Abstract
The precise physiological effects of antidepressant drugs, and in particular their actions at non-monoamine transporter targets, are largely unknown. We have recently identified the tricyclic antidepressant drug desipramine (DMI) as a direct ligand at the α2A adrenergic receptor (AR) without itself driving heterotrimeric G protein/downstream effector activation (Cottingham et al, J. Biol. Chem. 286: 36063–75). In this study, we report our novel finding that DMI modulates α2AAR signaling in response to the endogenous agonist norepinephrine (NE). DMI acted as a signaling potentiator, selectively enhancing NE-induced α2AAR-mediated ERK1/2 MAPK signaling. This potentiation of ERK1/2 activation was observed as an increase in NE response sensitivity and a prolongation of the activation kinetics. DMI in a physiologically relevant ratio with NE effectively turned on ERK1/2 signaling that is lacking in response to physiological NE alone. Further, the DMI-induced ERK1/2 potentiation relied on heterotrimeric Gi/o proteins and was arrestin-independent. This modulatory effect of DMI on NE signaling provides novel insight into the effects of this antidepressant drug on the noradrenergic system which it regulates, insight which enhances our understanding of the therapeutic mechanism for DMI.
Keywords: α2 adrenergic receptor, antidepressant, Akt, ERK1/2, norepinephrine
1. Introduction
A complete picture regarding the mechanism of action of antidepressant medications remains elusive. Indeed, although more than half a century has passed since the introduction of the tricyclic antidepressant drug class [1], a mechanistic physiological model for how these drugs function is nonexistent. These drugs are, speaking generally, understood to be antidepressants by virtue of their ability to inhibit the reuptake of the monoamine neurotransmitters norepinephrine (NE)1 and serotonin via blockade of the NE and serotonin transporters [1]. However, reuptake inhibition alone is not sufficient to explain the antidepressant effects of these medications.
Our previous work has characterized the interaction of the tricyclic antidepressant drug desipramine (DMI), a class member with the strongest specificity for the noradrenergic over the serotonergic system [1], with the α2A adrenergic receptor (AR). The α2AAR, a prototypical GPCR, is a well-established key player in noradrenergic transmission as a regulator of NE synthesis and release from noradrenergic neuronal terminals [2] and of neuronal activity within the locus coeruleus [3, 4], the primary brain region from which central noradrenergic neurons originate. Our work has established that DMI, as a direct α2AAR ligand, selectively drives recruitment of the important interacting regulator protein arrestin to the receptor leading to receptor trafficking responses, while not driving coupling/activation of heterotrimeric G proteins to the receptor and classical signaling responses [5].
The present work is aimed at investigating the potential impact of DMI, acting as a direct α2AAR ligand, on receptor-mediated signaling by the endogenous agonist NE. Given that DMI was shown to bind as an orthosteric ligand at the α2AAR, we hypothesized that DMI would act as a competitive antagonist and block signaling induced by the endogenous α2AAR agonist NE. Further, any antagonism will be dependent on the relative concentrations of DMI and NE when given together (e.g. the [DMI]/[NE] ratio), given that the α2AAR has essentially identical intrinsic affinities for the two ligands (Ki values of 4.62 and 3.63 μM for DMI and NE, respectively) [5], contrasting with typical antagonist affinities that are 100- to 1000-fold higher. The α2AAR classically couples to heterotrimeric G proteins of the Gi/o family, leading to inhibition of adenylyl cyclase and voltage-gated Ca2+ channels, and activation of downstream effectors including inwardly-rectifying K+ channels, MAPKs, and Akt [5–10]. Here, we have utilized activation of the ERK1/2 MAPK and Akt pathways as straightforward and reliable readouts for α2AAR signaling. As well, these signaling pathways have both been more generally implicated in physiological antidepressant responses [11, 12]. By utilizing an in vitro heterologous cell model, we have been able to isolate and specifically study α2AAR-mediated signal transduction in the absence complex confounding effects from the full complement of endogenous DMI molecular targets.
Through a combination of kinetic and response sensitivity analyses, we report here that DMI is in fact a potentiator that specifically facilitates NE-induced α2AAR-mediated ERK1/2 signaling. We further show that this DMI-potentiated signaling remains dependent on heterotrimeric Gi/o proteins and does not simply represent a switch to arrestin-mediated signal transduction. By comparison, we observed a general inhibitory effect of DMI on cellular Akt signaling. These data provide a novel example of a complex ligand/receptor relationship between DMI and the α2AAR which cannot be explained by classical agonist/antagonist regulation of receptor function. As well, in the context of antidepressant pharmacotherapy, our findings add valuable and novel information to the understanding of the physiological mechanism of the antidepressant drug DMI.
2. Materials and methods
2.1 Cell culture
Mouse embryonic fibroblast (MEF) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen), and maintained in a humidified 5% CO2 incubator. MEFs isolated from wild-type and arrestin2,3-null (Arr2,3−/−) [13] animals and stably expressing N-terminal hemagglutinin (HA) epitope-tagged murine α2AARs were used for this study. Generation of HA-α2AAR MEF lines expressing the receptor at an average density of 400 fmol/mg has been described [5].
2.2 Drugs and treatments
All drugs were obtained from Sigma unless otherwise noted. NE and DMI stocks of 10 mM were prepared freshly in distilled water prior to each experiment. For antagonist experiments, cells were pretreated with the selective α2AAR antagonist BRL44408 (1 μM) for 5 minutes prior to stimulation with an NE/BRL combination. For pertussis toxin (PTx) experiments, cells were pretreated with PTx (List Biological Laboratories, Inc.) at a final concentration of 200 ng/ml or vehicle (in serum-free DMEM) for 24 hours, and PTx was maintained during drug stimulation. All signaling experiments were done in the presence of 1 μM propranolol (βAR antagonist) and prazosin (α1 and α2B/CAR antagonist) to pharmacologically isolate the α2AAR.
2.3 SDS-PAGE and Western blot
SDS-PAGE (10% acrylamide gel) and Western blot were performed as previously described [5]. MEF cells were serum-starved overnight prior to all signaling assays. At least three independent samples were analyzed for each experimental group.
The following primary antibodies and dilutions were used: phospho-ERK1/2, p44/42 MAPK (T202/Y204) mouse monoclonal antibody (Cell Signaling), 1:16000; phospho-Akt (T308) rabbit polyclonal antibody (Cell Signaling), 1:4000; β-tubulin mouse antibody (University of Iowa Hybridoma Bank), 1:50000; total ERK, p44/42 MAPK rabbit polyclonal antibody (Cell Signaling). HRP-conjugated secondary antibodies and Immobilon Western detection system were obtained from Millipore. β-tubulin was selected as total protein/loading control for most experiments as both phospho-ERK1/2 and phospho-Akt were being analyzed simultaneously. Total ERK blots were performed after membrane stripping as previously described [5].
2.4 Data analysis
Densitometric analysis of dose response Western blot results was done using Scion Image software. Kinase activation for both ERK1/2 and Akt was determined as the ratio of optical density values obtained for phosphorylated kinase to those for total protein. All statistical analyses were carried out using GraphPad Prism software (GraphPad, San Diego, California, using Student’s t-tests with p<0.05 considered statistically significant.
3. Results and discussion
3.1 DMI alone does not initiate signaling but potentiates NE-induced α2AAR-mediated ERK1/2 activation
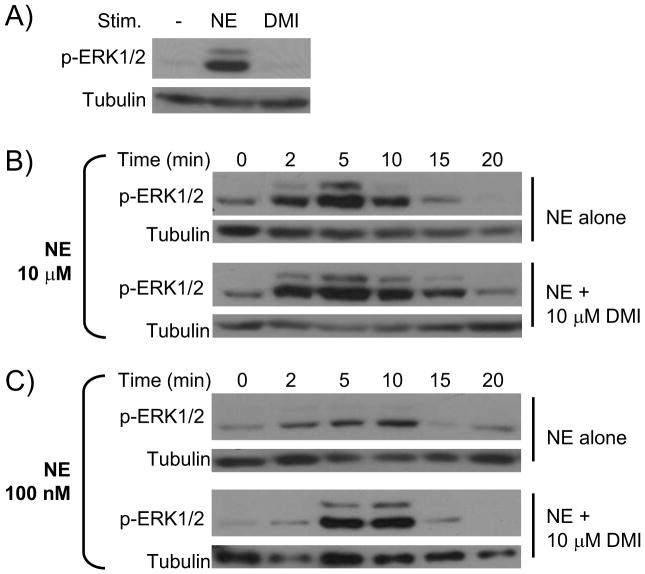
We have previously established that NE, in the absence of input from β, α1, and α2B/C ARs, drives activation of ERK1/2 MAPK in an α2AAR-dependent and Gi-dependent fashion [9, 10]. α2AAR-mediated ERK1/2 activation proceeds through the Gβγ subunits and Ras [14, 15]. For this study, we began confirming the absence of signal activation by DMI alone, as we have previously reported [5]. As shown in Figure 1A, DMI alone does not initiate ERK1/2 signaling. We next assayed the kinetics of NE-induced signaling, alone or in combination with DMI, through time course analysis. 10 μM NE alone induces a robust activation of ERK1/2, peaking at 5 minutes and fully desensitized to baseline by 15 minutes (Figure 1B). The kinetics of ERK1/2 activation observed here are consistent with our previous findings on NE-induced α2AAR signaling [9, 10]. When DMI was added (NE and DMI together at 10 μM), signaling was not blocked, but rather the time course was prolonged, with ERK1/2 activation elevated above baseline through 15 minutes (Figure 1B). These data seem to contradict our initial hypothesis that DMI acts as a competitive antagonist.
Figure 1.
DMI impacts the kinetics of NE-induced α2AAR-mediated ERK1/2 signaling. All NE stimulations were done in the presence of propranolol (βAR antagonist) and prazosin (α1 and α2B/CAR antagonist). A. DMI does not itself drive ERK1/2 signaling. MEF cells were stimulated for 5 minutes with NE alone (as a positive control) or DMI alone. Whole cell homogenates were then analyzed by SDS-PAGE/Western blot, probing for phospho-ERK1/2 and tubulin (loading control). B. & C. MEF cells were stimulated for the indicated times with either 100 nM or 10 μM NE, alone or in combination with 10 μM DMI, and analyzed for ERK1/2 activation as in panel A. Blots shown are representative of 3 independent experiments.
The ability of competitive antagonists to block agonist actions is typically observed either when receptor/antagonist affinity greatly exceeds receptor/agonist affinity or at elevated [antagonist]/[agonist] ratios. Given the essentially identical affinity values for NE and DMI at the α2AAR [5], we therefore chose to assay a much lower level of NE (100 nM), which alone induced a weak and transient activation of ERK1/2 having similar kinetics to those observed at 10 μM (Figure 1C). Surprisingly, the addition of DMI (now at a 100-fold higher level than NE, 10 μM against 100 nM) did not block NE-induced signaling but rather resulted in a markedly enhanced level of ERK1/2 activation (Figure 1C). Clearly, these data indicate that the interaction of DMI with the α2AAR is not that of a classical antagonist. Although DMI itself binds to the orthosteric site without driving heterotrimeric G protein/downstream effector activation, it lacks the ability to block agonist-induced signaling at the receptor. In fact, it appears to function as a signaling potentiator, and so our hypothesis has already been refuted.
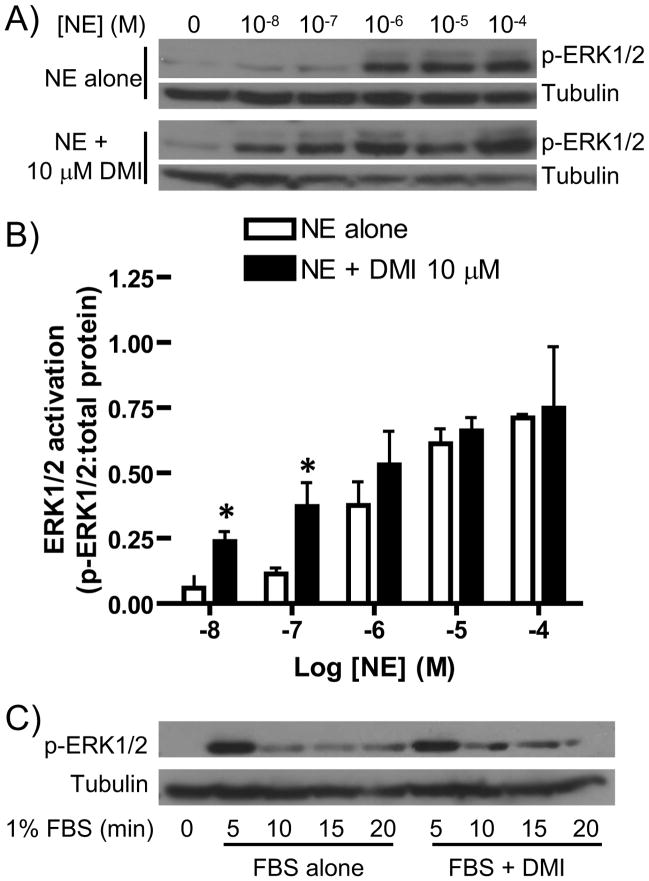
We further investigated this modulation of NE-induced signaling by dose response analysis, performed at the 5 minute (peak activation) time point. This analysis was intended to determine if DMI alters the response sensitivity of NE-induced ERK1/2 activation by the α2AAR. NE dose-dependently induced ERK1/2 activation, with modest activation beginning at 100 nM and more robust activation observed at 1, 10, and 100 μM, while the addition of DMI caused a clear leftward shift in the dose response (Figure 2A). Densitometric analysis confirmed significantly greater ERK1/2 activation at by NE at 10 and 100 nM in the presence of DMI (Figure 2B). The maximum efficacy of NE-induced ERK1/2 activation was unaffected by the addition of DMI (Figures 2A, 2B). These findings correspond nicely with the data presented in Figure 1, wherein the 5-minute ERK1/2 response was clearly potentiated by DMI at 100 nM NE but not at 10 μM NE. Overall, our dose response analysis indicates that DMI enhances the ERK1/2 activation response sensitivity of the α2AAR to NE stimulation and provides further evidence that the interaction of DMI with the α2AAR has a potentiating rather than antagonizing effect on agonist-induced signaling.
Figure 2.
DMI enhances α2AAR-mediated ERK1/2 response sensitivity to NE. A. MEF cells were stimulated for 5 minutes with NE at varying concentrations, ranging from 10 nM (10−8 M) to 100 μM (10−4 M), alone or in combination with 10 μM DMI, as indicated. Whole cell homogenates were then analyzed by SDS-PAGE/Western blot, probing for phospho-ERK1/2 and tubulin (loading control). Blots shown are representative of 3 independent experiments. B. Densitometric quantitation for panel A, with signaling activation calculated as a ratio of phospho-kinase:total protein. Data are mean ± SEM obtained over 3 independent experiments. *p<0.05 vs. NE alone. C. MEF cells were subjected to stimulation with 1% FBS for the indicated times, alone or in combination with 10 μM DMI, and analyzed as in panel A. Blots are representative of 3 independent experiments.
Our observations at 10 nM NE have particular physiological relevance, given that this is the same concentration reached in the brain following reuptake inhibition by antidepressants like DMI [3, 16]. The addition of DMI to 10 nM NE initiates ERK1/2 activation which is lacking with 10 nM NE alone. As well, the NE/DMI ratios at the 10 and 100 nM data points (100- to 1000-fold higher DMI) are representative of the physiological situation, given that therapeutic levels of DMI reach the micromolar range [5]. These data, then, are strongly supportive of a physiological and pharmacological role for the DMI-mediated potentiation of ERK1/2 signaling.
The possibility exists that DMI is modulating ERK1/2 signal transduction via a non-selective mechanism at some level other than the receptor. As well, ERK1/2 activation is not coupled exclusively to the α2AAR, and can be activated and modulated by a number of upstream mediators. To address this issue of selectivity, we tested the ability of DMI to modulate the growth factor receptor-mediated signaling activity associated with acute FBS exposure. FBS stimulation alone induced a robust and transient ERK1/2 activation, signaling which was unaffected by the addition of DMI (Figure 2C). Although these data do not definitively rule out an α2AAR-independent mechanism for the potentiation of NE-induced ERK1/2 activation by DMI, they do lend support to the contention that DMI is selectively modulating α2AAR-mediated ERK1/2 signaling.
3.2 Modulation of Akt signaling by DMI lacks selectivity
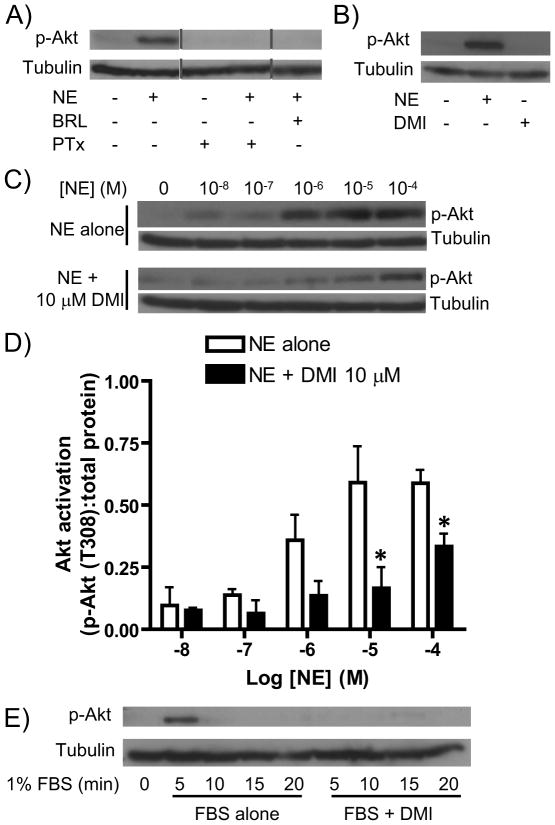
We next expanded our investigation to include a separate downstream effector activated by α2AAR signal transduction, the Akt kinase. We confirmed that the observed Akt response to NE is both α2AAR-mediated (blocked by the α2AAR antagonist BRL44408) and Gi-dependent (PTx-sensitive) (Figure 3A). Although G protein-mediated Akt signaling by the α2AAR has not been specifically reported, based on previous studies at other Gi/o-coupled receptors [17], it seems likely that the receptor couples to Akt via Gβγ subunit-mediated activation of PI3K. Additionally, we again confirmed our previous finding [5] that DMI alone does not drive activation of this downstream effector (Figure 3B).
Figure 3.
Modulation of Akt signaling by DMI lacks selectivity. A. MEF cells were subjected to 5 minute stimulation with 10 μM NE alone, NE in combination with the α2AAR antagonist BRL44408, or NE following treatment with pertussis toxin (PTx) as described in the methods section. Whole cell lysates were analyzed by SDS-PAGE/Western blot for phospho-Akt and tubulin. Note that the lanes shown are from a single experiment but not all adjacent lanes. B. MEF cells were stimulated for 5 minutes with 10 μM NE alone (positive control) or 10 μM DMI alone and analyzed as in panel A. C. MEF cells were stimulated for 5 minutes with NE at varying concentrations, ranging from 10 nM (10−8 M) to 100 μM (10−4 M), alone or in combination with 10 μM DMI, as indicated, and analyzed as in panel A. D. Densitometric quantitation for panel A, with signaling activation calculated as a ratio of phospho-kinase:total protein. Data are mean ± SEM obtained over 3 independent experiments. *p<0.05 vs. NE alone. E. MEF cells were subjected to stimulation with 1% FBS for the indicated times, alone or in combination with 10 μM DMI, and analyzed as in panel A. All blots are representative of 3 independent experiments.
Analyzing the samples from our dose response analyses above for Akt activation revealed yet another surprise. While NE alone dose-dependently induced α2AAR-mediated Akt activation, the addition of DMI attenuated this signaling (Figure 3C), contrasting with its effects on ERK1/2 (Figure 2). Densitometric analysis confirmed significantly reduced Akt activation at the highest NE levels of 10 and 100 μM (Figure 3D). These data initially suggested that DMI selectively modulates NE-induced signaling through the α2AAR, altering the signaling profile of the receptor to favor activation of ERK1/2 over Akt. However, in our FBS assay for selectivity, we found that the Akt activation driven by FBS was blocked by the addition of DMI (Figure 3E). Therefore, DMI appears to inhibit cellular Akt activation through a general mechanism that lacks selectivity for the agonist-induced α2AAR-mediated signaling.
Nevertheless, a more general physiological role for the observed Akt inhibitory effect should not be ruled out. Although a mechanistic explanation of course remains to be seen, that DMI exerts a general inhibitory effect on cellular Akt activation is itself a novel finding with potential importance to antidepressant pharmacology. Activated Akt targets and phosphorylates (inactivates) a downstream kinase, GSK3, and this Akt/GSK3 pathway has been extensively implicated in antidepressant mechanisms of action [12]. Therefore, further study of the DMI/Akt phenomenon reported here seems to be warranted.
3.3 Desipramine-modulated α2AAR-mediated ERK1/2 signaling is Gi-dependent and arrestin-independent
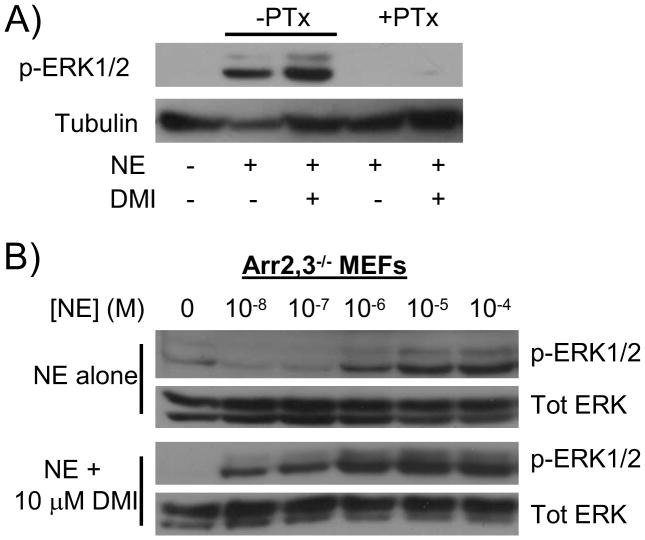
We have previously identified DMI as an arrestin-biased ligand at the α2AAR, selectively driving arrestin recruitment to the receptor without inducing G protein coupling [5], and demonstrated critical involvement of the α2AAR/arrestin complex for the in vivo antidepressant behavioral effects of DMI [18]. Therefore, a straightforward mechanistic explanation for the data presented thus far could be a switch from classical G protein-mediated to arrestin-mediated signal transduction by the α2AAR in the presence of DMI. Although the α2AAR-mediated ERK1/2 signaling reported thus far in the present and previous studies has been exclusively G protein-dependent, it is now a well-established general fact that GPCRs can signal via G protein-independent mechanisms utilizing arrestin for signal transduction [19, 20].
Therefore, we postulated that a DMI-dependent switch from classical G protein-mediated signaling to novel arrestin-mediated signaling might provide a mechanistic explanation for the observed potentiation of NE-induced ERK1/2 signaling by DMI. Such a mechanism could also provide an attractive potential biochemical basis for our recent in vivo findings mentioned above [18]. However, our data unfortunately do not support this postulated mechanism, as the DMI-potentiated ERK1/2 signaling remains PTx-sensitive and therefore dependent on Gi/o proteins (Figure 4A). This finding also rules out the possibility of a change in the identity of the heterotrimeric G proteins coupling to the receptor, as PTx is inhibitory toward Gi/o subfamily G proteins only. Additionally, repeating the dose response experiment in MEF cells lacking both ubiquitously-expressed arrestins (arrestin2 and 3, also known as β-arrestin 1 and 2) revealed that both NE-stimulated ERK1/2 activation and the potentiation of ERK1/2 signaling by DMI are preserved in the absence of arrestins. The mechanistic details of this G protein-dependent and arrestin-independent potentiation of NE-induced ERK1/2 by DMI remain to be determined.
Figure 4.
DMI-modulated ERK1/2 signaling through the α2AAR remains Gi-dependent and arrestin-independent. A) MEF cells were subjected to pertussis toxin (+PTx) or vehicle (-PTx) treatment as described in methods, then stimulated with 10 μM NE alone or in combination with 10 μM DMI for 5 minutes. Whole cell homogenates were analyzed by SDS-PAGE/Western blot, probing for phospho-ERK1/2 and tubulin. B) Arrestin2,3 double knockout (Arr2,3−/−) MEFs were stimulated for 5 minutes with NE at varying concentrations, ranging from 10 nM (10−8 M) to 100 μM (10−4 M), alone or in combination with 10 μM DMI, as indicated. Homogenates were analyzed as in panel A, with the addition of stripping/probing for total ERK. Blots are representative of 3 independent experiments.
3.4 Conclusions and implications
The data presented here provide the first evidence that the antidepressant drug DMI can selectively potentiate ERK1/2 signaling through the α2AAR induced by its endogenous agonist NE. The potentiation effect is observed as enhanced activation by an NE/DMI combination when [DMI] exceeds [NE] (Figures 1C, 2A, 2B), and a prolongation of the activation time course when [DMI] equals [NE] (Figure 1B). The potentiation presents mainly as an enhancement of response sensitivity, as DMI does not have any significant effect the maximal NE-induced ERK1/2 activation (Figures 2A, 2B), and is G protein-dependent (Figure 4A) and arrestin-independent (Figure 4B). Selectivity is supported by the findings that while α2AAR-mediated ERK1/2 activation is enhanced, an opposite and more general effect of DMI on Akt is observed (Figure 3).
Our original hypothesis that DMI would serve as a functional antagonist of endogenous agonist-mediated signaling at the α2AAR is soundly refuted by our data. In fact, it seems that the interaction of DMI with the α2AAR is much more complex than any associated with classical ligands. Given that a switch in the signaling from G protein-mediated to arrestin-mediated transduction does not occur (Figure 4), a mechanistic model of this complex interaction remains to be elucidated. The functional signaling data in this study could be interpreted as representing a positive allosteric modulation of the receptor by DMI. Although our past analysis indicates that DMI binds to the α2AAR orthosterically [5], it is possible that DMI may interact with both the orthosteric site and an allosteric site, a phenomenon which has been reported for ligands at the M2 muscarinic receptor [21]. DMI may bind to an allosteric site which is available only within the active conformation of the receptor, given that our previous analysis was carried out with an antagonist as the competing radioligand. These possibilities raised by our present data suggest a more rigorous analysis of the DMI/α2AAR interaction is warranted, utilizing such approaches as the kinetic receptor binding techniques described by Limbird and more specifically designed to detect allosterism [22].
Alternatively, DMI may in fact be binding to the orthosteric site on the α2AAR and modulating signaling in some other way, for example through a dimerization of DMI-bound receptor with NE-bound receptor which alters the receptor signaling profile. α2AARs are among the many GPCRs which have been shown to homodimerize [23], and dimerization has the potential to alter the signaling profiles of GPCRs as a kind of allosterism [24].
Regardless of the underlying mechanism, our findings have potential implications for antidepressant pharmacology. Our previous study [5] postulated a model whereby DMI exerts its therapeutic antidepressant effects through a downregulation of central nervous system α2AAR expression, correcting the pathobiological increases in α2AAR density and activity associated with clinical depression (reviewed in [25]). The present data demonstrate that DMI in a physiologically relevant ratio with NE can effectively turn on MAPK signaling at that is lacking in response to the physiological level (e.g. 10 nM, see Figures 2A, 2B). This α2AAR-activating effect could serve to counterbalance the beneficial α2AAR-decreasing effect until a certain level of α2AAR downregulation is attained. Such a model may represent an important component of the mechanistic basis underlying the significant delay of 3–6 weeks between the start of clinical antidepressant therapy and onset of symptom relief.
Highlights.
Effects of desipramine on α2A adrenergic receptor signaling are investigated.
Norepinephrine-induced ERK1/2 signaling is potentiated by desipramine.
The potentiating effect is selective for α2A adrenergic receptor-mediated ERK1/2.
Desipramine-potentiated ERK1/2 remains dependent on heterotrimeric G proteins.
A novel modulation of receptor signaling by an antidepressant drug is discovered.
Acknowledgments
Role of the funding sources
The funding sources had no involvement in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the article for publication.
We thank Dr. Robert J. Lefkowitz (Duke University) for providing the Arr2,3−/− MEF line. This work has been supported by a National Institute of Mental Health grant (MH081917, QW), the UAB Training Program in Neurobiology of Cognition and Cognitive Disorders (National Institutes of Heath T32 grant NS061788-03, CC), and the McNair Scholars Program (AJ).
Footnotes
The abbreviations used are: AR, adrenergic receptor; DMI, desipramine; ERK, extracellular signal-regulated kinase; FBS, fetal bovine serum; GPCR, G protein-coupled receptor; MAPK, mitogen-activated protein kinase; MEF, mouse embryonic fibroblast; NE, norepinephrine; PTx, pertussis toxin
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill, Inc; New York: 2006. pp. 429–460. [Google Scholar]
- 2.Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes--unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51(5):277–281. doi: 10.1016/j.neuint.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Mateo Y, Pineda J, Meana JJ. Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem. 1998;71(2):790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- 4.Linner L, Arborelius L, Nomikos GG, Bertilsson L, Svensson TH. Locus coeruleus neuronal activity and noradrenaline availability in the frontal cortex of rats chronically treated with imipramine: effect of alpha 2-adrenoceptor blockade. Biol Psychiatry. 1999;46(6):766–774. doi: 10.1016/s0006-3223(99)00126-2. [DOI] [PubMed] [Google Scholar]
- 5.Cottingham C, Chen Y, Jiao K, Wang Q. The antidepressant desipramine is an arrestin-biased ligand at the alpha2A adrenergic receptor driving receptor downregulation in vitro and in vivo. J Biol Chem. 2011;286:36063–36075. doi: 10.1074/jbc.M111.261578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limbird LE. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1988;2(11):2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- 7.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annual review of neuroscience. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 8.Richman JG, Regan JW. Alpha 2-adrenergic receptors increase cell migration and decrease F-actin labeling in rat aortic smooth muscle cells. The American journal of physiology. 1998;274(3 Pt 1):C654–662. doi: 10.1152/ajpcell.1998.274.3.C654. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304(5679):1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem. 2006;281(36):25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- 11.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61(5):661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35(11):2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98(4):1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alblas J, van Corven EJ, Hordijk PL, Milligan G, Moolenaar WH. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by alpha 2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993;268(30):22235–22238. [PubMed] [Google Scholar]
- 15.Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci USA. 1994;91(26):12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateo Y, Fernandez-Pastor B, Meana JJ. Acute and chronic effects of desipramine and clorgyline on alpha(2)-adrenoceptors regulating noradrenergic transmission in the rat brain: a dual-probe microdialysis study. Br J Pharmacol. 2001;133(8):1362–1370. doi: 10.1038/sj.bjp.0704196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinasegamma. J Biol Chem. 1998;273(30):19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 18.Cottingham C, Li X, Wang Q. Noradrenergic antidepressant responses to desipramine in vivo are reciprocally regulated by arrestin3 and spinophilin. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.02.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redka DS, Pisterzi LF, Wells JW. Binding of orthosteric ligands to the allosteric site of the M(2) muscarinic cholinergic receptor. Molecular pharmacology. 2008;74(3):834–843. doi: 10.1124/mol.108.048074. [DOI] [PubMed] [Google Scholar]
- 22.Limbird LE. Cell Surface Receptors: A Short Course on Theory and Methods. Springer Science+Business Media, Inc; New York: 2005. [Google Scholar]
- 23.Small KM, Schwarb MR, Glinka C, Theiss CT, Brown KM, Seman CA, Liggett SB. Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry. 2006;45(15):4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- 24.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacological reviews. 2010;62(4):701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottingham C, Chen H, Chen Y, Peng Y, Wang Q. Genetic variations of alpha2-adrenergic receptors illuminate the diversity of receptor functions. In: Wang Q, editor. Current Topics in Membranes. Vol. 67. Elsevier Inc; Amsterdam: 2011. [DOI] [PubMed] [Google Scholar]