Obesity is clearly associated with insulin resistance and chronic low-grade inflammation.1,2 Macrophages appear to be especially important in this relationship, as they infiltrate adipose tissue and produce a variety of inflammatory cytokines.1 Exploring the contribution of other immune cells to the development of obesity, Liu, et al., described a role for mast cells in the development of obesity and diabetes in mice.3 Using genetically modified mice and pharmacologic stabilizers of mast cells, they demonstrated that mast cells and mast cell-mediated protease expression may promote the growth of white adipose tissue (WAT). Importantly, the idea that mast cells may function in a similar manner in human obesity was suggested by the finding of increased numbers of mast cells in human WAT from obese compared to lean subjects in their study. Furthermore, mean serum tryptase levels were higher in obese (13.1 ng/ml) than lean (7.7 ng/ml) individuals using an in-house tryptase assay. We attempted to replicate this data by comparing serum tryptase levels in obese, overweight, and lean individuals from a pediatric population.
As the serum total tryptase level, comprised primarily of α and β protryptases, seems to correlate with the total-body burden of mast cells,4 we measured the level of this protein in individuals 8–18 years old, recruited through newspaper advertisement for research participation. The cohort contained a diverse group of children and adolescents with and without obesity, impaired glucose tolerance, and/or diabetes mellitus (see Figure 1A and Tables E1 and E2). As the body mass index (BMI) is age- and gender-specific in children and teens,5 we divided the subjects based on the BMI percentiles by age and sex into those of healthy weight (BMI ≥5th– <85th percentile), overweight (≥85th – <95th percentile), and obese (≥95th percentile). Comparisons were made using the non-parametric Kruskal-Wallis test, as the data were not normally distributed after standardization to percentiles. No statistical difference in the median serum tryptase levels was seen among the groups (3.1, 3.5, and 2.7 ng/ml, respectively; P = 0.068) (see Figure 1B). While the BMI varies with age and sex in pediatric patients, the raw values still accurately reflect total-body fatness5 and thus should directly correlate with the serum tryptase levels according to the data presented by Liu, et al. Therefore, to directly compare our results to those of Liu, et al., lean individuals (BMI<26) were compared to overweight (BMI ≥ 26, but <32) and obese (BMI ≥32) individuals; these data were normally distributed. Again, no statistical difference among the groups was observed by one-way analysis of variance (ANOVA, P = 0.449; see Figure E1A). Because some of the individuals in the cohort demonstrated impaired glucose tolerance or overt type 2 diabetes mellitus, the above comparisons were repeated with data from only those individuals with normal glucose tolerance (NGT). Once again, no statistical difference was seen between lean, overweight, and obese individuals, as defined either by BMI percentile (Kruskal-Wallis, P = 0.138; see Figure 1C) or by BMI alone (ANOVA, P = 0.386; see Figure E1B). Finally, multiple linear regression analysis was performed on the entire cohort to determine the association of multiple different obesity-related parameters (BMI, BMI percentile, age, race, height, weight, fat mass, percent body fat, waist circumference, hip circumference, waist:hip ratio, visceral adipose tissue, percent visceral adipose tissue, subcutaneous adipose tissue, percent subcutaneous adipose tissue, and total adipose tissue) to serum tryptase levels. The combined model was unable to significantly predict serum tryptase levels (R2 = 0.043, P = 0.997, for all individuals; R2 = 0.110, P = 0.974, for only NGT individuals). Moreover, none of the individual parameters were found to significantly correlate with serum tryptase levels.
Figure 1.
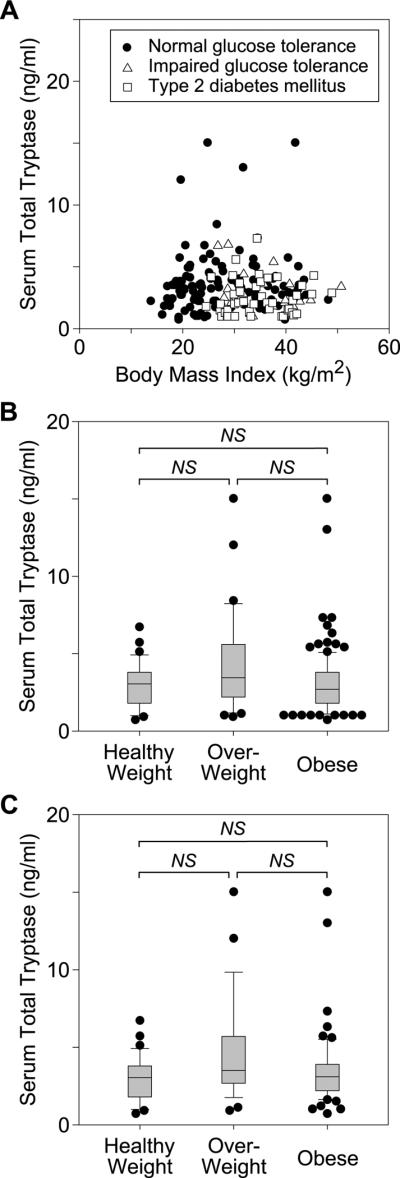
Serum total tryptase levels in healthy weight, overweight, and obese pediatric patients. A, Serum total tryptase levels were measured in a cohort of pediatric patients with varying degrees of body fat, approximated here by body mass index (BMI). Individuals with normal glucose tolerance, impaired glucose tolerance, and overt type 2 diabetes mellitus are indicated by filled circles, open triangles, and open squares, respectively. B, Serum total tryptase levels were compared among all healthy weight (n= 34), overweight (n= 26), and obese (n=117) individuals, as defined by BMI percentile. C, Serum total tryptase levels were compared among only those healthy weight (n= 34), overweight (n= 21), and obese (n=59) individuals, defined by BMI percentile, with normal glucose tolerance. Box plots (B,C) demonstrate the median and 10th, 25th, 75th, and 90th percentiles, with all outliers (those data outside the 10th through 90th percentiles) plotted as filled circles. NS, no significant difference.
We did not perform histologic examination of the adipose tissue, and thus we cannot comment on the numbers of mast cells within the adipose tissue. It is quite possible that they are indeed elevated as in the Liu, et al., report. However, despite any possible increase in mast cells in the adipose tissue, our data indicate that obese youth do not have a significantly increased whole body mast cell burden that would manifest as an increased serum total tryptase. There are several potential reasons for the conflicting results, the most obvious being the age difference of the subjects— in our study, ages ranged from 8 to 18 years, whereas in the Liu, et al., report, ages ranged from 20 to 66 years.3 Another recent study involving subjects aged 15–69 showed a positive correlation between BMI and serum tryptase levels (median tryptase=4.7 ng/ml [BMI<25 kg/m2], 5.2 ng/ml [BMI 25–30 kg/m2] and 5.1 ng/ml [BMI>30 kg/m2]),6 albeit with a more modest effect than in Liu, et al. In that same study, age was also found to correlate with serum tryptase levels, with greater than three times the standardized effect of BMI. However, the link between obesity and chronic low-grade systemic inflammation has been established in children as well as adults, with similar immunologic mechanisms and cytokine profiles found in both populations.7,8 One should therefore expect that similar mechanisms for mast cell recruitment and activation should be present in both age groups, though this does not seem to be the case. Another potential cause for the difference in findings is unintentional selection bias. While Liu, et al., attempted to exclude those with evidence of infection or inflammatory disease, the significantly elevated serum tryptase levels seen in the obese group (up to 73 ng/ml) suggest that one or more of the subjects may have had an undiagnosed clonal mast cell disorder, such as systemic mastocytosis.9 We would also note that the tryptase assay used by Liu, et al., has been utilized in a limited number of studies, and thus may not be well characterized or validated in a clinical setting. Indeed, the mean serum tryptase for lean individuals in their study appears to be substantially higher than the mean level in normal individuals as established by other groups (3.8 ng/ml) using the commercially available assay (see http://www.phadia.com/en/Health-Care-Providers/Allergy/Products1/ImmunoCAp-Tryptase/). Finally, while the differences in median tryptase levels among patients in our cohort grouped by BMI percentile did not meet statistical significance, they indeed approached it, and thus one could argue that our study lacks sufficient power to pick up obesity-related differences in serum tryptase. However, the median tryptase level for the obese group was lower than that of both the healthy weight and overweight groups, not higher. Thus, additional power would be unlikely to support a positive correlation between tryptase levels and obesity in our study population.
In their report, Liu, et al. provided an elegant argument for the role of mast cells in mouse obesity and diabetes. As inflammatory mechanisms are known to be associated with obesity and insulin resistance in humans, the stated implication of their study was that mast cells may have a similar roles in human and mouse obesity. We agree that the potential role for mast cells in human obesity and diabetes is intriguing, but using serum tryptase to show an increase in total-body mast cell burden in children with increased body fat is not supported by our data. Clearly additional studies are needed to fully establish and characterize the potential relationship between mast cells and obesity in humans.
Capsule Summary.
Recent studies found a positive correlation between obesity and serum tryptase levels in humans. Contrary to this report, serum tryptase levels are not increased in overweight and obese children.
Acknowledgments
Declaration of all sources of funding: This work was supported in part by a grant (RO1 AI27517) from the National Institutes of Health (LBS). Additional funding was provided by grants (RO1 HD27503, K24 HD01357, UL1 RR024153 [CTSA]) from the National Institutes of Health and by the Richard L. Day Endowed Chair (SAA).
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- NGT
normal glucose tolerance
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: Virginia Commonwealth University receives royalties from Phadia for the ImmunoCAP tryptase assay that are shared with Lawrence B Schwartz. The rest of the authors have declared that they have no conflicts of interest.
References
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signaling mechanisms to clinical implications. J Intern Med. 2007;262:431–438. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 6.Fenger RV, Linneberg A, Vidal C, Vizcaino L, Husemoen LL, Aadahl M, et al. Determinants of serum tryptase in a general population: the relationship of serum tryptase to obesity and asthma. Int Arch Allergy Immunol. 2011;157:151–158. doi: 10.1159/000327535. [DOI] [PubMed] [Google Scholar]
- 7.Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60:445–452. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Maffeis C, Silvagni D, Bonadonna R, Grezzani A, Banzato C, Tato L. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr. 2007;151:647–652. doi: 10.1016/j.jpeds.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 9.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]


