporting standards.17 Categoric variables were analyzed by Pearson χ2 and the Fisher exact test. Median length of stay was compared using the Wilcoxon rank sum test. Treatment outcomes during the course of follow-up were analyzed using Kaplan-Meier methodology, and time-to-failure curves were compared with the log rank test. Univariate and multivariable Cox regression models were used to assess predictor variables for time-dependent outcomes. Statistical significance was defined as P < .05. All statistical tests were done using STATA 8 software (StataCorp, College Station, Tex).
Results
Patient Characteristics
There were a total of 113 bypass grafts and 105 PTA/S on 218 patients meeting our inclusion and exclusion criteria. The average age in the bypass group was 63, which was significantly younger than mean age of 69 in the PTA/S group (P<.01). Congestive heart failure (CHF) was more common in bypass patients (7% vs. 0% P<.01) while hypertension (HTN) was more common in PTA/S patients 66% vs. 78%). There were no other differences in comorbidities and patient characteristics between the two groups. Perioperative aspirin and clopidogrel were used less in those having a bypass than those undergoing PTA/S (77% vs. 90%, P<.01), (23% vs. 96%, P<.01). There was no significant difference in the use of warfarin or statins (4.4 vs. 5.7%, NS), (62% vs. 69%, NS). Bypass patients had a significantly higher rate of β-blocker use (75% vs. 55%, P<.01) (Table 1). Mean follow-up for bypass was an average of 25 months (median 31, range <1–91). For PTA/S, the mean follow-up was 18 months (median 15 months, range <1–49 months.
Table 1.
Demographics and Comorbidities
| Bypass (113 patients) | PTA/S (105 patients) | P value | |
|---|---|---|---|
| Age | 63 (+/− 11.2) | 69 (+/− 11.3) | <.01 |
| Males | 68% (77) | 63% (66) | NS |
| Coronary artery disease | 39% (44) | 41% (43) | NS |
| Dialysis | 0 (0) | 0 (0) | NS |
| Congestive heart failure | 7% (8) | 0 | <.01 |
| COPD | 12% (14) | 7% (7) | NS |
| Diabetes Mellitus | 36% (41) | 35% (37) | NS |
| Hypertension | 66% (75) | 78% (82) | <.05 |
| Previous Stroke | 8% (9) | 9% (9) | NS |
| Creatinine | 1 (+/−0.46) | 1.07 (+/−0.34) | NS |
| Renal Transplant | 1% (1) | 3% (3) | NS |
| Former tobacco | 36% (41) | 44% | NS |
| Current tobacco | 29% (33) | 22% (23) | NS |
| Hyperlipidemia | 59% (67) | 50% (52) | NS |
| Aspirin | 77% | 90% | <0.01 |
| Clopiogrel | 23% | 96% | <0.01 |
| Warfarin | 4% | 6% | NS |
| Statin | 62% | 69% | NS |
| β-blocker | 75% | 55% | <0.01 |
Patients treated with bypass had significantly fewer TASC A lesions (17% vs. 40%, P<.01) compared to the PTA/S group, while TASC B lesions were similar (34% vs. 47%) (Table 2). There were more TASC C (36% vs. 11%, P<.01) and TASC D (13% vs. 3%, P<.01) lesions treated by bypass. Patients undergoing a bypass were more likely to have disease free run-off vessels to the foot than patients undergoing PTA/S (40 vs. 27%, P<.01). There were no other differences in the run-off between the two groups (Table 3).
Table 2.
TASC II classification of lesions
| TASC II Class | Bypass (113 patients) | PTA/S (105 patients) | P value |
|---|---|---|---|
| A | 17% (19) | 40% (42) | <.01 |
| B | 34% (38) | 47% (49) | NS |
| C | 36% (40) | 10% (11) | <.01 |
| D | 13% (16) | 3% (3) | <.01 |
Table 3.
Lower Extremity Runoff
| Runoff | Bypass (112 patients) | PTA/S (105 patients) | P value |
|---|---|---|---|
| 0 vessel disease | 40% (45) | 27% (28) | <.05 |
| 1 vessel disease | 30% (34) | 35% (37) | NS |
| 2 vessel disease | 24% (27) | 27% (28) | NS |
| 3 vessel disease | 5% (6) | 11% (12) | NS |
Perioperative Outcomes
Technical success in the PTA/S and bypass groups was 100%. We had two patients in the bypass group, one TASC C and one TASC D, who had a previously failed attempt at endovascular intervention. There was a higher complication rate overall with surgical bypass compared to PTA/S, mostly due to wound infection (16% vs. 0%, P<.01). However, the majority (82%) were superficial wound infections that were treated successfully with a short course of antibiotics, with the remaining deeper infections (Szilagyi class II) not involving the grafts (Table 4). Wound infections were distributed to 71% vein bypasses and 29% prosthetic bypass. All involved veins were non-reversed that were harvested with a continuous incision. Our perioperative antibiotic regimen has changed over time from Cephazolin to Vancomycin and Gentamicin. Recently, after the scope of this study, we have switched to Cephazolin and Vancomycin. Patients receiving a bypass had a significantly longer length of stay (LOS) (3.9 vs. 1.2, P<.01). Two bypass patients died after discharge within thirty days, both of suspected myocardial infarctions. There were no perioperative deaths in the PTA/S group. Vein was used 73% of the time and prosthetic material 27% of the time in bypass patients. Sixty-two percent of the bypasses were above the knee and 38% were below the knee. Vein was used in 45% of above the knee bypasses and 100% below the knee bypasses. Fifty-seven percent on the patients in the PTA/S group were stented.
Table 4.
Outcomes/Complications
| Bypass | PTA/S | P value | |
|---|---|---|---|
| Length of stay (mean days) | 3.9 (2–11) | 1.2 (1–3) | <.01 |
| Acute myocardial infarction | 1% | 0 | NS |
| Pseudoaneurysm | 0 | 4% | NS |
| Wound infection | 16% | 0% | <.01 |
| Renal failure - >20% increase in creatinine | 3% | 3% | NS |
| Return to Operating Room | 3% | 0 | NS |
| Bleeding complication | 2% | 1% | NS |
| Hematoma | 0 | 2% | NS |
| Post-op occlusion | 2% | 0 | NS |
Long-Term Outcomes
Bypass had a higher freedom from restenosis when compared to PTA/S (73% vs. 42% at 3 years, HR 0.4, 95% CI 0.23–0.71) (Figure 1). Multivariable predictors of restenosis for all patients in the study included PTA/S intervention (HR 2.5, 95% CI 1.4–4.4), TASC D lesions (HR 3.7, 95% CI 3.5–9), while statin use was protective (HR 0.58, 95% CI 0.35–0.97). Three year secondary freedom from restenosis was 89% vs. 79% (NS) (Figure 2). When outcomes were stratified by TASC class C-D lesions had a higher freedom from of restenosis with bypass (HR 4.2, 95% CI 1.7–10.8, p<0.01), with no significant difference seen for TASC class A-B lesions. Freedom from complete occlusion was 81% for bypass vs. 85% for PTA/S at 3 years (NS).
Figure 1.
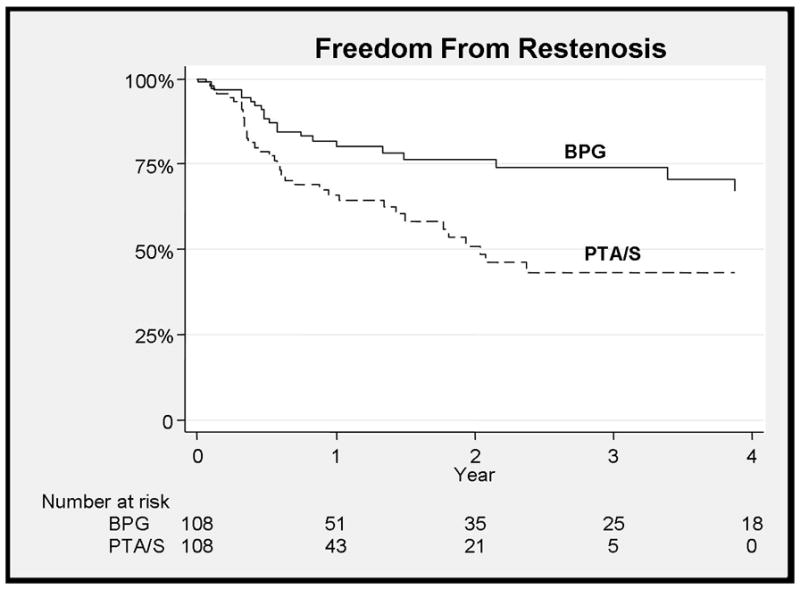
Improved freedom from restenosis with bypass (BPG) when compared to PTA/S (73% vs. 42% - 3 years, HR 0.4, 95% CI 0.23–0.71)
Figure 2.
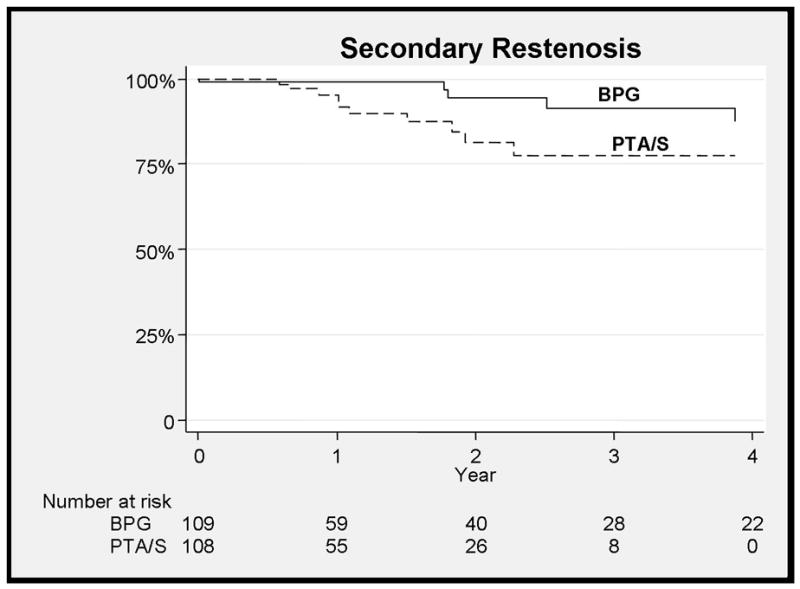
No difference between bypass and PTA/S for secondary restenosis at 3 years
Bypass patients were more likely to remain free from claudication symptom recurrence at three years (70% vs. 36% at 3 years, HR 0.37, 95% CI 0.2–0.56) (Figure 3) and more likely at have freedom from symptoms at last follow-up when compared to PTA/S (83% vs. 49% at 3 years, HR 0.18, 95% CI 0.08–0.40), taking into account subsequent interventions (Figure 4). Predictors of symptom recurrence mirrored the predictors of restenosis with PTA/S intervention (HR 3.0, 95% CI 1.8–5.0), TASC D lesions (HR 3.1, 95% CI 1.4–7), while statin use was again protective (HR 0.6, 95% CI 0.36–0.93). Ankle-brachial index (ABI) values preoperatively in patients undergoing bypass were 0.64 (+/− 0.15) and 0.69 (+/− 0.13) for PTA/S (NS). Most recent postoperative ABIs for those without symptom recurrence were 0.93 (+/−0.15) compared to 0.68 (+/−0.18) for patients with symptoms (P<.01). TASC class, C-D lesions had a higher freedom recurrence of symptoms (HR 3.9, 95% CI 1.5–10.0, p<0.01) with bypass compared to PTA/S as did TASC A-B lesions (HR 2.5, 1.3–4.7, p<0.01).
Figure 3.
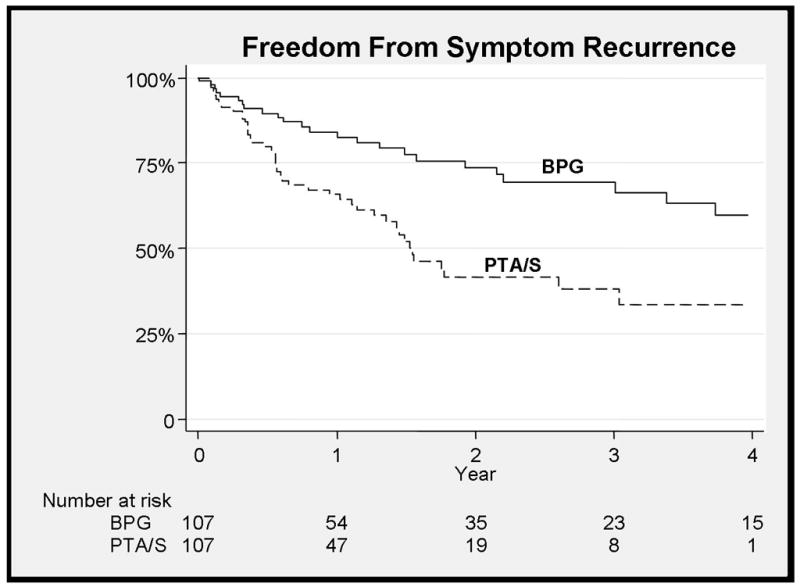
Improved freedom from recurrence of symptoms with bypass (70% and 36% at 3 years, HR 0.37, 95% CI 0.2–0.56)
Figure 4.
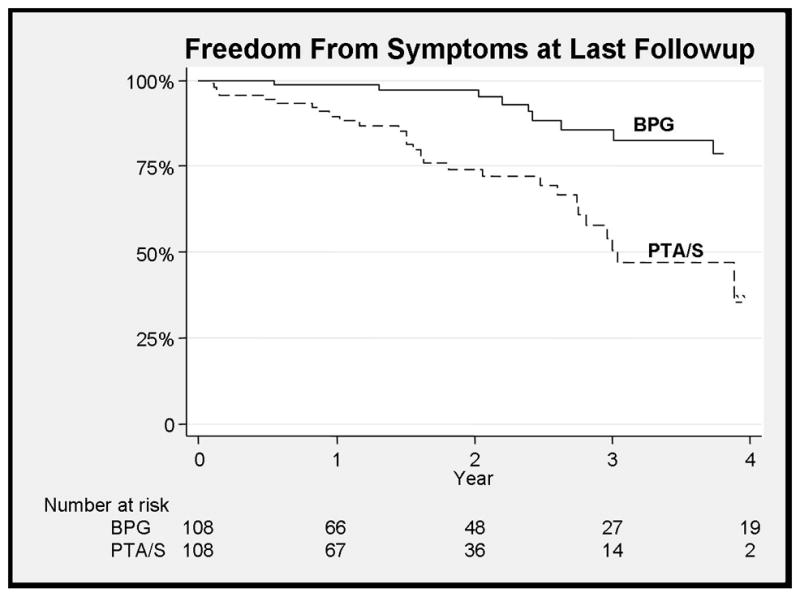
Improved freedom from symptoms at last follow-up with bypass
Freedom from rest pain at three years was 87% for bypass and 97% in the PTA/S (NS). Freedom from tissue loss at three years was 98% for bypass compared with 99% for PTA/S (NS). There were no major lower extremity amputations in either group. Freedom from reintervention was similar for bypass (77%) and PTA/S (66%) at 3 years (Figure 5). For the 21 bypass patients with reinterventions, there were 12 endovascular interventions of grafts, no endovascular interventions of native vessels, 12 redo bypasses, nine open revisions, five femoral to anterior tibial bypasses, one femoral to posterior tibial bypass, and one jump graft to an anterior tibial bypass. For the 21 PTA/S patients with reinterventions, there were 18 endovascular reinterventions, four above the knee popliteal bypasses, two below the knee popliteal bypasses, one femoral to posterior tibial bypass, one femoral to anterior tibial bypass, and two endovascular interventions of bypasses. No distal targets were altered postoperatively in PTA/S patients with subsequent below the knee bypass. Statin use, by multivariate analysis, was associated with freedom from reintervention, but did not reach statistical significance (HR 0.6, 95% CI 0.29–1.03). Freedom from bypass at three years for PTA/S patients was 87%. For PTA/S patients that subsequently underwent bypass, half were TASC B and the remaining 50% were evenly divided between TASC A and TASC C. Rest pain developed in 13% and none developed tissue loss. The remainder had bypass for persistent claudication.
Figure 5.
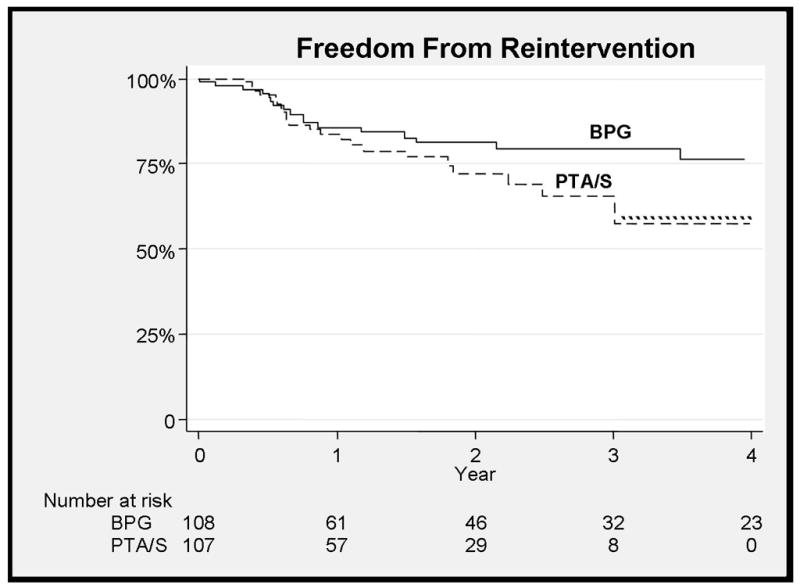
There is no difference between reinterventions between the two treatments at 3 years
There were no differences in any of the primary outcomes between vein versus prosthetic graft or above versus below the knee bypass. There was also no difference in outcomes between those that were stented and those that were not.
Long-term survival (9% vs. 8% - 3 years, NS) was not significantly different for bypass vs. PTA/S (Figure 6). Multivariable predictors of mortality at three years include chronic obstructive pulmonary disease (COPD) (HR 3.0, 95% CI 1.1–8%), coronary artery disease (CAD) (HR 2.3, 95% CI 1–5.2%), and diseased three-vessel runoff (HR 3.7, 95% CI 1.1–11.9).
Figure 6.
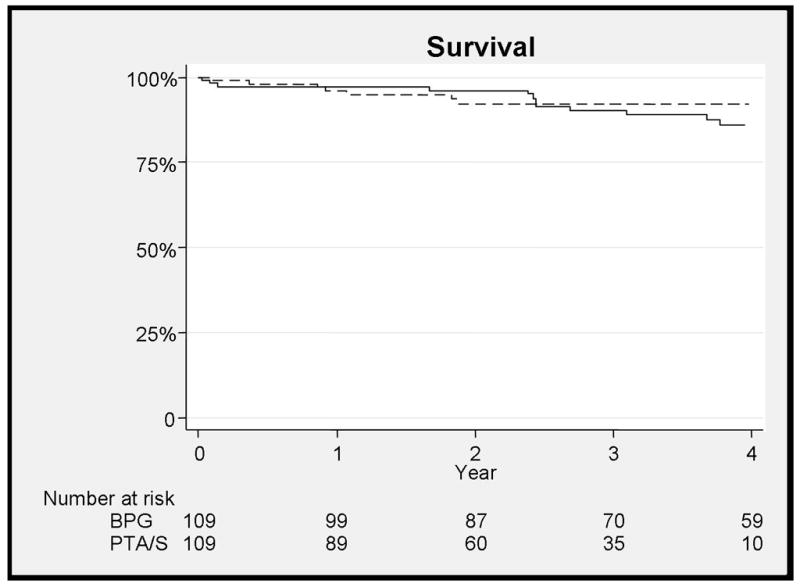
There is no difference in mortality between bypass and PTA/S at 3 years
Discussion
This is the largest study comparing first-time interventions for claudicants. Those who had PTA/S had a significantly decreased length of stay (3.9 vs. 1.2 days) and an overall lower perioperative complication rate. However, most of these complications are attributed to superficial wound infections, the majority of which were adequately treated with a course of antibiotics. Patients who had PTA/S had significantly higher rates of restenosis and recurrent symptoms. Surprisingly, this did not result in more reinterventions as had been seen previously.9 This may be due to the higher likelihood of our group reintervening on a graft to maintain patency with a restenosis or a recurrence of symptoms and a more conservative attitude toward multiple percutaneous re-interventions after early restenosis. For the lesions with more extensive disease with restenosis, there was thought to be a low likelihood of long-term success.
The younger average age of bypass patients may be attributed to selection bias since the less invasive procedure may have been offered to an older patient with claudication who previously would have had conservative medical treatment. Not surprisingly, the patients in the PTA/S group had more TASC A lesions and fewer TASC C-D lesions. PTA/S has been increasingly used with favorable results and is being seen as a more viable option for TASC C-D lesions.18, 20, 21 However, in our study bypass was superior to PTA/S for restenosis and recurrence of symptoms for TASC C-D lesions. TASC D lesions were an independent risk factor for restenosis and recurrence of symptoms overall. Bypass failure of TASC D lesions may represent more aggressive atherosclerotic disease in these patients and is consistent with our previous data in critical limb ischemia patients showing a higher failure rate in TASC D patients.23 The need for subsequent bypass after PTA/S may pose a long-term challenge as distal targets can be altered from percutaneous interventions, although we did not see this in our study.24 There have been two randomized control trials comparing Viabahn stents and prosthetic bypass with mixed results in patients that including both claudicants and those with critical limb ischemia. We do not think that the cost and need for larger sheath size mandates their use in our population with the limited data available.25, 26 Other comparisons between bypass and endovascular intervention have not specified first ipsilateral intervention, include those with chronic limb ischemia, do not comment on TASC II classification, and do not include perioperative medications.7, 9, 10–12
Statins have been increasingly used in cardiovascular disease. They are thought to block inflammation and have a beneficial effect independent of Low-Density Lipoprotein (LDL) cholesterol reduction, halt atherosclerosis progression, stabilize plaques, and improve endothelial function.27, 28 We saw a beneficial effect of statin use in our population. Statin use was a positive predictive factor of both graft patency and symptom relief, while also trending towards significance for freedom from reintervention. Henke et al and Abruzzese et al have shown that their lower extremity bypass patients, the majority of which were done for limb threat, benefited by statin use in regard to graft patency and limb salvage.29,30 Statin use also been shown to reduce mortality after bypass for critical limb ischemia, however we did not see this in our population.31 Statins alone have been shown to improve walking distances and symptoms in claudication.32, 33 There was no significant difference in statin use between our two groups, so this did not factor into one population having an advantage over the other. Our results reinforce the importance of statins in cardiovascular disease and all patients undergoing an intervention for claudication should be on a statin if possible.
There are limitations to this study. It was a retrospective single center review with a small group of patients. Patients were allocated to treatment based on surgeon preference and which has changed over time. However, it is the largest reported analysis of initial treatment of claudicants comparing surgical bypass and PTA/S.
Conclusions
Our results show that, despite the national trend towards endovascular intervention as the first line treatment for claudication, that surgical bypass remains an effective and durable option for patients with a low post-operative morbidity and mortality. Statin use was beneficial in both our treatment arms and their use should be standard in all patients being assessed for claudication. Future work focusing on costs and cumulative morbidity of primary and subsequent interventions, particularly multiple sequential vascular procedures or bypass after PTA/S needs to be done.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schermerhorn ML, Cronenwett JL, Baldwin JC. Open surgical repair versus endovascular therapy for chronic lower-extremity occlusive disease. Annu Rev Med. 2003;54:269–83. doi: 10.1146/annurev.med.54.101601.152509. Epub 2001 Dec 3. Review. [DOI] [PubMed] [Google Scholar]
- 2.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002 Dec 12;347(24):1941–51. doi: 10.1056/NEJMra021135. Review. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). TASC II Working Group. J Vasc Surg. 2007 Jan;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaï SP, Hendriks EJ, Prins MH, Teijink JA EXITPAD study group. Optimizing supervised exercise therapy for patients with intermittent claudication. J Vasc Surg. 2010 Nov;52(5):1226–33. doi: 10.1016/j.jvs.2010.06.106. Epub 2010 Aug 8. [DOI] [PubMed] [Google Scholar]
- 5.Byrne J, Darling RC, 3rd, Chang BB, Paty PS, Kreienberg PB, Lloyd WE, Leather RP, Shah DM. Infrainguinal arterial reconstruction for claudication: is it worth the risk? An analysis of 409 procedures. J Vasc Surg. 1999 Feb;29(2):259–67. doi: 10.1016/s0741-5214(99)70379-4. discussion 267–9. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka K, Esato K, Zempo N, Katoh T, Fujimura Y, Yoshimura K. Arterial reconstruction: justified for patients with intermittent claudication? World J Surg. 1998 Oct;22(10):1039–42. doi: 10.1007/s002689900513. [DOI] [PubMed] [Google Scholar]
- 7.Jämsén TS, Manninen HI, Tulla HE, Jaakkola PA, Matsi PJ. Infrainguinal revascularization because of claudication: total long-term outcome of endovascular and surgical treatment. J Vasc Surg. 2003 Apr;37(4):808–15. doi: 10.1067/mva.2003.148. [DOI] [PubMed] [Google Scholar]
- 8.Scott EC, Biuckians A, Light RE, Scibelli CD, Milner TP, Meier GH, 3rd, Panneton JM. Subintimal angioplasty for the treatment of claudication and critical limb ischemia: 3-year results. J Vasc Surg. 2007 Nov;46(5):959–64. doi: 10.1016/j.jvs.2007.06.031. Epub 2007 Oct 1. [DOI] [PubMed] [Google Scholar]
- 9.Taylor SM, Kalbaugh CA, Healy MG, Cass AL, Gray BH, Langan EM, 3rd, Cull DL, Carsten CG, 3rd, York JW, Snyder BA, Youkey JR. Do current outcomes justify more liberal use of revascularization for vasculogenic claudication? A single center experience of 1,000 consecutively treated limbs. J Am Coll Surg. 2008 May;206(5):1053–62. doi: 10.1016/j.jamcollsurg.2007.12.033. discussion 1062–4. Epub 2008 Mar 17. [DOI] [PubMed] [Google Scholar]
- 10.van der Zaag ES, Legemate DA, Prins MH, Reekers JA, Jacobs MJ. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. European Journal of Vascular and Endovascular Surgery. 2004;28(2):132–7. doi: 10.1016/j.ejvs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Holm J, Arfvidsson B, Jivegard L, Lundgren F, Lundholm K, Schersten T, et al. Chronic lower limb ischaemia. A prospective randomised controlled study comparing the 1-year results of vascular surgery and percutaneous transluminal angioplasty (PTA) European Journal of Vascular Surgery. 1991;5(5):517–22. doi: 10.1016/s0950-821x(05)80338-x. [DOI] [PubMed] [Google Scholar]
- 12.Wolf GL, Wilson SE, Cross AP, Deupree RH, Stason WB. Surgery or balloon angioplasty for peripheral vascular disease: a randomised clinical trial. Journal of Vascular and Interventional Radiology. 1993;4(5):639–48. doi: 10.1016/s1051-0443(93)71939-9. [DOI] [PubMed] [Google Scholar]
- 13.Kalbaugh CA, Taylor SM, Blackhurst DW, Dellinger MB, Trent EA, Youkey JR. One-year prospective quality-of-life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg. 2006 Aug;44(2):296–302. doi: 10.1016/j.jvs.2006.04.045. discussion 302–3. Epub 2006 Jul 11. [DOI] [PubMed] [Google Scholar]
- 14.DeRubertis BG, Faries PL, McKinsey JF, Chaer RA, Pierce M, Karwowski J, Weinberg A, Nowygrod R, Morrissey NJ, Bush HL, Kent KC. Shifting paradigms in the treatment of lower extremity vascular disease: a report of 1000 percutaneous interventions. Ann Surg. 2007 Sep;246(3):415–22. doi: 10.1097/SLA.0b013e31814699a2. discussion 422–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsi PJ, Manninen HI, Söder HK, Mustonen P, Kouri J. Percutaneous transluminal angioplasty in femoral artery occlusions: primary and long-term results in 107 claudicant patients using femoral and popliteal catheterization techniques. Clin Radiol. 1995 Apr;50(4):237–44. doi: 10.1016/s0009-9260(05)83478-6. [DOI] [PubMed] [Google Scholar]
- 16.Dormandy JA, Rutherford RB Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000 Jan;31(1 Pt 2):S1–S296. Review. [PubMed] [Google Scholar]
- 17.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007 Jan;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Conrad MF, Cambria RP, Stone DH, Brewster DC, Kwolek CJ, Watkins MT, Chung TK, LaMuraglia GM. Intermediate results of percutaneous endovascular therapy of femoropopliteal occlusive disease: a contemporary series. J Vasc Surg. 2006 Oct;44(4):762–9. doi: 10.1016/j.jvs.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 19.de Vries SO, Visser K, de Vries JA, Wong JB, Donaldson MC, Hunink MG. Intermittent claudication: cost-effectiveness of revascularization versus exercise therapy. Radiology. 2002 Jan;222(1):25–36. doi: 10.1148/radiol.2221001743. [DOI] [PubMed] [Google Scholar]
- 20.Rabellino M, Zander T, Baldi S, Garcia Nielsen L, Aragon-Sanchez FJ, Zerolo I, Llorens R, Maynar M. Clinical follow-up in endovascular treatment for TASC C-D lesions in femoro-popliteal segment. Catheter Cariovasc Interv. 2009 Apr 1;73(5):701–5. doi: 10.1002/ccd.21971. [DOI] [PubMed] [Google Scholar]
- 21.Baril DT, Chaer RA, Rhee RY, Makaroun MS, Marone LK. Endovascular interventions for TASC II D femoropopliteal lesions. Vasc Surg. 2010 Jun;51(6):1406–12. doi: 10.1016/j.jvs.2010.01.062. Epub 2010 Apr 10. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997 Sep;26(3):517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 23.Giles KA, Pomposelli FB, Spence TL, Hamdan AD, Blattman SB, Panossian H, Schermerhorn ML. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008 Jul;48(1):128–36. doi: 10.1016/j.jvs.2008.02.027. Epub 2008 May 23. [DOI] [PubMed] [Google Scholar]
- 24.Joels CS, York JW, Kalbaugh CA, Cull DL, Langan EM, 3rd, Taylor SM. Surgical implications of early failed endovascular intervention of the superficial femoral artery. J Vasc Surg. 2008 Mar;47(3):562–5. doi: 10.1016/j.jvs.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Lepäntalo M, Laurila K, Roth WD, Rossi P, Lavonen J, Mäkinen K, Manninen H, Romsi P, Perälä J, Bergqvist D Scandinavian Thrupass Study Group. PTFE bypass or thrupass for superficial femoral artery occlusion? A randomised controlled trial. Eur J Vasc Endovasc Surg. 2009 May;37(5):578–84. doi: 10.1016/j.ejvs.2009.01.003. Epub 2009 Feb 20. [DOI] [PubMed] [Google Scholar]
- 26.McQuade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg. 2010 Sep;52(3):584–90. doi: 10.1016/j.jvs.2010.03.071. discussion 590–1, 591.e1–591.e7. [DOI] [PubMed] [Google Scholar]
- 27.Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH. Simvastatin Lowers C-Reactive Protein Within 14 Days An Effect Independent of Low-Density Lipoprotein Cholesterol Reduction. Circulation. 2002 Sep 17;106(12):1447–52. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 28.Otsuka F, Hibi K, Kusama I, Endo M, Kosuge M, Iwahashi N, Okuda J, Tsukahara K, Ebina T, Kojima S, Sugiyama S, Ogawa H, Umemura S, Kimura K. Impact of statin pretreatment on the incidence of plaque rupture in ST-elevation acute myocardial infarction. Atherosclerosis. 2010 Dec;213(2):505–11. doi: 10.1016/j.atherosclerosis.2010.09.005. Epub 2010 Sep 21. [DOI] [PubMed] [Google Scholar]
- 29.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, Upchurch GR, Jr, Stanley JC, Eagle KA. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg. 2004 Feb;39(2):357–65. doi: 10.1016/j.jvs.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, Conte MS. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004 Jun;39(6):1178–85. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008 Apr;47(4):774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 32.Momsen AH, Jensen MB, Norager CB, Madsen MR, Vestersgaard-Andersen T, Lindholt JS. Drug Therapy for Improving Walking Distance in Intermittent Claudication: A Systematic Review and Meta-analysis of Robust Randomised Controlled Studies. Eur J Vasc Endovasc Surg. 2009 Oct;38(4):463–74. doi: 10.1016/j.ejvs.2009.06.002. Epub 2009 Jul 7. [DOI] [PubMed] [Google Scholar]
- 33.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003 Sep 23;108(12):1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. Epub 2003 Sep 2. [DOI] [PubMed] [Google Scholar]


