Abstract
Vasoconstriction is a major adverse effect of HBOCs. The use of a single drug for attenuating HBOC-induced vasoconstriction has been tried with limited success. Since HBOC causes disruptions at multiple levels of organization in the vascular system, a systems approach is helpful to explore avenues to counteract the effects of HBOC at multiple levels by targeting multiple sites in the system. A multi-target approach is especially appropriate for HBOC-induced vasoconstriction, because HBOC disrupts the cascade of amplification by NO-cGMP signaling and protein phosphorylation, ultimately resulting in vasoconstriction. Targeting multiple steps in the cascade may alter the overall gain of amplification, thereby limiting the propagation of disruptive effects through the cascade. As a result, targeting multiple sites may accomplish a relatively high overall efficacy at submaximal drug doses. Identifying targets and doses for developing a multi-target combination HBOC regimen for oxygen therapeutics requires a detailed understanding of the systems biology and phenotypic heterogeneity of the vascular system at multiple layers of organization, which can be accomplished by successive iterations between experimental studies and mathematical modeling at multiple levels of vascular systems and organ systems. Towards this goal, this article addresses the following topics: a) NO-scavenging by HBOC, b) HBOC autoxidation-induced reactive oxygen species generation and endothelial barrier dysfunction, c) NO- cGMP signaling in vascular smooth muscle cells, d) NO and cGMP-dependent regulation of contractile filaments in vascular smooth muscle cells, e) phenotypic heterogeneity of vascular systems, f) systems biology as an approach to developing a multi-target HBOC regimen.
Keywords: Endothelium, HBOC, Hemoglobin, Hypertension, Nitric Oxide, Systems Biology, Vasoconstriction, Vascular Smooth Muscle
INTRODUCTION
Hemoglobin-based oxygen carriers (HBOCs) are designed to carry oxygen to all organ systems via the circulation. Unfortunately, HBOCs also scavenge nitric oxide (NO) with high affinity - an important relaxing factor released by endothelial cells, and thereby reduces the availability of NO to vascular smooth muscle cells, disrupts NO-mediated cascade of signaling pathways in vascular smooth muscle cells, and causes vasoconstriction [1–3]. Furthermore, in the presence of oxygen, HBOCs can undergo autoxidation with the generation of reactive oxygen species (ROS), which can lead to endothelial barrier dysfunction. HBOCs can then permeate through the leaky endothelial cell-cell junctions to the interstitial space near vascular smooth muscle cells, thereby further reducing the bioavailability of NO to vascular smooth muscle cells and causes vasoconstriction.
This article proposes that HBOC-induced vasoconstriction is a systems problem, because the effect of NO-scavenging by HBOC is experienced by multiple organ systems at multiple layers of organization from hemoglobin reactions in solution to activation of contractile proteins in vascular smooth muscle cells (Fig. 1). As elaborated in this article, the endothelium-vascular smooth muscle system is highly heterogeneous and complex, rich in interconnected signaling pathways and feedback regulation. Predicting the behavior of such heterogeneous and complex system by intuition is extremely difficult, if not impossible. Systems biology is an approach that combines experimental studies with mathematical modeling for quantitative and multi-scale understanding of complex systems [4, 5]. This article proposes that systems biology approach is needed for understanding HBOC-induced vasoconstriction in a complex and highly heterogeneous vascular system, in order to develop an efficacious multi-target combination HBOC regimen for oxygen therapeutics.
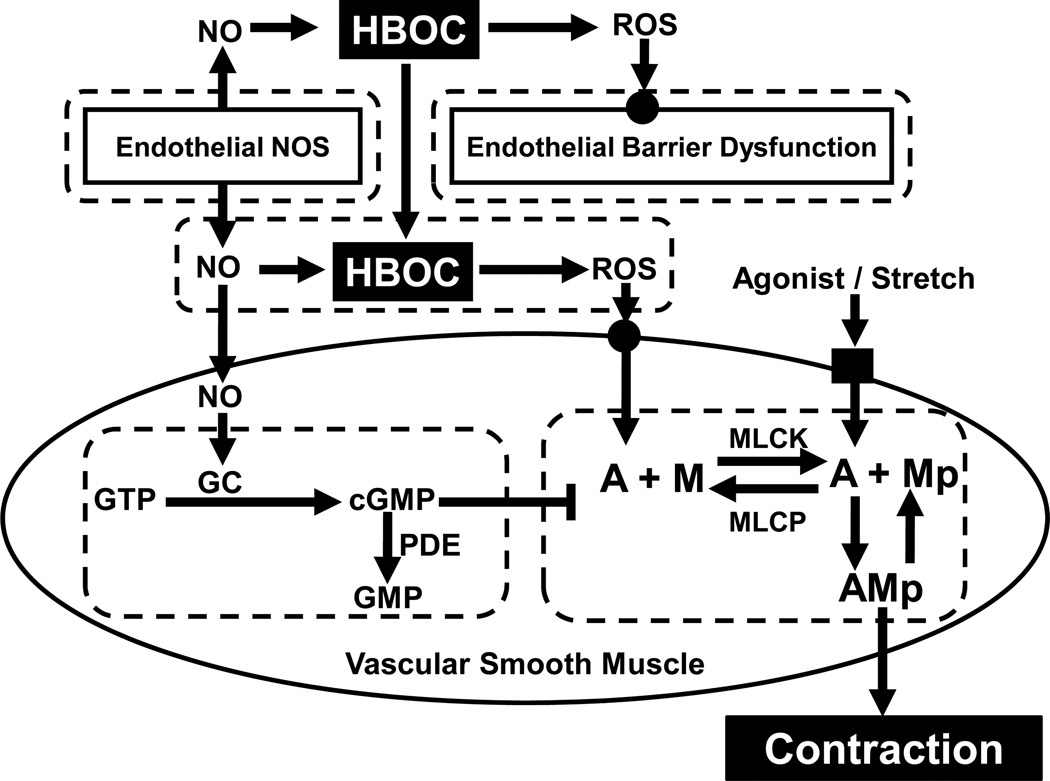
Figure 1.
Systems model of HBOC-induced vasoconstriction. HBOC scavenges NO produced by endothelial NO synthase (NOS) and mediates the production of reactive oxygen species (ROS), which can induce endothelial barrier dysfunction. As a result, HBOC may extravasate into the interstitial space, and further reduce the bioavailability of NO to vascular smooth muscle cells. The loss of NO lowers the activity of guanylate cyclase (GC), relative to phosphodiesterase (PDE) activity, and leads to a decrease in intracellular [cGMP] for inhibiting smooth muscle contraction. In addition, ROS, contractile agonists (neurotransmitters and hormones), and mechanical stretch exert stimulatory effects on smooth muscle contraction. A major regulatory mechanism of smooth muscle contraction is phosphorylation of the 20,000 dalton myosin light chain, which is determined by the myosin light chain kinase (MLCK) / phosphatase (MLCP) activity ratio. Phosphorylated myosin crossbridges (Mp) then interact with actin (A) to form attached crossbridges (AMp) for contraction.
NO-SCAVENGING BY HBOC
HBOC, being an acellular protein, can diffuse freely in plasma, in direct proximity to the endothelial surface, and scavenge NO released by endothelial cells. Indeed, NO-scavenging is generally recognized as an important mechanism underlying HBOC-induced vasoconstriction [1, 6]. Similarly, disruption in NO homeostasis caused by hemolysis is recognized to be a major mechanism underlying vasculopathy in sickle cell disease. However, sickle cell disease is a complex and chronic vasculopathy, and is therefore not fully applicable as a model for a single administration of an HBOC in a normal subject. Also, normal recipients of an HBOC are also different from those with endothelial dysfunction. The physiological significance of endothelial release of NO is further suggested by the experimental observation that abolishing endothelial release of NO by genetic deletion of endothelial nitric oxide synthase (eNOS) is sufficient to cause hypertension in mice [7–11]. Therefore, in principle, HBOC administration may provide the benefit of oxygen delivery, but also carry the risk of NO scavenging. A mathematical modeling study analyzed HBOC-mediated O2 transport and NO consumption in the microcirculation [12]. Furthermore, mathematical modeling of O2 transport from capillary to muscle in the absence or presence of HBOCs has led Patton and Palmers [13] to conclude that HBOC normalizes O2 transport to muscle in hypoxemia and anemia. Another modeling study actually suggested that hemoglobin solutions induce oversupply of O2 to arteriolar walls and causes vasoconstriction in arterioles [14]. Oxygen transport has two phases: a convective and a diffusive. The former depends on blood flow (both velocity and bulk flow). The latter two studies did not consider the vasoconstrictive effect of HBOC-induced changes in bulk flow and NO consumption.
Kavdia et al. [15] modeled NO diffusion and consumption in the presence of HBOC in an arteriole, and concluded that HBOC would significantly reduce [NO] near vascular smooth muscle cells, especially when extravasation of HBOC was considered. A similar conclusion was reached by Gundersen et al. [16], who modeled the transport of NO and O2 in an arteriole containing a mixture of HBOC and erythrocytes. Gundersen et al. [16] further concluded that significant NO-scavenging by HBOC is an unavoidable consequence of HBOC transfusion and probable cause of HBOC-induced vasoconstriction. Consistent with the latter findings, Jeffers et al. [17] modeled NO-scavenging by hemoglobin in hemolytic anemia, and concluded that as little as 1 µM heme is sufficient to reduce NO bioavailability significantly, especially when extravasation of hemoglobin is considered, perhaps because of the high binding affinity and rapid reaction rate of NO with hemoglobin. Altogether, these studies indicate that: a) HBOC enhances diffusive O2 transport in the microcirculation, b) HBOC significantly reduces NO bioavailability, especially when HBOC extravasation is considered, and c) a relatively low [HBOC] – 1 µM heme - can significantly reduce NO bioavailability to vascular smooth muscle cells.
HBOC AUTOXIDATION-INDUCED ROS GENERATION AND ENDOTHELIAL BARRIER DYSFUNCTION
Autoxidation refers to the spontaneous oxidation of hemoglobin (the heme iron Fe++) to methemoglobin (heme iron Fe+++) in the presence of oxygen, accompanied by the generation of ROS such as superoxide ions [1]. Superoxide can undergo dismutation to produce hydrogen peroxide, which reacts with methemoglobin to produce ferryl hemoglobin, a highly reactive species that can cause oxidant damages to cells. Within the circulation, HBOC-derived ROS can lead to endothelial barrier dysfunction and allow macromolecules, including HBOCs, to permeate across the endothelium to the interstitial space near vascular smooth muscle cells, where HBOCs can further reduce the availability of NO to vascular smooth muscle cells, and induces vasoconstriction [18]. Indeed, Baldwin [19] showed that intravascular injection of polyethylene glycol-hemoglobin induced the formation of endothelial junctional gaps and caused the leakage of albumin in the rat mesentery. Furthermore, Dull et al. [20] showed that hemoglobin, hemoglobin-Polytaur (molecular mass: 500 kDa), and hemoglobin-(Polytaur)n (molecular mass: ~ 1,000 kDa) increased permeability of microvascular endothelial cell monolayers to large molecules, including the three hemoglobin molecules. These findings suggest that, despite the relatively large molecular size, HBOCs can extravasate to the interstitial space by inducing endothelial barrier dysfunction. Recently, Yu et al. [21] reported that diabetic mice having endothelial dysfunction exhibited exaggerated HBOC-induced vasoconstriction. This finding suggests that patients having vascular diseases associated with compromised endothelial function such as atherosclerosis and diabetes mellitus are also likely to have elevated risks of HBOC extravasation and HBOC-induced vasoconstriction. Endothelium-derived NO appears to be essential for maintaining endothelial barrier integrity [22]; therefore, NO-scavenging by HBOC may be sufficient to induce endothelial barrier dysfunction. Endothelial cell-cell junctions are regulated by multiple signaling pathways involving Ca2+, protein kinase C, tyrosine kinases, myosin light chain kinase, and small Rho-GTPases [23]. Understanding the mechanisms by which HBOCs perturb these signaling pathways may reveal molecular targets for preserving endothelial barrier function during HBOC administration and ameliorating the HBOC-related side effects.
NO- cGMP SIGNALING in VASCULAR SMOOTH MUSCLE CELLS
Soluble guanylyl cyclase and phosphodiesterase-5 are the two major enzymes of NO-cGMP signaling in vascular smooth muscle cells. Soluble guanylyl cyclase is the intracellular NO receptor-enzyme that catalyzes the generation of cGMP for initiating the cascade of protein phosphorylation that leads to vascular smooth muscle relaxation [24–26]. A recent report indicates that smooth muscle-specific deletion of NO-sensitive guanylyl cyclase was sufficient to induce hypertension in mice, suggesting that NO-dependent guanylyl cyclase activity is essential for maintaining normal vasoregulation and blood pressure in mice [27]. Phosphodiesterase-5 is the enzyme that catalyzes the degradation of cGMP to GMP [28]. Therefore, the level of intracellular [cGMP] is determined by the guanylyl cyclase/phosphodiesterase-5 activity ratio.
NO-cGMP signaling is regulated by both negative and positive feedback mechanisms in vascular smooth muscle cells [29]. One negative feedback mechanism is cGMP-dependent inhibition of guanylyl cyclase via phosphorylation [30]. Another negative feedback mechanism is cGMP-dependent activation of phosphodiesterase -5 either directly via binding or indirectly via phosphorylation by cGMP-dependent kinase [31–34]. In addition, cGMP-dependent activation of eNOS functions as a positive feedback mechanism in NO-cGMP signaling [35, 36]. NO-scavenging by oxyhemoglobin has been shown to decrease the level of cGMP significantly in vascular smooth muscle cells [37]. However, relatively little, if any, has been published on the effect of HBOCs on cGMP levels in vascular smooth muscle cells. This knowledge gap is important for differentiating the effect of HBOC on NO-cGMP signaling from other adverse effects of HBOC on vascular smooth muscle.
Some mathematical studies have tried to resolve the temporal dynamics of activation and inactivation of guanylyl cyclase and phosphodiesterase-5 that underlie the complex temporal dynamics of cGMP transients during NO administration in solution and cells [38]. For example, by modeling the kinetics of NO-induced guanylyl cyclase activation in vitro, Condorelli and George [39] resolved the Michaelis constant and Hill coefficients for NO-induced cGMP generation, and concluded that activation of guanylyl cyclase is more rapid than its inactivation. By measuring and modeling the kinetics of both NO-dependent cGMP generation and downstream protein phosphorylation in platelets, Mo et al. [40] concluded that dynamics of both guanylyl cyclase desensitization and phosphodiesterase-5 activation contribute to the cGMP transients. Furthermore, by varying parameters in the NO-cGMP signaling pathways, Mo et al. [40] showed that the model could also explain the different temporal dynamics of cGMP transients in different cell types.
To understand the function of cGMP transients in vascular smooth muscle cells during NO-induced vasorelaxation, Tsoukias et al. [41] modeled the frequency and amplitude effects of NO production by endothelial cells on cGMP formation in vascular smooth muscle cells in the presence of NO scavengers in an arteriole, and concluded that burst-like NO production by endothelial cells minimizes NO-scavenging by hemoglobin, and maximizes NO delivery to vascular smooth muscle cells. The same authors [41] further suggested that the frequency of Ca2+ oscillations in endothelial cells may be a limiting factor in cGMP formation in vascular smooth muscle cells. This concept of frequency-dependent modulation of NO-cGMP signaling is intriguing, because it suggests the possibility that cGMP transients may also be efficient in maintaining cGMP-dependent protein phosphorylation and relaxation in vascular smooth muscle cells. A potential clinical implication of this concept is that oscillatory delivery of NO donors may be an effective approach to attenuating vasoconstriction during HBOC administration. However, for this approach to be realistic, the frequency, delivery means, and synchronization need to be considered.
NO AND cGMP-DEPENDENT REGULATION OF CONTRACTILE FILAMENTS IN VASCULAR SMOOTH MUSCLE CELLS
Ca2+, calmodulin-dependent activation of myosin light chain kinase (MLCK) for catalyzing phosphorylation of the 20 kDa myosin light chains is a well documented mechanism of smooth muscle contraction. The reverse of this reaction – myosin light chain dephosphorylation – is catalyzed by myosin light chain phosphatase (MLCP). Thus, the level of crossbridge activation, as measured by myosin light chain phosphorylation, is determined by the MLCK/MLCP activity ratio in vascular smooth muscle cells. Accordingly, NO may induce vascular smooth muscle relaxation by down-regulating Ca2+-calmodulin- MLCK activity and/or up-regulating MLCP activity [42]. Some but not all investigators have reported down-regulation of intracellular [Ca2+] during NO-induced vascular smooth muscle relaxation [43–47]. One mechanism of NO-dependent down-regulation of intracellular [Ca2+] is NO-dependent direct inhibition of voltage-gated Ca2+ channels. Another mechanism is NO-induced hyperpolarization via activation of calcium-sensitive potassium channels [48]. Several investigators have reported NO-dependent up-regulation of MLCP activity [46, 49–52]. Specific mechanisms of NO-dependent MLCP activation include inhibition of PKC and RhoA/Rho kinase, which normally function to inhibit MLCP in agonist-induced vascular smooth muscle contractions [53–56]. In addition, NO has also been shown to inhibit assembly and activation of actin filaments, thereby decreasing the number of functional cross-bridge binding sites on actin filaments and decreasing force-generating capacity of vascular smooth muscle cell [42, 57–60]. Contractile filament activation in vascular smooth muscle cells is the final step of HBOC-induced vasoconstriction. However, relatively little, if any, has been published on the effect of HBOCs on contractile filament activation in vascular smooth muscle cells. This knowledge gap is important for differentiating actin and myosin as the molecular targets of HBOC-induced vascular smooth muscle contraction.
Some mathematical modeling studies have contributed to the understanding of the integration of multiple mechanisms in NO-induced vascular smooth muscle relaxation. By modeling norepinephrine and NO-induced intracellular Ca2+ dynamics in vascular smooth muscle cells, Kapela et al. [61] concluded that intracellular Na+ may play a modulatory role in intracellular Ca2+ dynamics. By integrating NO-cGMP signaling, intracellular Ca2+ dynamics, myosin light chain phosphorylation, and actomyosin interactions, Yang et al. [62] explored the following hypotheses: a) rapid inactivation of guanylyl cyclase is caused by cGMP-dependent negative feedback, b) NO activates calcium-dependent potassium channels via cGMP-dependent and independent mechanisms, and c) Ca2+-desensitization of contractile filaments is distinct from Ca2+-sensitivity of myosin phosphorylation. This integrative model is potentially useful as a framework for building a systems model of HBOC-induced vasoconstriction; however, new experimental data on the effects of HBOC at multiple levels are needed for constraining model parameters and boundary conditions.
PHENOTYPIC HETEROGENEITY OF VASCULAR SYSTEMS
Phenotypic heterogeneity of vascular systems is a well established concept in physiology. For example, hypoxic vasoconstriction is a unique characteristic of pulmonary circulation, whereas hypoxic vasodilation is a general characteristic of systemic circulation [63–65]. Normal capillary permeability also varies widely among various organs, and converting enzyme activity (for both angiotensin and bradykinin) is specific to the endothelium of the pulmonary vasculature. Similarly, adverse effects of HBOC appear to be organ-specific [3]. For example, findings from one clinical trial showed that intravenous infusion of HBOC-201 to patients resulted in systemic and pulmonary hypertension without significant coronary vasoconstriction [66]. Consistent with this finding, a recently published ex vivo study indicated differential sensitivities of isolated pulmonary and coronary vessels to HBOCs and nitrovasodilators [67], suggesting that phenotypic differences between pulmonary and coronary vascular smooth muscle cells or endothelial function could explain the observed differential hypertensive effects of HBOC on pulmonary and coronary circulation in patients. Furthermore, pulmonary and coronary circulations exhibit differential compensatory responses to the loss of eNOS function. In eNOS-deficient mice, up-regulation of neuronal NO synthase (nNOS) appears to compensate fully for the loss of eNOS for the coronary circulation, but not in the pulmonary circulation [68–70].
Endothelial and vascular smooth muscle cells are the major cell types in a vascular system, and therefore major contributors to the phenotypic heterogeneity of vascular systems. Molecular heterogeneity of endothelial cells is indicated by data on organ-specificity of gene expression as measured by DNA arrays, membrane protein expression as measured by gel electrophoresis, and surface epitope expression as measured by in vivo screening of phage-displayed peptide libraries [71–73]. Functional heterogeneity of endothelial cells is indicated by data on organ-specificity of basal endothelial permeability, inducible endothelial permeability, inflammatory cell transmigration, vasomotor tone, and others [74]. Site-specific epigenetics and local environment have been proposed as major determinants of the phenotypic heterogeneity of endothelial cells [75–78].
If NO-scavenging by HBOC induces vasoconstriction in a vascular system, then endogenous NO release must contribute to the regulation of vascular resistance in this particular vascular system. For example, NO-scavenging by HBOC causes significant vasoconstriction in the pulmonary circulation, probably because endogenous NO release is responsible for maintaining the low vascular resistance in pulmonary circulation. Conversely, if endogenous NO release is absent in a vascular system, then NO-scavenging by HBOC cannot occur in this particular vascular system. This analysis suggests that phenotypic heterogeneity in the expression and function of eNOS (and possibly nNOS) is an important determinant of the organ-specificity of HBOC-induced vasoconstriction. However, relatively little, if any, has been published in this important area. In addition to releasing NO as a relaxing factor, some endothelial cells are capable of releasing hyperpolarizing factors (EDHFs) for inducing vascular smooth muscle relaxation [79–81]. The term EDHF includes multiple mediators, but Ca2+-activated potassium channels appear to be a common target that mediates the relaxing effect of EDHF in vascular smooth muscle cells [82]. Thus, EDHF is a potential compensatory mechanism in HBOC-induced vasoconstriction.
Vascular smooth muscle is another element of phenotypic heterogeneity of vascular systems. Molecular heterogeneity of vascular smooth muscle cells is indicated by data on tissue-specific protein expression of myosin (light and heavy chain) isoforms, and muscle mechanics, which are the criteria for classifying smooth muscles into phasic and tonic phenotypes [83]. For example, the portal vein contains phasic vascular smooth muscle cells, which predominantly express the SM-B myosin heavy chain isoform, and have a capacity for high shortening velocity. In contrast, the aorta contains tonic smooth muscle cells, which predominantly express the SM-A myosin heavy chain isoform, and have a capacity for low shortening velocity. Lineage mapping studies indicated that vascular smooth muscle cells in different organ systems are derived from differential embryonic origins [84]. For example, the ascending aorta develops from the neural crest, whereas the descending thoracic aorta, coronary arteries, and pulmonary arteries develop from non-neural crest regions of an embryo. Indeed, the concept of phenotypic heterogeneity of vascular smooth muscle is important for understanding the clonal nature of vascular smooth muscle cells in human atherosclerotic plaques [85]. For example, cross-transplantation of atherosclerosis-resistant and atherosclerosis-susceptible aortic segments in animal studies indicated that transplanted vascular segments retain the same resistance to atherosclerosis, independent of the site of transplantation. This observation suggests that intrinsic cell phenotype is more significant than regional hemodynamic environment in determining the atherosclerosis-susceptibility of a given vascular segment [86]. In addition to having innate phenotypic diversity, vascular smooth muscle cells are capable of phenotypic modulation from contractile to synthetic phenotype in inflammatory vascular diseases such as atherosclerosis [87]. Thus, phenotypic modulation of vascular smooth muscle cells in diseased organs represents another element of organ-specific phenotypic heterogeneity of HBOC-induced vasoconstriction.
It is conceivable that phenotypic heterogeneity in the expression and function of guanylyl cyclase and phosphodiesterase-5 in vascular smooth muscle cells may contribute to organ-specificity of HBOC-induced vasoconstriction, because these two enzymes determine the level of cGMP in vascular smooth muscle cells in response to NO and HBOC. For example, vascular smooth muscle cell (or activity) of guanylyl cyclase are likely to produce high levels of cGMP and robust vasorelaxation in response to NO. Conversely, vascular smooth muscle cells expressing low levels (or activity) of guanylyl cyclase may be more likely to produce low levels of cGMP and weak vasorelaxation in response to NO. Therefore, NOS function in endothelial cells and NO-cGMP signaling in vascular smooth muscle cells are both necessary for NO-induced vasorelaxation. It is intriguing to speculate that matched phenotypic heterogeneity of endothelial and vascular smooth muscle cells as an integrated system determines organ-specificity of HBOC-induced vasoconstriction.
A clear understanding of organ-specific phenotypic heterogeneity of endothelial and vascular smooth muscle cells is necessary for predicting organ-specificity of HBOC-induced vasoconstriction. Systematic studies are particularly needed in the following areas of research: a) organ-specific expression and function of eNOS and nNOS, b) organ-specificity of EDHF function, and c) organ-specific expression of guanylyl cyclase and phosphodiesterase-5 in vascular smooth muscle cells.
SYSTEMS BIOLOGY AS AN APPROACH TO DEVELOPING A MULTI-TARGET HBOC REGIMEN
Vasoconstriction is a major adverse effect of HBOCs. To overcome the vasoconstriction, one approach would be multi-targeted combination therapeutics. The other is to create a new HBOC that does not induce vasoconstriction. Many researchers are struggling for the latter one. The use of a single drug in combination with HBOC for attenuating HBOC-induced vasoconstriction has been tried with limited success. For example, the administration of inhaled NO and NO donor before and/or during HBOC infusion has been used to attenuate HBOC-induced vasoconstriction in animal studies with some success [88, 89]. However, there is some concern that the maintenance of HBOC-NO reactions by exogenous NO may sustain the production of ROS. Other candidate drugs for co-administration with HBOC include phosphodiesterase-5 inhibitors and guanylate cyclase activators [90–93]. However, a clinical trial for testing the efficacy of the phosphodiesterase-5 inhibitor - sildenafil - for the treatment of pulmonary hypertension in patients with Sickle Cell Disease has been stopped early due to safety concerns (http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2650).
Erythrocyte hemoglobin is physically separated from the endothelial surface by the red cell membrane and unstirred layers on endothelial cells and erythrocytes; therefore, there is minimal consumption of endothelium-derived NO by hemoglobin within erythrocytes in circulation [94–97]. Based on this observation, shielding of HBOC chemically by polyethylene glycol conjugation or physically by encapsulation is being developed to minimize NO-scavenging by HBOC [98–100]. However, chemical stability of polyethylene glycol-conjugated hemoglobin is an important issue to be addressed [101–102].
One potential problem in the single drug-HBOC combination approach is the necessity of using a relatively high dose of drug in order to counteract completely the effect of HBOC at a single site, because even a small residual effect of HBOC at a single site may be amplified by a cascade of enzymatic reactions. High drug doses are likely to have large side effects. This article proposes that HBOC-induced vasoconstriction is a systems problem, somewhat analogous to vasculopathy caused by hemolysis in sickle cell anemia, which is also becoming recognized as a systems problem [103]. From the systems point of view, since HBOC causes disruptions at multiple levels of organization in the vascular system (Fig. 1), then a systems approach is useful to explore pathways to counteract the effects of HBOC at multiple levels by targeting multiple sites in the system. A multi-target approach is especially appropriate for HBOC-induced vasoconstriction, because HBOC disrupts the cascade of amplification by NO-cGMP signaling and protein phosphorylation, where the product of one enzymatic reaction activates the enzyme for the next reaction. Targeting multiple steps in the cascade may alter the overall gain of amplification, thereby limiting the propagation of disruptive effects through the cascade. As a result, targeting multiple sites may accomplish a relatively high overall efficacy at submaximal drug doses.
The concept of multi-target combination therapeutics has led to the successful development of a highly active antiretroviral therapy (HAART) for treating AIDS patients [104]. This example suggests that the development of a multi-target combination HBOC regimen for oxygen therapeutics may also be achievable. In principle, finding a solution to a systems problem requires understanding of the complexity of the biological system at multiple layers or organization [105–106]. Therefore, identifying targets and doses for developing a multi-target combination HBOC regimen for oxygen therapeutics requires a detailed understanding of the systems biology and phenotypic heterogeneity of the vascular system within the context of HBOC-induced vasoconstriction, which can be accomplished by successive iterations between experimental studies and mathematical modeling at multiple levels of vascular systems and organ systems.
REFERENCES
- 1.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nature Rev Drug Discovery. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 2.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med. 2010;30:381–389. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion. 2009;49:2495–2515. doi: 10.1111/j.1537-2995.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 4.Kohl P, Crampin EJ, Quinn TA, Noble D. Systems biology: an approach. Clin Pharmacol Therap. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- 5.Pujol A, Mosca R, Farres J, Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol Sc. 2010;31:115–123. doi: 10.1016/j.tips.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Buehler PW, D’Agnillo F. Toxicological consequences of extracellular hemoglobin: biochemical and physiological perspectives. Antioxidants Redox Signal. 2010;12:275–291. doi: 10.1089/ars.2009.2799. [DOI] [PubMed] [Google Scholar]
- 7.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Eng J Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 8.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intracellular hemolysis and extracellular plasma hemoglobin. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 10.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Rad Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsui M, Shimokawa H, Otsuji Y, Ueta Y, Sasaguri Y, Yanagihara N. Nitric oxide synthases and cardiovascular diseases. Insights from genetically modified mice. Circ J. 2009;73:986–993. doi: 10.1253/circj.cj-09-0208. [DOI] [PubMed] [Google Scholar]
- 12.Tsoukias NM. Nitric oxide bioavailability in the microcirculation: insights from mathematical models. Microcirc. 2008;15:813–834. doi: 10.1080/10739680802010070. [DOI] [PubMed] [Google Scholar]
- 13.Patton JN, Palmer AF. Numerical simulation of oxygen delivery to muscle tissue in the presence of hemoglobin-based oxygen carriers. Biotechnol Prog. 2006;22:1025–1049. doi: 10.1021/bp060022a. [DOI] [PubMed] [Google Scholar]
- 14.Cole RH, Vandegriff KD, Szeri AJ, Savas O, Baker DA, Winslow RM. A quantitative framework for the design of acellular hemoglobins as blood substitutes: implications of dynamic flow conditions. Biophys Chem. 2007;128:63–74. doi: 10.1016/j.bpc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen SI, Chen G, Palmer AF. Mathematical model of NO and O2 transport in an arteriole facilitated by hemoglobin based O2 carriers. Biophys Chem. 2009;143:1–17. doi: 10.1016/j.bpc.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radical Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin AL. Modified hemoglobins produce venular interendothelial gaps and albumin leakage in the rat mesentery. Am J Physiol. 1999;277:H650–H659. doi: 10.1152/ajpheart.1999.277.2.H650. [DOI] [PubMed] [Google Scholar]
- 20.Dull RO, DeWitt BJ, Dinavahi R, Schwartz L, Hubert C, Pace N, Fronticelli C. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol. 2004;97:1930–1937. doi: 10.1152/japplphysiol.00102.2004. [DOI] [PubMed] [Google Scholar]
- 21.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiol. 2010;112:586–594. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 26.Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21:149–156. doi: 10.1016/j.niox.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Groneberg D, Konig P, Wirth A, Offermanns S, Koesling D, Friebe A. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation. 2010;121:401–409. doi: 10.1161/CIRCULATIONAHA.109.890962. [DOI] [PubMed] [Google Scholar]
- 28.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 29.Cawley SM, Sawyer CL, Brunelle KF, van der Vliet A, Dostmann WR. Nitric oxide-evoked transient kinetics of cyclic GMP in vascular smooth muscle cells. Cell Signal. 2007;19:1023–1033. doi: 10.1016/j.cellsig.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z, Sayed N, Pyriochou A, Roussos C, Fulton D, Beuve A, Papapetropoulos A. Protein kinase G phosphorylates soluble guanylyl cyclase on serine 64 and inhibits its activity. Arterioscler Thromb Vasc Biol. 2008;28:1803–1810. doi: 10.1161/ATVBAHA.108.165043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koesling D, Mullershausen F, Lange A, Friebe A, Mergia E, Russwurm M. Negative feedback in NO/cGMP signaling. Biochem Soc Transactions. 2005;33:1119–1122. doi: 10.1042/BST20051119. [DOI] [PubMed] [Google Scholar]
- 32.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:3310–3317. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 33.Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J Cell Biol. 2003;160:719–727. doi: 10.1083/jcb.200211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodisterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol. 2001;155:271–278. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 36.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Gruetter CA, Gruetter DY, Lyon JE, Kadowitz PJ, Ignarro LJ. Relationship between cyclic guanosine 3’:5’-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Exp Pharmacol Exp Therap. 1981;219:181–186. [PubMed] [Google Scholar]
- 38.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. doi: 10.1007/s11010-009-0318-8. [DOI] [PubMed] [Google Scholar]
- 39.Condorelli P, George SC. In vivo control of soluble guanylate cyclase activation by nitric oxide: a kinetic analysis. Biophys J. 2001;80:2110–2119. doi: 10.1016/S0006-3495(01)76184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo E, Amin H, Bianco IH, Garthwaite J. Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. J Biol Chem. 2004;279:26149–26158. doi: 10.1074/jbc.M400916200. [DOI] [PubMed] [Google Scholar]
- 41.Tsoukias NM, Kavdia M, Popel A. A theoretical model of nitric oxide transport in arterioles: frequency- vs. amplitude-dependent control of cGMP formation. Am J Physiol Heart Circ Physiol. 2004;286:H1043–H1056. doi: 10.1152/ajpheart.00525.2003. [DOI] [PubMed] [Google Scholar]
- 42.Murphy RA, Walker JS. Inhibitory mechanisms for cross-bridge cycling: the nitric oxide-cGMP signal transduction pathway in smooth muscle relaxation. Acta Physiol Scand. 1998;164:373–380. doi: 10.1046/j.1365-201X.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 43.Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Intracellular smooth muscle [Ca2+] in acetylcholine and nitric oxide-mediated relaxation of human small arteries. Eur J Pharmacol. 2006;535:243–247. doi: 10.1016/j.ejphar.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 44.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 45.Fellner SK, Arendshorst WJ. Complex interactions of NO/cGMP/PKG systems on Ca2+ signaling in afferent arteriolar vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2010;298:H144–H151. doi: 10.1152/ajpheart.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDaniel NL, Chen XL, Singer HA, Murphy RA, Rembold CM. Nitrovasodilators relax arterial smooth muscle by decreasing [Ca2+]I and uncoupling stress from myosin phosphorylation. Am J Physiol. 1992;263:C461–C467. doi: 10.1152/ajpcell.1992.263.2.C461. [DOI] [PubMed] [Google Scholar]
- 47.Van Hove CE, Van der Donckt C, Herman AG, Bult H, Fransen P. Vasodilator efficacy of nitric oxide depends on mechanisms of intracellular calcium mobilization in mouse aortic smooth muscle cells. Br J Pharmacol. 2009;158:920–930. doi: 10.1111/j.1476-5381.2009.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2010;47:93–107. doi: 10.1159/000235964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem. 2001;276:34681–34685. doi: 10.1074/jbc.M104737200. [DOI] [PubMed] [Google Scholar]
- 50.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- 51.Soloviev A, Lehen’kyi V, Zelensky S, Hellstrand P. Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell Calcium. 2004;36:165–173. doi: 10.1016/j.ceca.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Wu X, Somylo AV, Somlyo AP. Cyclic GMP-dependent reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Comm. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- 53.Bolz SS, Vogel L, Sollinger D, Serwand R, de Wit C, Loirand G, Pohl U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003;107:3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 54.Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol. 2009;587:3587–3603. doi: 10.1113/jphysiol.2009.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phoshorylation of myosin phosphatase. Circ Res. 2007;101:712–722. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 56.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 57.Rembold CM, Foster DB, Strauss JD, Wingard CJ, Van Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscel relaxation without myosin light chain phosphorylation in swine carotid artery. J Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rembold CM, O’Connor M, Clarkson M, Wardle RL, Murphy RA. Selected Contribution: HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid media tone. J Appl Physiol. 2001;91:1460–1466. doi: 10.1152/jappl.2001.91.3.1460. [DOI] [PubMed] [Google Scholar]
- 59.Sandau KB, Gantner F, Brune B. Nitric oxide-induced F-actin disassembly is mediated via cGMP, cAMP, and protein kinase A activation in rat mesangial cells. Exp Cell Res. 2001;271:329–336. doi: 10.1006/excr.2001.5378. [DOI] [PubMed] [Google Scholar]
- 60.Woodrum D, Pipkin W, Tessier D, Komalavilas P, Brophy CM. Phosphorylation of the heat shock-related protein, HSP20, mediates cyclic nucleotide-dependent relaxation. J Vasc Surg. 2003;27:874–881. doi: 10.1067/mva.2003.153. [DOI] [PubMed] [Google Scholar]
- 61.Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: Agonist and NO stimulation. J Theoretical Biol. 2008;253:238–260. doi: 10.1016/j.jtbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Clark JW, Bryan RM, Robertson CS. Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. Am J Physiol. Heart Circ Physiol. 2005;289:H886–H897. doi: 10.1152/ajpheart.00216.2004. [DOI] [PubMed] [Google Scholar]
- 63.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 64.Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schulz R, Seeger W, Grimminger F, Weissmann N. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J. 2008;32:1639–1651. doi: 10.1183/09031936.00013908. [DOI] [PubMed] [Google Scholar]
- 65.Ward JPT, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9:287–296. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serruys PW, Vranckx P, Slagboom T, Regar E, Meliga E, de Winter RJ, Heyndrickx G, Schuler G, van Remortel EAM, Dube GP, Symons J for the COR-0001 trial investigators. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. EuroIntervention. 2008;3:600–609. doi: 10.4244/eijv3i5a108. [DOI] [PubMed] [Google Scholar]
- 67.Fonseca V, Avizinis J, Moon-Massat P, Freilich D, Kim HW, Hai CM. Differential sensitivity of pulmonary and coronary arteries to hemoglobin-based oxygen carriers and nitrovasodilators: study in a bovine ex vivo model of vascular strips. Vascul Pharmacol. 2010;52:215–223. doi: 10.1016/j.vph.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chlopicki S, Kozlovski VI, Lorkowska B, Drelicharz L, Gebska A. Compensation of endothelium-dependent responses in coronary circulation of eNOS-deficient mice. J Cardiovasc Pharmacol. 2005;46:115–123. doi: 10.1097/01.fjc.0000164093.88821.00. [DOI] [PubMed] [Google Scholar]
- 69.Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr, Huang PL, McMurtry IF, Rodman DM. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–L478. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 70.Talukder MAH, Fujiki T, Morikawa K, Motoishi M, Kubota H, Morishita T, Tsutsui M, Takeshita A, Shimokawa H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- 71.Chi JT, Chang HY, Haraldsen G, Jahnsen FJ, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Nat Acad Sc USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghitescu L, Robert M. Diversity in unity: the biochemical composition of the endothelial cell surface varies between the vascular beds. Microscopy Res Technique. 2002;57:381–389. doi: 10.1002/jemt.10091. [DOI] [PubMed] [Google Scholar]
- 74.Aird WC. Phenotypic heterogeneity of the endothelium I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 75.Aird WC. Endothelium as an organ system. Crit Care Med. 2004;32(Suppl):S271–S279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 76.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 77.Aird WC. Phenotypic heterogeneity of the endothelium II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 78.Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JGN, Herbel RP, Tuder RM, Garfinkel S. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol. 2001;281:C1422–C1433. doi: 10.1152/ajpcell.2001.281.5.C1422. [DOI] [PubMed] [Google Scholar]
- 79.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarizing factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 80.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sc. 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 81.Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses – relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010 doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 85.Haimovici H. The role of arterial tissue susceptibility in atherogenesis. Texas Heart Inst J. 1991;18:81–83. [PMC free article] [PubMed] [Google Scholar]
- 86.Haimovici H, Maier N. Fate of aortic homografts in canine atherosclerosis. 3. Study of fresh abdominal and thoracic aortic implants into thoracic aorta: role of tissue susceptibility in atherogenesis. Arch Surg. 1964;89:961–969. doi: 10.1001/archsurg.1964.01320060029006. [DOI] [PubMed] [Google Scholar]
- 87.Rensen SSM, Doevendans PAFM, van Eys GJJM. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katz LM, Manning JE, McCurdy S, Sproule C, McGwin G, Jr, Moon-Massat P, Cairns CB, Freilich D. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation. 2010;81:481–487. doi: 10.1016/j.resuscitation.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 89.Yu B, Bloch KD, Zapol WM. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med. 2009;19:103–107. doi: 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boerrigter G, Burnett JC., Jr Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41–2272 in cardiovascular disease. Cardiovasc Drug Rev. 2007;25:30–45. doi: 10.1111/j.1527-3466.2007.00003.x. [DOI] [PubMed] [Google Scholar]
- 91.Caretti A, Fantacci M, Caccia D, Perrella M, Lowe KC, Samaja M. Modulation of the NO/cGMP pathway reduces the vasoconstriction induced by acellular and PEGylated haemoglobin. Biochim Biophys Acta. 2008;1784:1428–1434. doi: 10.1016/j.bbapap.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 92.Hoenicka M, Schmid C. Cardiovascular effects of modulators of soluble guanylyl cyclase activity. Cardiovas Hematol Agents Medicinal Chem. 2008;6:287–301. doi: 10.2174/187152508785909555. [DOI] [PubMed] [Google Scholar]
- 93.Priviero FBM, Webb RC. Heme-dependent and independent soluble guanylate cyclase activators and vasodilation. J Cardiovas Pharmacol. 2010;56:229–233. doi: 10.1097/FJC.0b013e3181eb4e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J. Biol Chem. 2005;280:39024–39032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- 95.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Nat Acad Sc. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Samouilov A, Lancaster JR, Jr, Zweier J. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J. Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 97.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 98.Portoro I, Kocsis L, Herman P, Caccia D, Perrella M, Ronda L, Bruno S, Bettati S, Micalella C, Mozzarelli A, Varga A, Vas M, Lowe KC, Eke A. Towards a novel haemoglobin –based oxygen carrier: Euro-PEG-Hb, physic-chemical properties, vasoactivity and renal filtration. Biochim Biophys Acta. 2008;1784:1402–1409. doi: 10.1016/j.bbapap.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artificial Organs. 2009;33:139–145. doi: 10.1111/j.1525-1594.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 100.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4: a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 101.Caccia D, Ronda L, Frassi R, Perrella M, Del Favero E, Bruno S, Pioselli B, Abbruzzetti S, Viappiani C, Mozzarelli A. PEGylation promotes hemoglobin tetramer dissociation. Bioconjugate Chem. 2009;20:1356–1366. doi: 10.1021/bc900130f. [DOI] [PubMed] [Google Scholar]
- 102.Hu T, Li D, Manjula BN, Brenowitz M, Prabhakaran M, Acharya SA. PEGylation of Val-1(α) destabilizes the tetrameric structure of hemoglobin. Biochemistry. 2009;48:608–616. doi: 10.1021/bi801880y. [DOI] [PubMed] [Google Scholar]
- 103.Hebbel RP, Vercellotti GM, Nath KA. A systems biology consideration of the vasculopathy of sickle cell anemia: The need for multi-modality chemo-prophylaxis. Cardiovasc. Hematol. Disord. Drug Targets. 2009;9:271–292. doi: 10.2174/1871529x10909040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hellerstein MK. Exploiting complexity and the robustness of network architecture for drug discovery. J Pharmacol Exp Therap. 2008;325:1–9. doi: 10.1124/jpet.107.131276. [DOI] [PubMed] [Google Scholar]
- 106.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nature Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]