Abstract
Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) is the major enzyme in the prostate that reduces 4-androstene-3,17-dione (Δ4-Adione) to the androgen receptor (AR) ligand testosterone. AKR1C3 is upregulated in prostate cancer (PCa) and castrate resistant prostate cancer (CRPC) that develops after androgen deprivation therapy. PCa and CRPC often depend on intratumoral androgen biosynthesis and upregulation of AKR1C3 could contribute to intracellular synthesis of AR ligands and stimulation of proliferation through AR signalling. To test this hypothesis, we developed an LNCaP prostate cancer cell line overexpressing AKR1C3 (LNCaP-AKR1C3) and compared its metabolic and proliferative responses to Δ4-Adione treatment with that of the parental, AKR1C3 negative LNCaP cells. In LNCaP and LNCaP-AKR1C3 cells, metabolism proceeded via 5α-reduction to form 5α-androstane-3,17-dione and then (epi)androsterone-3-glucuronide. LNCaP-AKR1C3 cells made significantly higher amounts of testosterone-17β-glucuronide. When 5α-reductase was inhibited by finasteride, the production of testosterone-17β-glucuronide was further elevated in LNCaP-AKR1C3 cells. When AKR1C3 activity was inhibited with indomethacin the production of testosterone-17β-glucuronide was significantly decreased. Δ4-Adione treatment stimulated cell proliferation in both cell lines. Finasteride inhibited LNCaP cell proliferation, consistent with 5α-androstane-3,17-dione acting as the major metabolite that stimulates growth by binding to the mutated AR. However, LNCaP-AKR1C3 cells were resistant to the growth inhibitory properties of finasteride, consistent with the diversion of Δ4-Adione metabolism from 5α-reduced androgens to increased formation of testosterone. Indomethacin did not result in differences in Δ4-Adione induced proliferation since this treatment led to the same metabolic profile in LNCaP and LNCaP-AKR1C3 cells. We conclude that AKR1C3 overexpression diverts androgen metabolism to testosterone that results in proliferation in androgen sensitive prostate cancer. This effect is seen despite high levels of uridine glucuronosyl transferases suggesting that AKR1C3 activity can surmount the effects of this elimination pathway. Treatment options in prostate cancer that target 5α-reductase where AKR1C3 co-exists may be less effective due to the diversion of Δ4-Adione to testosterone.
Keywords: type 5 17β-hydroxysteroid dehydrogenase, 4-androstene-3, 17-dione, 5α-reductase, indomethacin, castrate resistant prostate cancer
1. Introduction
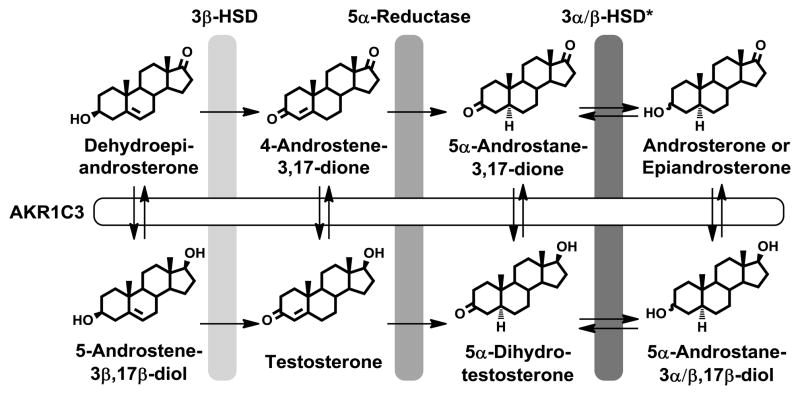
A critical step in the synthesis of androgen receptor (AR) ligands involves the conversion of 4-androstene-3,17-dione (Δ4-Adione) to testosterone, which is catalyzed by 17β-hydroxysteroid dehydrogenases type 3 (HSD17B3) and type 5 (aldo-keto reductase (AKR) 1C3, Fig. 1) [1–4]. Testosterone can then be converted to 5α-dihydrotestosterone (5α-DHT) by either type 1 or type 2 5α-reductase [5]. In testis, HSD17B3 is the predominant enzyme in catalyzing testosterone formation, in prostate however, synthesis of biologically active androgens proceeds via AKR1C3 [6–8]. Several studies indicate that AKR1C3 is overexpressed in prostate cancer and that expression increases with the progression of the disease [9–12].
Fig. 1.
Intratumoral androgen biosynthesis in prostate cancer that leads to the formation of the active androgens testosterone and 5α-dihydrotestosterone from the circulating inactive androgens dehydroepiandrosterone and/or 4-androstene-3,17-dione. The key role of AKR1C3 is shown. *The primary reductive 3α-HSD in prostate is thought to be AKR1C2, while RODH-like short chain dehydrogenase/reductase (17β-HSD Type 6) is the oxidative 3α-HSD in the prostate. AKR1C1 converts 5α-androstanes to 3β-hydroxysteroids but there is no evidence that it also catalyzes the reverse reaction.
Prostate cancer is driven by androgens and can be effectively suppressed by blocking androgen synthesis through orchiectomy or the use of a luteinizing hormone-releasing hormone agonist (e.g. leuprolide). However, in patients receiving androgen deprivation therapy, the androgen signaling pathway is eventually reactivated, resulting in castrate resistant prostate cancer (CRPC), which is fatal [13]. Two general mechanisms that result in the reactivation of the androgen axis in CRPC have been proposed [14]. One includes changes in AR signaling, such as AR mutations that result in ligand promiscuity [15,16], expression of a constitutively active AR [17], post-translational modification of the AR so it becomes ligand-independent [18], or AR gene amplification so that it can respond to trace amounts of ligand [19]. The other mechanism focuses on changes in intratumoral androgen synthesis, involving de novo synthesis of AR ligands from cholesterol, or increased conversion of adrenal androgens (e.g. dehydroepiandrosterone or Δ4-Adione) to active androgens. In support of the latter, affymetrix microarray data, validated by qRT-PCR, suggested reprogramming of the androgen biosynthetic pathway in advanced CRPC [9,10]. A dramatic increase in AKR1C3 expression was accompanied by a net decrease in 5α-reductase expression. Furthermore, measurement of intratumoral testosterone and 5α-DHT levels in castrate resistant disease showed that, consistent with the transcript levels, the ratio of these metabolites now favored testosterone over 5α-DHT synthesis [9] and that the key enzyme involved may be AKR1C3. By virtue of its 17β-HSD activity, AKR1C3 also converts 5α-androstane-3,17-dione to 5α-DHT. Thus irrespective of whether testosterone or 5α-DHT drives advanced disease the formation of these androgens must proceed through AKR1C3 (Fig. 1).
To gain a deeper understanding of the putative role AKR1C3 plays in androgen responsive prostate cancer, we explored the influence of upregulated AKR1C3 on intracellular androgen synthesis and cell proliferation in the LNCaP prostate cancer cell line. We monitored androgen metabolism and cell growth upon addition of the precursor Δ4-Adione in AKR1C3-negative LNCaP cells and in stably transfected LNCaP-AKR1C3 cells. We find that AKR1C3 overexpression resulted in the redirection of androgen metabolism which now favored testosterone-17β–glucuronide formation. We also find that LNCaP-AKR1C3 cells are now less sensitive to the growth inhibitory effects of the 5α-reductase inhibitor finasteride suggesting that sufficient testosterone is produced to override the effect of the drug, and overcome the elimination pathway catalyzed by uridine glucuronosyl transferases. Our data may have clinical utility, since they suggest that if AKR1C3 is overexpressed on a background of 5α-reductase activity this will lead to the unintended diversion of Δ4-Adione to testosterone with a resultant growth phenotype.
2. Methods
2.1. Chemicals and Reagents
Unlabeled steroids were purchased from Steraloids (Newport, RI). [4-14C]- Δ4-Adione was purchased from Perkin-Elmer Life Sciences (Waltham, MA). Indomethacin, finasteride, bicalutamide and β-glucuronidase (Type VII-A from E.coli; 5292000 units/g) were purchased from Sigma (St. Louis, MO). Media and cell culture reagents were from Invitrogen (Carlsbad, CA), except where noted otherwise. Organic solvents were from Fisher Scientific (Fair Lawn, NJ).
2.2. Stable Transfection of LNCaP Cells
Stable transfection of LNCaP cells with a pLNCX2-AKR1C3 vector containing the coding sequence for AKR1C3 (human type 5 17β-HSD) was accomplished using the methods previously described for MCF-7 cells [20], except that following infection, cells were maintained in RPMI with 10% FBS and 0.5 mg/mL geneticin. AKR1C3 expression levels in the parental and transfected cells were examined by RT-PCR using the isoform specific primer set: (forward primer) 5′ dGTA AAG CTT TGG AGG TCA C 3′ and (reverse primer) 5′ dCAC CCA TCG TTT GTC TCG T 3′, and by Western blot analysis using our isoform specific mouse monoclonal antibody against AKR1C3, as previously described [20].
2.3. Cell Culture and Western Blot of VCaP and CWR22Pc
VCaP cells were maintained in RPMI1640 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT), 1 % L-glutamate and 100 units/ml penicillin/streptomycin. CWR22Pc cells were maintained in DMEM medium supplemented with 10 % FBS, 1 % L-glutamate and 100 units/ml penicillin/streptomycin. Cells were collected and a cell lysate prepared for Western Blot analysis as described before [21]. Protein concentration was determined by Bradford assay.
2.4. Radiometric Determination of Androgen Metabolism
To monitor the metabolism of radiolabeled Δ4-Adione in LNCaP and LNCaP-AKR1C3 cells, 1 × 106 cells were plated in 6 well dishes and incubated overnight. Medium was replaced with 2 mL phenol-free RPMI with 1% charcoal stripped FBS (HyClone Laboratories, Inc., Logan, NV). For inhibitor studies, this medium contained indomethacin (30 μM) and/or finasteride (2 μM). After a 30 min equilibration period, [4-14C]-Δ4-Adione (0.096 μCi) in 0.25 % DMSO was added alone or mixed with unlabeled Δ4-Adione to attain a final concentration of 0.1 or 5 μM steroid, respectively. After incubation for 0, 6, 24, or 48 h, 1 mL medium was removed from the cells and extracted twice with 2 mL cold ethyl acetate. In order to determine the radioactivity in each phase, 100 μL each of the organic and aqueous fractions were added to 5 mL Ultima Gold (Perkin Elmer Life Sciences) scintillation fluid and analyzed with a TriCarb 2100 (Packard Instrument, Perkin Elmer Life Sciences) scintillation counter which detected [14C]-radioactivity with a machine efficiency of 99 %. The percentage of the blank corrected cpm in each phase was determined. The remainder of the organic fraction was dried under reduced pressure. The dried extracts were re-dissolved in 100 μl ethyl acetate containing 25 μg each of steroid reference standards and applied to LK6D Silica TLC plates (Whatman Inc., Clifton, NJ). TLC plates were developed using a dichloromethane/ethyl acetate (80:20 v/v) solution and were counted with a Bioscan System 200 plate reader (Washington, DC). Identification of products was determined by visualizing the reference standards with an acetic acid/sulfuric acid/anisaldehyde (100:2:1, v/v/v) solution and heating. This method did not achieve separation of androsterone and epiandrosterone. Quantification of the individual steroid analytes was achieved by multiplying the proportion of the radioactivity corresponding to each peak in the radiochromatogram by the proportion of the corrected cpm for the organic fraction from which it was derived. Three independent experiments were performed in triplicate for all data sets. Statistics were performed using a two-sided Student’s t-test.
The aqueous fraction was acidified to pH 6.5 with acetic acid and then subjected to treatment with 400 units of β-glucuronidase in 2 mL total volume at 37 °C for 24 h. Steroid extraction and analysis of the fractions were performed as described above.
2.5. Determination of Cell Proliferation
Medium from LNCaP and LNCaP-AKR1C3 cells was removed, cells were washed twice with DPBS (Dulbecco’s phosphate buffered saline) and then maintained in phenol-free RPMI containing 5 % charcoal stripped FBS (HyClone Laboratories, Inc., Logan, NV) for 72 h. Cells were seeded at 1 × 104 cells per well in a 96-well plate and kept in culture for another 48 h. To monitor the growth response of cells to androgens alone, steroid dissolved in DMSO was added to yield the final concentration of steroid in 0.1 % DMSO. To monitor the effects of enzyme inhibitors, 0.1 μM Δ4-Adione was added together with increasing concentrations of finasteride (0–30 μM), indomethacin (0–30 μM), or bicalutamide (0–10 μM), in a final concentration of 0.3 % DMSO. Nine days after addition of steroids, proliferation was stopped and quantified with an MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell proliferation kit (Roche), according to manufacturer’s protocol, using a Synergy 2 multi-detection microplate reader (BioTek). Growth response to androgens was normalized to the lowest concentration of added androgen (which was not significantly different from cells treated with either 0.1 or 0.3 % DMSO).
3. Results
3.1. Establishment of the LNCaP Prostate Cancer Cell Line Stably Expressing AKR1C3
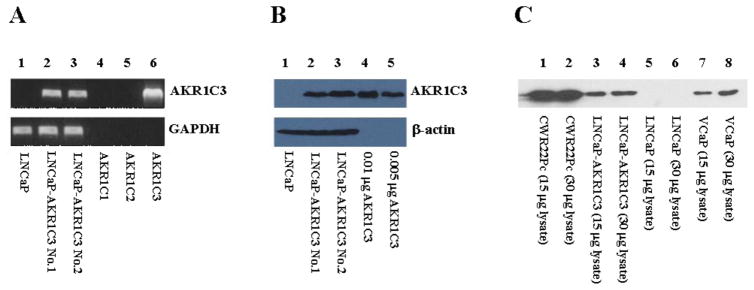
LNCaP cells are a widely used and well established cell line model to study prostate cancer and contain a mutated AR (T877A) [22]. However, AKR1C3 expression is either very low or completely absent in these cells as reported [1] (see also Fig. 2). As we sought to determine the effect of high, constitutive AKR1C3 expression in a prostate cancer cell setting, we generated an LNCaP cell line stably expressing AKR1C3 so that its properties could be compared to the parental LNCaP cells. We stably transfected parental LNCaP cells with a pLNCX2-AKR1C3 lentiviral vector and verified AKR1C3 expression at the transcript and protein level by using RT-PCR and immunoblot analyses, respectively (Fig. 2). RT-PCR gave a clear signal for AKR1C3 mRNA expression in two LNCaP-AKR1C3 cell lines (clone 1 and clone 2), while the parental cell line was negative (Fig. 2A). The primers used for RT-PCR were AKR1C3 specific and did not amplify the closely related isoforms AKR1C1 and AKR1C2 [3]. Western blot analysis using an AKR1C3 specific, monoclonal antibody [11], confirmed the expression of AKR1C3 in stably transfected cell lines and its absence in the parental cell line (Fig. 2B). Based on the intensity of the band from the LNCaP-AKR1C3 cells relative to those observed with 0.05 μg or 0.1 μg recombinant AKR1C3, we estimate that the level of expression achieved is approximately 0.1% of the soluble protein in the transfected cells. The level of AKR1C3 expression was comparable to that in VCaP and lower than in CWR22Pc cells (Fig. 2C). VCaP cells are derived from advanced prostate cancer and CWR22Pc cells can give rise to CRPC in murine xenograft models. All further experiments were carried out with the LNCaP-AKR1C3 clone 2 cell line.
Fig. 2.
AKR1C3 expression in the parental LNCaP and stably expressing LNCaP-AKR1C3 cell lines. (A) RT-PCR with AKR1C3 specific primers on cDNA generated from LNCaP and two LNCaP-AKR1C3 cell lines (lanes 1–3, respectively), and control plasmids containing the complete coding sequence for AKR1C1-3 (lanes 4–6, respectively). GAPDH was used as positive control. (B) Western Blots for AKR1C3 protein expression using 45 μg of LNCaP and LNCaP-AKR1C3 cell lysates (lanes 1–3, respectively), and signal intensities of different amounts of pure recombinant AKR1C3 (lanes 4–5, respectively). As a loading control, β-actin was used. (C) Western Blot detection of AKR1C3 protein expression in lysate of different prostate cancer cell lines.
3.2. Metabolism of Δ4-Adione in LNCaP and LNCaP-AKR1C3 Cells
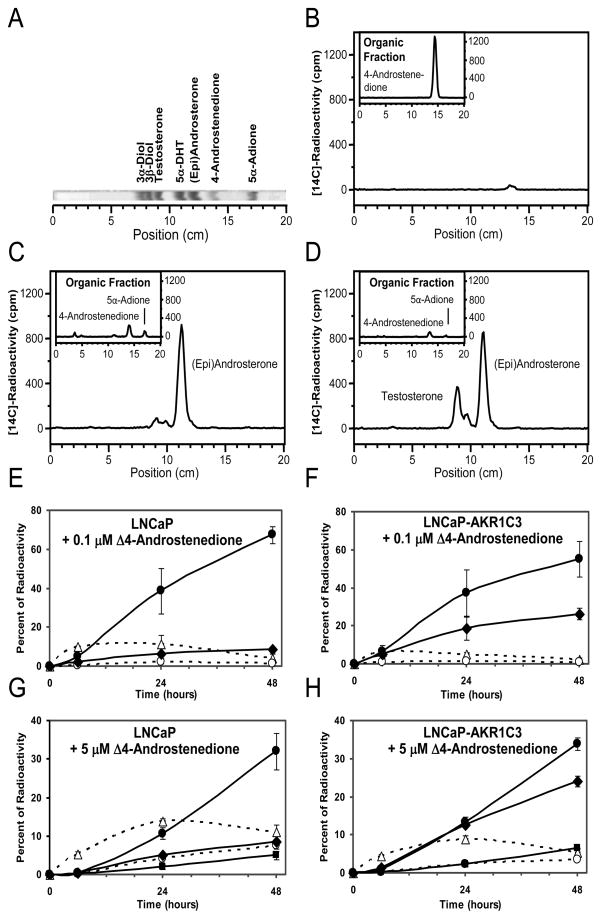
We followed the metabolism of 0.1 μM and 5.0 μM Δ4-Adione in LNCaP and LNCaP-AKR1C3 cells over 48 h using radio-TLC to separate (Fig. 3A) and quantify the downstream metabolites. At the 0 h time point, Δ4-Adione was the only detectable steroid in the medium (Fig. 3B). Within 48 h of incubation in LNCaP cells, 0.1 μM Δ4-Adione was almost completely consumed and concomitant formation of 5α-androstane-3,17-dione and the appearance of a large amount of radioactivity in the aqueous phase was observed (Fig. 3C). The aqueous radioactivity could be recovered primarily as either androsterone or epi-androsterone (which co-migrate), with a small amount of testosterone, following β-glucuronidase treatment (Fig. 3C and E). When 5.0 μM Δ4-Adione was used as substrate, (epi)androsterone could now be detected in the organic phase. Following β-glucuronidase treatment, a large amount of (epi)androsterone and small amounts of testosterone and 5α-DHT could be detected (Fig. 3G). At 5.0 μM, a larger proportion of radioactivity remained in the aqueous fraction after β-glucuronidase treatment, suggesting the presence of sulfate conjugates.
Fig. 3.
Metabolism of Δ4-Adione in LNCaP and LNCaP-AKR1C3 cells. (A) Authentic standards run on TLC and imaged as described in Materials and Methods. (B–D) Downstream metabolites generated from 0.1 μM [14C]-Δ4-Adione were identified at (B) time 0 or after 48 h incubation with (C) LNCaP and (D) LNCaP-AKR1C3 cells. Main panel shows metabolites recovered from the aqueous phase after β-glucuronidase treatment and re-extraction while insets show chromatogram of the organic fractions before β-glucuronidase treatment. Identification and quantification of steroids from both phases using 0.1 μM [14C]-Δ4-Adione as substrate gave rise to the time courses in LNCaP (E) and LNCaP-AKR1C3 cells (F). Identification and quantification of steroids from both phases using 5 μM [14C]-Δ4-Adione as substrate gave rise to the time courses in LNCaP (G) and LNCaP-AKR1C3 cells (H). Note the increase in testosterone-17β-glucuronide in panels (F) and (H). Error bars represent standard deviation of the mean n =3. 4-Androstenedione: Δ4-Adione; 5α-Adione: 5α-androstane-3,17-dione; 3α-Diol: 3α-androstane-3,17-diol; 3β-Diol: 3β-androstane-3,17-diol; solid line: glucuronidated steroid; broken line: free steroid; ●, (Epi)Androsterone-glucuronide; ◆, Testosterone-17β-glucuronide; ■, 5α-DHT-17β-glucuronide; ❍ (Epi)Androsterone; △, 5α-androstane-3,17-dione.
Metabolism of 0.1 μM Δ4-Adione in LNCaP-AKR1C3 cells also led to its complete consumption within 48 h and a large portion of radioactivity was recovered in the aqueous phase (Fig. 3D), similar to that seen in the parental cells (Fig. 3C). Treatment with β-glucuronidase now gave two large peaks, corresponding to (epi)androsterone and testosterone, showing that AKR1C3 functioned as an efficient 17β-hydroxysteroid dehydrogenase in this cell environment, (Fig. 3D and F). A similar pattern of metabolism was observed with 5.0 μM Δ4-Adione except that a small amount of 5α-DHT could now be detected as its glucuronide and there was more radioactivity remaining in the aqueous fraction after β-glucuronidase treatment (Fig. 3H). The LNCaP-AKR1C3 cells consistently made large amounts of testosterone-17β-glucuronide with both 0.1 μM and 5.0 μM Δ4-Adione, whereas the parental cells did not.
3.3. Metabolism of Δ4-Adione in LNCaP Cells and LNCaP-AKR1C3 Cells in the Presence of Finasteride
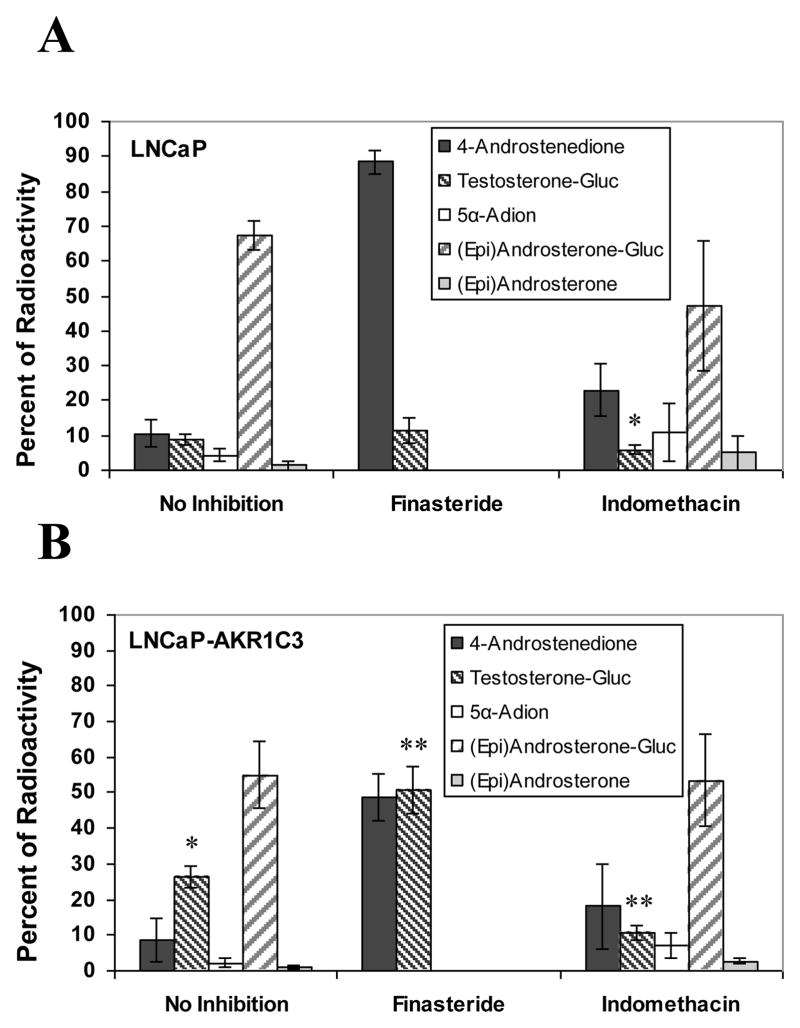
Finasteride is a mechanism based inhibitor of type 2 5α-reductase that also competitively inhibits type 1 5α-reductase at high nanomolar concentrations [23,24]. Addition of 2 μM finasteride to LNCaP cells blocked metabolism of 0.1 μM Δ4-Adione to 5α-andostane-3,17-dione and no (epi)androsterone was released from the aqueous phase following β-glucuronidase treatment (Fig. 4A). Finasteride treatment had little effect on the small amount of testosterone-17β-glucuronide formed in the parental cells. When the experiment was replicated in the LNCaP-AKR1C3 cells (Fig. 4B), we observed similar effects. However, testosterone-17β-glucuronide was now formed at much higher levels than in cells that did not receive finasteride. This suggests that in the presence of AKR1C3, Δ4-Adione metabolism was diverted from the formation of 5α-androstane-3,17-dione and the inactive metabolite (epi)androsterone in favor of the formation of testosterone-17β-glucuronide and that this was exacerbated by finasteride treatment.
Fig. 4.
Formation of androgens from Δ4-Adione in (A) LNCaP and (B) LNCaP-AKR1C3 cells in the presence or absence of finasteride and indomethacin. Cells were treated with 0.1 μM Δ4-Adione alone or in combination with 2.0 μM finasteride or 30 μM indomethacin. Error bars represent standard deviation of the mean n=3. Gluc: glucuronidase treatment; * p<0.05 vs LNCaP cells without inhibitor; ** p<0.05 vs LNCaP-AKR1C3 cells without inhibitor. 4-Androstenedione: Δ4-Adione; 5α-Adione: 5α-androstane-3,17-dione; -Gluc: glucuronidated form.
3.4. Metabolism of Δ4-Adione in LNCaP Cells and LNCaP-AKR1C3 Cells in the Presence of Indomethacin
Indomethacin and indomethacin analogues are selective competitive inhibitors for AKR1C3 yielding KI values in the low micromolar range [25]. We therefore measured the metabolism of 0.1 μM Δ4-Adione in LNCaP and LNCaP-AKR1C3 cells in the presence and absence of 30 μM indomethacin to determine the contribution of AKR1C3 to the observed metabolic profiles (Fig. 4). We found that indomethacin only slightly reduced the metabolism of Δ4-Adione in parental LNCaP cells (Fig. 4A). By contrast, indomethacin greatly reduced the formation of testosterone-17β-glucuronide in the LNCaP-AKR1C3 cells, down to the levels observed in parental cells (Fig. 4B).
3.5. Effect of Finasteride and Indomethacin on Δ4-Adione Induced Proliferation of LNCaP and LNCaP-AKR1C3 Cells
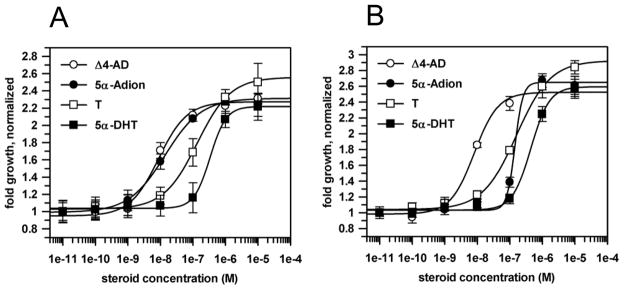
We studied the proliferation of LNCaP and LNCaP-AKR1C3 cells in steroid depleted medium in response to increasing concentrations of ketoandrogens (Δ4-Adione and 5α-androstane-3,17-dione) and hydroxyandrogens (testosterone and 5α-DHT). LNCaP cells were more responsive to the ketoandrogens than the hydroxyandrogens, with the trend being: Δ4-Adione = 5α-androstane-3,17-dione ≫ testosterone > 5α-DHT. In LNCaP-AKR1C3 cells the dose-response curve for 5α-androstane-3,17-dione was now significantly shifted to the right, consistent with AKR1C3 enhancing reduction of the 3- and/or 17-ketones leading to the glucuronidation of the corresponding alcohols. Surprisingly, there was no significant difference in the ability of Δ4-Adione to induce proliferation in the LNCaP and LNCaP-AKR1C3 cells and EC50 values observed were 8.3 nM and 8.9 nM, respectively (Fig. 5).
Fig. 5.
Growth response of LNCaP cells (A) and LNCaP-AKR1C3 (B) cells to a panel of androgens. Cell proliferation was measured by MTT assay. Error bars represent the standard deviation of the mean n =3. Δ4-AD: Δ4-Adione; 5α-Adion: 5α-androstane-3,17-dione; T: testosterone.
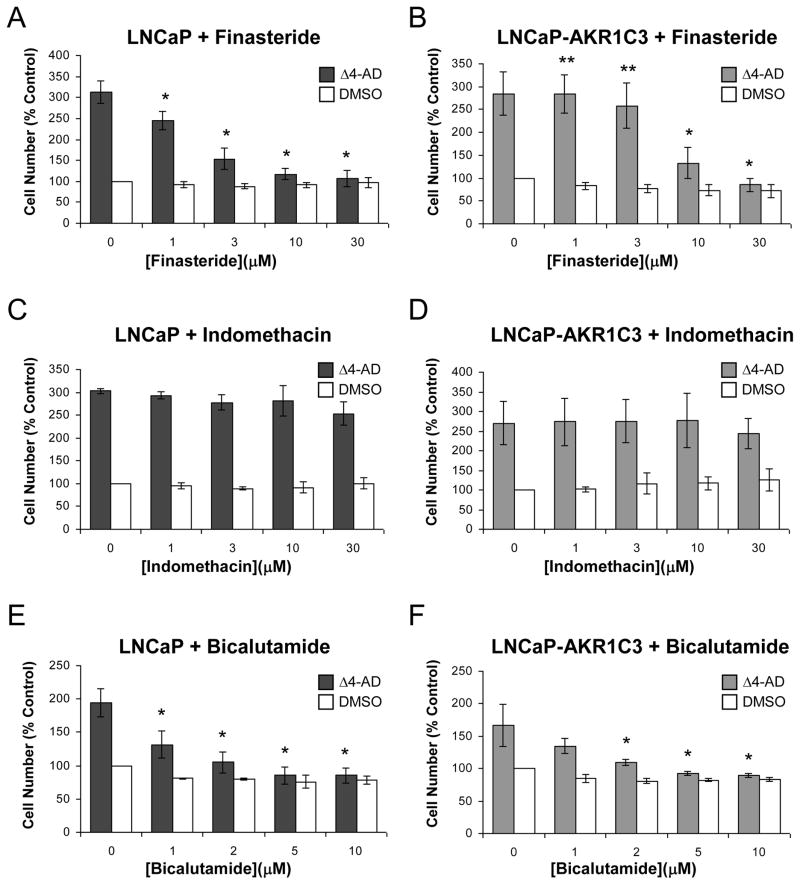
Upon addition of increasing concentrations of finasteride (0–30 μM), Δ4-Adione induced proliferation was significantly inhibited in both cell lines, and at 30 μM finasteride, the effect of the drug was identical to the no-steroid control (Fig. 6A and B). However, LNCaP-AKR1C3 cells were significantly less susceptible than parental cells to the inhibitory effects of finasteride and required higher concentrations to achieve the same level of inhibition. This observation was consistent with the increased production of testosterone observed in these cells. Upon addition of increasing concentrations of indomethacin, Δ4-Adione induced growth in LNCaP cells was unaffected (Fig. 6C), and LNCaP-AKR1C3 cell growth was only slightly susceptible to inhibition of AKR1C3 by indomethacin (Fig. 6D). This suggests that indomethacin treatment redirected Δ4-Adione metabolism in these cells back towards 5α-androstanedione-3,17-dione and thus the growth properties were indistinguishable from the parental cells treated with Δ4-Adione. In both cell lines, Δ4-Adione induced growth could be inhibited to a similar extent by administration of bicalutamide (Fig. 6E and F).
Fig. 6.
Δ4-Adione induced growth of LNCaP and LNCaP-AKR1C3 cells in response to finasteride, indomethacin and bicalutamide. Cell growth was induced by addition of 0.1 μM Δ4-Adione (Δ4-AD) and the effects of increasing concentrations of inhibitor were monitored. Values were normalized to 100% for growth response observed in the absence of inhibitor. (A) LNCaP cells treated with finasteride; *p <0.01 LNCaP with Δ4-Adione vs LNCaP with Δ4-Adione and finasteride; (B) LNCaP-AKR1C3 cells treated with finasteride; *p <0.01 LNCaP-AKR1C3 with Δ4-Adione vs LNCaP-AKR1C3 with Δ4-Adione and finasteride; **p <0.01 LNCaP-AKR1C3 with Δ4-Adione and finasteride vs LNCaP with Δ4-Adione and finasteride; (C) LNCaP cells treated with indomethacin; (D) LNCaP-AKR1C3 cells treated with indomethacin; (E) LNCaP cells treated with bicalutamide; and (F) LNCaP-AKR1C3 cells treated with bicalutamide; *p <0.05 cells with Δ4-Adione vs cells with Δ4-Adione and bicalutamide. Error bars are mean +/− SD, n=3–11. Δ4-AD: Δ4-Adione.
4. Discussion
AKR1C3 has been implicated in catalyzing the conversion of Δ4-Adione to testosterone in prostate cancer, based on expression data and measurement of intratumoral androgens [9,10]. It is also one of the most highly expressed genes in advanced prostate cancer and CRPC [9–12]. However, functional studies on its role in prostate cancer have been lacking. In our study we sought to illuminate the functional role of AKR1C3 in prostate cancer by investigating the effects of its overexpression on androgen metabolism and cell proliferation in the androgen sensitive LNCaP prostate cancer cell line. In this model we find that AKR1C3 redirects the metabolism of Δ4-Adione to testosterone-17β-glucuronide, and cells also become resistant to the growth inhibitory effects of finasteride. This implies that AKR1C3 produces sufficient testosterone to override the effects of finasteride on growth, and override the elimination of testosterone catalyzed by the uridine glucuronosyl transferases (UGTs) which are also highly overexpressed in CRPC [10]. We also find that indomethacin, a selective AKR1C3 inhibitor, can block the formation of testosterone in this setting. Thus, LNCaP-AKR1C3 cells provide a useful tool in which to screen AKR1C3 inhibitors.
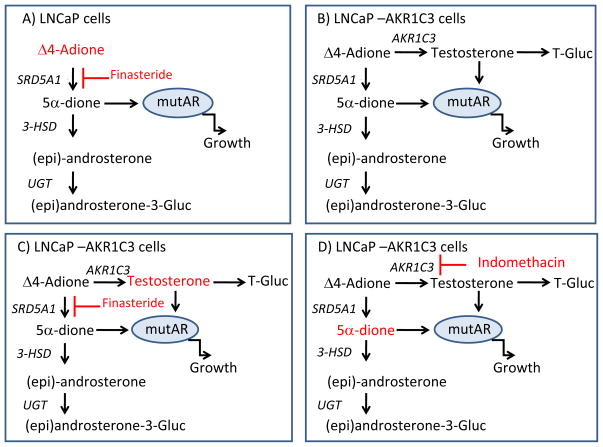
Recently, a study of Δ4-Adione metabolism in different prostate cancer cell lines showed that the synthesis of 5α-DHT by-passed testosterone since the immediate precursor 5α-androstane-3,17-dione was formed [26]. However, that study did not directly conduct metabolism using 5α-androstane-3,17-dione as a precursor of 5α-DHT, and the expression level of AKR1C3 was not addressed. In agreement with this study we find that metabolism of Δ4-Adione in LNCaP cells mainly proceeds via the 5α-reduction pathway and identified 5α-androstane-3,17-dione as the major organic soluble metabolite formed. However, due to presence of androgen conjugating UGT2B15 and UGTB2B17 in LNCaP cells [27], additional examination of glucuronidated metabolites in the aqueous phase was necessary. Our results revealed that (epi)androsterone-glucuronide is the principle product formed by LNCaP cells and that only basal amounts of testosterone-17β-glucuronide are formed (Fig. 7A).
Fig. 7.
Androgen metabolism and AR stimulated growth in LNCaP and LNCaP-AKR1C3 cells. (A) The major routes of Δ4-Adione mediated metabolism and AR activation in LNCaP cells. In these cells 5α-androstane-3,17-dione (5α-dione) likely stimulates the mutated AR. In the presence of finasteride, Δ4-Adione accumulates leading to growth suppression; the role of the minor amount of testosterone formed is not shown for clarity. (B) The major routes of Δ4-Adione mediated metabolism and AR activation in LNCaP-AKR1C3 cells; two ligands for the AR are now produced, testosterone and 5α-dione. (C) When LNCaP-AKR1C3 cells are treated with finasteride, Δ4-Adione metabolism to 5α-reduced steroids is blocked, testosterone accumulates and cells are resistant to the growth inhibitory effects of this drug. (D) When LNCaP-AKR1C3 cells are treated with indomethacin, testosterone is no longer produced and metabolism and growth effects revert back to that seen for the parental cells, where 5α-dione drives proliferation.
These findings suggest that LNCaP cells contain endogenous 5α-reductase, reductive 17β-HSD, and reductive 3α- or 3β-HSD activities. Real-time PCR analyses in these cells detected 5α-reductase type 1, but not type 2, as the 5α-reductase responsible and also detected 17β-HSD types 1, 4, 7 and 10 [28]. However, of these only the type 1 enzyme has been shown to have a low capacity to reduce Δ4-Adione to testosterone [29,30]. AKR1C isoforms can act as reductive 3α/β-HSD and could be responsible for the formation of (epi)androsterone but their expression could not be detected by RT-PCR (data not shown). Therefore, the identity of the enzymes responsible for the observed reductive 17β-HSD and 3α/β-HSD activities in parental LNCaP cells remains unknown.
Growth of LNCaP cells in response to Δ4-Adione relies on the 5α-reduced steroids. Lack of substantial 17β-HSD activity leads to the formation of 5α-androstane-3,17-dione that activates the mutated AR present in LNCaP cells. It has been shown that the T877A mutation in the AR ligand binding domain present in LNCaP is a common mutation that occurs in about 18% of all prostate cancer [15,31], and it renders the receptor ligand permissive [28,32]. The significance of 5α-reduced steroids in regulating LNCaP cell growth is demonstrated by the finding that finasteride treatment inhibited cell growth (Fig. 7A). Although testosterone formation in LNCaP cells was increased slightly upon finasteride treatment this was insufficient to surmount the effects of 5α-reductase inhibition. Testosterone formation in LNCaP cells treated with finasteride has been observed before [26] and has been ascribed to background levels of AKR1C3. However, indomethacin treatment inhibited less than one- third of the testosterone formed suggesting involvement of another 17β-HSD isoform in the basal formation of this hormone.
Expression of AKR1C3 in LNCaP cells redirected Δ4-Adione metabolism so that a significant amount of testosterone, in the form of testosterone-17β-glucuronide, was now produced (Fig. 7B). The lack of effect of AKR1C3 on cell proliferation in response to Δ4-Adione might be explained by the formation of the active androgen testosterone offsetting the decreased levels of 5α-androstane-3,17-dione produced. While testosterone was not as effective at inducing proliferation as either Δ4-Adione or 5α-androstane-3,17-dione when added directly to the cells, it is likely that the slow generation of low levels of testosterone over time might be more efficacious than adding a single bolus of testosterone that would be rapidly glucuronidated. Notably, proliferation of LNCaP and LNCaP-AKR1C3 cells was found to be less responsive to 17β-hydroxyandrogens (testosterone and 5α-DHT) probably due to high glucuronidation activity, which will lead to rapid inactivation of added testosterone and 5α-DHT.
Effects of AKR1C3 expression on cell proliferation were however evident by the loss of sensitivity in growth response to 5α-androstane-3,17-dione. AKR1C3 has a high catalytic efficiency for this substrate and can reduce the 3- and 17-ketone groups to alcohols leading to their glucuronidation [3,6] and rapid clearance of 5α-androstane-3,17-dione which acts as a ligand for the mutated AR in this cell line.
AKR1C3 expression led to the increased production of testosterone which was doubled in the presence of finasteride. As a result, the levels of testosterone observed in the LNCaP-AKR1C3 cells treated with this drug were now elevated by five-fold over the parental cells (Fig. 4). Consistent with the metabolism data, LNCaP-AKR1C3 cells were now more resistant to the growth inhibiting properties of finasteride, most likely due to redirection of Δ4-Adione metabolism away from the 5α-reduced androstanes to testosterone formation mediated by AKR1C3 (Fig. 7C).
Importantly, the conversion of Δ4-Adione to testosterone-17β-glucuronide was blocked by indomethacin, indicating that this drug blocks the formation of active androgens in a prostate cancer cell setting. However, indomethacin had only a limited effect on LNCaP-AKR1C3 cell growth mediated by Δ4-Adione because the overall metabolic profile had now reverted to that of the parental cells (Fig. 7D). As a result, formation of 5α-androstane-3,17-dione once again became the preferred ligand responsible for activating the mutated AR [28].
CRPC is characterized by changes in AR signaling and/or adaptive intratumoral androgen biosynthesis that includes increased expression of AKR1C3 [9,10]. Consistent with these expression profiles intratumoral androgens showed a shift from 5α-DHT to testosterone as the predominant AR ligand present. Overexpression of AKR1C3 in LNCaP cells provides a functional explanation for this clinical finding, since it leads to a shift in the dominant 5α-reductase pathway that leads to 5α-androstane-3,17-dione to a significant production of testosterone. Most importantly, although AKR1C3 expression in itself did not result in changed growth in response to Δ4-Adione, production of an alternative AR ligand (e.g. testosterone) decreased susceptibility of the cells to the growth inhibitory effects of the 5α-reductase inhibitor finasteride. The growth effects observed in the LNCaP and LNCaP-AKR1C3 cells were blocked by the AR antagonist bicalutamide showing that they were AR mediated.
Our results may have therapeutic consequences for the use of inhibitors that target type 1 and/or type 2 5α-reductase, e.g. finasteride or dutasteride, to block intratumoral androgen synthesis in prostate cancer. Prevention of the sequential 5α- and 3α/β-reduction of Δ4-Adione to (epi)androsterone by 5α-reductase inhibitors could cause a build-up of substrate that would promote the formation of the AR ligand testosterone when AKR1C3 is also present. However, inhibition of AKR1C3 would prevent the conversion of Δ4-Adione to testosterone and the conversion of 5α-androstane-3,17-dione to 5α-DHT which are potent ligands for the AR. Under these conditions Δ4-Adione and 5α-androstane-3,17-dione would accumulate. These steroids are poor ligands for the wild type AR but could still activate the mutated AR receptor (T877A) present in prostate cancer. This sequence would explain why indomethacin blocks the conversion of Δ4-Adione to testosterone but has little or no effect on cell growth in LNCaP-AKR1C3 cells.
We conclude that the beneficial effects of 5α-reductase inhibitors in the treatment of prostate cancer will depend on whether AKR1C3 is expressed or not. In its presence Δ4-Adione metabolism will be redirected to testosterone. We also conclude that an AKR1C3 inhibitor will be beneficial for the treatment of prostate cancer if the AR is wild type since the AR will be deprived of the AKR1C3 products testosterone and 5α-DHT. However, an AKR1C3 inhibitor would be deleterious in the presence of 5α-reductase and mutated, ligand promiscuous AR since 5α-androstane-3,17-dione would now accumulate and activate this form of the receptor. Thus personalized approaches to androgen-deprivation therapy will require a determination of 5α-reductase, AKR1C3 and wild type AR expression within the tumor.
Acknowledgments
We thank Dr. Marja T. Nevalainen for the kind gift of CWR22Pc cells. We thank Christopher Yarosh for help with Western Blotting. Supported by grants R01-CA90744, P30-ES013508 and a Prostate Cancer Foundation Challenge grant awarded to TMP. MCB was funded in part by NIH training grants T32-DK007314-25 and T32-HD007305-22.
Footnotes
Disclosure
No potential conflict of interest to disclose for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fung KM, Samara EN, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JV, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13(1):169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 2.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology. 1999;140(2):568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 3.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DW, Wilson JD. Steroid 5 α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 6.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 α/17 β-HSD activity and cellular distribution. Mol Endocrinol. 1997;11(13):1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 7.Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 β-hydroxysteroid dehydrogenase 3. Nat Genet. 1994;7(1):34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 8.El-Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. Localization of type 5 17β-hydroxysteroid dehydrogenase, 3β-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology. 1999;140(3):1481–1491. doi: 10.1210/endo.140.3.6585. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69(13–14):795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, Schroder FH, de Jong FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70(3):1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010;21(5):315–324. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan G, Greenberg NM, Scher HI, Harris JM, Marshall VR, Tilley WD. Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res. 2001;7(5):1273–1281. [PubMed] [Google Scholar]
- 17.Lapouge G, Marcias G, Erdmann E, Kessler P, Cruchant M, Serra S, Bergerat JP, Ceraline J. Specific properties of a C-terminal truncated androgen receptor detected in hormone refractory prostate cancer. Adv Exp Med Biol. 2008;617:529–534. doi: 10.1007/978-0-387-69080-3_53. [DOI] [PubMed] [Google Scholar]
- 18.Reddy GP, Barrack ER, Dou QP, Menon M, Pelley R, Sarkar FH, Sheng S. Regulatory processes affecting androgen receptor expression, stability, and function: potential targets to treat hormone-refractory prostate cancer. J Cell Biochem. 2006;98(6):1408–1423. doi: 10.1002/jcb.20927. [DOI] [PubMed] [Google Scholar]
- 19.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 20.Byrns MC, Duan L, Lee SH, Blair IA, Penning TM. Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J Steroid Biochem Mol Biol. 2010;118(3):177–187. doi: 10.1016/j.jsbmb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drury JE, Mindnich R, Penning TM. Characterization of disease-related 5β-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency. J Biol Chem. 2010;285(32):24529–24537. doi: 10.1074/jbc.M110.127779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 23.Iehle C, Delos S, Guirou O, Tate R, Raynaud JP, Martin PM. Human prostatic steroid 5 α-reductase isoforms--a comparative study of selective inhibitors. J Steroid Biochem Mol Biol. 1995;54(5–6):273–279. doi: 10.1016/0960-0760(95)00134-l. [DOI] [PubMed] [Google Scholar]
- 24.Bull H, Garcia-Calvo M, Andersson S, Baginsky W, Chan H, Ellsworth D, Miller R, Stearns R, Bakshi R, Rasmusson G, Tolman R, Myers R, Kozarich J, HGS Mechanism-based inhibition of human steroid 5α-reductase by finasteride: Enzyme-catalyzed formation of NADP-dihydrofinasteride, a potent bisubstrate analog inhibitor. Journal of the American Chemical Society. 1996;118:2359–2365. [Google Scholar]
- 25.Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3α-HSD, type 5 17β-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75(2):484–493. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouinard S, Barbier O, Belanger A. UDP-glucuronosyltransferase 2B15 (UGT2B15) and UGT2B17 enzymes are major determinants of the androgen response in prostate cancer LNCaP cells. J Biol Chem. 2007;282(46):33466–33474. doi: 10.1074/jbc.M703370200. [DOI] [PubMed] [Google Scholar]
- 28.Laplante Y, Poirier D. Proliferative effect of androst-4-ene-3,17-dione and its metabolites in the androgen-sensitive LNCaP cell line. Steroids. 2008;73(3):266–271. doi: 10.1016/j.steroids.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Nokelainen P, Puranen T, Peltoketo H, Orava M, Vihko P, Vihko R. Molecular cloning of mouse 17 β-hydroxysteroid dehydrogenase type 1 and characterization of enzyme activity. Eur J Biochem. 1996;236(2):482–490. doi: 10.1111/j.1432-1033.1996.00482.x. [DOI] [PubMed] [Google Scholar]
- 30.Puranen T, Poutanen M, Ghosh D, Vihko R, Vihko P. Origin of substrate specificity of human and rat 17β-hydroxysteroid dehydrogenase type 1, using chimeric enzymes and site-directed substitutions. Endocrinology. 1997;138(8):3532–3539. doi: 10.1210/endo.138.8.5303. [DOI] [PubMed] [Google Scholar]
- 31.Shi XB, Ma AH, Xia L, Kung HJ, de Vere White RW. Functional analysis of 44 mutant androgen receptors from human prostate cancer. Cancer Res. 2002;62(5):1496–1502. [PubMed] [Google Scholar]
- 32.Grigoryev DN, Long BJ, Njar VC, Brodie AH. Pregnenolone stimulates LNCaP prostate cancer cell growth via the mutated androgen receptor. J Steroid Biochem Mol Biol. 2000;75(1):1–10. doi: 10.1016/s0960-0760(00)00131-x. [DOI] [PubMed] [Google Scholar]