Abstract
Objective
The aim of this study was to identify common trajectories of lipid levels across childhood and early adulthood life span.
Design
The sample was a subpopulation of 824 young adults (3 – 9 years at baseline in 1980) of the on-going population-based prospective Cardiovascular Risk in Young Finns Study. Lipid levels were determined in 1980, 1983, 1986 and 2001.
Main outcome measures
Depressive symptoms were assessed using a modified version of Beck’s Depression Inventory in 1992 and 2001.
Results
The two triglycerides trajectories (steeply vs moderately increasing) were differently related to depressive symptoms in adulthood. The trajectory showing steep increase over time was associated with higher level of depressive symptoms (mean 2.18, 95% CI 2.08 - 2.28 vs 1.99, 95% CI 1.95–2.04). This relationship persisted after adjustments for various risk factors. These triglycerides trajectories accounted for part of the association between high BMI and depressive symptoms.
Conclusion
A pattern of steeply increasing triglyceride levels throughout childhood and adulthood may be associated with increased the risk of depressive symptoms in adulthood. This pattern may also be one link between obesity and depressive symptoms.
Keywords: depression, CHD, psychosocial factors, lipids
Public health significance of depression has been emphasized due to the impact depression has on the quality of life and especially on the course and mortality associated with other medical diseases (Kessler, Zhao, Blazer, & Swartz, 1997). A number of psychosocial factors such as disturbed family environment, predisposing personality traits, exposure to traumatic events, low social support, recent stressful life events and work stress are suggested to increase the risk of depressive symptoms(Bruce, 2002; Elovainio et al., 2006; Kendler, Neale, Kessler, Heath, & Eaves, 1993; Virtanen et al., 2008). It has also repeatedly been shown that lipid metabolism may be associated with depression or depressive symptoms (J. M. Kim et al., 2006; Troisi, 2009).
The hypotheses on the biological mechanisms between cholesterol and mental health are based on the findings that cholesterol forms part of cell membranes and is a component of myelin. The development, function and stability of synapses are also affected by cholesterol (Chattopadhyay & Paila, 2007; Chattopadhyay et al., 2007). It has been suggested that serum cholesterol may directly influence brain lipids and the fluidity of the cell membrane, with secondary effects on serotonergic neurotransmission (Engelberg, 1992). It has also been proposed that a decrease in serum total cholesterol or low density lipoprotein cholesterol (LDL-c) would induce a relative increase in brain cell membrane fluidity with increased presynaptic serotonin reuptake and decreased postsynaptic serotonin function (Diebold et al., 1998), which in turn could affect psychological states. It has been suggested that the lipid fraction associated with neuroendocrine indices of reduced serotonin function is low high density lipoprotein cholesterol (HDL-c) rather than total cholesterol or LDL-c (Buydens-Branchey, Branchey, Hudson, & Fergeson, 2000). Very little is known, however, about the biological role of triglycerides. There are also preliminary findings suggesting that the same genetic factor (i.e., the APOE promoters and the serotonin transporter polymorphism) contributes to lipid metabolism and psychiatric vulnerability (Elovainio et al., 2008; Fischer, Gruenblatt, Pietschmann, & Tragl, 2006).
Low levels of HDL-c has repeatedly been found to be a risk factor for incident depression (Horsten, Wamala, Vingerhoets, & Orth-Gomer, 1997; J. M. Kim et al., 2006) as well as a correlate of prevalent depression, mood disorders (Chen, Lu, Wu, & Chang, 2001; Dimopoulos et al., 2007; J. M. Kim, Stewart, Shin, & Yoon, 2004; Y. K. Kim & Myint, 2004; Lehto et al., 2008; Vogelzangs et al., 2007) and bi-polar disorders (Sagud, Mihaljevic-Peles, Pivac, Jakovljevic, & Muck- Sleer, 2009). However, for total cholesterol and LDL-c, both lower and higher levels have been found to be associated with depression (Aijanseppa et al., 2002; Partonen, Haukka, Virtamo, Taylor, & Lonnqvist, 1999; Shin, Suls, & Martin, 2008). Terao and others found more cases of depression among subjects having total cholesterol levels between 4.8 mmol/l and 5.0 mmol/l compared to those having total cholesterol levels higher than 6.1 mmol/l (Terao et al., 2000). Also null findings have been reported (Blazer, Burchett, & Fillenbaum, 2002; Brown, 1995; Ergun et al., 2004). There is also evidence suggesting positive association between high triglycerides and depression, bipolar disorders or affective disorders (Ergun et al., 2004; Glueck, Tieger et al., 1994; Lehto et al., 2008; Sagud et al., 2009). For instance, Almeida et al (Almeida et al., 2007) showed that high triglycerides, but not other lipids, may be associated with depression in older men.
There are many potential reasons for these mixed findings. Cholesterol and triglycerides circulate in various carrier proteins, each with unique features and are separated by their density. LDL-c is the major carrier of cholesterol and is needed for the synthesis of steroid hormones and cell membranes. Elevated levels of LDL-c accumulate subendothelially in arteries, but HDL is responsible for the reverse cholesterol transport. The independent role of triglycerides on various health outcomes is less clear due to the collinearity of elevated triglycerides with reduced HDL-c and other lipids. A recent review suggested, however, that non-fasting triglyceride levels may strongly predict the risk of cardiovascular events and other health outcomes (Abdel-Maksoud, Sazonov, Gutkin, & Hokanson, 2008). Most of the previous studies have examined only the effect of LDL-c or total cholesterol on depressive symptoms although the effects may differ for other lipids, such as HDL-cholesterol or triglycerides.
Furthermore, many of the previous studies have used clinical or elderly populations and cross-sectional designs. These studies presuppose that lipid levels have rather immediate impact on mood or depressive symptoms. According to a previous study using the same cohort (Pulkki-Raback et al., 2009) depressive symptoms were associated with increased risk of the metabolic syndrome in adulthood and the metabolic syndrome in childhood, in turn, predicted higher levels of depressive symptoms in adulthood. The authors concluded that process linking depressive symptoms with the components of metabolic syndrome may go into both directions and may begin early in life. The authors also found a cross-sectional association between triglycerides and depression in adulthood. The problem with this study was the inability to model the change in metabolic syndrome components and especially the inability to model the unobserved heterogeneity in the population, in other words, differently shaped developmental trajectories of lipid levels, which was the particular aim of this study. There are age- and time-related patterns of lipid levels and thus it is reasonable to assume that long term patterns of lipid levels over time may be more important for developing mental health problems than lipid levels at single points in time.
The current study examined patterns of lipid levels through childhood and early adulthood and their associations with depressive symptoms in adulthood. We used a new statistical approach (Jones, Nagin, & Roeder, 2001; Marin, Chen, & Miller, 2008) to indentify common trajectories of LDL-c, HDL-c and triglycerides across childhood and early adulthood in a random sample of Finnish men and women. These trajectories were used to predict depressive symptoms in adulthood. The lipid levels trajectories were identified at four time points during the 21-year follow-up.
Methods
Study sample
The participants were derived from two samples of the on-going population based study of “Cardiovascular Risk in Young Finns“(Young Finns) (Raitakari et al., 2008). In this prospective, epidemiological study, a randomized sample of 3596 healthy Finnish children and adolescents in age cohorts of 3, 6, 9, 12, 15, and 18 years have been followed since 1980. During the sixth follow-up of the Young Finns in 2001 (21 years after the baseline), 2229 participants were re-examined by measuring depressive symptoms using the modified Beck Depression Inventory. The sample of this study consisted of 824 boys and girls who were 3, 6 or 9 years of age in 1980 and had complete follow-up data (i.e., lipids measured at 1980, 1983, 1986 and 2001 and depression measured at 1992 and 2001). As 1765 participants belonging to the three youngest age cohorts had lipids measured at baseline, the final sample was 47% of the baseline population.
Plasma lipids
All measurements of lipid levels were performed in duplicate in the same laboratory. All venous blood samples were taken after 12 hours of fasting. Serum samples were stored frozen at −20°C for no more than 6 months until analyzed. All lipid determinations were performed in duplicate in the same season (fall) and as simultaneously as possible. Frozen serum was used both in sample analyses and in the determination of method correction equations. Standardized enzymatic methods were used for measuring levels of serum total cholesterol, triglycerides, and high density lipoprotein cholesterol. Low density lipoprotein cholesterol concentration was calculated by the Friedewald formula (Friedewald, Levy, & Fredrickson, 1972). Similar methods were used in all study phases. Those with having triglycerides higher that 4 were excluded form the analyses (n=4).
Depressive symptoms
Depressive symptoms were self-rated using a modified version of the Beck Depression Inventory (Beck, 1967). The questionnaire pins down negative mood, sadness, pessimism, indecisiveness and somatic problems such as insomnia and fatigue. The original BDI is a 21-item questionnaire that offers 4 alternative statements for each item. In the present study, the BDI was modified so that each statement represents the second mildest level of depression in the original BDI. The participants were asked to choose between 1 (totally disagree) and 5 (totally agree) (Elovainio et al., 2006). Cronbach’s α for the modified BDI in 1992 and 2001 was .0.89 and 0.92. The Pearson correlation between depressive tendencies in 1992 and 2001 was r=0.58 (p<0.0001).
Confounding and mediating factors
The potential confounders were parental socioeconomic position, childhood body mass index and childhood C-reactive protein (CRP), all measured in 1980. Parental socioeconomic position was defined as family income and it was calculated as gross annual income, ranging from 1 (corresponding to <USD 3,000) to 8 (corresponding to >USD 22,000) (Kivimaki et al., 2006). These criteria correspond to the median income per household in Finland which was 12 920 USD in 1983. Childhood serum high sensitive C-reactive protein (hsCRP) was analyzed by an automated analyzer (Olympus AU400, Olympus, USA) and a highly sensitive turbidimetric immunoassay kit ("CRP-UL"-assay, Wako Chemicals, Neuss, Germany). Detection limit of the assay was 0.06 mg/L. Physical activity was a sum index of five questions assessing the intensity, duration, and frequency of sports.
The potential mediating factors were adulthood indicators of health behavior including body mass index, alcohol consumption, smoking, physical activity and metabolic syndrome. Body mass index was calculated at both times (1980 and 2001) as weight (kg)/[height]2. Height was measured with a wall-stated statiometer and weight with Seca scales. Alcohol consumption was measured by one question (number of occasions per week when alcoholic beverages are consumed more than six units during one day). Smoking status was assessed by a questionnaire. Those smoking on daily basis were classified as smokers. All measure s have been reported previously in Raitakari et al. (Raitakari et al., 2008). Metabolic syndrome was defined following the criteria of National Institute of Health Adult Treatment Panel III (NCEP). Metabolic syndrome was diagnosed as 3 or more of the following conditions: waist ≥102 cm in men and ≥88 cm in women, serum triglycerides ≥1.695 mmol/l (150 mg/dl), HDL cholesterol <1.036 mmol/l (40 mg/dl) in men and <1.295 mmol/l (50 mg/dl) in women, blood pressure ≥130 or ≥85 mmHg or treated, and plasma glucose ≥5.6 mmol/l (100 mg/dl).
Statistical analyses
Statistical calculations were done using SAS (version 9.1) statistical package. In the first wave of analyses, we modelled trajectories of LDL-c, HDL-c and triglyceride levels across childhood and adulthood by using a SAS procedure PROC TRAJ (Jones et al., 2001) that separates individuals into trajectory groups. TRAJ is a semiparametric, group-based modelling strategy that identifies clusters of individual trajectories. Model estimation produces posterior probabilities of membership in each trajectory group for each participant. These probabilities are used to assign individuals to the trajectory group to which they are most likely to belong. First, we determined the number of trajectories that best represented patterns of lipids in our sample. The way of doing this was using the change in the Bayesian information criteria (BIC) as an approximation to the log of the Bayes factor. Next, we looked at the parameter estimates to determine the shape (linear, quadratic, or cubic) of each trajectory. Finally, we examined the relationship between trajectory group membership and depressive symptoms in adulthood in 2001 using analyses of variance. Depressive symptoms measured at 1992 were treated as baseline measure of depressive symptoms. This set of analyses could be thought of as an inductive empirical approach based on what the data reveal about the types of life-course patterns that are present in this sample.
Results
The sample characteristics are shown in Table 1. Almost half of the sample was lost during the follow-up. Compared to the baseline population, the participants in the present sample were more often women (χ2 = 110.24, df = 1, p < 0.001). The other baseline differences were not statistically significant. Depressive symptoms slightly decreased from 1992 to 2001
Table 1.
Sample characteristics (N=824)
| Variable | Mean | (SE) | N | (%) |
|---|---|---|---|---|
| In childhood | ||||
| Age (range 3–9 years) | 5.9 | (2.5) | ||
| Sex | ||||
| Women | 487 | (59) | ||
| Men | 336 | (41) | ||
| Body-mass index in childhood (kg/m2) | 15.9 | (1.8) | ||
| C-reactive protein (mg/L)a | −1.9 | (1.3) | ||
| Family’s annual income a | 4.9 | (1.9) | ||
| In adulthood | ||||
| Body-mass index in adulthood (kg/m2) | 24.4 | (4.3) | ||
| Smoking | ||||
| No | 615 | (80) | ||
| Yes | 159 | (20) | ||
| Alcohol consumptionc | 2.5 | (1.3) | ||
| Physical activity d | 9.9 | (2.4) | ||
| Metabolic syndrome (NCEP) | ||||
| No | 627 | (76) | ||
| Yes | 31 | (4) | ||
| Missing | 166 | (20) | ||
| Depressive symptoms in 1992 | 2.2 | (0.6) | ||
| Depressive symptoms in 2001 | 2.0 | (0.7) | ||
Logarithmic
Range from 1 (less than 15000FIM) to 8 (more than 100 000)
Range 1–6
Physical activity index
We tested the number of trajectories following the Bayesian Information Criterion (BIC) and continued to increase the number of trajectories as long as the BIC increased, indicating a better model fit of the model: For LDL-c the BIC was −6969.0 for 1 group, −6181.4 for 2 groups, and −5931.0 for 3 groups. For HDL-c the BIC was −1345.0 for 1 group, −841.6 for 2 groups and −686.2 for 3 groups and -641.9 for 4 groups. For triglycerides the BIC was −2712.9 for 1 group, −1932.2 for 2 groups and −1768.4 for 3 groups. Because the fit did not much improve after 2 group solutions in LDL-c (group sizes 75%25%) and triglycerides (83%17%) we retained the 2 trajectory models. In HDL-c the fit improved after three group solution only marginally and to prevent the trajectory group sizes from getting too small, we did not exceed the 4-group model. Both the 3- and 4-group models yielded similar information. In the interest of parsimony, we retained the 3-trajectory model (43%/48%/9%).
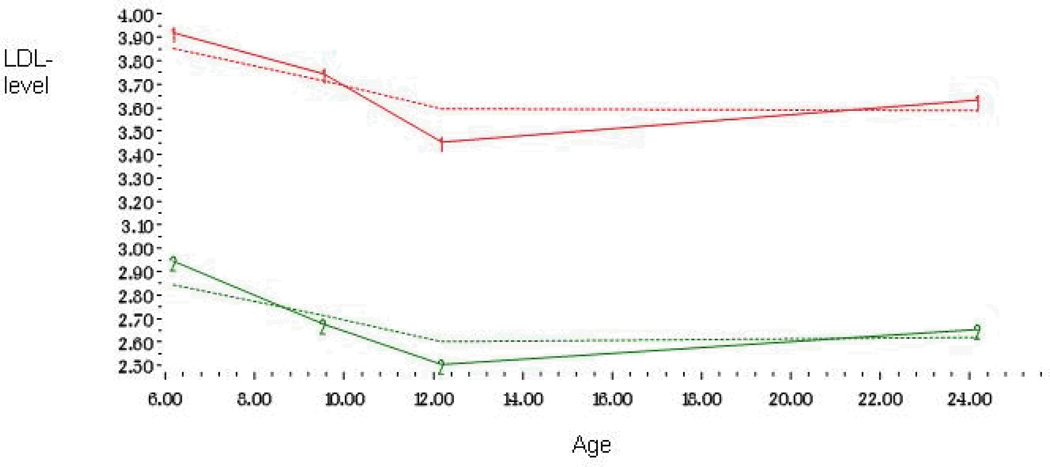
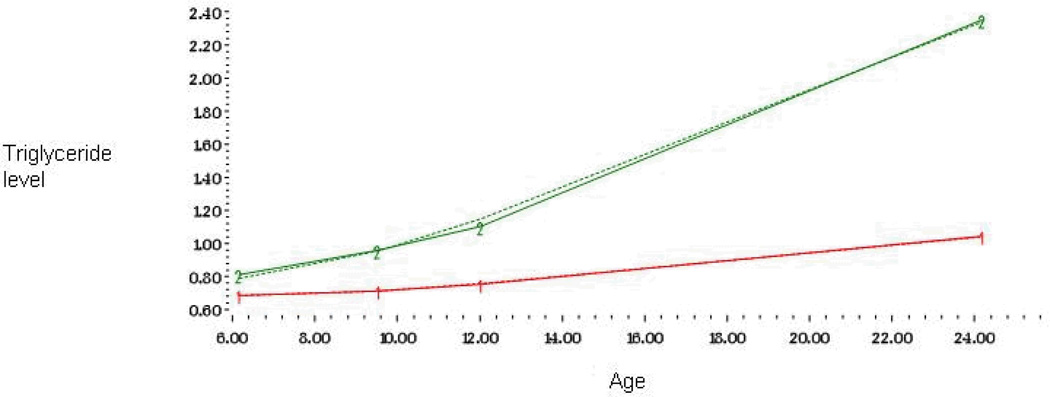
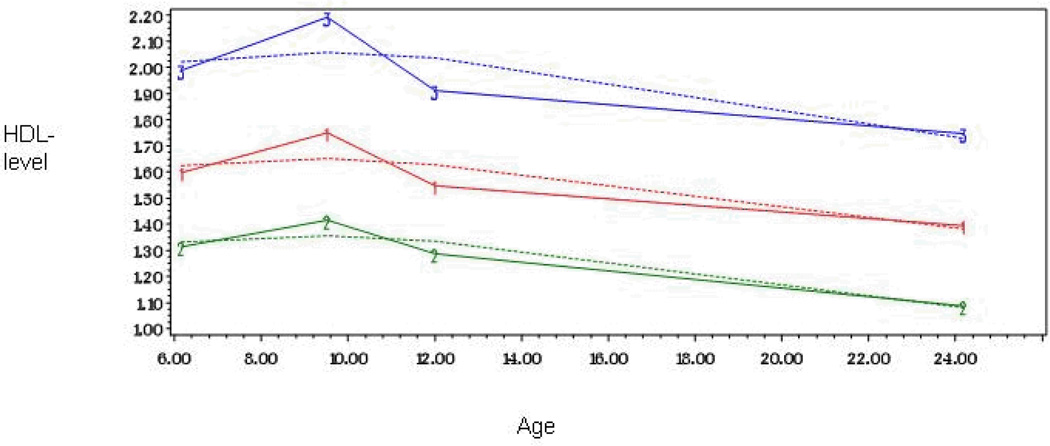
The shape of each trajectory was determined separately for LDL-c, HDL-c and triglycerides by initially including linear, quadratic, and cubic parameters for both trajectories, and then dropping the nonsignificant ones. A parameter estimate divided by its standard error results in a t statistic, which was used to determine statistical significance. The shape of each trajectory was identified by the highest order term included in the model. In Model 1, linear, quadratic, and cubic parameters were included for each of the four trajectories. In LDL-c, HDL-c and triglycerides the cubic parameters were significant for all trajectories and thus Model 1 was adopted as our final model for these lipids (Table 2). In LDL-c the groups were called high (25%) and low (75%) and triglycerides groups were called moderately increasing (83%) and steeply increasing (17%) trajectories. In HDL-c the trajectory groups were called high (9%), medium (48%) and low (43%) (Figures 1–3).
Table 2.
Model selection for the shape of the LDL, HDL and Triglycerides trajectories
| Trajectory 1 | Trajectory 2 | Trajectory 3 | ||
|---|---|---|---|---|
| Model and parameter | Parameter estimate |
Parameter estimate |
Parameter estimate |
BIC |
| HDL –cholesterol trajectories | −532.8 | |||
| Intercept | 6.12 | 6.62 | 22.2 | |
| Linear | 0.12*** | 0.10*** | 0.11*** | |
| Quadratic | −0.01*** | −0.01*** | −0.01*** | |
| Cubic | 0.00*** | 0.00*** | 0.00*** | |
| LDL−cholesterol trajectories | −6181.4 | |||
| Intercept | −0.00 | 22.8 | NA | |
| Linear | −0.02 | −0.01* | NA | |
| Quadratic | −0.01*** | −0.01*** | NA | |
| Cubic | 0.00*** | −0.01*** | NA | |
| Triglycerides-trajectories | −1918.6 | |||
| Intercept | −12.9 | −94.4 | NA | |
| Linear | −0.04*** | −0.08*** | NA | |
| Quadratic | 0.00*** | 0.01*** | NA | |
| Cubic | −0.00*** | −0.00*** | NA | |
Function tested is Cubic
Figure 1.
Developmental trajectories of LDL-cholesterol level from childhood to adulthood among 824 men and women
Figure 3.
Developmental trajectories of triglyceride level from childhood to adulthood among 824 men and women
As shown in Table 3, no statistically significant differences in baseline-adjusted depressive symptoms between LDL-c or HDL-c trajectory groups were observed. However, the steeply increasing triglycerides trajectory group showed higher baseline-adjusted means of depressive symptoms than the moderately increasing group. Table 4 presents the effects of various childhood confounders and adulthood mediators in the association between triglycerides trajectories and depressive symptoms. The association seemed to be quite robust to adjustments for any childhood or adulthood factors or for their combination. We additionally adjusted the association between triglycerides trajectories and depressive symptoms for triglyceride levels in 2001 and although it somewhat attenuated the effect, the association remained statistically significant (p=0.02).
Table 3.
Means (95% CI) of depressive symptoms in the LDL, HDL and triglyceride trajectory groups.
| Adjusted for baseline depressive symptoms |
Adjusted for baseline depressive symptoms, age and sex |
|||
|---|---|---|---|---|
| Trajectory group | Mean (95% CI ) | p-value | Mean (95% CI ) | p-value |
| LDL | ||||
| Low (N=550) | 2.00 (1.96, 2.06) | 2.01 (1.96, 2.06) | ||
| High (N=273) | 2.06 (1.99, 2.13) | 0.206 | 2.05 (1.98, 2.12) | 0.335 |
| HDL | ||||
| Low (N=340) | 2.04 (1.98, 2.11) | 2.04 (1.98, 2.11) | ||
| Medium (N=417) | 2.00 (1.95, 2.06) | 2.00 (1.95, 2.06) | ||
| High (N=66) | 2.08 (1.94, 2.22) | 0.442 | 2.07 (1.93, 2.21) | 0.450 |
| Triglycerides | ||||
| Moderate increase (N=692) | 2.00 (1.95, 2.04) | 1.99 (1.95, 2.04) | ||
| Steep increase (N=131) | 2.18 (2.08, 2.27) | 0.001 | 2.18 (2.08, 2.28) | 0.001 |
Table 4.
Means and 95% Cl of depressive symptoms in 2 triglyceride trajectory groups (moderate and steep increase) with depressive symptoms. All models adjusted for depressive symptoms in 1992, age and sex)
| Moderate increase group |
Steep increase group | ||
|---|---|---|---|
| Adjusted in addition to baseline depressive symptoms, age and sex |
Mean (95% Cl) | Mean (95% Cl) | p-value |
| Parental socioeconomic position | 2.00 (1.95 – 2.04) | 2.19 (2.08 – 2.29) | <0.001 |
| Childhood BMI | 2.00 (1.96 – 2.04) | 2.18 (2.08 – 2.28) | 0.001 |
| Childhood CRP | 1.96 (1.91 – 2.01) | 2.20 (2.10 – 2.30) | <0.001 |
| Adulthood metabolic syndrome | 1.96 (1.91 – 2.01) | 2.19 (2.07 – 2.30) | <0.001 |
| Adulthood health behaviour (BMI, physical activity, smoking and alcohol consumption) |
2.00 (1.95 – 2.04) | 2.19 (2.09 – 2.30) | 0.001 |
| All | 1.97 (1.92 – 2.01) | 2.21 (2.10 – 2.33) | <0.001 |
We confirmed the results using linear regression analyses using triglycerides levels at baseline and at follow-up predictors of depressive symptoms. The results showed that triglycerides at both time points were associated with depressive symptoms in models where baseline depressive symptoms and other baseline variables were adjusted for, but only the effect of baseline triglycerides (B=0.18, t(1)=2.61, p=0.009) was robust to adjustment for combination of all childhood and adulthood factors. Using triglycerides change score (subtracting baseline from follow-up) produced comparable results. The association between triglyceride change and depressive symptoms was robust to adjustments for childhood and adulthood risk factors including metabolic syndrome (B=0.11, t(1)=2.48, p=0.013).
High childhood BMI (B=0.02, t(1)= 3.63, p<0.001) and greater increase of BMI from childhood to adulthood (B=0.01, t(1)=2.85, p=0.005) predicted increased depressive symptoms, even when adjusted for other childhood and adulthood factors covariates except triglycerides. However, when triglycerides trajectory group was added to the model, neither childhood BMI (B=0.00, t(1)=1.67, p=0.095) nor BMI change from childhood to adulthood (B=0.01, t(1)=0.87, p=0.385) were associated with adulthood depressive symptoms.
Discussion
Previous studies have reported mixed findings on the associations between LDL-c, HDL-c, triglycerides and depressive symptoms, but these studies have not taken into account the age-related changes in lipid trajectories and their effects on mental health problems. In this 21-year follow-up of a randomly selected group of Finnish boys and girls aged 3 to 9 years at baseline a pattern of steeply increasing triglycerides levels from childhood to adulthood was associated with depressive symptoms in adulthood. This association was not explained by confounding factors, such as childhood or adulthood health behaviours, earlier depressive symptoms, or adulthood triglycerides. We observed no associations of LDL-c or HDL-c trajectories with depressive symptoms.
High LDL- and total cholesterol levels have been shown to be associated with serious physical diseases, such atherosclerosis, coronary heart disease and diabetes, but they are suggested to be associated with more favourable mental health outcomes (Shin, Suls & Martin, 2008). Although some studies suggest no association between cholesterol and depression or depressive symptoms (Deisenhammer et al., 2004), several studies have found low or lowered LDL-cholesterol/total cholesterol to predict increased risk of depression and suicidal behaviour (Borgherini, Dorz, Conforti, Scarso, & Magni, 2002; Terao et al., 2000). Early findings on the association between triglycerides and depressive symptoms, on the other hand, suggest that low triglycerides may be associated with less depressive symptoms (Glueck, Kuller et al., 1994; Glueck, Tieger et al., 1994). Recently some studies have found similar positive associations between depression or depressive symptoms and elevated triglycerides but not with other lipids (Almeida et al., 2007; Glueck, Kuller et al., 1994; Glueck, Tieger et al., 1994; Toker, Shirom, Shapira, Berliner, & Melamed, 2005). Our findings are consistent with this evidence. We took into account age-related changes in lipid levels and found triglycerides to have two clearly different-shaped trajectories (steep and moderate increase) whereas trajectories of LDL-c and HDL-c showed group differences in average levels but not in the shapes of the age-related trajectories. Interestingly, only steeply increasing triglycerides trajectory was associated with depressive symptoms.
The importance of cholesterol in the function of neural system has been underlined. Barres and Smith suggested that cholesterol is needed to activate the signaling pathway that triggers synaptogenesis (Barres & Smith, 2001). Engelberg (Engelberg, 1992) suggested a more specific physiological mechanism that might explain the association between low LDL –cholesterol and total cholesterol and depressive symptoms. He concluded that low membrane cholesterol decreases the number of serotonin receptors. Since membrane cholesterol exchanges freely with cholesterol in the surrounding medium, a lowered serum cholesterol concentration may contribute to a decrease in brain serotonin, which then may affect mood. However, the role of triglycerides in physiological background of depression remains unclear (Troisi, 2009).
The most obvious confounder in our analyses was body mass index due to its strong associations with both lipid levels and depressive symptoms, a finding also observed in previous studies (Cortese, Faussard, Angriman, Pigaiani, & al., 2008). However, the associations between triglycerides trajectories and depressive symptoms were quite robust to adjustments for childhood BMI. This is in line with recent studies suggesting that the direction of the association between mental health and BMI may be dominantly from mental disorder to obesity rather than vice verse (Kivimäki et al., 2009). Moreover, the link between steep increase in triglycerides and subsequent depressive symptoms was not explained by markers of childhood systemic inflammation, or by adulthood health risk behavior, both of which have been found to be associated with lipids and depressive symptoms (Elovainio et al., 2006; Maes, 1999). In contrast, we found some evidence that the association of childhood BMI and weight gain from childhood to adulthood, with depressive symptoms in adulthood attenuated when steeply increasing triglyceride levels were adjusted for.
In the current study, we applied semi-parametric, group-based modeling. The approach is intended to complement two well-established methods for analyzing developmental trajectories - hierarchical modeling (Goldstein, Healy, & Rasbash, 1994) and latent growth curve modeling (Bollen & Curran, 2006). These methods model variation in the parameters of developmental trajectories using continuous multivariate density function. The group based approach employs a multinomial modeling strategy and is especially useful for modeling unobserved heterogeneity in a population. In contrast to the homogeneous case, it is assumed that there are unobserved subpopulations differing in their parameter values dependent on time.
We additionally, however, performed latent growth curve analyses with intercept and slope latent variables of triglycerides predicting depression at follow up. Latent factors representing intercept (baseline status) and slope (rate of change) components are extracted from the four observations of triglycerides across time, here identified as wave 1 (baseline), wave 2 and wave 3. Factor loadings of the latent intercept component to all three observation were fixed to 1, and the linear slope component was defined by fixing the parameters to 0 (baseline), 1 (wave 2), 2 (wave 3) and 3 (wave 4). The estimated variance of the intercept growth factor was 0.63 (SE 0.46), and the variance of the slope growth factor was 0.17 (SE 0.09) indicating that there was significant individual heterogeneity around the group means for both initial status and rate of change. Furthermore, previous analyses indicated nonlinear growth curves in later triglycerides, thus, we freed the fourth factor loading to capture this nonlinear growth curve The fit indices suggested that the proposed model fit the data quite well Χ2 (5) = 15.12, p=0.009, RMSEA=0.053. In the second stage of model development we tested whether the latent factors were associated with depressive symptoms at follow up and found that there was a significant association between depressive symptoms and triglyceride intercept (equation = 8.66, t=10.44) and slope (equation = 8.66, t=10.44). When the model was additionally adjusted for earlier depressive symptoms only the association between depressive symptoms and triglyceride slope remained statistically significant (t=10.54).
In interpreting the present results, it is important to note some limitations. First, assignment into trajectory groups is probabilistic and some individuals had a high probability of membership in the particular trajectory group, whereas others had a lower membership probability in that same trajectory group. Although this probabilistic assignment means that the trajectory groups should not be taken as real entities, any uncertainty in group membership is factored into the depressive symptoms comparisons. Second, the scale of depressive symptoms was not a measure of clinical depression and therefore our findings should be replicated using a standard measure of depression with clinical cut-off points. Our measure of mild depression may better bring out subtle variations in depressive symptoms in a population of healthy young adults among whom rates of clinical depression are rather low. Even mild, sub-clinical, levels of depression may, however, be important as they have been suggested to be associated with increased CHD risk if they persist over time. The validity of the modified BDI is supported by previous studies that have associated it with psychosocial characteristics known to be associated with depression, such as child’s difficult temperament (negative emotionality and low sociability), adulthood sentimentality and fatigability, and low social support and with cardiovascular risk factors such as C-reactive protein and pre-clinical atherosclerosis. Although we can never rule out the potential residual confounding, the fact that we did take into account the effect of earlier depressive symptoms in the relationship between lipid trajectories and later depressive symptoms offer some support to the conclusion that our results are not explained by confounders known to impact the level of depressive symptoms. Such factors would have already influenced the level of earlier depressive symptoms.
In conclusion, our results have to be considered as preliminary, and further research is needed to confirm these findings. Our results suggest that relatively rapid increase of triglycerides already at early age is associated with depressive symptoms in adulthood. No evidence on association of any change patterns of LDL- or HDL-cholesterol on depressive was found.
Figure 2.
Developmental trajectories of HDL-cholesterol level from childhood to adulthood among 824 men and women
Acknowledgements
This study was financially supported by the Signe and Ane Gyllenberg’s Foundation (LK-J), the Academy of Finland grants 111056, 209514, 123399 (LK-J), 123621 (LP-R) and 117604 (MK) and 7784 and 210283 (O.T.R), Emil Aaltonen Foundation (TL), Tampere University Hospital Medical Fund (TL), the Yrjö Jahnsson Foundation and Turku University Hospital Medical Funds (O.T.R and J.V.). ME was supported by the Work Environment Fund and Academy of Finland (128002). MK was supported by the National Heart, Lung, and Blood Institute (R01HL036310-20A2) and National Institute on Aging (R01AG034454-01), NIH, US; the Academy of Finland, Finland; and BUPA Foundation, UK.
Abbreviations
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- CHD
coronary heart disease
- Young Finns
Cardiovascular Risk in Young Finns
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/HEA
References
- Abdel-Maksoud M, Sazonov V, Gutkin SW, Hokanson JE. Effects of modifying triglycerides and triglyceride-rich lipoproteins on cardiovascular outcomes. Journal of Cardiovascular Pharmacology. 2008;51(4):331–351. doi: 10.1097/FJC.0b013e318165e2e7. [DOI] [PubMed] [Google Scholar]
- Aijanseppa S, Kivinen P, Helkala EL, Kivela SL, Tuomilehto J, Nissinen A. Serum cholesterol and depressive symptoms in elderly Finnish men.[see comment] International Journal of Geriatric Psychiatry. 2002;17(7):629–634. doi: 10.1002/gps.666. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Flicker L, Norman P, Hankey GJ, Vasikaran S, van Bockxmeer FM, et al. Association of cardiovascular risk factors and disease with depression in later life. American Journal of Geriatric Psychiatry. 2007;15(6):506–513. doi: 10.1097/01.JGP.0000246869.49892.77. [DOI] [PubMed] [Google Scholar]
- Barres BA, Smith SJ. Neurobiology. Cholesterol--making or breaking the synapse. Science. 2001;294(5545):1296–1297. doi: 10.1126/science.1066724. [DOI] [PubMed] [Google Scholar]
- Beck A. Depression. Clinical, experimental and theoretical aspects. New York, Harper & Row Publishers, 1967. New York: Harper & Row Publishers; 1967. [Google Scholar]
- Blazer DG, Burchett BB, Fillenbaum GG. APOE epsilon4 and low cholesterol as risks for depression in a biracial elderly community sample. American Journal of Geriatric Psychiatry. 2002;10(5):515–520. [PubMed] [Google Scholar]
- Bollen KA, Curran PJ. Latent curve models: A structural equation perspective. Hoboken, New Jersey: Wiley-Interscience; 2006. [Google Scholar]
- Borgherini G, Dorz S, Conforti D, Scarso C, Magni G. Serum cholesterol and psychological distress in hospitalized depressed patients. Acta Psychiatrica Scandinavica. 2002;105(2):149–152. doi: 10.1034/j.1600-0447.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- Brown SL. Cholesterol concentrations and depression in elderly people. Annals of Medicine. 1995;27(2):141–142. doi: 10.3109/07853899509031950. [DOI] [PubMed] [Google Scholar]
- Bruce ML. Psychosocial risk factors for depressive disorders in late life. Biol Psychiatry. 2002;52(3):175–184. doi: 10.1016/s0006-3223(02)01410-5. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Fergeson P. Low HDL cholesterol, aggression and altered central serotonergic activity. Psychiatry Res. 2000;93(2):93–102. doi: 10.1016/s0165-1781(99)00126-2. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochemical & Biophysical Research Communications. 2007;354(3):627–633. doi: 10.1016/j.bbrc.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Paila YD, Jafurulla M, Chaudhuri A, Singh P, Murty MR, et al. Differential effects of cholesterol and 7-dehydrocholesterol on ligand binding of solubilized hippocampal serotonin1A receptors: implications in SLOS. Biochemical & Biophysical Research Communications. 2007;363(3):800–805. doi: 10.1016/j.bbrc.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lu FH, Wu JS, Chang CJ. Correlation between serum lipid concentrations and psychological distress. Psychiatry Research. 2001;102(2):153–162. doi: 10.1016/s0165-1781(01)00231-1. [DOI] [PubMed] [Google Scholar]
- Cortese S, Faussard B, Angriman M, Pigaiani Y, et al. The relationship between body size and depressive symptoms in adolescents. Journal of Pediatrics. 2008:1–5. doi: 10.1016/j.jpeds.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Deisenhammer EA, Kramer-Reinstadler K, Liensberger D, Kemmler G, Hinterhuber H, Fleischhacker WW. No evidence for an association between serum cholesterol and the course of depression and suicidality. Psychiatry Research. 2004;121(3):253–261. doi: 10.1016/j.psychres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Diebold K, Michel G, Schweizer J, Diebold-Dorsam M, Fiehn W, Kohl B. Are psychoactive-drug-induced changes in plasma lipid and lipoprotein levels of significance for clinical remission in psychiatric disorders? Pharmacopsychiatry. 1998;31(2):60–67. doi: 10.1055/s-2007-979300. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Mitsonis C, Liappas I, et al. Characterization of the lipid profile in dementia and depression in the elderly. Journal of Geriatric Psychiatry & Neurology. 2007;20(3):138–144. doi: 10.1177/0891988707301867. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Keltikangas-Jarvinen L, Pulkki-Raback L, Kivimaki M, Puttonen S, Viikari L, et al. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychological Medicine. 2006;36(6):797–805. doi: 10.1017/S0033291706007574. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Puttonen S, Pulkki-Raback L, Kivimaki M, Viiri LE, Lehtimaki T, et al. Association of the apolipoprotein E gene, its promoter polymorphisms and haplotypes with depressive symptoms. The Cardiovascular Risk in Young Finns Study. Neuropsychobiology. 2008;58(2):91–96. doi: 10.1159/000162355. [DOI] [PubMed] [Google Scholar]
- Engelberg H. Low serum cholesterol and suicide. Lancet. 1992;339(8795):727–729. doi: 10.1016/0140-6736(92)90609-7. [DOI] [PubMed] [Google Scholar]
- Ergun UG, Uguz S, Bozdemir N, Guzel R, Burgut R, Saatci E, et al. The relationship between cholesterol levels and depression in the elderly. International Journal of Geriatric Psychiatry. 2004;19(3):291–296. doi: 10.1002/gps.1078. [DOI] [PubMed] [Google Scholar]
- Fischer P, Gruenblatt E, Pietschmann P, Tragl KH. Serotonin transporter polymorphism and LDL-cholesterol. Molecular Psychiatry. 2006;11(8):707–709. doi: 10.1038/sj.mp.4001837. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Glueck CJ, Kuller FE, Hamer T, Rodriguez R, Sosa F, Sieve-Smith L, et al. Hypocholesterolemia, hypertriglyceridemia, suicide, and suicide ideation in children hospitalized for psychiatric diseases. Pediatric Research. 1994;35(5):602–610. [PubMed] [Google Scholar]
- Glueck CJ, Tieger M, Kunkel R, Hamer T, Tracy T, Speirs J. Hypocholesterolemia and affective disorders. American Journal of the Medical Sciences. 1994;308(4):218–225. doi: 10.1097/00000441-199430840-00002. [DOI] [PubMed] [Google Scholar]
- Goldstein H, Healy MJ, Rasbash J. Multilevel time series models with applications to repeated measures data.[see comment] Statistics in Medicine. 1994;13(16):1643–1655. doi: 10.1002/sim.4780131605. [DOI] [PubMed] [Google Scholar]
- Horsten M, Wamala SP, Vingerhoets A, Orth-Gomer K. Depressive symptoms, social support, and lipid profile in healthy middle-aged women.[erratum appears in Psychosom Med 1998 May-Jun;60 (3)257] Psychosomatic Medicine. 1997;59(5):521–528. doi: 10.1097/00006842-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods & research. 2001;20:374–393. [Google Scholar]
- Katainen S, Räikkönen K, Keltikangas-Järvinen L. Adolescent temperament, perceived social support, and depressive tendencies as predictors of depressive tendencies in young adulthood. European Journal of Personality. 1999;13(3):183–207. [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and phobias: the genetic and environmental sources of comorbidity. Psychol Med. 1993;23(2):361–371. doi: 10.1017/s0033291700028464. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. Journal of Affective Disorders. 1997;45(1–2):19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Vascular risk factors and incident late-life depression in a Korean population. British Journal of Psychiatry. 2006;189:26–30. doi: 10.1192/bjp.bp.105.015032. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Yoon JS. Vascular disease/risk and late-life depression in a Korean community population. British Journal of Psychiatry. 2004;185:102–107. doi: 10.1192/bjp.185.2.102. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM. Clinical application of low serum cholesterol as an indicator for suicide risk in major depression. Journal of Affective Disorders. 2004;81(2):161–166. doi: 10.1016/S0165-0327(03)00166-6. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Smith GD, Elovainio M, Pulkki L, Keltikangas-Jarvinen L, Talttonen L, et al. Socioeconomic circumstances in childhood and blood pressure in adulthood: the cardiovascular risk in young Finns study. Annals of Epidemiology. 2006;16(10):737–742. doi: 10.1016/j.annepidem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Lawlor DA, Singh-Manoux A, Batty GD, Ferrie JE, Shipley MJ, et al. Common mental disorder and obesity -- Insight from 4 repeat measures over 19 years: The prospective Whitehall II cohort study. British Medical Journal. 2009;339:b3765. doi: 10.1136/bmj.b3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Hintikka J, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, et al. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(6):1557–1561. doi: 10.1016/j.pnpbp.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Advances in Experimental Medicine & Biology. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Marin TJ, Chen E, Miller GE. What do trajectories of childhood socioeconomic status tell us about markers of cardiovascular health in adolescence? Psychosomatic Medicine. 2008;70(2):152–159. doi: 10.1097/PSY.0b013e3181647d16. [DOI] [PubMed] [Google Scholar]
- Partonen T, Haukka J, Virtamo J, Taylor PR, Lonnqvist J. Association of low serum total cholesterol with major depression and suicide.[see comment] British Journal of Psychiatry. 1999;175:259–262. doi: 10.1192/bjp.175.3.259. [DOI] [PubMed] [Google Scholar]
- Pulkki-Raback L, Elovainio M, Kivimaki M, Mattsson N, Raitakari OT, Puttonen S, et al. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychology. 2009;28(1):108–116. doi: 10.1037/a0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, et al. Cohort profile: The Cardiovascular risk in young Finns study. International Journal of Epidemiology. 2008:1–7. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- Sagud M, Mihaljevic-Peles A, Pivac N, Jakovljevic M, Muck-Sleer D. Lipid levels in female patients with affective disorders. Psychiatry Research. 2009;168:218–221. doi: 10.1016/j.psychres.2008.06.048. [DOI] [PubMed] [Google Scholar]
- Shin JY, Suls J, Martin R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Annals of Behavioral Medicine. 2008;36(1):33–43. doi: 10.1007/s12160-008-9045-8. [DOI] [PubMed] [Google Scholar]
- Terao T, Iwata N, Kanazawa K, Takano T, Takahashi N, Hayashi T, et al. Low serum cholesterol levels and depressive state in human dock visitors. Acta Psychiatrica Scandinavica. 2000;101(3):231–234. [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. Journal of Occupational Health Psychology. 2005;10(4):344–362. doi: 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- Troisi A. Cholesterol in coronary heart disease and psychiatric disorders: same or opposite effects on morbidity risk? Neuroscience & Biobehavioral Reviews. 2009;33(2):125–132. doi: 10.1016/j.neubiorev.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Virtanen M, Pentti J, Vahtera J, Ferrie JE, Stansfeld SA, Helenius H, et al. Overcrowding in hospital wards as a predictor of antidepressant treatment among staff. American Journal of Psychiatry. 2008;165:1482–1486. doi: 10.1176/appi.ajp.2008.07121929. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late-life.[see comment] Psychoneuroendocrinology. 2007;32(2):151–159. doi: 10.1016/j.psyneuen.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]