Abstract
Introduction
A significant proportion of patients undergoing endovascular aneurysm repair (EVAR) have common iliac artery aneurysms (CIAA). Aneurysmal involvement at the iliac bifurcation potentially undermines long-term durability.
Methods
Patients who underwent EVAR with CIAA were identified in two teaching hospitals. Bell-bottom technique (iliac limb ≥ 20mm) (BBT) or internal iliac artery embolization and limb extension to the external iliac artery (IIE+EE) were used. Outcome between these two approaches are compared.
Results
One hundred and eighty five patients were identified. . Indication for EVAR included asymptomatic AAA (n=157), symptomatic or ruptured aneurysm (n=19), and common iliac artery aneurysm (n=9). Mean AAA diameter was 59 mm. A total of 260 large CIAAs were treated. One hundred and sixty six CIAA limbs were treated with BBT, 94 limbs underwent IIE+EE. Total reintervention rates were similar for BBT (n=19, 11%) and IIE+EE (n=18, 19.1%) (p=0.149). Similar rates of reintervention for type 1b or 3 endoleak are reported, BBT (n=7, 4%) versus IIE+EE (n=4, 4%) (p=1.0). There was no significant difference in limb patency rates. Thirty-day mortality was 1%. Median follow-up was 22 months. While there was no significant difference in complications between the two groups the combined incidence of perioperative complications and reinterventions was higher in the IIE+EE group (49% versus 22%, p-0.002).
Conclusion
The combined incidence of perioperative complications and reinterventions is significantly higher in the IIE+EE when compared with the BB technique. Therefore, when feasible, BB is desirable..
INTRODUCTION
Endovascular abdominal aortic aneurysm repair (EVAR) has evolved as a feasible, less invasive alternative to open repair.1-3 Limiting the long-term complications specific to EVAR is a challenge to interventionalists. Poor patient or stent-graft selection undermines the effectiveness of EVAR.4 Various pre-, intra-, and postoperative factors may compromise repair. To date, most studies report the outcome of EVAR in patients with adverse morphological features at the proximal seal, including neck angulation, diameter, and thrombus.5-7 Postoperative aortic remodeling following successful sac exclusion and late progression of aneurysmal degeneration may predispose to late endoleaks.8,9
The common iliac artery serves as the distal stent-graft implantation site. Concomitant CIAAs are present in 15-40% of patients with AAA.10 Like the proximal landing zone, a durable distal seal is essential. Alternatively, deploying additional stent-grafts into external iliac arteries may compromise long-term stent-graft patency.11 There are conflicting results on whether concomitant ectasis or CIAA limits full exclusion of the aneurysm and increases the complexity of EVAR.10-14 A variety of open and endovascular techniques are available to treat these patients. In the absence of many comparative studies, standardization of treatment is poor.15,16 The two most commonly performed procedures are internal iliac embolization/occlusion with extension of stent-graft to the external iliac extension (IIE+EE) and flared limb or bell-bottom technique to the CIA (BBT). In this present study, outcome between these two approaches is compared.
MATERIALS AND METHODS
A retrospective review was performed of all patients who underwent EVAR in two large university teaching hospitals, Northwestern Memorial Hospital (NMH), Chicago, IL, USA, and St. James’s Hospital (SJH), Dublin, Ireland, between January 2004 and December 2009. Both hospitals are high volume vascular centers with an annual caseload of over 50 EVAR and more than 350 EVARs have been performed in each institution since their endovascular programs were initiated. All patients treated by EVAR with an iliac stent-graft diameter ≥20 mm (BBT) or IIE+EE were identified. Patients were identified from a prospectively collected database documenting demographics, presentation, procedure, and outcome. Data collection was performed according to approved Institutional Review Board protocols.
A pre-interventional computed tomography angiogram (CTA) with intravenous contrast and multiplanar reconstruction was used in all patients to assess extent of aneurysmal disease, tortuosity of the iliac vessels, and patency of the internal and external iliac arteries. All EVAR were completed in the operating angio-suite equipped with a fixed fluoroscopic unit. EVAR was performed under general anesthesia (n = 122), spinal anesthesia (n = 58), or local anesthesia (n=5). Access was achieved by entirely percutaneous access using the suture-mediated closure “Preclose” technique in 65 patients (35%),the remaining patients needed cut-down femoral artery exposure.17
In the IIE+EE group, embolization was performed preferentially via a contra-lateral approach before EVAR. Technique used depended on anatomy and operator preference. IIE was achieved using an Amplatzer vascular plug (AGA Medical, Golden Valley, MN) delivered though an appropriate sized guiding sheath or by 0.035 inch coils (MR Eye or Nestor Coils, Cook Medical Inc., Bloomington, IN) delivered through a selection of 5 - 6 French catheters. The IIA was occluded at the origin except when the presence of an IIA aneurysm precluded flush occlusion and proximal embolization; in these circumstances, the primary branches of the IIA were embolized.
Initially, the BBT involved deploying an aortic extension cuff or a reverse-mounted iliac limb stent-graft in the distal CIA landing zone using techniques previously described.16,18 Recently this technique has been superseded by the introduction of commercially available large diameter iliac extension limbs of up to 28 mm diameter.19
Follow-up
During the postoperative period, the patient’s progress was closely monitored and complications noted by the operating team. Acute renal failure (ARF) was defined as any increase in the creatinine level greater than 3.0 mg/dL with or without the need for dialysis. Respiratory failure was defined as any patient with postoperative pneumonia, respiratory insufficiency or prolonged intubation. Myocardial infarction was defined by at least two of the following criteria: typical chest pain lasting 20 minutes or more; serum levels of creatine kinase, creatine kinase MB, or troponin at least twice the upper limit of the normal range; and new Q wave on at least two adjacent derivations or predominant R waves in V1 (R wave ≥1 mm >S wave in V1). Clinically significant hematoma is any hematoma that prolonged hospital stay or necessitated reintervention.
All patients had clinical examination and CTA at one month and annually thereafter in NMH. In SJH, duplex ultrasound scanning with selective CTA surveillance was performed as previously validated.20
Statistics
The SPSS® 18.0 software package (SPSS Inc., Chicago, IL) was used for statistical analysis. Normally distributed continuous data were expressed as mean ± standard deviation (SD), while median (inter-quartile range) was used to describe the non-normally distributed continuous data. Student t-tests were used, as appropriate, for comparison of continuous variables and the Chi-square test was employed for analysis of categorical variables. All tests were two sided and a result was considered significant if the calculated P value was <0.05.
Differences in limb patency and rate of type 1b/3 endoleak between the two groups were determined with Kaplan-Meier survival analysis and compared by log-rank testing
RESULTS
Demographics
Among the 185 patients treated with EVAR, 260 concomitant large CIA were treated. Mean age was 73±8.1 years and 93.5% were men. Demographics and comorbid conditions were similar between the BBT and IIE+EE groups (Table 1).
Table 1.
Patient demographics
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Patients Characteristics | Total (N=260) | BBT N=166 |
IIE+EE N=94 |
P valuea | |||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 73 | 8.1 | 72.7 | 8.0 | 73.5 | 8.4 | 0.468 |
| Diameter AAA (mm) | 59.2 | 14 | 59.5 | 13.3 | 58.6 | 16 | 0.627 |
| (B) | |||||||
|---|---|---|---|---|---|---|---|
| Patients Characteristics | Total (N=260) | BBT N=166 |
IIE+EE N=94 |
P valueb | |||
| n | % | n | % | n | % | ||
| Gender (male) | 243 | 93.5% | 156 | 94 | 87 | 92.6 | 0.656 |
| Hypertension | 196 | 75.7% | 119 | 72.1 | 77 | 81.9 | 0.077 |
| COPD | 22 | 8.5% | 18 | 10.8 | 4 | 4.3 | 0.067 |
| Diabetes | 36 | 13.9% | 18 | 10.9 | 18 | 19.1 | 0.065 |
| Smoking | 88 | 34.2% | 58 | 35.2 | 30 | 32.6 | 0.680 |
| Hyperlipidaemia | 147 | 56.8% | 96 | 58.2 | 51 | 34.3 | 0.540 |
| IHD | 107 | 41.2% | 74 | 44.6 | 33 | 35.1 | 0.136 |
| Renal disease | 6 | 2.3% | 3 | 1.8 | 3 | 3.2 | 0.475 |
| (C) | |||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Total (N=260) | BBT N=166 |
IIE+EE N=94 |
P valueb | |||
| n | % | n | % | n | % | ||
| 30 days mortality | 2 | 1.1% | 2 | 1.7% | 0 | 0% | 0.286 |
|
Combined reinterventions &
Complications |
51 | 28.3% | 26 | 22% | 25 | 49% | 0.002* |
Student t-test
Chi-square or Fisher exact test
Procedural results
EVAR and CIAA exclusion was achieved using a number of commercially available stent-graft devices; 107 had Excluder (W. L. Gore, Flagstaff, AZ); 67 had Talent or Endurant (Medtronic, Minneapolis, MN); 56 had Zenith (Cook Medical Inc, Bloomington, IN), and 26 had other devices (AneuRx, Ancure, and Endologix).
One hundred and sixty-six (64%) of patients were treated by BBT, 94 (36%) underwent IIE+EE. Interestingly, a higher proportion of patients treated with the Excluder device were treated by IIE+EE (52%) compared to Talent/Endurant (18%) or Zenith (19%) (p<0.0001) (Table 2). IIE was completed using an Amplatzer vascular plug (n=41), coils (n=47), and a combination of coils and plug (n=6). In two patients with bilateral CIAA an external to internal iliac artery bypass was performed prior to bilateral IIE. Both patients were young, active males who opted for this additional procedure to preserve pelvic perfusion rather than undergo staged IIE. An additional patient who underwent IIE was noted to have a tight stenosis at the origin of the contralateral IIA that was treated with angioplasty and deployment of a balloon-expandable stent at the time of EVAR. In the BBT group, the average distal limb diameter was 20 mm (range 20-28).
Table 2.
Type of Stent-Graft Device used in the Two Treatment Groups
| Type of stent | BBT | IIE+EE |
|---|---|---|
| Cook | 46 (82.1%) | 10 (17.9%) |
| Medtronic | 57 (85.1%) | 10 (14.9%) |
| Gore | 51 (47.7%) | 56 (52.3%) |
| Others | 12 (46.2%) | 14 (53.8%) |
Perioperative Complications
The overall complication rate was 28%. Although not statistically significant, more procedure-specific, access related, and general complications were noted in the internal iliac artery embolization group compared to the BBT group.
Specific complication rate was 9% in the BBT group compared to 10.6% in the internal iliac embolization group (p=0.674). Specific complications reported in the IIE patients included buttock claudications (2.1%), limb occlusion (1.1%), and pelvic ischemia (1.1%); while in BBT patients, complications included kinked limb (0.6%) and limb occlusion (2.4%). When the IIE group was further analyzed, more specific complications were noted in patients who underwent bilateral embolization compared to those who had unilateral artery embolization (38.5% vs. 6.2%, p<0.001).
Analysis of access related complications showed no significant difference between the two groups (11.7% in IIE vs. 7.2% in BBT, p=0.222). Hematoma was the most common access related complication (6.4% and 6% of access related complications in IIE and BBT, respectively). In addition, 5.4% of access related problems were wound pain and infection in IIE compared to 1.8% in BBT group.
General complications included acute myocardial infarction (n=3), acute renal failure (n=2), non-specific bowel conditions (pain, diarrhea) (n=3), respiratory tract infection (6), urinary tract infection (n=1). Comparing both groups, no significant difference was noted in general complications (15.7% in BBT vs. 25.5%, p=0.052).
There were only two 30-day mortalities. Both of these patients (one from each treatment group, 1.1%) presented with ruptured AAA.
Reintervention
Median follow-up was 22 months (inter-quartile range 9-38 months). Overall reintervention rate was 14%, 11 patients (4%) had reintervention for a type 1b or III endoleak and 7 (3%) to maintain iliac limb patency (Figure 1). Comparing outcome of the BBT to IIE+EE groups, there was no significant difference in total reintervention (11.6 % versus 19%, p=0.15) (Figure 2), type 1b or III endoleak (4% versus 4%, p= 0.888) (Figure 3), or limb patency (3% versus 2%, p=0.566) (Figure 4). On subgroup analysis, there was no difference in outcome in the BBT group comparing subgroups that were treated with iliac limbs diameter 20 or 22 mm (n=146) compared to those treated with 24 or 28 mm (n=20) limbs (p>0.05).
Figure 1.
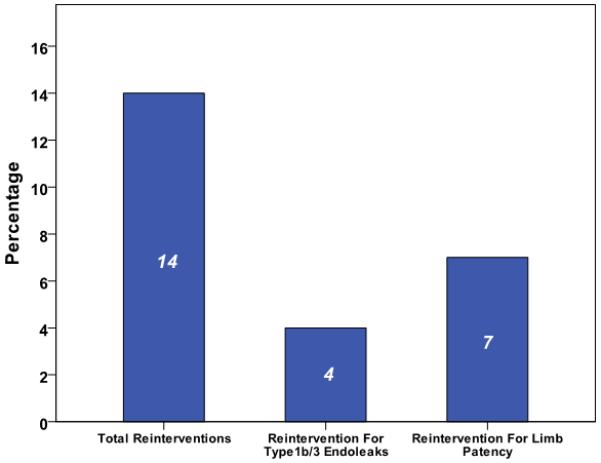
Reintervention rates in our cohort of patients.
Figure 2.
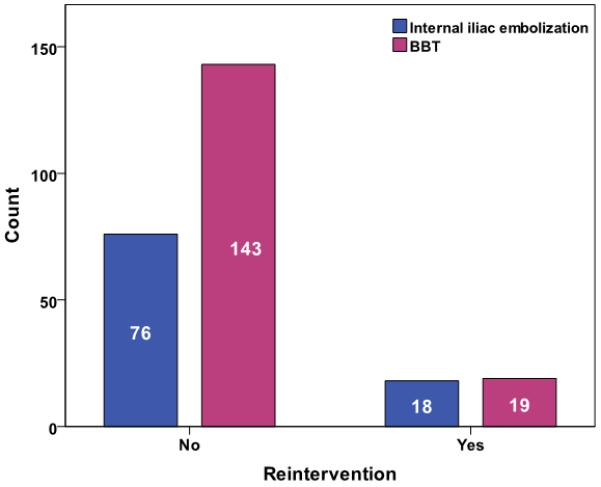
Overall reinterventions comparing BBT to ILE+EE
Figure 3.
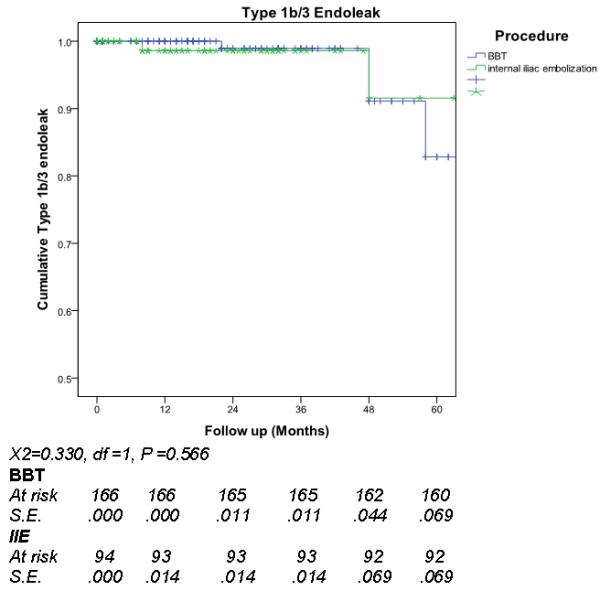
Reintervention for type 1b/3 endoleaks comparing large iliac (BBT) to internal iliac embolization (IIE)
Figure 4.
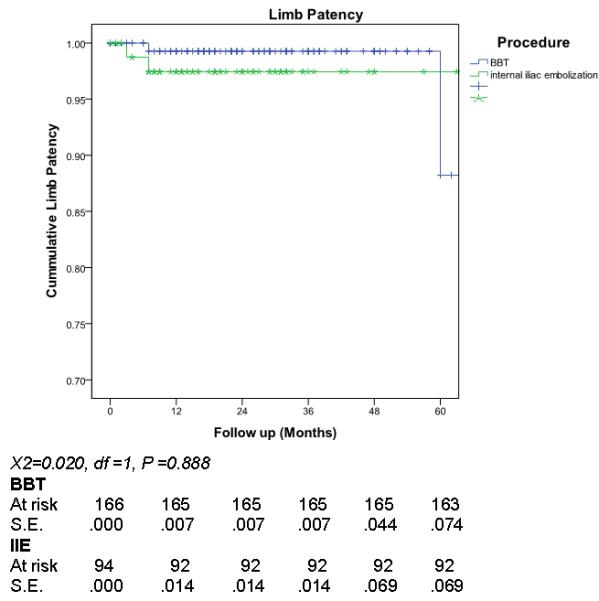
Limb patency comparing large iliac (BBT) to internal iliac embolization (IIE)
DISCUSSION
EVAR is based on the successful exclusion of blood flow into the aneurysmal sac. A significant number of patients undergoing EVAR have concomitant CIAA. These CIAA may undermine the benefit of EVAR if a robust distal seal is not achieved. Patients with CIAA may have unfavorable anatomical features and more extensive medical comorbidities.13
The importance of the distal seal zone is highlighted in three large single-center studies examining reintervention following EVAR.21-23 Problems at the distal landing zone were a more common indication for reintervention than proximal seal compromise in all three studies. Mehta et al report the largest single-center experience of EVAR (n=1768) with a reintervention rate of 19.2%.23 Progressive iliac artery aneurysm formation was the third most common indication for reintervention (11.5%) and 7.4% of reinterventions were for iliac limb thrombosis. Iliac limb occlusion was the second most common indication for reintervention in a series of 832 EVAR patients.22 In our experience of endovascular reintervention following EVAR, type 1b endoleak was the most common indication for reintervention.4
Few studies focus on the outcome of patients with concomitant CIAA during EVAR and results are conflicting.10,12-14 Increased complexity of procedure, AAA-related complications, and reinterventions are reported in three studies.12-14 Hobo et al (n=6668) report that those with concomitant CIAA had a higher incidence of type 1b endoleaks, iliac limb occlusion, reinterventions, and aneurysm rupture.13 Albertini et al had similar results in patients with CIAA extending to the distal third of the CIA.12 CIAA renders EVAR more complex with longer operative and fluoroscopic time and more contrast used.14 In contrast, the Cook Zenith trial (n=736) failed to show any significant difference in technical success, AAA related complications, or reintervention in patients with concomitant CIAA.10 Thirty percent of all patients developed CIA expansion; this enlargement was not related to the baseline diameter of CIA but to stent-graft oversizing.
A number of techniques are available to treat patients with concomitant CIAA during EVAR. Options include IIE+EE, BBT, iliac side branch device (IBD), open advancement of the CIA bifurcation by internal iliac artery bypass/transposition, and aorto-uniiliac stent-graft with femoral-femoral bypass (AUI) with a retrograde endovascular EIA-IIA bypass.24-32 In the absence of randomized controlled studies, standardization of treatment is poor.
Patient anatomy, operator preference, availability of appropriate stent-graft, and financial constraints influence the technique adopted. IIE+EE and BBT are the two most commonly performed procedures. In our earlier experience, there was preference to perform IIE+EE but, with the introduction of commercially available larger iliac limbs, BBT was often favored when anatomically feasible. Anatomical factors favoring IIE+EE included CIA bifurcation >25mm, significant thrombus in the CIA, or the presence of aneurysm of the IIA. Factors favoring BBT included contralateral IIA occlusion and CIA of ≤ 25mm. Ischemic complications caused by coil misplacement or pelvic malperfusion limit the appeal of IIE+EE.33-35 A number of strategies devised to diminish this risk of ischemia by preserving the pelvic collateral vascular network include: interruption of the IIA as proximally as possible, thereby preserving the IIA bifurcation; preferential use of an Amplatzer vascular plug with its deployment system, enabling accurate positioning of occlusion at the target site; staging IIE prior to EVAR; and taking precautions to preserve the contralateral IIA.36 However, there is emerging evidence that these measures fail to diminish the ischemic complications of IIE.18, 37 In view of the unpredictability of complications, techniques to preserve IIA flow should always be considered.
BBT facilitates a distal seal while preserving pelvic flow. This technique has been facilitated by commercially available large diameter iliac extension limbs. The long-term durability of deploying large diameter iliac extension limbs into a CIAA is uncertain as data on the progression of CIA diameter after open AAA repair show that growth is directly proportional to baseline diameter.38
The present study is the first comparative study between IIE+EE and BBT. In this series, low rates of reintervention for type 1b/III endoleak and iliac limb patency are reported. There was no significant difference in reintervention between the two treatment groups. IIE+EE had a higher combined incidence of perioperative complications and long-term reintervention. Similar to Kirkwood et al, use of large iliac limbs in BBT was not associated with increased risk of type 1b endoleaks.10 In the absence of non-inferiority of BBT and lower complications, these results suggest that BBT may be preferable to IIE+EE as it preserves pelvic flow.
There are a number of limitations of this study. Firstly, it is retrospective; therefore, we are unable to analysis all factors that contributed to the decision of technique adopted to treat concomitant CIAA and, furthermore, analysis of all the anatomical factors that contributed to treatment failure is limited. Secondly, combining two centers’ experience risks comparing heterogenous patient groups and interventionalists. Analysis failed to demonstrate significant differences in patient demographics or treatment outcome between the two centers. Finally, with low incidence of reintervention and treatment failure, perhaps greater patient numbers and longer follow-up may reveal differences between the treatment groups. In addition, certain complications like buttock claudication may be more accurately assessed via prospectively recorded quality of life studies.
CONCLUSION
This is the first reported comparative study comparing IIE+EE to BBT in patients undergoing EVAR with concomitant CIAA. Both treatment groups had low rates of reintervention. There was no significant difference in the incidence of distal endoleaks or iliac limb patency. BBT preserves pelvic perfusion and has a lower incidence of combined complications and reintervention. A multicenter randomized study is appropriate but present results support the preference of BBT when feasible.
Acknowledgments
Note: Stipend for Michael S. Park, M.D., is partially supported by National Institutes of Health Grant #5T32HL094293.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 2011 Vascular Annual Meeting, The Society for Vascular Surgery, Chicago, IL, June 16-18, 2011.
REFERENCES
- (1).Bush RL, Johnson ML, Collins TC, Henderson WG, Khuri SF, Yu HJ, et al. Open versus endovascular abdominal aortic aneurysm repair in VA hospitals. J Am Coll Surg. 2006;202:577–87. doi: 10.1016/j.jamcollsurg.2006.01.005. [DOI] [PubMed] [Google Scholar]
- (2).Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–8. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- (3).Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- (4).Naughton PA, Garcia-Toca M, Rodriguez HE, Keeling AN, Resnick SA, Eskandari MK. Endovascular treatment of delayed type 1 and 3 endoleaks. Cardiovasc Intervent Radiol. 2011;34:751–7. doi: 10.1007/s00270-010-0020-y. [DOI] [PubMed] [Google Scholar]
- (5).Zayed HA, Attia R, Modarai B, Clough RE, Bell RE, Carrell T, et al. Predictors of reintervention after endovascular repair of isolated iliac artery aneurysm. Cardiovasc Intervent Radiol. 2011;34:61–6. doi: 10.1007/s00270-010-9876-0. [DOI] [PubMed] [Google Scholar]
- (6).White GH, May J, Petrasek P, Waugh R, Stephen M, Harris J. Endotension: an explanation for continued AAA growth after successful endoluminal repair. J Endovasc Surg. 1999;6:308–15. doi: 10.1583/1074-6218(1999)006<0308:EAEFCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- (7).Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Bower TC, Karla M, et al. Proximal type I endoleak after endovascular abdominal aortic aneurysm repair: predictive factors. Ann Vasc Surg. 2004;18:621–8. doi: 10.1007/s10016-004-0100-z. [DOI] [PubMed] [Google Scholar]
- (8).Harris P, Brennan J, Martin J, Gould D, Bakran A, Gilling-Smith G, et al. Longitudinal aneurysm shrinkage following endovascular aortic aneurysm repair: a source of intermediate and late complications. J Endovasc Surg. 1999;6:11–6. doi: 10.1177/152660289900600104. [DOI] [PubMed] [Google Scholar]
- (9).Matsumura JS, Pearce WH, McCarthy WJ, Yao JS. Reduction in aortic aneurysm size: early results after endovascular graft placement. EVT Investigators. J Vasc Surg. 1997;25:113–23. doi: 10.1016/s0741-5214(97)70327-6. [DOI] [PubMed] [Google Scholar]
- (10).Kirkwood ML, Saunders A, Jackson BM, Wang GJ, Fairman RM, Woo EY. Aneurysmal iliac arteries do not portend future iliac aneurysmal enlargement after endovascular aneurysm repair for abdominal aortic aneurysm. J Vasc Surg. 2011;53:269–73. doi: 10.1016/j.jvs.2010.08.062. [DOI] [PubMed] [Google Scholar]
- (11).Hobo R, Laheij RJ, Buth J. The influence of aortic cuffs and iliac limb extensions on the outcome of endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;45:79–85. doi: 10.1016/j.jvs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- (12).Albertini JN, Favre JP, Bouziane Z, Haase C, Nourrissat G, Barral X. Aneurysmal extension to the iliac bifurcation increases the risk of complications and secondary procedures after endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg. 2010;24:663–9. doi: 10.1016/j.avsg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- (13).Hobo R, Sybrandy JE, Harris PL, Buth J. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR Experience. J Endovasc Ther. 2008;15:12–22. doi: 10.1583/07-2217.1. [DOI] [PubMed] [Google Scholar]
- (14).Parlani G, Zannetti S, Verzini F, De Rango P, Carlini G, Lenti M, et al. Does the presence of an iliac aneurysm affect outcome of endoluminal AAA repair? An analysis of 336 cases. Eur J Vasc Endovasc Surg. 2002;24:134–8. doi: 10.1053/ejvs.2002.1669. [DOI] [PubMed] [Google Scholar]
- (15).Verzini F, Parlani G, Romano L, De Rango P, Panuccio G, Cao P. Endovascular treatment of iliac aneurysm: Concurrent comparison of side branch endograft versus hypogastric exclusion. J Vasc Surg. 2009;49:1154–61. doi: 10.1016/j.jvs.2008.11.100. [DOI] [PubMed] [Google Scholar]
- (16).Lee WA, Nelson PR, Berceli SA, Seeger JM, Huber TS. Outcome after hypogastric artery bypass and embolization during endovascular aneurysm repair. J Vasc Surg. 2006;44:1162–8. doi: 10.1016/j.jvs.2006.08.047. [DOI] [PubMed] [Google Scholar]
- (17).Morasch MD, Kibbe MR, Evans ME, Meadows WS, Eskandari MK, Matsumura JS, et al. Percutaneous repair of abdominal aortic aneurysm. J Vasc Surg. 2004;40:12–6. doi: 10.1016/j.jvs.2004.03.019. [DOI] [PubMed] [Google Scholar]
- (18).Bratby MJ, Munneke GM, Belli AM, Loosemore TM, Loftus I, Thompson MM, et al. How safe is bilateral internal iliac artery embolization prior to EVAR? Cardiovasc Intervent Radiol. 2008;31:246–53. doi: 10.1007/s00270-007-9203-6. [DOI] [PubMed] [Google Scholar]
- (19).van Keulen JW, de Vries JP, Dekker H, Goncalves FB, Moll FL, Verhagen HJ, et al. One-year multicenter results of 100 abdominal aortic aneurysm patients treated with the Endurant stent graft. J Vasc Surg. 2011 doi: 10.1016/j.jvs.2011.02.053. available online May 28, 2011. [DOI] [PubMed] [Google Scholar]
- (20).Manning BJ, O’Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg. 2009;49:60–5. doi: 10.1016/j.jvs.2008.07.079. [DOI] [PubMed] [Google Scholar]
- (21).Becquemin JP, Kelley L, Zubilewicz T, Desgranges P, Lapeyre M, Kobeiter H. Outcomes of secondary interventions after abdominal aortic aneurysm endovascular repair. J Vasc Surg. 2004;39:298–305. doi: 10.1016/j.jvs.2003.09.043. [DOI] [PubMed] [Google Scholar]
- (22).Conrad MF, Adams AB, Guest JM, Paruchuri V, Brewster DC, LaMuraglia GM, et al. Secondary intervention after endovascular abdominal aortic aneurysm repair. Ann Surg. 2009;250:383–9. doi: 10.1097/SLA.0b013e3181b365bd. [DOI] [PubMed] [Google Scholar]
- (23).Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, et al. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010;52:1442–9. doi: 10.1016/j.jvs.2010.06.110. [DOI] [PubMed] [Google Scholar]
- (24).Torsello G, Schonefeld E, Osada N, Austermann M, Pennekamp C, Donas KP. Endovascular treatment of common iliac artery aneurysms using the bell-bottom technique: long-term results. J Endovasc Ther. 2010;17:504–9. doi: 10.1583/10-3112.1. [DOI] [PubMed] [Google Scholar]
- (25).Mehta M, Veith FJ, Ohki T, Cynamon J, Goldstein K, Suggs WD, et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg. 2001;33:S27–S32. doi: 10.1067/mva.2001.111678. [DOI] [PubMed] [Google Scholar]
- (26).Lin PH, Bush RL, Chaikof EL, Chen C, Conklin B, Terramani TT, et al. A prospective evaluation of hypogastric artery embolization in endovascular aortoiliac aneurysm repair. J Vasc Surg. 2002;36:500–6. doi: 10.1067/mva.2002.127350. [DOI] [PubMed] [Google Scholar]
- (27).Kritpracha B, Pigott JP, Russell TE, Corbey MJ, Whalen RC, DiSalle RS, et al. Bell-bottom aortoiliac endografts: an alternative that preserves pelvic blood flow. J Vasc Surg. 2002;35:874–81. doi: 10.1067/mva.2002.123326. [DOI] [PubMed] [Google Scholar]
- (28).Karthikesalingam A, Hinchliffe RJ, Holt PJ, Boyle JR, Loftus IM, Thompson MM. Endovascular aneurysm repair with preservation of the internal iliac artery using the iliac branch graft device. Eur J Vasc Endovasc Surg. 2010;39:285–94. doi: 10.1016/j.ejvs.2009.11.018. [DOI] [PubMed] [Google Scholar]
- (29).Karch LA, Hodgson KJ, Mattos MA, Bohannon WT, Ramsey DE, McLafferty RB. Management of ectatic, nonaneurysmal iliac arteries during endoluminal aortic aneurysm repair. J Vasc Surg. 2001;33:S33–8. doi: 10.1067/mva.2001.111659. [DOI] [PubMed] [Google Scholar]
- (30).Greenberg RK, West K, Pfaff K, Foster J, Skender D, Haulon S, et al. Beyond the aortic bifurcation: branched endovascular grafts for thoracoabdominal and aortoiliac aneurysms. J Vasc Surg. 2006;43:879–86. doi: 10.1016/j.jvs.2005.11.063. [DOI] [PubMed] [Google Scholar]
- (31).Faries PL, Morrissey N, Burks JA, Gravereaux E, Kerstein MD, Teodorescu VJ, et al. Internal iliac artery revascularization as an adjunct to endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2001;34:892–9. doi: 10.1067/mva.2001.118085. [DOI] [PubMed] [Google Scholar]
- (32).Bergamini TM, Rachel ES, Kinney EV, Jung MT, Kaebnick HW, Mitchell RA. External iliac artery-to-internal iliac artery endograft: a novel approach to preserve pelvic inflow in aortoiliac stent grafting. J Vasc Surg. 2002;35:120–4. doi: 10.1067/mva.2002.120038. [DOI] [PubMed] [Google Scholar]
- (33).Karch LA, Hodgson KJ, Mattos MA, Bohannon WT, Ramsey DE, McLafferty RB. Adverse consequences of internal iliac artery occlusion during endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2000;32:676–83. doi: 10.1067/mva.2000.109750. [DOI] [PubMed] [Google Scholar]
- (34).Kritpracha B, Comerota AJ. Unilateral lower extremity paralysis after coil embolization of an internal iliac artery aneurysm. J Vasc Surg. 2004;40:819–21. doi: 10.1016/j.jvs.2004.07.043. [DOI] [PubMed] [Google Scholar]
- (35).Marty B, Perruchoud C, Wicky S, Guillou L, Von Segesser LK. Atheroembolization: a harmful complication of therapeutic internal iliac artery occlusion. J Vasc Surg. 2002;36:1062–5. doi: 10.1067/mva.2002.127531. [DOI] [PubMed] [Google Scholar]
- (36).Resnick SA, Eskandari MK. Outcomes of Amplatzer vascular plugs for occlusion of internal iliacs during aortoiliac aneurysm stent grafting. Ann Vasc Surg. 2008;22:613–7. doi: 10.1016/j.avsg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- (37).Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, London NJ, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol. 2008;31:728–34. doi: 10.1007/s00270-008-9319-3. [DOI] [PubMed] [Google Scholar]
- (38).Ballotta E, Da Giau G, Gruppo M, Mazzalai F, Toniato A. Natural history of common iliac arteries after aorto-aortic graft insertion during elective open abdominal aortic aneurysm repair: a prospective study. Surgery. 2008;144:822–6. doi: 10.1016/j.surg.2008.07.011. [DOI] [PubMed] [Google Scholar]


