Abstract
Several known trans-splicing RNA structures were used to define a canonical trans-splicing structure which was then used to perform a computer search of the EMBL nucleotide database. In addition to most known trans-splicing structures, many putative new trans-splicing sites were detected. These were found in a broad range of organisms including the vertebrates. Control experiments indicate that the search predicts known false positives at a rate of only 20%. Trans-splicing may therefore be a very wide-spread phenomenon.
Full text
PDF
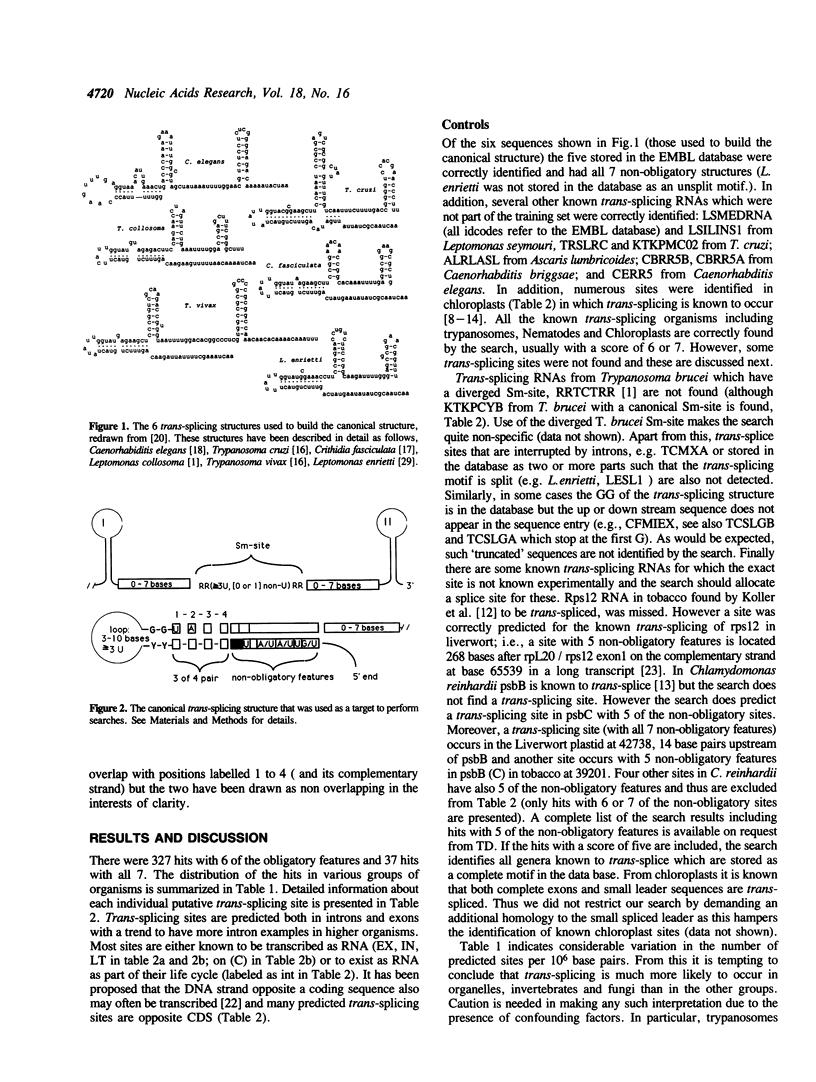
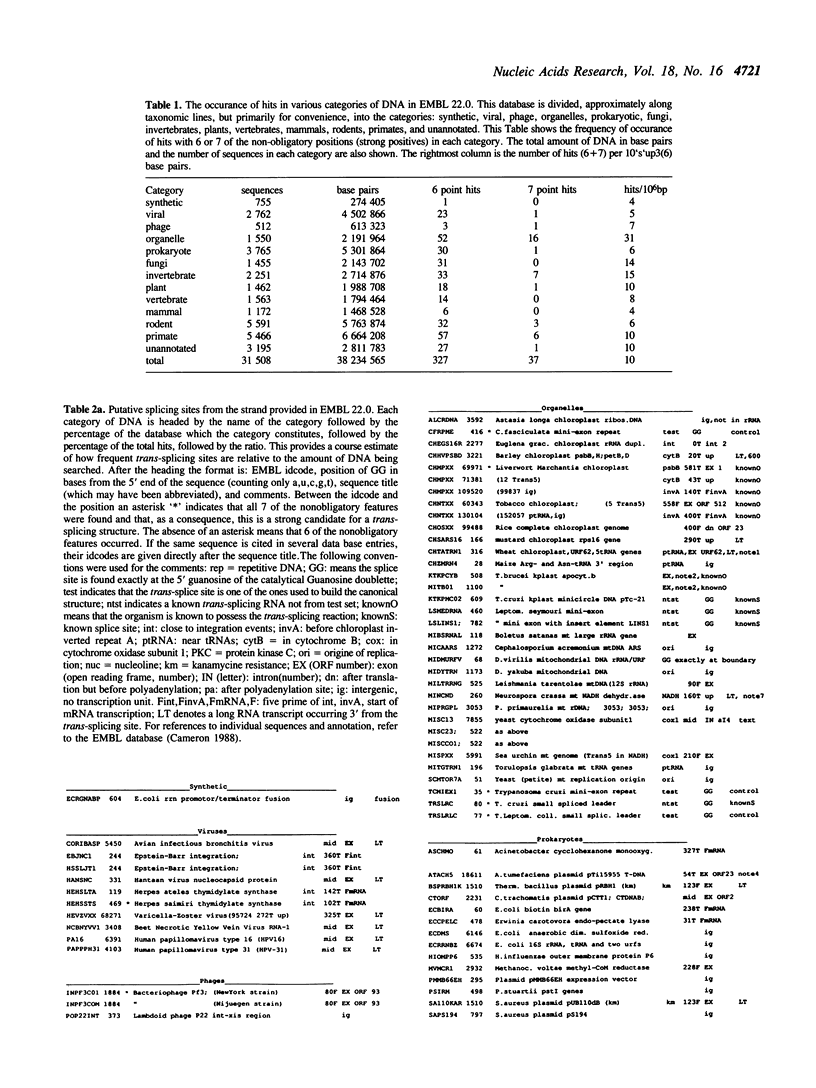
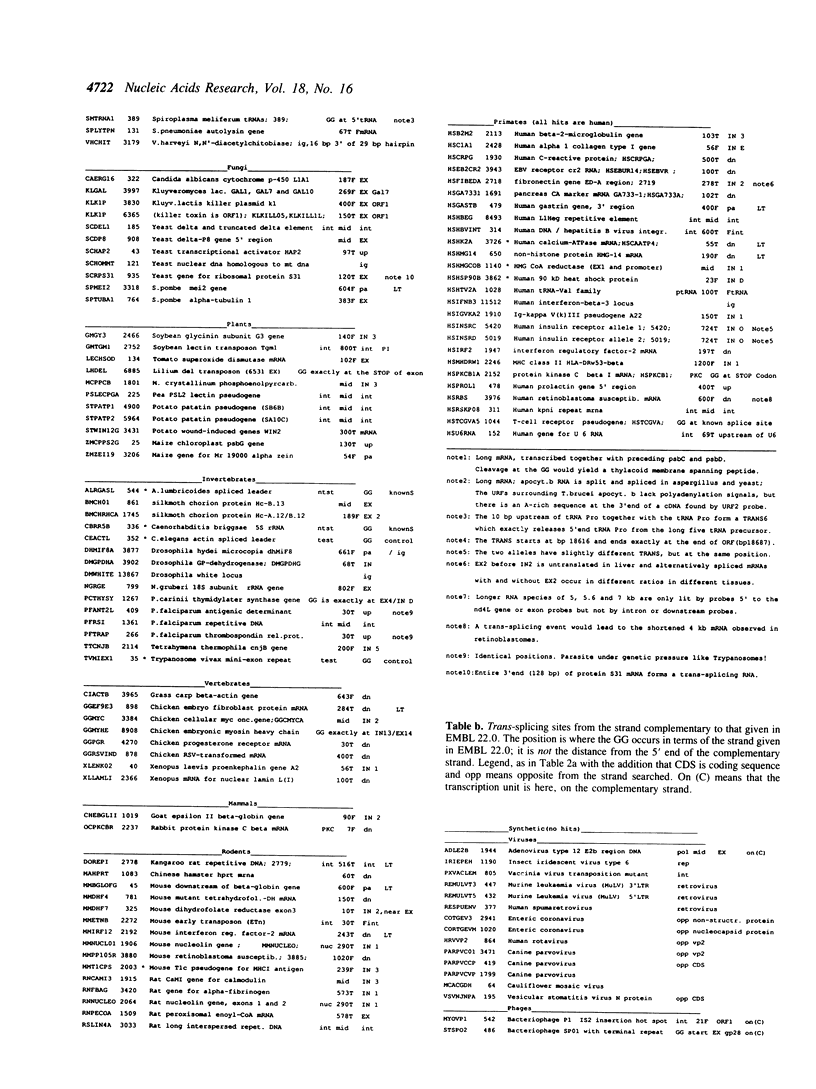
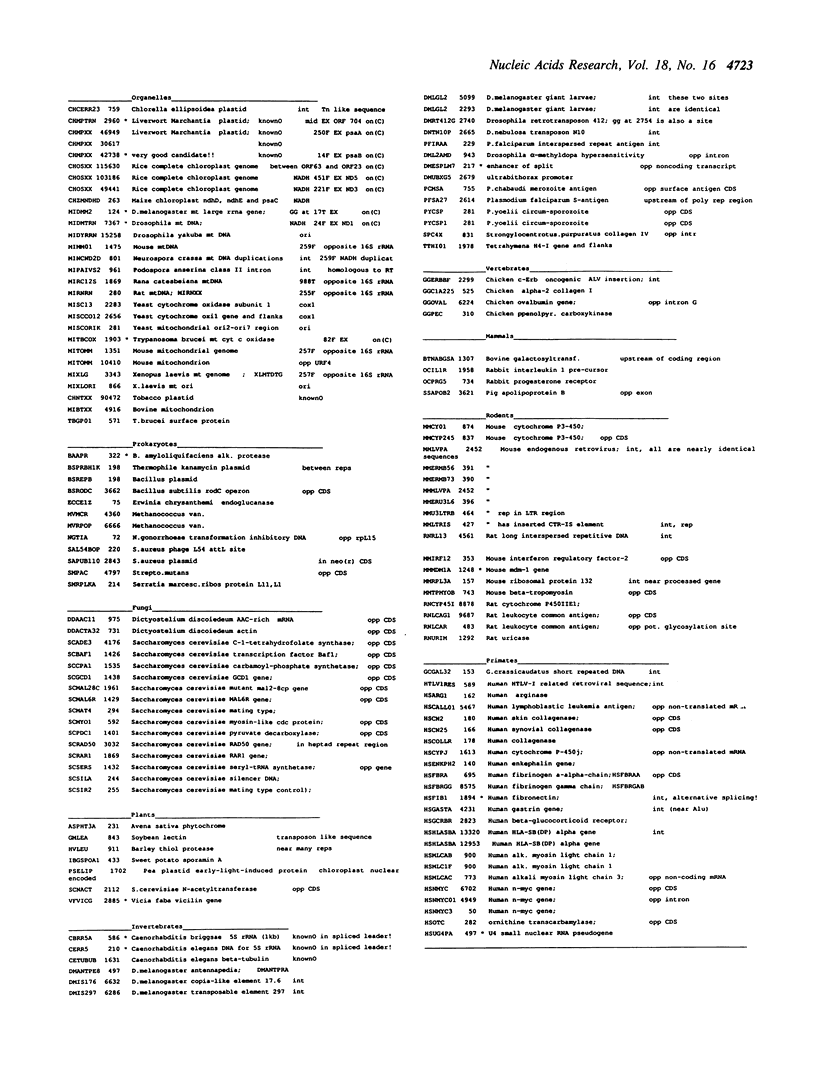
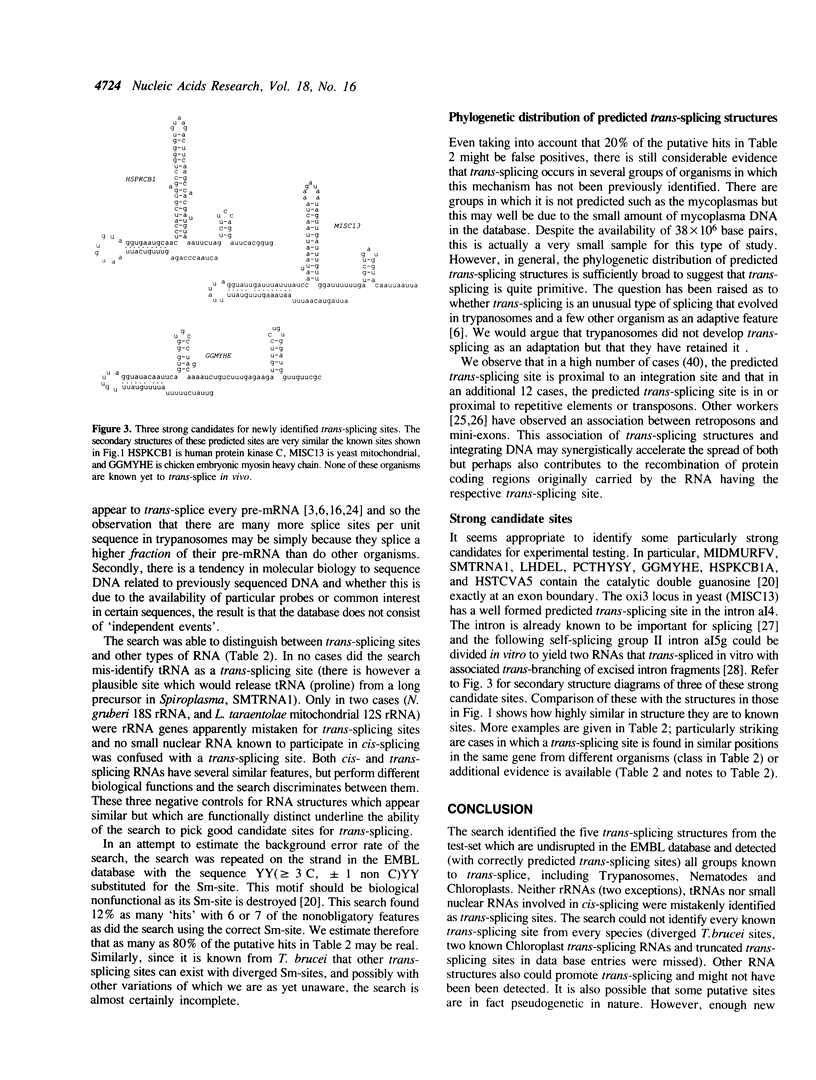

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Rindisbacher L., Braun R. The tubulin gene cluster of Trypanosoma brucei starts with an intact beta-gene and ends with a truncated beta-gene interrupted by a retrotransposon-like sequence. Gene. 1989 Aug 1;80(1):177–183. doi: 10.1016/0378-1119(89)90264-3. [DOI] [PubMed] [Google Scholar]
- Aksoy S., Lalor T. M., Martin J., Van der Ploeg L. H., Richards F. F. Multiple copies of a retroposon interrupt spliced leader RNA genes in the African trypanosome, Trypanosoma gambiense. EMBO J. 1987 Dec 1;6(12):3819–3826. doi: 10.1002/j.1460-2075.1987.tb02718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C. Antigenic variation in African trypanosomes. Annu Rev Microbiol. 1985;39:475–502. doi: 10.1146/annurev.mi.39.100185.002355. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Braun R. Discontinuous RNA synthesis through trans-splicing. Bioessays. 1986 Nov;5(5):223–227. doi: 10.1002/bies.950050509. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Cameron G. N. The EMBL data library. Nucleic Acids Res. 1988 Mar 11;16(5):1865–1867. doi: 10.1093/nar/16.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Berkvens T. M., Veerman H. J., Frasch A. C., Barry J. D., Borst P. Comparison of the genes coding for the common 5' terminal sequence of messenger RNAs in three trypanosome species. Nucleic Acids Res. 1984 Jun 11;12(11):4431–4443. doi: 10.1093/nar/12.11.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Michels P. A., Veerman H. J., Cornelissen A. W., Borst P. Many trypanosome messenger RNAs share a common 5' terminal sequence. Nucleic Acids Res. 1984 May 11;12(9):3777–3790. doi: 10.1093/nar/12.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin G., Jacq C., Slonimski P. P. Single base substitution in an intron of oxidase gene compensates splicing defects of the cytochrome b gene. Nature. 1982 Aug 12;298(5875):628–632. doi: 10.1038/298628a0. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi T., Ogura Y., Umesono K., Yamada Y., Komano T., Ozeki H., Ohyama K. Ordered processing and splicing in a polycistronic transcript in liverwort chloroplasts. Curr Genet. 1988 Aug;14(2):147–154. doi: 10.1007/BF00569338. [DOI] [PubMed] [Google Scholar]
- Koller B., Fromm H., Galun E., Edelman M. Evidence for in vivo trans splicing of pre-mRNAs in tobacco chloroplasts. Cell. 1987 Jan 16;48(1):111–119. doi: 10.1016/0092-8674(87)90361-8. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U., Choquet Y., Schneider M., Dron M., Bennoun P. Structural and transcription analysis of two homologous genes for the P700 chlorophyll a-apoproteins in Chlamydomonas reinhardii: evidence for in vivo trans-splicing. EMBO J. 1987 Aug;6(8):2185–2195. doi: 10.1002/j.1460-2075.1987.tb02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Sather S., Selkirk M., Agabian N. Identification of a small RNA containing the trypanosome spliced leader: a donor of shared 5' sequences of trypanosomatid mRNAs? Cell. 1984 Oct;38(3):721–729. doi: 10.1016/0092-8674(84)90267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Landfear S. M., Wirth D. F. Cloning and characterization of a Leishmania gene encoding a RNA spliced leader sequence. Nucleic Acids Res. 1986 Sep 25;14(18):7341–7360. doi: 10.1093/nar/14.18.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Hughes D. E., Simpson A. M., Simpson L. The monogenetic kinetoplastid protozoan, Crithidia fasciculata, contains a transcriptionally active, multicopy mini-exon sequence. Nucleic Acids Res. 1987 Apr 10;15(7):3141–3153. doi: 10.1093/nar/15.7.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing in nematodes. Exp Parasitol. 1989 Nov;69(4):413–416. doi: 10.1016/0014-4894(89)90191-4. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Trans splicing: variation on a familiar theme? Cell. 1987 Jul 17;50(2):147–148. doi: 10.1016/0092-8674(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano A., Scarlato V., Barni N., Cipollaro M., Franzè A., Macchiato M. F., Cascino A. Statistical evaluation of the coding capacity of complementary DNA strands. Nucleic Acids Res. 1984 Jun 25;12(12):5049–5059. doi: 10.1093/nar/12.12.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H. Discontinuous transcription and splicing in trypanosomes. Cell. 1986 Nov 21;47(4):479–480. doi: 10.1016/0092-8674(86)90608-2. [DOI] [PubMed] [Google Scholar]



