Abstract
Background
Increasing the threshold to define a positive D-dimer could reduce unnecessary computed tomographic pulmonary angiography (CTPA) for suspected PE but might increase rates of missed PE and missed pneumonia, the most common nonthromboembolic diagnosis seen on CTPA.
Objective
Measure the effect of doubling the standard D-dimer threshold for “PE unlikely” Revised Geneva (RGS) or Wells’ scores on the exclusion rate, frequency and size of missed PE and missed pneumonia.
Methods
Patients evaluated for suspected PE with 64-channel CTPA were prospectively enrolled from EDs and inpatient units of four hospitals. Pretest probability data were collected in real time and the D-dimer was measured in a central laboratory. Criterion standard was CPTA interpretation by two independent radiologists combined with clinical outcome at 30 days.
Results
Of 678 patients enrolled, 126 (19%) were PE+ and 93 (14%) had pneumonia. Use of either Wells≤4 or RGS≤6 produced similar results. For example, with RGS≤6 and standard threshold (<500 ng/mL), D-dimer was negative in 110/678 (16%), and 4/110 were PE+ (posterior probability 3.8%), and 9/110 (8.2%) had pneumonia. With RGS≤6 and a threshold <1000 ng/mL, D-dimer was negative in 208/678 (31%) and 11/208 (5.3%) were PE+, but 10/11 missed PEs were subsegmental, and none had concomitant DVT. Pneumonia was found in 12/208 (5.4%) with RGS≤6 and D-dimer<1000 ng/mL.
Conclusions
Doubling the threshold for a positive D-dimer with a PE unlikely pretest probability could reduce CTPA scanning with a slightly increased risk of missed isolated subsegmental PE, and no increase in rate of missed pneumonia.
Keywords: Fibrin fragment D, venous thromboembolism, medical decision making, tomography, spiral computed
INTRODUCTION
In the past decade, the D-dimer has become an integral part of the evaluation of patients with suspected acute pulmonary embolism (PE). Published clinical guidelines recommend that PE can be safely excluded with the combination of a normal quantitative D-dimer value and a low pretest probability assessment. 1;2 The first quantitative D-dimer assay to receive marketing clearance by the Food and Drug Administration for this indication used a threshold of 500 ng/mL (VIDAS, Biomérieux, Durham, NC). In conjunction with a low pretest probability this 500 ng/mL threshold produces nearly 100% diagnostic sensitivity for detection of acute PE, and thus has provided the benchmark for subsequent commercial D-dimer assays.3 Simultaneously, in the past decade, multidetector-row computerized tomographic pulmonary angiography (CTPA) has become the imaging mainstay in the diagnosis and exclusion of acute pulmonary embolism. The CTPA can also disclose clinically important alternative diagnoses, most often pulmonary infiltrates suggestive of pneumonia. 4;5 However, CTPA can cause direct and indirect adverse effects. Two direct adverse effects of CTPA are increased lifetime risk of cancer from radiation exposure and risk of contrast nephropathy. 6–9 An indirect effect of CPTA stems from the increasing resolution afforded by 64-channel CT equipment, which has increased the frequency of PE diagnosis based upon an isolated subsegmental filling defect—a finding that definitely causes clinical uncertainty, and in the absence of concomitant DVT, may lead to overtreatment. 9–14 Additionally, at one US hospital, almost 40% of patients who had one CTPA , underwent a second CTPA that was negative for PE within the next few years. 15 These concerns prompted the National Quality Forum to issue an imaging efficiency measure (NQF #IEP-005-10) intended to reduce CTPA usage in low-risk and D-dimer negative patients.
Judicious use of D-dimer-based screening can decrease unnecessary CTPA usage, but some clinicians may be reticent to use D-dimer in patients with risk factors for a false positive D-dimer, including age >70, active malignancy, or pregnancy.16–18 Several authors have proposed increasing the D-dimer threshold based upon the pretest probability, or the patient’s age, or comorbidity.19–22 The central hypothesis of this work stems from the concept that one D-dimer threshold does not fit all patients equally, and that the threshold for abnormal can be safely increased under two circumstances: 1. In patients who have a condition that predict an elevated D-dimer concentration even in the absence of PE, or 2. Those with a low enough pretest probability. Increasing the D-dimer threshold would likely increase the exclusion rate, but potentially at the cost of increasing the false negative rate. No previous study has enrolled a large, prospective, multicenter sample of inpatients and outpatients who have all undergone CTPA testing, standardized quantitative D-dimer testing, and with methodology to determine how many of these false negative cases are isolated subsegmental filling defects Moreover, a sensible study of D-dimer should also acknowledge the potential clinical significance of alternative diagnoses revealed by CTPA, most often infiltrate suggesting pneumonia.4;5;23–26 The primary goal of this project was to measure the change in the frequency of PE exclusion, and the coincident change in the frequency and size of missed PE, and the posterior probability of pneumonia produced when the D-dimer threshold is doubled for the subset of patients aged ≥70 years or for patients with a PE unlikely pretest probability.
METHODS
Study Design
This was a four center, prospective, noninterventional study of diagnostic accuracy, conducted in accordance with the guidelines set out by the STARD criteria.27
Study Setting and Population
Prospective enrollment occurred in the emergency department, inpatient wards, intensive care units and radiology suites of 4 academic medical centers in the US (Carolinas Medical Center, Charlotte, NC; Northwestern Memorial Hosptial, Chicago, IL; Wake Forest University, Baptist Hospital, Winston-Salem, NC; Baystate Hospital, Springfield, MA). The study protocol was registered on clinicaltrials.gov prior to enrollment (NCT00368836). Experienced research coordinators initiated the screening process based upon discovery of an order entry for a CTPA from anywhere within the hospital, 6 days per week, 12 hours per day. Based upon preliminary work that showed CTPA scans were ordered in equal proportions for inpatients and ED patients, the screening process was designed to produce equal proportions of enrolled inpatients and outpatients. Inclusion criteria required that CTPA was ordered, and to further restrict the population to those patients with one of 15 signs or symptoms of PE and one of 21 known risk factors for PE (Table 1).10 All patients provided written informed consent. Patients were excluded if they were unlikely to provide follow-up (imprisonment, homelessness, no telephone, history of noncompliance) or if they were hemodynamically unstable, intubated, had fibrinolytic treatment within 48 hours, had PE diagnosed within the last 6 months and were currently receiving systemic anticoagulation, or had known active tuberculosis.
Table 1.
Inclusion criteria for signs, symptoms, and risk factors for PE
| Signs or symptoms for PE (at least one required for inclusion) | |
|---|---|
| New onset dyspnea (58%*) | Pulse ≥ 90 beats/minute (34%) |
| Dyspnea worse than baseline (16%) | Dizziness (19%) |
| Pleuritic chest pain (39%) | Confusion/Altered mental status (4%) |
| Upper abdominal pain (9%) | Respiratory rate > 20 breaths/minute |
| Upper back pain (12%) | Cough (38%) |
| Syncope (3%) | Patient or clinician observation of unilateral limb swelling (9%) |
| Near Syncope (4%) | Any pulse oximetry reading < 95% (13%) |
| Hemoptysis (3%) | |
| Risk Factors for PE (at least one required for inclusion) | |
| Age >49 years (67%) | Chronic neuromuscular disease with immobility (<1%) |
| Surgery (within previous 4 weeks requiring general endotracheal anesthesia) (16%) | Body mass index > 36 kg/m2 (24%) |
| Bed rest of hospitalization > 72 hours(18%) | Stroke, MI or arterial embolism within previous 30 days (2%) |
| Current hospitalization for > 11 hours trauma (2%) | Congestive heart failure (8%) |
| Trauma requiring hospitalization within the previous 2 weeks (2%) | Active intravenous recreational drug use (<1%) |
| Personal history of thrombophilia (2%) | Current DVT within past 3 months without known PE (5%) |
| Active malignancy (currently under the care of an oncologist for treatment) (14%) | Active connective tissue disease (lupus, MCTD, scleroderma) (5%) |
| Any exogenous estrogen use (9%) | Focal infection requiring hospitalization or observation (8%) |
| Post-partum status (within past 2 weeks) (2%) | Indwelling deep vein catheter or port (excludes pacemaker wire) (9%) |
| Immobilization of an ankle, knee, hip or shoulder for > 48 hours within the past 7 days (4%) | Hemodialysis-dependent renal failure (within past 2 weeks) (2%) |
| Paralysis of one or more limbs from prior stroke or spinal cord injury (<1%) | Any prior venous thromboembolism (16%) |
Percentage of n=678 with complete data with the feature present
Study Protocol
CTPA images were obtained at each site as part of standard care with 64 channel, multi-detector equipment capable of ≤2.5mm collimation.10
Clinical data, blood collection and D-dimer assay
Blood was drawn by a qualified phlebotomist into two (blue-top) tubes containing sodium citrate dihydrate (0.11mM). These tubes were drawn and transported on ice and immediately centrifuged at 2500g × 15 minutes and the plasma fraction separated from red cell mass. Plasma was stored at −80°C.(26) The plasma D-dimer concentration was measured in batches of single-freeze-thawed aliquots of plasma at a central site clinical laboratory using a well-validated commercial assay (VIDAS ELISA, Biomérieux, Durham NC).3 Study D-dimer results were not provided to any member of the clinical care team or to radiologists, but all sites had the ability to order a stat D-dimer at all times during the study at the discretion of the attending physician. Key elements of clinical data were obtained in real time, as opposed to chart review, including all data in Table 1. Each data field had an explicit definition and coordinators were trained by the PI according to an explicit set of standard operating procedures (SOPs) on how to collect these in a standardized method. Vital signs recorded were those closest in time to when the patient was enrolled, and all pulse oximetry readings were obtained with the patient breathing room air. Moreover, the factor termed by Wells et al as “An alternative diagnosis is less likely than PE” that confers 3 points was determined by interview with the physician who ordered the CTPA prior to the time when CTPA results were known and was considered present if the physician answered yes to the question of whether he or she believed that PE was the most likely diagnosis at the time the CTPA was ordered or if the clinical team initiated empiric heparin prior to knowing the results of the CTPA. 28
CTPA interpretation
An independent reference reading (IRR) laboratory (Medical Metrics Inc., Houston, TX) interpretation and the site interpretation were both integrated in this analysis. At the site hospitals, images were interpreted by board-certified hospital radiologists who had completed a fellowship in emergency radiology or body imaging. Later, at the IRR laboratory, images that were stripped of protected health information and any added comments or markings were interpreted by one of two board-certified radiologists who had completed fellowship training in body imaging. The IRR radiologists interpreted images as “No PE”, “Positive for acute PE”, “Positive for chronic PE”, “Positive for other finding,” which was further graded including a specific code for pulmonary infiltrate suggestive of pneumonia. Interpretations of CTPAs at each site were performed as part of standard care, and the final, written interpretation entered into the medical record was abstracted using a standardized approach to determine the site reading. All scans read as positive for PE were further evaluated by the IRR radiologist for the location of the filling defect and the percentage obstruction of the vessel(s) using a modified version of the method described by Mastora et al.29 If the IRR read no PE for a scan read as PE+ at the site, the percentage obstruction was set at 2.5%.
Clinical follow-up
All patients had telephone follow-up at 30 and 90 days using scripted query and a specific data collection template designed to discover an interval diagnosis of PE and treatment status.
Criterion standard for acute PE and pneumonia and contrast induced nephropathy
The criterion standard defined PE+ if the initial CTPA was interpreted as positive for any filling defect consistent with PE by either the IRR or the site radiologist, or a repeat CTPA performed within 30 days was found to be positive for PE and the patient treated for 90 days with systemic anticoagulation. All other patients were considered PE (−). PE was considered subsegmental if the total filling defect were less than 5%.29 Pneumonia was considered present if either radiologist indicated presence of an infiltrate and all three of the following were true: the patient received a new prescription or order for antibiotics within 24 hours of enrollment and both the written discharge summary and the ICD-9 billing codes indicated a diagnosis of pneumonia. We assessed the frequency of contrast induced nephropathy (CIN) in patients who had a serum creatinine (sCr) measured as part of standard care on the same day as CTPA was then repeated at 2–7 days post enrollment. We defined CIN as present as follows: [(sCrbaseline – sCrrepeat)/sCrbaseline]>0.5 (50% relative increase).
Data archiving
Clinical data, centralized D-dimer results, radiological interpretations and follow up data were abstracted from source documentation or obtained directly from the patient using study specific data templates by qualified clinical research associates in accordance with Good Clinical Practice guidelines. Data were then transferred in duplicate by independent coordinators to paper case report forms and then manually uploaded to a central server in an SQL database (Microsoft Inc, Redmond, WA). Each data element on each CRF was inspected regularly during the conduct of the study against source documents for completeness, validity and between-CRF reproducibility by an experienced independent clinical study monitor (Southshore Clinical, Cornelius, NC). The study monitor issued a written report that contained any queries about data fields. Data were exported from SQL to a spreadsheet to facilitate analysis for this manuscript.
Data analysis
Prior literature has suggested that for age >70 years or for a PE unlikely pretest probability, the D-dimer threshold could be doubled without compromising the posterior probability. We confirmed these cutoffs in our data by comparing the posterior probability of PE observed at increasing intervals of age and intervals of pretest probability at 8 increasing D-dimer thresholds, starting with the standard threshold of 500 ng/mL. A PE unlikely pretest probability was determined by dichotomizing the Wells score and the revised Geneva score (RGS).30;31 Ninety five percent confidence intervals (95% CI) were calculated from the exact (Clopper Pearson) method (StatsDirect v. 2.7.8, Cheshire, England). Quadratic regression was used to produce curves to show the relationship between the D-dimer concentration and patient age (SigmaPlot, v11.0, Systat Software, Chicago, IL).
RESULTS
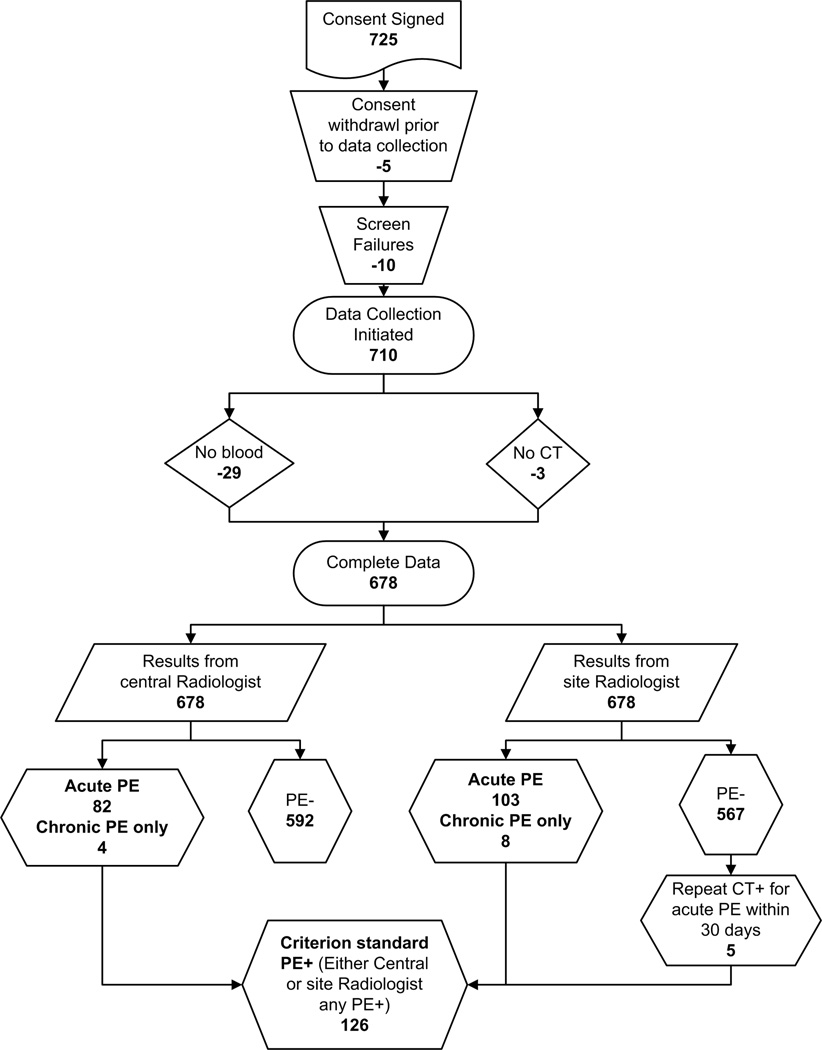
Patients were enrolled from January 30, 2007 to April 27, 2008. Figure 1 shows the flow diagram of patients starting with those subjects screened for inclusion to the point of final outcome with respect to PE diagnosis. Seven hundred twenty-five patients signed a consent form and 678 had complete data. Table 1 presents the percentages of the 678 patients who were enrolled with complete data who had each inclusion criterion and Table 2 presents the demographic data and additional clinical characteristics of these 678 patients. One hundred eleven (18%) patients were aged >70 years.
Figure 1.
Flow diagram showing the number of patients screened, enrolled and reasons for exclusions.
Table 2.
Clinical characteristics of the study population
| Variable | mean or N | SD or % |
|---|---|---|
| Vital sign data | ||
| Pulse rate (beats/min) | 84 | 18 |
| Respiratory rate (breaths/min) | 19 | 4 |
| Pulse oximetry | 97 | 2 |
| Systolic blood pressure (mm Hg) | 128 | 21 |
| Patient location | ||
| Inpatient | 335 | 49% |
| Emergency Department | 334 | 49% |
| Rehabilitation unit | 9 | 1% |
| Demographic data | ||
| Age (years) | 55 | 16 |
| Body weight (lbs) | 194 | 56 |
| Black race | 218 | 32% |
| Native American race | 2 | 0% |
| Caucasian race | 446 | 66% |
| Asian race | 2 | 0% |
| other race | 10 | 1% |
| Male sex | 258 | 38% |
| Risk factors for PE | ||
| Wells score | 3.0 | 1.9 |
| Revised Geneva Score | 6.7 | 3.4 |
| Chronic obstructive pulmonary disease | 74 | 11% |
| Warfarin within past 7 days | 89 | 13% |
| Current smoker | 258 | 38% |
| Sickle cell disease | 2 | 0% |
| Other diagnostic tests done | ||
| Ultrasound of leg | 144 | 21% |
| Ultrasound of arm | 13 | 2% |
| D-dimer at site | 286 | 42% |
| Echocardiography | 286 | 42% |
The criterion standard for any filling defect consistent with PE was found in126/678 patients (19%, 95% CI: 16 to 22%), including 108 with acute PE only, nine with acute and chronic PE and four with chronic PE only. On follow-up, 60 (9%) patients had a repeat CTPA of which five (8%) showed interval development of new, acute PE. None of these five had been treated with systemic anticoagulation prior to the second CTPA. By 30 days follow-up, clinicians treated 124 patients who had any PE observed on CTPA with systemic anticoagulation, withholding anticoagulation in 2 patients with subsegmental PE, neither of whom developed any thrombotic event within 90 days. Fifty-four of the 126 PE cases (42%) were isolated subsegmental (total pulmonary vascular occlusion <5%). The criterion standard for pneumonia was found in 93/678 patients (14%, 95% CI: 11 to 17%). The posterior probabilities of PE and pneumonia in the presence of a normal D-dimer using the conventional cutoff of 500 ng/mL, was 8/152 (5%) and 16/152 (10.5%) respectively.
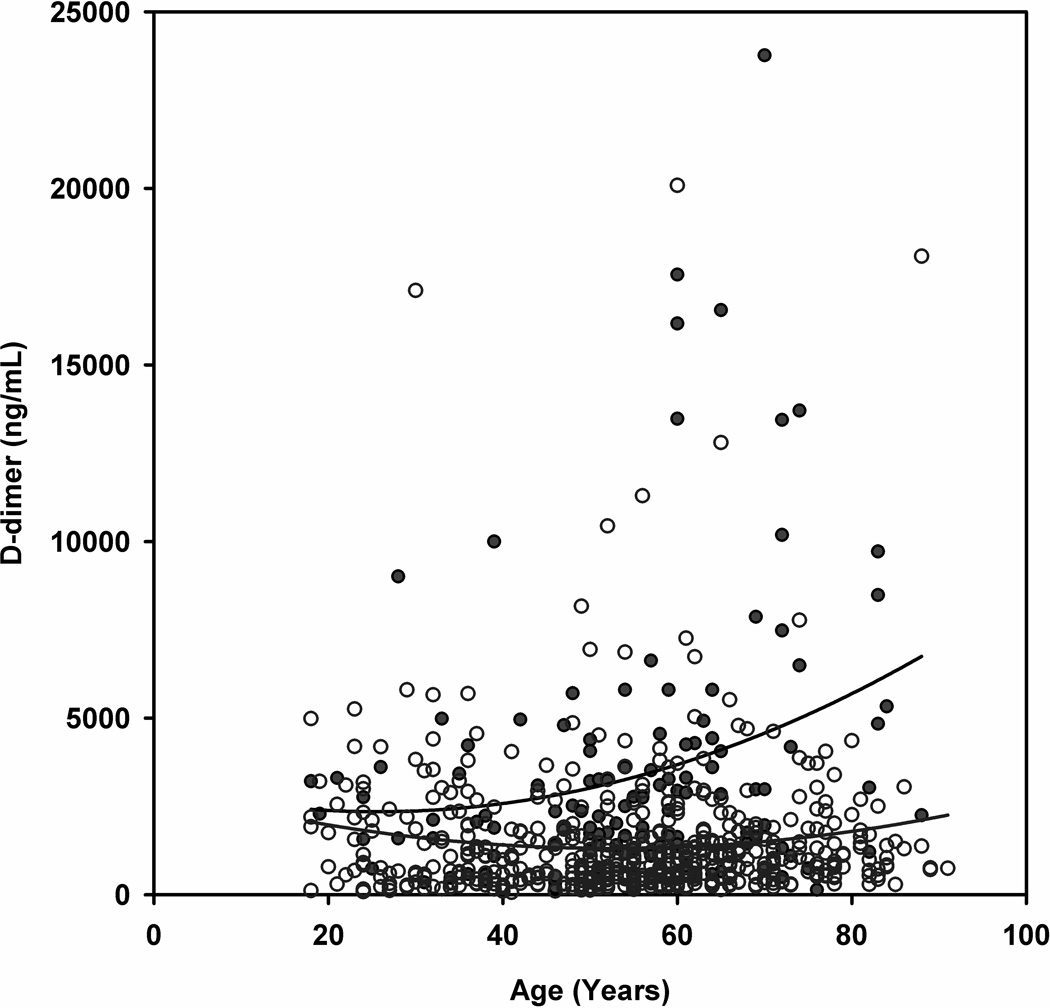
To illustrate the relationship between D-dimer and age, Figure 2 plots the D-dimer concentration versus the age and shows the best fit quadratic equation for D-dimer values for patients with and without PE. The splay in the curves shows widening at 70 years of age, providing support to the decision to increase the D-dimer threshold at this age.
Figure 2.
Scatter plot of the plasma D-dimer concentration versus patient age for patients. Red circles represent PE+ patients and open blue circles represent PE− patients. The curved lines are best fit quadratic equations (PE+: D-dimer = 3096 - 58*age +1.13*age2; PE-: D-dimer = 3052 – 66*age +0.63*age2)
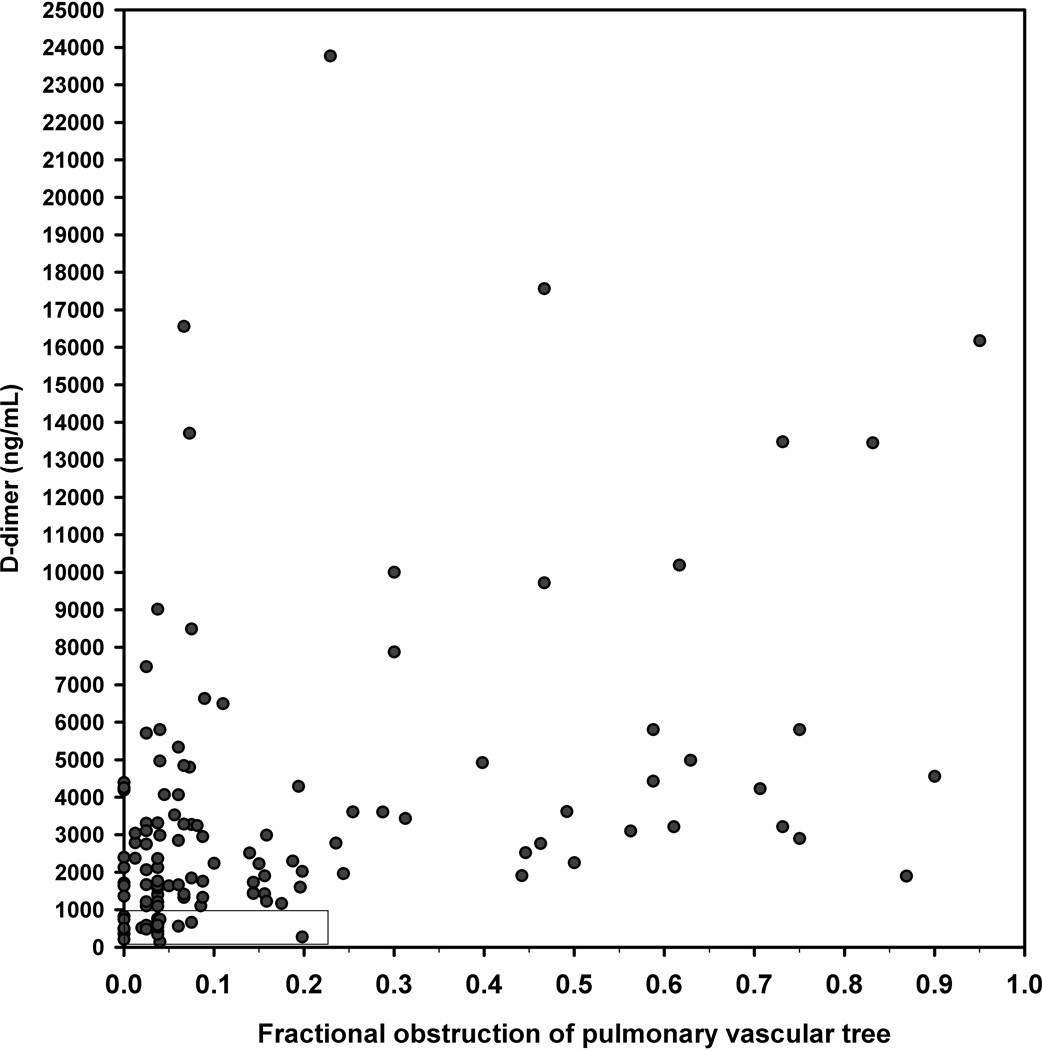
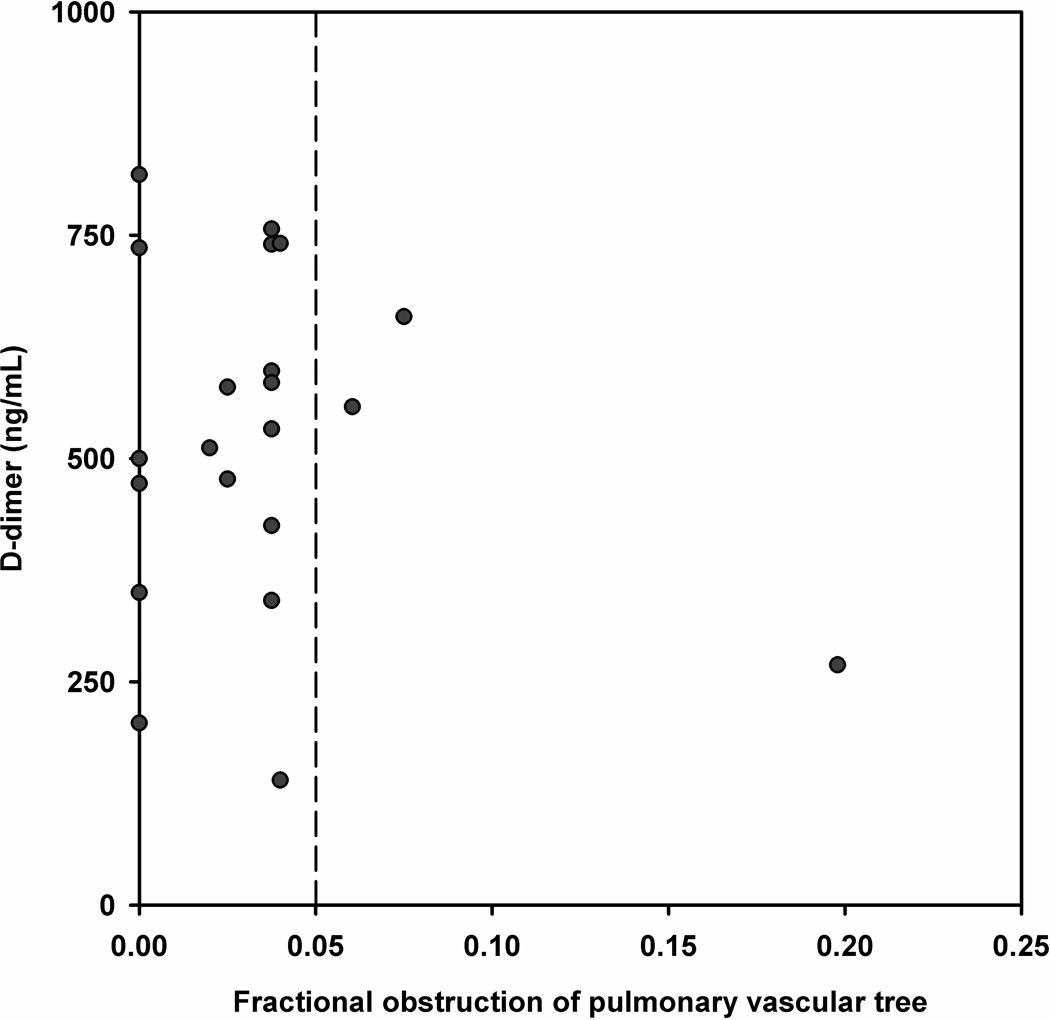
To assess the relationship between size of PE and D-dimer, Figure 3A plots the D-dimer concentration as a function of the fraction of pulmonary vascular obstruction for the entire range of D-dimer concentrations. The plot shows no evidence of overall correlation (Pearson’s correlation coefficient [r] value <0.1), even with multiple attempts at data transformation. However, Figure 3B, which displays the area shown in the lower left corner of Figure 2A does convey important information about the size of PEs in the range of D-dimer values that might be used for exclusion. Dots to the left of the vertical dotted line represent subsegmental PEs. The major point of this plot is that the majority of patients with PE and a D-dimer concentration below 1000 ng/mL have subsegmental PEs.
Figure 3.
A and B. (A) Scatter plot of the D-dimer versus the percentage of pulmonary vasculature obstructed on CTPA for all patients with PE and (B) shows data for the rectangle at the lower left corner; all red circles to the left of the vertical dotted line represent subsegmental PE.
Table 3 presents a matrix designed to systematically examine the posterior probability of PE in the presence of eight increasing D-dimer thresholds according to the age of each patient, stratified into four ranges intervals (<30, 30–49, 50–69 and ≥70 years) and according to the Wells and RGS scores, stratified into three intervals (<2, 2 to 6, >6 for the Wells score and <4, 4–10 or >10 for RGS ) and then at the single most useful cutoff for each (≤4 for Wells and ≤6 for RGS).The purpose of Table 3 was to inspect each cell for the posterior probability to estimate the optimal cutoff in D-dimer and age, or pretest probability score respectively.
Table 3.
Posterior probabilities for pulmonary embolism stratified for age, pretest probability at increasing thresholds for a normal D-dimer
| Adjustment Criteria | Cutoff for an abnormal D-dimer concentration (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 500 | 750 | 1000 | 1250 | 1500 | 5000 | 10000 | 25000 | ||
| Age | 0–29 | 0/12 (0%) | 1/18 (6%) | 1/22 (5%) | 1/23 (4%) | 1/23 (4%) | 8/48 (17%) | 9/51 (18%) | 9/51 (18%) |
| 30–49 | 5/56 (9%) | 8/81 (10%) | 8/88 (9%) | 9/101 (9%) | 10/110 (9%) | 26/169 (15%) | 28/174 (16%) | 28/175 (16%) | |
| 50–69 | 2/69 (3%) | 6/125 (5%) | 8/148 (5%) | 12/187 (6%) | 18/213 (8%) | 58/312 (19%) | 63/323 (20%) | 67/331 (20%) | |
| 70–100 | 1/15 (7%) | 4/34 (12%) | 4/52 (8%) | 6/63 (10%) | 7/77 (9%) | 13/110 (12%) | 18/116 (16%) | 22/121 (18%) | |
| all ages | 8/152 (5%) | 19/258 (7%) | 21/310 (7%) | 28/374 (7%) | 36/423 (9%) | 105/639 (16%) | 118/664 (18%) | 126/678 (19%) | |
| Wells Score | <2 | 3/80 (4%) | 8/139 (6%) | 9/165 (5%) | 10/185 (5%) | 14/212 (7%) | 36/292 (12%) | 38/301 (13%) | 39/304 (13%) |
| 2 to 6 | 3/68 (4%) | 9/113 (8%) | 9/134 (7%) | 15/174 (9%) | 19/196 (10%) | 59/316 (19%) | 68/328 (21%) | 72/336 (21%) | |
| >6 | 2/4 (50%) | 2/6 (33%) | 3/11 (27%) | 3/15 (20%) | 3/15 (20%) | 10/31 (32%) | 12/35 (34%) | 15/38 (39%) | |
| ≤4 | 4/106 (4%) | 11/186 (6%) | 12/221 (5%) | 16/260 (6%) | 21/299 (7%) | 54/434 (12%) | 57/445 (13%) | 60/452 (13%) | |
| Revised Geneva Score |
<4 | 2/65 (3%) | 3/98 (3%) | 3/104 (3%) | 4/116 (3%) | 7/126 (6%) | 18/159 (11%) | 18/160 (11%) | 18/160 (11%) |
| 4 to 10 | 4/75 (5%) | 13/139 (9%) | 14/178 (8%) | 19/220 (9%) | 24/256 (9%) | 64/398 (16%) | 72/413 (17%) | 77/424 (18%) | |
| >10 | 2/12 (17%) | 3/21 (14%) | 4/28 (14%) | 5/38 (13%) | 5/41 (12%) | 23/82 (28%) | 28/91 (31%) | 31/94 (33%) | |
| ≤6 | 4/110 (4%) | 10/179 (6%) | 11/208 (5%) | 15/241 (6%) | 19/273 (7%) | 45/366 (12%) | 48/373 (13%) | 51/377 (14%) | |
Taken together, data in Figures 1 and 2 and Table 3 indicate that posterior probability could be maintained and rule out rate for PE could be increased if the D-dimer cutoff were increased up to 1000 ng/mL for age >70 or Wells score ≤4 or RGS ≤6. These cutoffs in D-dimer, age and pretest probability agree with prior work.19;22;32
Table 4 presents the main findings of this report, including the exclusion rate and posterior probability for eight different D-dimer-based screening strategies. Table 4 first shows the performance of the quantitative site D-dimer that was ordered as part of standard care in 286 patients. Curiously, the site D-dimer was negative in 40 patients (6% of the cohort) who underwent CTPA, only one of whom had PE, suggesting either an uncoupling of test ordering from subsequent behavior, or that clinicians may order the CTPA to diagnose other conditions besides PE. Table 4 then shows the diagnostic performance of the study D-dimer at standard threshold (<500 ng/mL) and at <1000 ng/mL alone and under three assumptions: age >70, a Wells’ score ≤4, or an RGS ≤6. With any of these three strategies, the net rule out rate could have been substantially increased by doubling the D-dimer threshold, and with minimal cost in terms of increased posterior probability of segmental or larger PE. For example, for patients with a Wells’ score ≤4 and the standard D-dimer threshold, the exclusion rate was 16% (95% CI: 13 to 19%) and the posterior probability was 4/106 or 3.8% (95% CI: 1 to 9.3%) whereas with a Wells’ score≤4 and a D-dimer <1000 ng/mL the exclusion rate increased to 33% (95% CI: 29 to 36%), and the posterior probability of PE was 12/221 or 5.4% (95% CI: 2.8 to 9.3%). Likewise, for patients with an RGS≤6 at standard D-dimer threshold, the exclusion rate was 110/678 (16%, 95% CI: 14 to 19%) with a 4/110 or 3.6% (95% CI 1 to 10%) posterior probability whereas for an RGS≤6 and D-dimer <1000 ng/mL, the exclusion rate increased to 208/678 (31%, 95% CI 27 to 44%) with a posterior probability of 11/208 (5.3%, 95% CI 3.0 to 9.2%). The same four patients with PE were missed by the Wells’ score ≤4, and the RGS ≤6 and D-dimer <500 ng/mL, and three of these four had subsegmental PE and one had a PE with 19% obstruction.
Table 4.
Diagnostic indexes produced by screening strategies for PE that include an increased D-dimer threshold
| Screening strategy | TP | TN | FN | FP | N | Exclusion rate |
Sens | Spec | LR(-) | Posterior Probability |
FN with Subseg* |
CIN** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site D-dimer, standard threshold* | 35 | 39 | 1 | 211 | 286 | 6% | 97.2% | 15.6% | 0.18 | 2.5% | 0 | 0 |
| D-dimer < 500 ng/mL | 118 | 144 | 8 | 408 | 678 | 22% | 93.7% | 26.1% | 0.24 | 5.3% | 7 | 3 |
| D-dimer < 1000 ng/mL | 105 | 289 | 21 | 263 | 678 | 46% | 83.3% | 52.4% | 0.32 | 6.8% | 17 | 11 |
| D-dimer < 500 or <1000 if Age >70 | 116 | 175 | 10 | 377 | 678 | 27% | 92.1% | 31.7% | 0.25 | 5.4% | 9 | 6 |
| Wells ≤4 and D-dimer <500 ng/mL | 122 | 102 | 4 | 450 | 678 | 16% | 96.8% | 18.5% | 0.17 | 3.8% | 4 | 2 |
| Wells≤4 and D-dimer <1000 ng/mL | 114 | 209 | 12 | 343 | 678 | 33% | 90.5% | 37.9% | 0.25 | 5.4% | 10 | 10 |
| RGS≤6 and D-dimer <500 ng/mL | 122 | 106 | 4 | 446 | 678 | 16% | 96.8% | 19.2% | 0.17 | 3.6% | 4 | 2 |
| RGS≤6 and D-dimer <1000 ng/mL | 115 | 197 | 11 | 355 | 678 | 31% | 91.3% | 35.7% | 0.24 | 5.3% | 10 | 9 |
Abbreviations: TP- true positive, TN- true negative, FN false negative, FP- false positive, Sens sensitivity, Spec- specificity, LR(-)- likelihood ratio negative, DVT- deep venous thrombosis, CIN- contrast induced nephropathy (defined as a 50% increase in serum creatinine within 2–7 days), RGS-revised Geneva score
number of false negative PEs that were subsegmental;
number of cases of contrast induced nephropathy in the true negative group
We then examined the size of the filling defects of patients present when the patient had a PE unlikely pretest probability and a D-dimer <1000 ng/mL. Among the 12 false negative cases produced by the Wells score ≤4 and a D-dimer threshold of 1000 ng/mL, 10 of these 12 false negative PEs were isolated, subsegmental filling defects and no patient with a D-dimer <1000 ng/mL was diagnosed with concomitant DVT and one had active malignancy. Among the 11 patients with an RGS≤6 and a D-dimer <1000 ng/mL, 10 of these 11 had isolated, subsegmental filling defects, and none had DVT and one had active malignancy. Seven of the patients with false negative PEs (12 with Wells ≤4 and 11 with RGS≤6) had a lower extremity ultrasound and three others underwent lower extremity CT venogram which was negative for DVT. The sole PE that larger than subsegmental that was missed by both strategies was the same PE with 19% obstruction missed by the D-dimer <500 ng/mL.
To assess the rate of contrast induced nephropathy, we examined data from 224 patients who had a serum creatinine measured prior to CTPA and again at 2–7 days post enrollment as part of standard care. Of the 224 patients with paired serum creatinine measurements, 37 (17%, 95% CI: 12 to 22%) developed contrast induced nephropathy, using the definition of a 50% relative increase in creatinine value. In the last column on the right, Table 4 presents the number of cases of CIN that could have been prevented with each screening strategy. Ten patients with CIN had a Wells score ≤4 and a D-dimer <1000 ng/mL and nine with CIN had RGS≤6 and D-dimer <1000.
When the data were restricted to inpatients only, we found a trend toward reduced protocol efficiency. For example, among inpatients, the combination of a Wells score ≤4 and a D-dimer <500 ng/mL would have allowed a rule out rate of 12% producing a posterior probability of PE of 6.8% whereas a Wells score ≤4 and a D-dimer <1000 ng/mL would have ruled out 26% producing a posterior probability of PE of 7.7%.
Because active cancer is known to elevate the D-dimer and sharply reduce the test specificity, we examined the impact of D-dimer at a threshold at 1000 ng/mL for patients with age >70 or active cancer and 500 ng/mL for all others.33 This strategy would have led to 195 (28.7%) patients with a negative screen, with 12 (6.1%) false negatives, and thus provided minimal incremental increase in rule out rate.
Regarding potential alternative diagnoses on CTPA, radiologists found at least one pathologic finding on 425/552 CTPA scans that did not have PE. Both radiologists agreed that infiltrate was present in 93 patients who received antibiotics within 24 hours and had a discharge diagnosis that included pneumonia. Thus, pneumonia was present in 14% (95% CI: 11 to 17%) of patients who underwent CTPA for suspected PE, and pneumonia accounted for most clinically important acutely life-threatening, alternative diagnoses seen on CTPA. Other diagnoses seen on CTPA included aortic dissection (9), thoracic aneurysm (10) and pneumothorax (3). Table 5 shows the diagnostic indexes for each putative strategy for pneumonia. The primary findings in Table 5 was that the strategy of doubling the D-dimer for age <70, was not an efficient screening tool for pneumonia because, whereas the combination of an RGS≤6 and a D-dimer <1000 led to the lowest posterior prprobability of pneumonia. Plain film chest radiographs were ordered on 565 patients (83%) and among these, 200 (35%) were interpreted as normal and 58 (10%) were interpreted as positive for infiltrate. Surprisingly, only 21 of these 58 were interpreted as positive for infiltrate on CTPA. These data suggest substantial discordance between plain film chest radiographs and the CTPA.
Table 5.
Diagnostic indexes for pneumonia using the proposed D-dimer-based screening strategies
| Screening strategy | TP | TN | FN | FP | N | Sensitivity | Specificity | LR(-) | Posterior Probability |
|---|---|---|---|---|---|---|---|---|---|
| D-dimer < 500 ng/mL | 77 | 136 | 16 | 449 | 678 | 82.8% | 23.2% | 0.74 | 10.5% |
| D-dimer < 1000 ng/mL | 66 | 283 | 27 | 302 | 678 | 71.0% | 48.4% | 0.60 | 8.7% |
| D-dimer < 500 or <1000 if Age >70 | 75 | 167 | 18 | 418 | 678 | 80.6% | 28.5% | 0.68 | 9.7% |
| Wells ≤4 and D-dimer <500 ng/mL | 83 | 96 | 10 | 489 | 678 | 89.2% | 16.4% | 0.66 | 9.4% |
| Wells≤4 and D-dimer <1000 ng/mL | 76 | 204 | 17 | 381 | 678 | 81.7% | 34.9% | 0.52 | 7.7% |
| RGS≤6 and D-dimer <500 ng/mL | 84 | 101 | 9 | 484 | 678 | 90.3% | 17.3% | 0.56 | 8.2% |
| RGS≤6 and D-dimer <1000 ng/mL | 81 | 196 | 12 | 389 | 678 | 87.1% | 33.5% | 0.39 | 5.8% |
Abbreviations are the same as Table 4
DISCUSSION
This study examines the hypothesis that selected use of an elevated threshold for a normal D-dimer concentration can decrease the need for CTPA scanning for PE in a moderate risk population. As has been reviewed recently, an extensive prior literature has been published to suggest that D-dimer can be elevated in the elderly, and in patients with certain comorbidities, and in patients with a low pretest probability.32 We designed this study specifically to address this question, after years of planning, and using methods designed to enhance the generalizability of results. We enrolled a sample of patients who represent the whole population of patients undergoing 64 channel CTPA at four large hospitals, including a balance of emergency department and hospitalized patients.34 We used a rigorous criterion standard inasmuch all CTPA scans were read by two independent radiologists, and the central radiologist graded the size of PE; we also included clinical follow-up. D-dimer concentrations were measured in a central laboratory using a well-studied D-dimer assay that was the first cleared by FDA as an aid to exclusion of PE. Recognizing the possibility that in clinical practice, some physicians eschew the D-dimer and order a CTPA to help them evaluate non-thromboembolic pathology, we also examined the ability of D-dimer to exclude pneumonia, the most common important alternative diagnosis seen on CTPA scans.4;5
These resultant data allow several clinically important observations. First, the data in Table 4 show that physicians sometimes order a D-dimer at the site and then disregard the result. In fact, the site D-dimer was negative in 40 patients who underwent CTPA, and only one of whom had PE. Second, most patients with PE in the presence of D-dimer concentrations below 1000 ng/mL had very small PEs. Had a high sensitivity D-dimer been ordered in all patients regardless of pretest probability and using the standard threshold of 500 ng/mL, 152 (22%) of CTPA scans could have been obviated. Importantly, these 152 patients included 8 (5.3%) possible false negative D-dimer patients who had filling defects on CTPA suggestive of PE, and only one of these eight patients had a filling defect in an artery that was larger than subsegmental. Had a more liberal definition of 1000 ng/mL been used, the exclusion rate would have increased to 310 (46%), but at a cost of a false negative rate of 21/310 (6.8%) including four patients with filling defect larger than subsegmental. This rate would appear unacceptably high, and underscores the need for additional criteria. The third main observation was that increasing the normal D-dimer threshold to <1000 ng/mL for patients with a “PE unlikely” pretest probability, based upon either the Wells’ score ≤4 or an RGS ≤6, would potentially obviate one-third of CTPA scans in this high risk population, with isolated subsegmental filling defects accounting for most of the false negatives. In addition to preventing radiation exposure, this strategy would have potentially prevented at least 10 cases of contrast induced nephropathy, using a very strict definition.35The potentially simpler strategy of elevating the D-dimer threshold to 1000 ng/mL only for patients over 70 years had similar results, but adding active malignancy had marginal value. Our data also show some support the D-dimer as a screening tool for pneumonia, particularly for an RGS≤6, and a D-dimer <1000 ng/mL the combination of which significantly lowered the posterior probability of pneumonia from a baseline of 14%, (95% CI: 11 to 17%) to 5.8% (95% CI 3 to 10%). The final salient observation was the remarkably high rate of subsegmental PE (42% of PEs), which raises a question as to whether the sample may have spectrum or severity bias, or if this reflects the effect of 64 channel multi-row detector CTPA and a criterion standard of PE seen by either the site radiologist or the IRR radiologist.
This work must be in interpreted with caution because of the absence of clear data on the significance of isolated subsegmental PE, which comprised almost all of the false negative cases, and most of these patients were treated with anticoagulation. Observational studies suggest the outcomes of patients with isolated subsegmental PE who are not treated have similar outcomes to those who are treated with anticoagulation, although the literature lacks a randomized trial to address this question.12–14;36 Indeed, the present report only tests the hypothetical contribution of elevating the threshold to define an abnormal D-dimer concentration, as opposed to examining outcomes of patients in a management study. Thus, the next step should be an outcomes trial that randomizes low pretest probability patients to one of two groups, both of which would have anticoagulation withheld: D-dimer at standard threshold or D-dimer at twice local threshold . The rational stating the D-dimer at local threshold as opposed to 500 ng/mL is that thresholds for abnormal vary with many quantitative D-dimer assays. Indeed, in the present study, each of the four hospitals used a different local D-dimer assay, each with a different threshold for abnormal. Another limitation involves the interpretation of the significance of the D-dimer as a screen for pneumonia, because we assume that some clinicians are motivated to order CTPA to evaluate for alternative diagnoses such as pneumonia, a point that is based upon experience of the authors. Lastly, in the interest of brevity, we did not use all potentially valid pretest probability methods.30
In conclusion, a screening strategy that doubles the threshold for an abnormal D-dimer concentration for patients over age 70 or patients with a Wells score ≤4 or an RGS ≤6 could significantly reduce the rate of CTPA with no net increase in the rate of missing larger than subsegmental PE and no increase the rate of missed pneumonia.
Acknowledgments
Funding Support
Supported by Grant K23HL077404 NHLBI, R42 HL086316-01, and BreathQuant Medical, LLC
Contributor Information
Jeffrey A. Kline, Email: JKline@carolinas.org, Department of Emergency Medicine, 1000 Blythe Boulevard, MEB 3rd floor, Room 306, Charlotte, NC 28203.
Melanie M. Hogg, Email: Melanie.Hogg@carolinashealthcare.org, Department of Emergency Medicine, MEB 1st floor, 1000 Blythe Boulevard, Charlotte, NC 28203.
D. Mark Courtney, Email: d-courtney@northwestern.edu, Department of Emergency Medicine, Feinberg School of Medicine, Northwestern University, 211 E. Ontario Suite 200, Chicago, IL 60611.
Chadwick D. Miller, Email: cmiller@wfubmc.edu, Department of Emergency Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27517-1089.
Alan E. Jones, Email: aejones@umc.edu, Department Emergency Medicine, University of Mississippi Medical Center, Jackson, MS.
Howard A Smithline, Email: howard.Smithline@bhs.org, Department of Emergency Medicine, Baystate Medical Center, 759 Chestnut Street, Springfield, MA 01199.
References List
- 1.Fesmire FM, Brown MD, Espinosa JA, Shih RD, Silvers SM, Wolf SJ, Decker WW. Critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected pulmonary embolism. Ann.Emerg.Med. 2011;57:628–652. doi: 10.1016/j.annemergmed.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Filippatos G, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur.Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 3.Carrier M, Righini M, Djurabi RK, Huisman MV, Perrier A, Wells PS, Rodger M, Wuillemin WA, Le GG. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb.Haemost. 2009;101:886–892. [PubMed] [Google Scholar]
- 4.Richman PB, Courtney DM, Kline JA. Prevalence and significance of non-thromboembolic findings on chest computerized tomography angiography performed to rule-out pulmonary embolism-A multi-center study of 1025 Emergency Department patients. Acad Emerg Med. 2004;11:642–647. [PubMed] [Google Scholar]
- 5.Hall WB, Truitt SG, Scheunemann LP, Shah SA, Rivera MP, Parker LA, Carson SS. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch.Intern.Med. 2009;169:1961–1965. doi: 10.1001/archinternmed.2009.360. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell AM, Kline JA. Contrast nephropathy following computed tomography angiography of the chest for pulmonary embolism in the emergency department. J Thromb Haemost. 2007;5:50–54. doi: 10.1111/j.1538-7836.2006.02251.x. [DOI] [PubMed] [Google Scholar]
- 8.Le GG, Righini M, Parent F, van SM, Couturaud F. Diagnosis and management of subsegmental pulmonary embolism. J Thromb.Haemost. 2006;4:724–731. doi: 10.1111/j.1538-7836.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunot S, Corneloup O, Latrabe V, Montaudon M, Laurent F. Reproducibility of multi-detector spiral computed tomography in detection of sub-segmental acute pulmonary embolism. Eur.Radiol. 2005;15:2057–2063. doi: 10.1007/s00330-005-2844-4. [DOI] [PubMed] [Google Scholar]
- 10.Courtney DM, Miller CD, Smithline HA, Klekowski N, Hogg MM, Kline JA. Prospective multi-center assessment of interobserver agreement for radiologist interpretation of multi-detector CT angiography for pulmonary embolism. Journal of Thrombosis & Haemostasis. 2010;8:533–540. doi: 10.1111/j.1538-7836.2009.03724.x. [DOI] [PubMed] [Google Scholar]
- 11.Richman PB, Kasper D, Chen F, Dominguez S, Friese JL, Wood JP, Kline JA. Interobserver agreement for the diagnosis of venous thromboembolism on CT chest angiography and indirect venography of the lower extremities in emergency department patients. Academic Emergency Medicine. 2006;13:295–301. doi: 10.1197/j.aem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, Pleasance S, Le GG. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb.Haemost. 2010;8:1716–1722. doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 13.Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb.Res. 2010;126:e266–e270. doi: 10.1016/j.thromres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Le GG, Righini M, Parent F, van SM, Couturaud F, Le Gal G, Righini M, Parent F, van Strijen M, Couturaud F. Diagnosis and management of subsegmental pulmonary embolism. Journal of Thrombosis & Haemostasis. 2006;4:724–731. doi: 10.1111/j.1538-7836.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 15.Kline JA, Courtney DM, Beam DM, King MC, Steuerwald M. Incidence and Predictors of Repeated Computed Tomographic Pulmonary Angiography in Emergency Department Patients. Ann.Emerg.Med. 2009;54:41–48. doi: 10.1016/j.annemergmed.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Drescher FS, Chandrika S, Weir ID, Weintraub JT, Berman L, Lee R, Van Buskirk PD, Wang Y, Adewunmi A, Fine JM. Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann.Emerg.Med. 2011;57:613–621. doi: 10.1016/j.annemergmed.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Kline JA, Webb WB, Jones AE, Hernandez J. Impact of a rapid rule-out protocol for pulmonary embolism on the rate of screening, missed cases, and pulmonary vascular imaging in an urban U. S. emergency department. Ann Emerg Med. 2004;44:490–502. doi: 10.1016/j.annemergmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Kabrhel C, Mark Court, Camargo CA, Jr, Plewa MC, Nordenholz KE, Moore CL, Richman PB, Smithline HA, Beam DM, Kline JA. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Academic Emergency Medicine. 2010;17:589–597. doi: 10.1111/j.1553-2712.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linkins LA, Bates SM, Ginsberg JS, Kearon C. Use of different D-dimer levels to exclude venous thromboembolism depending on clinical pretest probability. Journal of Thrombosis & Haemostasis. 2004;2:1256–1260. doi: 10.1111/j.1538-7836.2004.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Douma RA, Le GG, Sohne M, Righini M, Kamphuisen PW, Perrier A, Kruip MJ, Bounameaux H, Buller HR, Roy PM. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475. doi: 10.1136/bmj.c1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Righini M, Le Gal G, Perrier A, Bounameaux H. The challenge of diagnosing pulmonary embolism in elderly patients: influence of age on commonly used diagnostic tests and strategies. [Review] [42 refs] Journal of the American Geriatrics Society. 2005;53:1039–1045. doi: 10.1111/j.1532-5415.2005.53309.x. [DOI] [PubMed] [Google Scholar]
- 22.Kabrhel C, Courtney DM, Camargo CA, Jr, Moore CL, Richman PB, Plewa MC, Nordenholz KE, Smithline HA, Beam DM, Brown MD, Kline JA. Potential impact of adjusting the threshold of the quantitative D-dimer based upon pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325–342. doi: 10.1111/j.1553-2712.2009.00368.x. [DOI] [PubMed] [Google Scholar]
- 23.De Monye W, Sanson BJ, Mac GM, Pattynama PM, Buller HR, van den Berg-Huysmans AA, Huisman MV, Antelope-Study G. Embolus location affects the sensitivity of a rapid quantitative D-dimer assay in the diagnosis of pulmonary embolism. American Journal of Respiratory & Critical Care Medicine. 2002;165:345–348. doi: 10.1164/ajrccm.165.3.2104099. [DOI] [PubMed] [Google Scholar]
- 24.Agapakis DI, Tsantilas D, Psarris P, Massa EV, Kotsaftis P, Tziomalos K, Hatzitolios AI. Coagulation and inflammation biomarkers may help predict the severity of community-acquired pneumonia. Respirology. 2010;15:796–803. doi: 10.1111/j.1440-1843.2010.01773.x. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers JD, Singanayagam A, Scally C, Hill AT. Admission D-dimer can identify low-risk patients with community-acquired pneumonia. Ann.Emerg.Med. 2009;53:633–638. doi: 10.1016/j.annemergmed.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Milbrandt EB, Reade MC, Lee M, Shook SL, Angus DC, Kong L, Carter M, Yealy DM, Kellum JA. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol.Med. 2009;15:438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bussuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, De Vet HCW, Lijmer JG. The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Clin Chem. 2003;49:7–18. doi: 10.1373/49.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AGG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb.Haemost. 2000;83:416–420. [PubMed] [Google Scholar]
- 29.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. European Radiology. 2003;13:29–35. doi: 10.1007/s00330-002-1515-y. [DOI] [PubMed] [Google Scholar]
- 30.Lucassen W, Geersing GJ, Erkens PM, Reitsma JB, Moons KG, Buller H, van Weert HC. Clinical Decision Rules for Excluding Pulmonary Embolism: A Meta-analysis. Ann.Intern.Med. 2011;155:448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 31.Le GG, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, Perrier A. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann.Intern.Med. 2006;144:165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 32.Righini M, Bounameaux H, Perrier A. Plasma D-Dimer and Venous Thromboembolic Disease. In: van Beek EJR, Buller HR, Oudkerk M, editors. Deep Vein Thrombosis and Pulmonary Embolism. Chichester, West Sussex, UK: John Wiley & Sons; 2009. pp. 85–111. [Google Scholar]
- 33.Righini M, Le GG, De LS, Roy PM, Meyer G, Aujesky D, Bounameaux H, Perrier A. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb.Haemost. 2006;95:715–719. [PubMed] [Google Scholar]
- 34.Miron MJ, Perrier A, Bounameaux H, de Moerloose P, Slosman DO, Didier D, Junod A. Contribution to noninvasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients. Eur Respir J. 1999;13:1365–1370. doi: 10.1183/09031936.99.13613719. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyer BA, Goodman LR, Washington L. Clinicians' response to radiologists' reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. AJR Am. J Roentgenol. 2005;184:623–628. doi: 10.2214/ajr.184.2.01840623. [DOI] [PubMed] [Google Scholar]