Abstract
Background
Δ9-Tetrahydrocannabinol (THC) in oral fluid (OF) implies cannabis intake, but eliminating passive exposure and improving interpretation of test results requires additional research.
Methods
Ten adult cannabis users smoked ad libitum one 6.8% THC cigarette. Expectorated OF was collected for up to 22h, and analyzed within 24 h of collection. THC, 11-nor-9-carboxy-THC (THCCOOH), cannabidiol, and cannabinol were quantified by 2-dimensional-GCMS.
Results
Eighty specimens were analyzed; 6 could not be collected due to dry mouth. THC was quantifiable in 95.2%, cannabidiol in 69.3%, cannabinol in 62.3%, and THCCOOH in 94.7% of specimens. Highest THC, cannabidiol, and cannabinol concentrations were 22370, 1000, and 1964 μg/l, respectively, 0.25 h after the start of smoking; THCCOOH peaked within 2 h (up to 560 ng/l). Concentrations 6h after smoking were THC (0.9-90.4 μg/l) and THCCOOH (17.0-151 ng/l) (8 of 9 positive for both); only 4 were positive for cannabidiol (0.5-2.4 μg/l) and cannabinol (1.0-3.0 μg/l). By 22h, there were 4 THC (0.4-10.3 μg/l), 5 THCCOOH (6.0-24.0 ng/l), 1 cannabidiol (0.3 μg/l), and no cannabinol positive specimens.
Conclusions
THCCOOH in OF suggests no passive contamination, and CBD and CBN suggest recent cannabis smoking. Seventeen alternative cutoffs were evaluated to meet the needs of different drug testing programs.
Keywords: Oral fluid, expectoration, cannabinoids, Δ9-tetrahydrocannabinol, detection window, cannabis
1. INTRODUCTION
The value of oral fluid (OF) as an alternative matrix to document drug exposure in workplace, drug treatment, pain management, and driving under the influence of drugs (DUID) programs is clearly established [1-2]. OF sampling is directly observed, less invasive than blood collection, and may be obtained by direct expectoration (spitting) or absorption onto a permeable pad or sponge, with or without an agent that stimulates OF production. Expectoration is less expensive than utilizing a commercial collection device and offers the advantage of directly determining drug concentrations. A disadvantage is that many drugs reduce salivary flow [3], making expectoration collection difficult and of low volume. Low sample volumes frequently occur after cannabis smoking and stimulant ingestion [4-5].
The oral mucosa is immediately contaminated during cannabis smoking, as Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN) are contained in cannabis vapor; thus, THC OF concentrations often exceed 1000 μg/l for a short time after smoking [6-7]. Many prior studies did not investigate or were unable to quantify concentrations of THC metabolites and other cannabinoids in OF following controlled smoking [8,9]. Detection of the non-psychotropic THC metabolite THCCOOH provides evidence of active smoking [10], as it is not present in cannabis smoke [11-12] and occurs in OF due to passive diffusion from blood [13]. However, THCCOOH quantification requires a highly sensitive analytical method capable of ng/l detection.
Current regulatory guidelines for OF testing suggest screening and confirmation analysis for THC only. However, we documented that chronic therapeutic oral THC administration and illicit oral THC use are unlikely to be identified with current guidelines [14], as THC OF and blood concentrations decreased with chronic oral THC. Measurement of other cannabinoids may improve the detection and interpretation of OF cannabinoid tests and minimize the possibility of false positive results from OF contamination by passive inhalation of cannabis smoke. This report quantifies THC, 11-OH-THC, THCCOOH, CBD, and CBN in expectorated OF following controlled smoked cannabis.
2. Material and methods
2.1. Chemicals and reagents
THC, 11-OH-THC, THCCOOH, CBD, and CBN for calibrators and quality control samples and corresponding internal standards (d3-THC, d3-11-OH-THC , d3-THCCOOH, d3-CBD) were from Cerilliant Corporation (Round Rock, TX). N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane was from Thermo Fisher Scientific Inc. (Rockford, IL). Trifluoroacetic anhydride (TFAA) and hexafluoroisopropanol (HFIP) were from Campbell Science (Rockton, IL), and CEREX® Polycrom™ THC (3cc/35mg) solid-phase extraction (SPE) columns were from SPEware Corporation (Baldwin Park, CA).
2.2. Participants
Participants provided written informed consent for this Institutional Review Board-approved protocol. Inclusion criteria were ages 18-45 y, self-reported cannabis use with a minimum frequency of ≥2 times/month during the 3 months prior to study entry, and a cannabinoid-positive urine sample upon admission. Clinically significant medical or psychiatric disease, history of seizures or psychosis, or cannabis-related adverse effect, and interest in or participation in drug abuse treatment within 60 days preceding study enrollment were exclusionary.
2.3. Study design and specimens collection
Participants smoked ad libitum for 10 min one cannabis cigarette containing approximately 6.8% THC, 0.25% CBD, and 0.21% CBN. OF specimens were collected 0.5 h before and 0.25, 0.5, 1, 2, 3, 4, 6, and 22 h after the start of smoking. Participants could be discharged 6 h after cannabis smoking if their neuromotor examination was normal and vital signs returned to baseline, or could choose to stay overnight with an additional OF specimen collection at 22 h. OF was collected by expectoration into polypropylene tubes. Participants spit into the tube until at least 3 ml OF was collected or for 5 min, whichever occurred first. OF was centrifuged and stored at 4°C in Nunc® cryotubes until analysis within 24 h of collection.
2.4. Oral fluid analysis
OF specimens were analyzed by a previously validated two-dimensional gas chromatography mass spectrometry (2D-GCMS) method for THC, 11-OH-THC, THCCOOH, CBD and CBN, with separate injections on two analytical systems with different ionization techniques [14]. Briefly, 0.5 ml drug-free OF was fortified with calibrator or quality control solution. Deuterated internal standards (d3-THC, d3-11-OH-THC, d3-CBD and d3-THCCOOH; CBN utilized d3-THC) were added to calibrators, controls, and authentic OF samples. To reduce viscosity and precipitate proteins, expectorated specimens were diluted with deionized water (1 ml) and ice-cold acetonitrile (0.75 ml) prior to extraction. Following vortexing and centrifugation, supernatants were decanted onto preconditioned SPE extraction columns and washed with deionized water/acetonitrile/ammonium hydroxide (84:15:1, v/v/v). THC, 11-OH-THC, CBD and CBN were eluted with hexane/acetone/ethyl acetate (60:30:20, v/v/v), evaporated to dryness and derivatized with BSTFA prior to 2D-GCMS with electron ionization. THCCOOH was eluted into separate tubes with hexane/ethyl acetate/glacial acetic acid (75:25:2.5, v/v/v), evaporated and derivatized with HFIP (20 μL) and TFAA (40 μl) prior to 2D-GCMS with negative chemical ionization. The published method for analysis of cannabinoids in expectorated OF was fully validated [14]. Limits of quantification (LOQ) and dynamic ranges for THC and CBD were 0.25–50 μg/l, 1–50 μg/l for CBN, 0.25–25 μg/l for 11-OH-THC and 5–500 ng/l for THCCOOH. Samples exceeding the linear range were diluted with blank OF and reanalyzed. Intra- and inter-assay CVs were <6.5% and analytical recovery was within ±15.2% of target. Extraction efficiencies ranged between 54.4 and 97.4%. Electronic Supplementary Material Table S1 provides further detail and additional method validation data.
2.5. Data analysis
Statistical calculations utilized SPSS® 14.0 for Windows (SPSS, Chicago, IL). Means are presented for normally distributed data, and medians and ranges for non-normally distributed data. Median concentrations included only positive (≥LOQ) specimens. For comparative statistical analyses, OF specimens <LOQ were assigned values of 10% LOQ (0.025 μg/l for THC and CBD, 0.1 μg/l for CBN, and 0.5 ng/l for THCCOOH). Concentration differences between collections were evaluated by non-parametric Wilcoxon tests. Associations between variables were assessed by Spearman correlation. A two-tailed P<0.05 defined significance for all comparisons.
3. RESULTS
Ten (1 female Caucasian; 9 male (5 Caucasian, 4 African-American)) healthy research volunteers [mean±SD age 30.7±8.9 y] participated in the study. Body-mass index ranged from 18.1 to 32.0. Mean self-reported cannabis smoking was 4.9±3.2 joints/day and mean (±SD) cannabis smoking was 11.4±2.2 of the past 14 days prior to screening. All participants self-reported cannabis smoking within 1-4 days (mean±SD 2.0±1.1) prior to admission, and had a positive cannabinoid urine test upon admission.
80 OF specimens were collected. Six specimens from 4 individuals could not be collected between 0.25–1 h after cannabis smoking due to reduced salivary excretion (dry mouth). Four participants left 6 h after smoking and 6 provided a 22 h OF specimen by staying overnight. One participant (J) had visible bleeding gums and OF mixed with blood. These nine specimens contained highly elevated cannabinoid concentrations; thus, their data are described separately. For clarity, we describe cannabinoid concentrations in baseline specimens prior to smoking (n=9) separately from OF concentrations after cannabis smoking (n=63).
3.1. THC in oral fluid
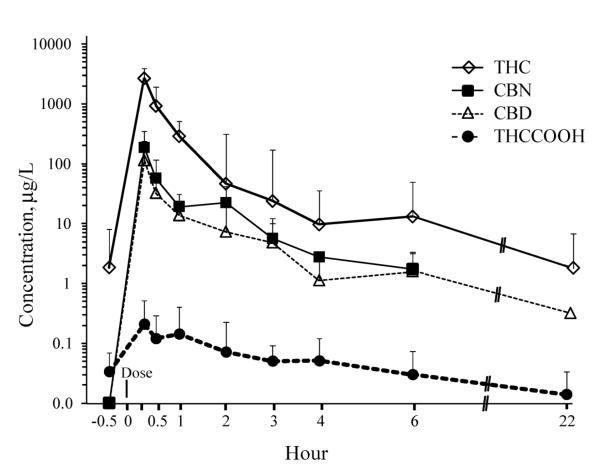
Four of 9 participants’ OF specimens were THC-positive 0.5 h prior to smoking cannabis, with a median concentration of 1.3 μg/l (range, 0.3–43.6) from previously self-administered smoked cannabis (Table 1). THC baseline (0.5 h prior to smoking) OF concentrations were significantly lower (Z=−2.6, p=0.008) than those collected 0.25 h after smoking. THC was present in 60 of 63 (95.2%) expectorated OF specimens from 9 participants after smoking, with a maximum concentration of 22370 μg/l. Maximum THC concentrations occurred at or prior to the first collection at 0.25 h, decreasing rapidly during the first 3 h after smoking. Median (range) THC concentration was 2629 μg/l (264–22370) at 0.25 h, decreasing 10-fold within 1h to 282 μg/l (35.4–1030) and 100-fold over 3 h to 13.5 μg/l (1.5–205) (Fig.1). Concentration decreased significantly only between 0.25–0.5 h (Z=−2.2, p=0.028), 0.5–1 h (Z=−2.2, p=0.03), and 2–3 h (Z=−2.8, p=0.005) collections. Four of 6 specimens were THC-positive 22 h after smoking, with a median concentration of 1.8 μg/l (0.4–10.3) μg/l. THC concentrations significantly decreased (Z=−2.2, p=0.028) from 6 to 22 h in these 4 participants.
Table 1.
THC (μg/l) concentrations in expectorated oral fluid from 10 participants before and after smoking a single 6.8% THC cigarette.
| Time (h) | −0.5 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 22 |
|---|---|---|---|---|---|---|---|---|---|
| A | <LOQ | 641 | NC | 116 | 20.7 | 2.9 | 6.5 | 4.1 | <LOQ |
| B | 43.6 | NC | NC | NC | 313 | 205 | 245 | 90.4 | 10.3 |
| C | <LOQ | 2634 | 1383 | 263 | 382 | 33.6 | 36.4 | 39.0 | 2.5 |
| D | <LOQ | 3384 | 311 | 301 | 12.0 | 4.0 | 8.3 | 2.1 | 0.4 |
| E | 0.7 | 4436 | 527 | 238 | 15.9 | 13.5 | 7.7 | <LOQ | DC |
| F | <LOQ | 2624 | 1524 | 471 | 59.9 | 55.8 | 10.8 | 14.4 | DC |
| G | <LOQ | 2103 | NC | 508 | 31.7 | 2.9 | 2.1 | 0.9 | <LOQ |
| H | 1.8 | 22370 | NC | 1030 | 186 | 186 | 22.6 | 13.0 | 1.0 |
| I | 0.3 | 265 | 101 | 35.4 | 11.6 | 1.5 | 1.9 | 0.9 | DC |
| J | 7.1 | 2735 | 1296 | 284 | 1310 | 206 | 40.4 | 42.4 | DC |
| Median a | 1.3 | 2629 | 527 | 282 | 31.7 | 13.5 | 8.3 | 8.5 | 1.8 |
NC- not collected due to dry mouth; DC -discharged at 6 h from the unit;
<LOQ – below limit of quantification: THC – 0.25 μg/l;
Median – participant J excluded due to blood in oral fluid
Figure 1.
Median and interquartile ranges THC, CBD, CBN, and THCCOOH concentrations in expectorated oral fluid from 9 participants after smoking a single 6.8% cannabis cigarette.
Participant J’s OF contained 7.1 μg/l THC at baseline, increasing to 2735 μg/l immediately after smoking and decreasing 10-fold within 1 h. At 2 h, the concentration increased four-fold to 1310 μg/l, but the color of that specimen indicated the highest presence of blood. The THC OF concentration was 42.4 μg/l at the last (6 h) collection.
3.2. 11-OH-THC in oral fluid
Two of 63 (2.9%) specimens were 11-OH-THC-positive from 2 individuals at the 0.25 μg/l LOQ. One was positive at 0.25 h (1.2 μg/l) and a second (0.3 μg/l) 1 h after smoking. Participant J produced two 11-OH-THC positive OF specimens 1 and 2 h after smoking, with concentrations of 0.4 and 1.3 μg/l, respectively.
3.3. THCCOOH in oral fluid
All 9 participants’ OF specimens were THCCOOH-positive at baseline, with a median concentration of 28.0 ng/l (range 8.4–98.3). THCCOOH was present in 54 of 57 (94.7%) expectorated OF specimens after cannabis smoking, at concentrations up to 560 ng/l. A 50-fold dilution was required for 6 low volume expectorated specimens collected between 0.25 and 1.0 h. Although THC concentrations fell within the linear range of the assay with this dilution, THCCOOH concentrations fell below the 50 fold higher LOQ. Thus, these data were not included in prevalence evaluations or in Table 2, where different cutoff concentrations were compared. Maximum THCCOOH concentrations occurred within 2 h after smoking, with a median Tmax of 1 h (0.25–2.0). Median THCCOOH concentration at 0.25 h was 147 ng/l (20.8–467), decreasing nonsignificantly (Z=−0.7, p=0.484) to 66.4 ng/l (22.8–560) within 1 h, and nonsignificantly (Z=−1.5, p=0.123) to 44.0 ng/l (9.1–127) after 3 h (Table 2). Significant concentration decreases were observed from 2–3 h (Z=−2.8, p=0.005) and 3–4 h (Z=−1.9, p=0.047). Five of 6 specimens were THCCOOH-positive 22 h after smoking, with a nonsignificant decrease (Z=−1.7, p=0.08) (median 14.2 ng/l; range 6.0–24.0) from 6 h concentrations.
Table 2.
THCCOOH (ng/l) concentrations in expectorated oral fluid from 10 participants before and after smoking a single 6.8% THC cigarette.
| Time (h) | −0.5 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 22 |
|---|---|---|---|---|---|---|---|---|---|
| A | 17.5 | <LOQ* | NC | 51.0 | 49.3 | 20.1 | 34.0 | 24.0 | 6.0 |
| B | 39.2 | NC | NC | NC | 64.8 | 12.3 | 18.5 | <LOQ | <LOQ |
| C | 8.4 | <LOQ* | <LOQ* | <LOQ* | 37.1 | <LOQ | 14.3 | 23.9 | 7.7 |
| D | 28.0 | 97.1 | 82.6 | 66.4 | 78.2 | 37.5 | 74.6 | 30.1 | 22.6 |
| E | 57.0 | 148 | 157 | 274 | 94.1 | 54.2 | 67.9 | 66.8 | DC |
| F | 56.9 | <LOQ* | <LOQ* | 220 | 314 | 127 | 125 | 151 | DC |
| G | 98.3 | 468 | NC | 561 | 244 | 62.7 | 96.6 | 23.7 | 24.0 |
| H | 26.8 | 271 | NC | 57.2 | 62.6 | 50.5 | 25.0 | 33.5 | 14.2 |
| I | 11.5 | 20.8 | 24.5 | 22.8 | 19.9 | 9.1 | 15.6 | 17.0 | DC |
| J | 581 | 537 | 488 | 462 | 3519 | 372 | 424 | 529 | DC |
| Median a | 28 | 148 | 82.6 | 66.4 | 64.8 | 44.0 | 34.0 | 27.0 | 14.2 |
NC- not collected due to dry mouth; DC -discharged at 6 h from the unit;
<LOQ – below limit of quantification: THCCOOH –5 ng/l;
<LOQ* – below limit of quantification in low volume specimens for THCCOOH (250 ng/l), due to a required 50-fold dilution.
Median – participant J excluded due to blood in oral fluid
At baseline (−0.5 h), participant J’s THCCOOH OF concentration was 581 ng/l. There was no change in THCCOOH concentrations within 1 h after smoking (range 462-537 ng/l). A 7-fold increase was observed at the 2 h collection (3519 ng/l), the specimen with the largest amount of blood, followed by a 10-fold decrease in the next 3 h collection. In the last (6 h) collection, THCCOOH concentration was 529 ng/l.
3.4. CBD and CBN in oral fluid
CBD was present in 43 (69.3%) of 62 and CBN in 38 (62.3%) of 61 specimens, always in conjunction with THC. No baseline specimens were positive for CBD or CBN. Maximum CBD and CBN concentrations occurred prior to or at the first collection after smoking, with maximum concentrations of 1000 and 1964 μg/l, respectively. CBD (Table 3) and CBN (Table 4) significantly (Z=−2.6, p= 0.008) decreased 10-fold between 0.25 h and 1 h collections. One CBD (0.3 μg/l) and no CBN positive specimens were found 22 h after smoking. CBD and CBN were highly (p<0.001) correlated (ρ = 0.963) in all specimen collections. Both analytes were significantly (p<0.001) correlated with THC concentrations (THC-CBD ρ = 0.952, THC-CBN 0.944).
Table 3.
CBD (μg/l) concentrations in expectorated oral fluid from 10 participants before and after smoking a single 6.8% THC cigarette.
| Time (h) | −0.5 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 22 |
|---|---|---|---|---|---|---|---|---|---|
| A | <LOQ | 28.5 | NC | 6.6 | 1.2 | <LOQ | 0.3 | <LOQ | <LOQ |
| B | <LOQ | NC | NC | NC | 23.1 | 6.4 | 5.6 | 2.4 | 0.3 |
| C | <LOQ | 213 | 42.5 | <LOQ* | 15.0 | 1.5 | 1.6 | 1.6 | <LOQ |
| D | <LOQ | 152 | 10.6 | 17.6 | <LOQ | 0.3 | 0.8 | <LOQ | <LOQ |
| E | <LOQ | 271 | 22.0 | 10.8 | <LOQ | <LOQ | <LOQ | <LOQ | DC |
| F | <LOQ | 90.0 | 49.0 | 14.5 | 3.9 | 3.2 | 0.5 | 0.6 | DC |
| G | <LOQ | 89.5 | NC | 25.0 | 1.8 | <LOQ | <LOQ | <LOQ | <LOQ |
| H | <LOQ | 1000 | NC | 38.0 | 10.7 | 10.6 | 1.1 | 0.5 | <LOQ |
| I | <LOQ | 8.8 | 3.6 | 1.5 | 0.4 | <LOQ | <LOQ | <LOQ | DC |
| J | <LOQ | 113 | 52.5 | 12.8 | 42.2 | 12.9 | 2.2 | 2.2 | DC |
| Mediana | - | 121 | 22.0 | 14.5 | 3.9 | 3.2 | 0.9 | 1.1 | 0.3 |
NC- not collected due to dry mouth; DC -discharged at 6 h from the unit;
<LOQ – below limit of quantification: CBD – 0.25 μg/l;
<LOQ* – below limit of quantification in low volume specimens for CBD (12.5 μg/l), due to a required 50-fold dilution.
Median – participant J excluded due to blood in oral fluid
Table 4.
CBN (μg/l) concentrations in expectorated oral fluid from 10 participants before and after smoking a single 6.8% THC cigarette.
| Time (h) | −0.5 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 22 |
|---|---|---|---|---|---|---|---|---|---|
| A | <LOQ | <LOQ* | NC | 8.1 | 1.4 | <LOQ | <LOQ | <LOQ | <LOQ |
| B | <LOQ | NC | NC | NC | 22.8 | 6.9 | 6.7 | 3.0 | <LOQ |
| C | <LOQ | 303 | 73.5 | <LOQ* | 22.2 | 1.8 | 2.8 | 1.7 | <LOQ |
| D | <LOQ | 230 | 14.6 | 20.5 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| E | <LOQ | 414 | 40.5 | 19.2 | <LOQ | 4.3 | 1.1 | <LOQ | DC |
| F | <LOQ | 142 | 85.0 | 15.6 | 4.8 | 2.9 | <LOQ | 1.0 | DC |
| G | <LOQ | 151 | NC | 39.8 | 2.3 | <LOQ | <LOQ | <LOQ | <LOQ |
| H | <LOQ | 1964 | NC | 115 | 23.7 | 21.6 | 2.8 | 1.2 | <LOQ |
| I | <LOQ | 12.7 | 5.8 | 2.7 | <LOQ | <LOQ | <LOQ | <LOQ | DC |
| J | <LOQ | 185 | 85.5 | 19.3 | 23.3 | 7.9 | 2.4 | 2.8 | DC |
| Mediana | - | 230 | 40.5 | 19.2 | 13.5 | 4.3 | 2.8 | 1.5 | - |
NC- not collected due to dry mouth; DC -discharged at 6 h from the unit;
<LOQ – below limit of quantification: CBN – 1 μg/l;
<LOQ* – below limit of quantification in low volume specimens for CBN (50 μg/l), due to a required 50-fold dilution.
Median – participant J excluded due to blood in oral fluid
Participant J’s baseline OF specimen was negative for CBD and CBN prior to smoking. At 0.25 h after smoking, CBD and CBN concentrations were 113 and 185 μg/l, respectively. A 10-fold decrease (CBD – 12.8 μg/l, CBN – 19.3 μg/l) was observed within 1 h. CBD and CBN concentrations in the specimen with visible blood (2 h) were 42.2 and 23.3 μg/l, respectively. Participant J’s last 6 h OF specimen was positive for CBD (2.2 μg/l) and CBN (2.8 μg/l).
3.5. Recommended and proposed OF cannabinoid concentration cutoffs
Table 5 summarizes OF results according to recommended THC concentration cutoffs from the Driving under the Influence of Drugs, Alcohol and Medicines (DRUID) (≥ 1 μg/l) and Substance Abuse and Mental Health Services Administration (SAMHSA) (≥ 2 μg/l) guidelines. In addition, we include results based on a number of proposed cutoff concentrations for one or more cannabinoids in OF. One specimen for CBD, 2 for CBN, and 6 for THCCOOH were not included in the cutoff evaluation, due to the need for a 50 fold dilution as a result of low specimen volumes. These negative results due to the higher LOQs of 12.5 μg/l, 50 μg/l, and 250 ng/l, respectively, are not appropriate for evaluation of different cutoff concentrations.
Table 5.
Recommended SAMHSA, DRUID and proposed cannabinoid concentration cutoffs for expectorated oral fluid specimens following controlled smoked cannabis.
| Cutoffs | # Positive a / Total b |
% Positive |
Time of last positive (range) h |
Median Time of last positive, h |
Positive baseline specimens |
|---|---|---|---|---|---|
| THC ≥THC ≥ 1 μg/l (DRUID) | 59/63 | 93.7% | 4 - >22 | 6 | 2 |
| THC ≥ 2 μg/l (SAMHSA) | 55/63 | 87.3% | 2 - >22 | 6 | 1 |
| CBD ≥ 1 μg/l | 35/62 | 56.5% | 1 - 6 | 2 | 0 |
| THC ≥ 1 μg/l & CBD ≥ 1 μg/l | 35/62 | 56.5% | 1 - 6 | 2 | 0 |
| THC ≥ 2 μg/l & CBD ≥ 1 μg/l | 35/62 | 56.5% | 1 - 6 | 2 | 0 |
| CBN ≥ 1 μg/l | 38/61 | 62.3% | 1 - 6 | 4 | 0 |
| THC ≥ 1 μg/l & CBN ≥ 1 μg/l | 38/61 | 62.3% | 1 - 6 | 4 | 0 |
| THC ≥ 2 μg/l & CBN ≥ 1 μg/l | 38/61 | 62.3% | 1 - 6 | 4 | 0 |
| THCCOOH ≥ 15 ng/l | 48/57 | 84.2% | 4 - >22 | 6 | 7 |
| THC ≥ 1 μg/l & THCCOOH ≥ 15 ng/l | 43/57 | 75.4% | 4 - 6 | 6 | 2 |
| THC ≥ 2 μg/l & THCCOOH ≥ 15 ng/l | 42/57 | 73.7% | 2 - 6 | 6 | 1 |
| THCCOOH ≥ 20 ng/l | 44/57 | 77.2% | 1 - >22 | 6 | 6 |
| THC ≥ 1 μg/l & THCCOOH ≥ 20 ng/l | 40/57 | 70.2% | 1 - 6 | 6 | 2 |
| THC ≥ 2 μg/l & THCCOOH ≥ 20 ng/l | 40/57 | 70.2% | 1 - 6 | 6 | 1 |
| THCCOOH ≥ 25 ng/l | 35/57 | 61.4% | ND - 6 | 5 | 6 |
| THC ≥ 1 μg/l & THCCOOH ≥ 25 ng/l | 34/57 | 59.6% | ND - 6 | 4 | 2 |
| THC ≥ 2 μg/l & THCCOOH ≥ 25 ng/l | 34/57 | 59.6% | ND - 6 | 4 | 1 |
ND – not detected; DRUID – Driving under the Influence of Drugs, Alcohol and Medicines; SAMHSA – Substance Abuse and Mental Health Services Administration
Number of positive specimens after cannabis smoking
Total number of specimens evaluated in the category
Data exclude specimens from participant J due to blood in oral fluid
4. DISCUSSION
This controlled cannabis smoking study describes the disposition of THC, 11-OH-THC, THCCOOH, CBD, and CBN in expectorated OF specimens analyzed within 24 h of collection. Several studies investigated THC OF concentrations after cannabis smoking with a variety of commercially-available collection devices [7, 15]. These devices employed buffers to extract drugs from the absorbent pad or sponge and stabilize analytes, but also diluted OF and reduced assay sensitivity. Expectoration provides undiluted OF, permitting direct determination of drug concentrations in excreted saliva. However, collection and analysis of expectorated specimens also has limitations, including low specimen volume, and presence of highly-viscous mucous. We previously published data on cannabinoids in expectorated OF specimens after controlled oral dosing that revealed challenges in utilizing this alternative biological specimen [14]. Cannabis smoking, as well as stimulant consumption, reduces salivary flow, leading to xerostomia, increased froth, and low-volume, viscous specimens [2,16]. In the current study, 6 participants could not provide expectorated OF within the 1st h after smoking due to dry mouth and decreased salivation. Specimens could not be collected 0.25 h (1), 0.5 h (4), and 1 h (1) after cannabis smoking. An additional 6 specimens had low specimen volumes, but were analyzed with dilution, increasing LOQs for these specimens.
Viscous OF specimens prevent flow and effective analyte binding to the sorbent bed of the SPE columns [14]. Freezing and thawing specimens prior to analysis helps decrease OF viscosity, improving pipetting accuracy. Sample centrifugation also removes some mucous, but may change concentrations if cannabinoids bind to precipitant; these factors are to date poorly characterized [17-19]. Expectorated specimens from the present study were stored at 4°C and analyzed within 24 h. Ice-cold acetonitrile was added to reduce viscosity, and centrifugation precipitated much of the mucous and mouth debris in expectorated OF, substantially improving SPE performance, as reported previously [14,20].
THC concentrations were highest 0.25 h after smoking and decreased on average 100-fold 3 to 6 h later. Similarly, Kauert et al. [7] observed highest THC concentrations in OF in the first collection at 0.25 h, with 900±589 and 1041±652 μg/l following 18.2±2.8 mg and 36.5±5.6 mg smoked THC, respectively. Concentrations decreased to 18±12 μg/l 6 h later. Niedbala et al. [9] reported mean±SEM peak OF THC concentrations of 83.4±18.6 and 75.9±19.5 μg/l for 5 chronic and 5 casual smokers, respectively, in the first collection 1 h following a single smoked cannabis cigarette. In our study, mean±SD THC concentration 1 h after smoking was 361±292 μg/l. This much higher mean concentration could be explained by the greater amount of THC in the smoked cannabis cigarette in our study, 54 mg THC, compared to 20-25 mg in the Niedbala study [9]. Furthermore, in the later study, OF was collected with the OraSure collection device, which dilutes OF 1:3 with extraction/stabilization buffer; analyte adsorption to the collection pad, recovery from the pad, and variability in OF specimen volume may have contributed to lower concentrations compared to expectorated specimens. Huestis and Cone [6] reported OF THC as high as 5800 μg/l 0.2 h after a cannabis cigarette containing 3.55% THC and 81 μg/l after 0.33 h. These samples were collected by expectoration under stimulated (citric acid-type sour candy) conditions and were frozen prior to analysis. Although stimulation enhances sample volume, it also changes salivary composition and pH, affecting cannabinoid OF concentrations [21]. In addition, inter-individual variability in cannabinoid concentrations might derive from different smoking topographies due to varied inhalation volumes, hold duration, puff number, time between puffs, and side-stream smoke losses [22,23].
In contrast to THC, THCCOOH was detected in all specimens 0.5 h prior to smoking, albeit at a much lower LOQ of 5 ng/l. Six specimens from 3 participants were THCCOOH-negative the 1st h after smoking; however, these specimens had low specimen volumes, and were diluted with negative OF prior to analysis. THCCOOH, a phase I metabolism product, is not present in cannabis smoke [11,12] and is quantified in OF in ng/l concentrations after active cannabis consumption [13,24]. THCCOOH is suggested as a promising biomarker for cannabinoid detection because it would minimize the possibility of external contamination from cannabis smoke [10] and detect oral THC (medications) or cannabis intake [13]. THCCOOH peak concentrations occurred within 2 h after smoking, while analytes present in cannabis smoke, THC, CBD, and CBN, peaked within the first 0.25 h after initiation of smoking. Peak THCCOOH concentrations varied considerably between participants, ranging from 24.5 to 314 ng/l. Residual THCCOOH from previously self-administered cannabis may contribute to the total measured THCCOOH, depending upon the frequency and chronicity of cannabis smoking.
For participant J, with clearly observable blood in the expectorated OF specimens, THC and THCCOOH OF concentrations were noticeably higher than other participants. Cannabinoids in blood presumably contaminated and increased expectorated OF concentrations. Cannabinoid concentrations could be misinterpreted in bloody expectorated OF specimens; thus, OF appearance should be noted and recorded. It is unknown whether small amounts of blood would be detectable in OF specimens collected with devices, rather than by expectoration. Although this is to date the only OF specimen we have collected in any of our controlled cannabis administration studies that contained visible blood, it is an important issue for the testing community to consider. OF specimens containing blood should be recollected at a different time or an alternative matrix should be considered, as blood will alter OF cannabinoid results.
THCCOOH OF concentrations significantly (Z=−2.803; p=0.005) decreased from 2 to 3 h, before and after lunch in all participants. Presumably, this decrease was induced by food and drink consumption, reducing drug concentrations in the oral cavity [25].
Four OF specimens from 3 participants had quantifiable 11-OH-THC. Two positive specimens contained blood in the expectorated OF and were collected from participant J. 11-OH-THC, a psychoactive phase I intermediate THC metabolite, generally is not found in OF with a 0.25 μg/l cutoff. Previously, we reported a single 11-OH-THC-positive specimen (0.5 μg/l) in expectorated OF collected after 36 oral THC doses that was detected concurrently with the highest THCCOOH (Cmax=1390.3 ng/l) concentration [14]. Analysis of 11-OH-THC in OF with a lower LOQ, similar to that employed for THCCOOH, would most likely yield additional 11-OH-THC-positive specimens. In plasma, 11-OH-THC concentrations range from 50 to 100% of THC concentrations following oral cannabinoid administration, compared to only 10% after smoked administration [26]. Further research should address the value of inclusion of 11-OH-THC in the interpretation of OF cannabinoid results.
CBD and CBN had similar concentration profiles as THC, but always at much lower concentrations. Unlike THC, no specimens were positive for CBD and CBN at baseline. Moore et al. [27] reported OF specimens positive for CBN for 2 h after cannabis smoking with no measurable CBD in OF collected with the Quantisal collection device from 3 chronic smokers. Previously, we reported 5 CBD- and 14 CBN-positive OF specimens from 28 chronic, daily cannabis smokers enrolled in a 30-day abstinence study [5]. OF specimens were collected with the Quantisal device; only specimens collected upon admission were CBD and CBN positive, demonstrating a detection window of <24 h. THC was always present concurrently with CBD and CBN, generally quantifiable for 48 h. In some subjects OF was THC positive for as long as 28 days in chronic daily smokers during sustained abstinence. In contrast, THCCOOH detection times were much longer, up to 29 days, with a median of 13 days. Data collected in the present study indicate that CBD and CBN concentrations decreased 10-fold within the 1st h after smoking in OF collected by expectoration, and were detectable for at least 22 and 6 h with LOQs of 0.25 and 1 μg/l, respectively. Participant J’s CBD and CBN concentrations in OF did not change, despite the obvious presence of blood in the 2 h collection. CBD and CBN were not quantifiable in participant J’s whole blood and plasma specimens collected at the same time. Maximum whole blood and plasma concentrations were 2.1 and 3.4 μg/l for CBD, and 2.9 and 4.7 μg/l for CBN, respectively [28]. Monitoring CBD and/or CBN at 1 μg/l in addition to THC reduced the detection window to no more than 6 h after smoking.
Quantifying multiple cannabinoids in OF and applying different cutoffs provides different detection windows and improves interpretation of cannabinoid OF results. We evaluated seventeen alternative cutoffs based on the needs of different drug testing programs. Monitoring THC with CBD and/or CBN provides valuable information about recent cannabis intake that is useful in DUID and accident investigations. Of the cutoffs tested, the current recommended SAMHSA (≥2 μg/l) and DRUID (≥1 μg/l) confirmation cutoffs yielded a greater number of positive specimens. Modifying the criteria to include THCCOOH ≥20 ng/l reduced positive specimens by 17 to 23%, but provided protection against false positive OF results due to passive inhalation [10].
These data increase our knowledge of cannabinoid pharmacokinetics in authentic expectorated OF specimens collected after controlled smoked cannabis administration. Low OF volume due to cannabinoid-induced dry mouth may hinder quantitative analysis close to the time of smoking. The present study demonstrates that quantification of THC together with CBD and CBN in OF can improve interpretation of results by suggesting recent cannabis smoking, helping distinguish this from residual cannabinoid excretion after chronic daily smoking. In addition, measurement of THCCOOH in OF reduces the potential for contamination by passive environmental cannabis smoke exposure. Finally, the presence of blood in OF can artificially increase cannabinoid concentrations in expectorated OF. Inclusion of additional cannabinoids during OF testing could improve test interpretation in drug treatment, workplace, driving under the influence of drugs (DUID), and pain-management programs.
Supplementary Material
THC quantification with CBD and CBN in oral fluid suggests recent cannabis smoking.
Quantification of multiple cannabinoids improves oral fluid interpretation results.
Blood in expectorated oral fluid artificially increase cannabinoid concentrations.
Low oral fluid volume due to dry mouth hinders quantitative analysis after smoking.
Acknowledgements
We acknowledge the contributions of the clinical staff of the National Institute on Drug Abuse, Intramural Research Program and the Behavioral Research Pharmacology Unit. Funding was provided by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health.
Abbreviations
- OF
oral fluid
- DUID
driving under the influence of drugs
- CBD
cannabidiol
- CBN
cannabinol
- 2D-GCMS
2-dimensional gas chromatography mass spectrometry
- BSTFA
N,O-bis(trimethylsilyl)trifluoroacetamide
- TFAA
trifluoroacetic anhydride
- HFIP
hexafluoroisopropanol
- DRUID
Driving under the Influence of Drugs, Alcohol and Medicines
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Moore C. Oral fluid for workplace drug testing: Laboratory implementation. Drug Test Anal. 2011 doi: 10.1002/dta.322. [DOI] [PubMed] [Google Scholar]
- [2].Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gjerde H, Normann PT, Christophersen A. The prevalence of alcohol and drugs in sampled oral fluid is related to sample volume. J Anal Tox. 2010;34:416–419. doi: 10.1093/jat/34.7.416. [DOI] [PubMed] [Google Scholar]
- [4].Kauert GF, Iwersen-Bergmann S, Toennes SW. Assay of delta-9-tetrahydrocannabinol (THC) in oral fluid-evaluation of the OraSure oral specimen collection device. J Anal Tox. 2006;30:274–277. doi: 10.1093/jat/30.4.274. [DOI] [PubMed] [Google Scholar]
- [5].Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- [6].Huestis MA, Cone EJ. Relationship of delta-9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Tox. 2004;28:394–399. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- [7].Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta-9-tetrahydrocannabinol in serum and oral fluid. J Anal Tox. 2007;31:288–293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- [8].Huestis MA, Cone EJ. Alternative testing matrices. In: Karch SB, editor. Drug Abuse Handbook. CRC Press; Boca Raton: 1998. pp. 799–857. [Google Scholar]
- [9].Niedbala RS, Kardos KW, Fritch DF, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Tox. 2001;25:289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- [10].Moore C, Coulter C, Uges D, et al. Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–230. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- [11].Davis KH, McDaniel IA, Cadwell l. W., Moody PL. Some smoking characteristics of marijuana cigarettes. In: Agurell S, Dewey WL, Willette RE, editors. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. Academic Press; Orlando: 1984. pp. 97–109. [Google Scholar]
- [12].Sachs H, Dressler U. Detection of THCCOOH in hair by MSD-NCI after HPLC clean-up. Forensic Sci Int. 2000;107:239–247. doi: 10.1016/s0379-0738(99)00167-x. [DOI] [PubMed] [Google Scholar]
- [13].Milman G, Barnes AJ, Schwope DM, et al. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56:1261–1269. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Milman G, Barnes A, Schwope D, et al. Cannabinoids and metabolites in expectorated oral fluid after 8 days of controlled around-the-clock oral THC administration. Anal Bioanal Chem. 2011;401:599–607. doi: 10.1007/s00216-011-5066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niedbala S, Kardos K, Salamone S, Fritch D, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. J Anal Tox. 2004;28:546–552. doi: 10.1093/jat/28.7.546. [DOI] [PubMed] [Google Scholar]
- [16].Verstraete AG. Oral fluid testing for driving under the influence of drugs: history, recent progress and remaining challenges. Forensic Sci Int. 2005;150:143–150. doi: 10.1016/j.forsciint.2004.11.023. [DOI] [PubMed] [Google Scholar]
- [17].Kintz P, Samyn N. Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Ther Drug Monit. 2002;24:239–246. doi: 10.1097/00007691-200204000-00006. [DOI] [PubMed] [Google Scholar]
- [18].Hold KM, De Boer D, Zuidema J, Maes RAA. Saliva as an analytical tool in toxicology. Int J Drug Test. 1996;1:1–31. [Google Scholar]
- [19].Lillsunde P. Analytical techniques for drug detection in oral fluid. Ther Drug Monit. 2008;30:181–187. doi: 10.1097/FTD.0b013e3181685088. [DOI] [PubMed] [Google Scholar]
- [20].Teixeira H, Proenca P, Verstraete A, Corte-Real F, Vieira DN. Analysis of delta-9-tetrahydrocannabinol in oral fluid samples using solid-phase extraction and high-performance liquid chromatography-electrospray ionization mass spectrometry. Forensic Sci Int. 2005;150:205–211. doi: 10.1016/j.forsciint.2004.11.026. [DOI] [PubMed] [Google Scholar]
- [21].Crouch DJ. Oral fluid collection: the neglected variable in oral fluid testing. Forensic Sci Int. 2005;150:165–173. doi: 10.1016/j.forsciint.2005.02.028. [DOI] [PubMed] [Google Scholar]
- [22].Perez-Reyes M, Owens SM, Di Guiseppi S. The clinical pharmacology and dynamics of marijuana cigarette smoking. J Clin Pharmacol. 1981;21:201S–207S. doi: 10.1002/j.1552-4604.1981.tb02596.x. [DOI] [PubMed] [Google Scholar]
- [23].Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: Effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261(1):114–122. [PubMed] [Google Scholar]
- [24].Moore C, Ross W, Coulter C, et al. Detection of the marijuana metabolite 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens and its contribution to positive results in screening assays. J Anal Tox. 2006;30:413–418. doi: 10.1093/jat/30.7.413. [DOI] [PubMed] [Google Scholar]
- [25].Maseda C, Hama K, Fukui Y, Matsubara K, Takahashi S, Akane A. Detection of delta-9-THC in saliva by capillary GC/ECD after marihuana smoking. Forensic Sci Int. 1986;32:259–266. doi: 10.1016/0379-0738(86)90202-1. [DOI] [PubMed] [Google Scholar]
- [26].Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- [27].Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chrom B. 2007;852:459–464. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- [28].Schwope D, Scheidweiler K, Huestis M. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2011:1–11. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.