Abstract
Objective
We examined whether the depiction of brown adipose tissue (BAT) with positron emission tomography/computed tomography (PET/CT) in pediatric patients is associated with anthropometric meaures.
Study design
We determined measures of body mass, adiposity, and musculature in 71 children and adolescents who underwent PET/CT examinations and compared patients with and without BAT. We used regression analyses to assess the relation between BAT and anthropometric measures.
Results
A total of 30 patients (42%) had BAT depicted on PET/CT, 10 of 26 girls (38%) and 20 of 45 boys (44%). Compared with patients without functional BAT, patients with BAT had significantly greater neck musculature (1880 ± 908 cm3 versus 1299 ± 806 cm3; P = .028 for boys and 1295 ± 586 cm3 versus 854 ± 392 cm3; P = .030 for girls) and gluteus musculature (1359 ± 373 cm3 versus 1061 ± 500 cm3; P = .032 for boys and 1138 ± 425 cm3 versus 827 ± 297 cm3; P = .038 for girls), but no differences in age, body mass index, or measures of subcutaneous fat. With logistic regression analyses, neck and pelvic musculature predicted the presence of BAT independently of age, sex, body size, and season of scan (P = .018 and .009, respectively).
Conclusion
Pediatric patients with visualized BAT on PET/CT examinations had significantly greater muscle volume than patients with no visualized BAT.
The adipose organ is a complex endocrine system, composed of white and brown adipose tissue (BAT). White adipose tissue (WAT) serves as the primary site of energy storage, storing triglycerides within individual adipocytes, and BAT stores little fat, burning it instead to produce heat and regulate body temperature.1–4 Rich in mitochondria, BAT generates heat through the uncoupling of oxidative respiration from the production of adenosine triphosphate, an action regulated by uncoupling protein-1, uniquely expressed in brown adipocytes.4–6 All newborns have considerable amounts of BAT, and recent data with fluoro-deoxyglucose-based positron emission tomography/computed tomography (PET/CT) found significant amounts of metabolically active BAT in as many as 8% of adults.1
Adipose and musculoskeletal tissues originate from the mesoderm, and the prevailing model has been that a common stem cell gives rise to WAT, BAT, bone, and muscle.7,8 In an intermediate developmental step, it was assumed that WAT and BAT share a common precursor because both forms of adipose tissue express a similar array of genes involved in triglyceride metabolism.9 However, although it is possible to convert WAT precursors into brown adipocyte-like cells,10 evidence also suggests that brown adipocytes are developmentally close to skeletal muscle.11–15 An in vivo study by Seale et al indicates that some brown fat cells arise from the same Myf5-expressing progenitor as skeletal muscle cells, whereas white fat cells do not.11 Consistent with this notion are data indicating that brown adipocytes and skeletal muscle cells share many features: an abundance of mitochondria, energy expenditure via oxidative phosphorylation, and sympathetically mediated adaptive thermogenesis.16,17 Moreover, brown adipocytes have been identified in skeletal muscle, and their abundance in different strains of mice correlates with differences in energy expenditure and protection against obesity.18
Studies in animals have shown that although reduced amounts or function of BAT lead to obesity, insulin resistance, and dyslipidemia,19,20 increased amounts or function of BAT protect against obesity and its co-morbidities.21–23 An inverse relation between body mass and body fat and the uptake of fluoro-deoxyglucose by BAT in PET examinations has also been suggested in humans.1,6,24,25 Obese and overweight adults and elderly patients exhibit less BAT uptake than lean and younger subjects. On the basis of these data, modulation of BAT activity has been proposed as a potential strategy to combat obesity and its associated co-morbidities.26–29
In this study, we examine whether the depiction of BAT in children, adolescents, and young adult male and female patients undergoing PET/CT examination is related to their body mass, adiposity, or skeletal musculature.
Methods
The institutional review board for clinical investigations at Children’s Hospital Los Angeles approved this retrospective study; informed consent was waived, and this study was compliant with the Health Insurance Portability and Accountability Act. The study population comprises 71 patients, aged 6 to 20 years old, 45 male and 26 female. Of the 71 patients, 56 had lymphoma (35 Hodgkin’s, 3 Burkitt’s, 8 B-cell, 3 anaplastic large cell, 7 lymphoma), 3 had neuroblastoma, 2 had Ewing’s sarcoma, 2 had acute lymphoblastic leukemia, and 1 had each of the following: adenosarcoma, adenocarcinoma, angiosarcoma, melanoma, nasopharyngeal carcinoma, rhabdomyosarcoma, and carcinomas of the thyroid and liver. Age, height, and weight measures were obtained at the time of each PET/CT examination. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters, and values were corrected via the Centers for Disease Control and Prevention’s calculator (http://apps.nccd.cdc.gov/dnpabmi/Calculator.aspx).
PET/CT scans were performed on a Gemini system (Philips Healthcare, GXL Release 3.3, Cleveland, Ohio) in patients after 12 hours fasting. Patients were injected with fluoro-deoxyglucose between 2.2 and 12.9 mCuries, depending on body weight. Oral contrast medium (meglumine diatrizoate, Gastrografin, Bristol-Myers Squibb, New York, New York) was administered in all patients. No muscle relaxants or additional agents were administered, nor were any patients subjected to cold and warm temperature preparations. All patients were indoors in room temperature (22°C) for >2 hours before examination. However, because of a lack of co-operation, children <6 years of age underwent general anesthesia and were excluded from this study. In patients who underwent repeated examinations, only initial PET/CT studies were used for analyses.
All PET/CT studies were reviewed independently by two radiologists to determine the presence or absence of metabolically active BAT in the neck, supraclavicular, paravertebral, and pararenal areas. A patient was considered to have BAT when both radiologists diagnosed its presence. Studies with discrepant determinations were re-evaluated by both radiologists together to arrive at a consensus.
Muscle and fat tissue volumes were measured semi-quantitatively on the basis of CT Hounsfield units (HU). In brief, the volume of neck adiposity was assessed by calculating all negative HU voxels30 (after manually excluding the airway), and the volume of cervical musculature was determined by calculating all positive HU voxels31 (after manually excluding the thyroid, bone, lymph nodes, and vessels) from the first cervical vertebrae to the upper portion of the sternum. The volume of gluteal musculature was determined by measuring all voxels with positive HU values (excluding bone) behind the ileac bones, from the ileac crest to the femoral heads. The volumes of subcutaneous fat in the buttocks were measured from the same scans by measuring all voxels with negative HU values adjacent to the gluteus muscles. All analyses were done with a workstation (Philips Extended Brilliance Workspace, Cleveland, Ohio) and Matlab (The Mathworks, Natick, Massachusetts).
Statistical analysis was carried out with Statview software (version 5.0.1; SAS Institute, Cary, North Carolina) by using the t test for unpaired samples and logistic linear regression analyses. Presence or absence of BAT was used as the outcome variable, and age, sex, BMI, season (defined as spring, summer, fall, or winter), and musculature were used as independent variables. In building the model, we included BMI to adjust for the confounding effects of body size, but excluded weight, height, and measures of subcutaneous adiposity to avoid multicollinearity in co-variates. All values are expressed as means plus or minus SD.
Results
A total of 30 of the 71 examinations (42%) depicted BAT. Visualized active BAT had standard uptake values ranging from 1.41 to 3.94 standard uptake values max. There were no significant sex differences in the prevalence of BAT (10 of 26 for female patients versus 20 of 45 for male patients; P = .63). Table I describes the age, anthropometric characteristics, and neck and buttock adiposity and musculature in BAT +/− studies for male and female patients. Male patients with BAT were taller than male patients without BAT (P = .034). In contrast, the heights of female patients with or without BAT were similar, and there were no differences in the age, weight, BMI, and measures of adiposity in the neck or buttocks of patients with and without BAT (all P values >.05), regardless of sex.
Table I.
Age, anthropometric measures, and measures of muscle and fat in brown adipose tissue-positive and −negative male and female patients
| Male patients | Female patients | |||||
|---|---|---|---|---|---|---|
| BAT+ (n = 20) |
BAT− (n = 25) |
P value | BAT+ (n = 10) |
BAT− (n = 16) |
P value | |
| Age, years | 15.0 ± 3.5 | 13.5 ± 3.9 | .187 | 14.9 ± 3.5 | 13.9 ± 4.2 | .532 |
| Weight, kg | 64.1 ± 19.6 | 52.0 ± 24.8 | .082 | 61.2 ± 26.8 | 49.0 ± 19.4 | .190 |
| Height, cm | 165 ± 20 | 152 ± 21 | .034 | 157 ± 13 | 149 ± 12 | .138 |
| BMI | 22.9 ± 4.0 | 21.6 ± 6.8 | .443 | 24.6 ± 10.5 | 21.5 ± 6.3 | .365 |
| Neck fat, cm3 | 544 ± 443 | 513 ± 707 | .867 | 835 ± 836 | 514 ± 500 | .230 |
| Buttock fat, cm3 | 799 ± 449 | 867 ± 636 | .686 | 1120 ± 883 | 823 ± 775 | .377 |
| Neck muscle, cm3 | 1880 ± 908 | 1299 ± 806 | .028 | 1295 ± 586 | 854 ± 392 | .030 |
| Gluteus muscle, cm3 | 1359 ± 373 | 1061 ± 500 | .032 | 1138 ± 425 | 827 ± 297 | .038 |
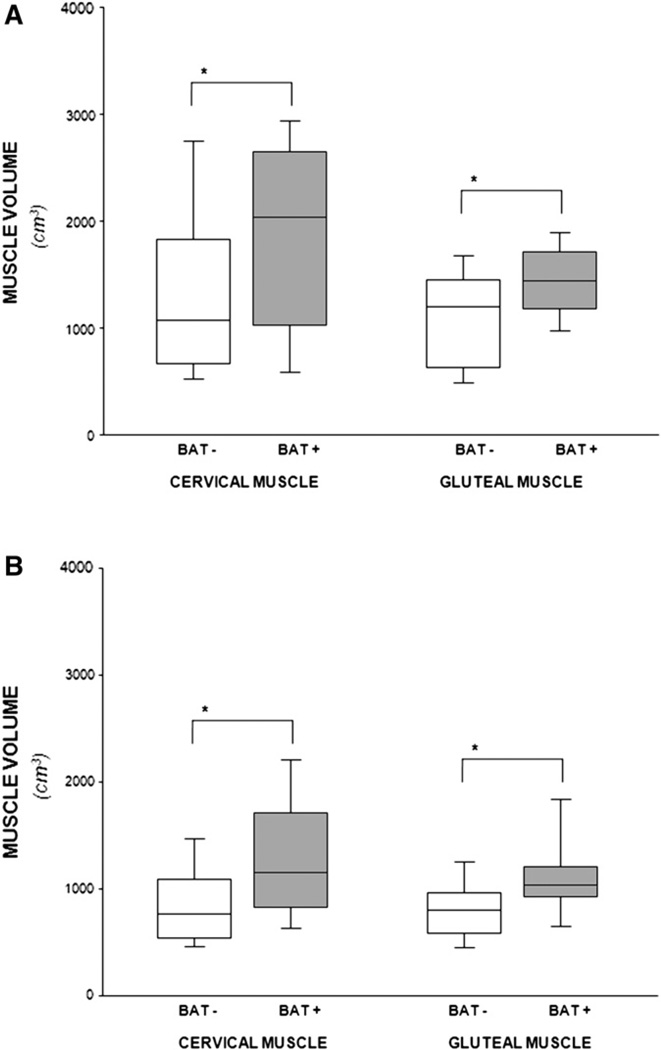
The Figure shows box plots for measures of cervical and gluteal muscle volumes for male and female patients. Regardless of sex or anatomical site, there were significant differences in muscle volume between patients with and patients without functional BAT. For all comparisons, the upper 50th percentile of values for muscle in patients with BAT was >75th percentile of the measures in patients without BAT.
Figure.
Box plot of cervical and gluteal muscle volume comparing patients with and without visualized BAT. A, Male patients; B, Female patients. *P < .04.
Table II shows the correlations among age, anthropometric measures, and values for adiposity and musculature in male and female patients. Regardless of sex, there were significant correlations seen between BAT +/− and neck and gluteus musculature (r values between 0.32 and 0.43; all P values between .028 and .037). In contrast, neither age, weight, height, BMI, or neck or buttock fat were associated with the presence or absence of BAT (Table II). Multiple logistic regression analyses indicated that both appendicular and axial musculature were independently associated with the presence of BAT, after accounting for age, sex, BMI, and season (Table III).
Table II.
Simple correlations of age, anthropometric measures, and measures of fat and muscle in male and female patients
| Age | Weight | Height | BMI | Neck fat | Buttock fat | Neck muscle | Gluteus muscle | BAT +/− | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | 0.74* | 0.86* | 0.38* | 0.28 | 0.11 | 0.82* | 0.77* | 0.20 | |
| Weight | 0.63* | 1.00 | 0.79* | 0.82* | 0.66* | 0.42* | 0.83* | 0.88* | 0.26 | |
| Height | 0.73* | 0.60* | 1.00 | 0.34* | 0.21 | 0.17 | 0.78* | 0.81* | 0.32 | |
| BMI | 0.44* | 0.93* | 0.27 | 1.00 | 0.85* | 0.54* | 0.56* | 0.61* | 0.12 | Male patients (n = 45) |
| Neck fat | 0.50* | 0.90* | 0.36 | 0.92* | 1.00 | 0.53* | 0.42 | 0.41* | 0.03 | |
| Buttock fat | 0.37 | 0.86* | 0.28 | 0.90* | 0.77* | 1.00 | 0.25 | 0.24 | −0.06 | |
| Neck muscle | 0.45* | 0.79* | 0.52* | 0.69* | 0.77* | 0.56* | 1.00 | 0.86* | 0.34* | |
| Gluteus muscle | 0.57* | 0.91* | 0.53* | 0.86* | 0.80* | 0.79* | 0.75* | 1.00 | 0.32* | |
| BAT +/– | 0.13 | 0.27 | 0.30 | 0.19 | 0.24 | 0.18 | 0.43* | 0.41* | 1.00 | |
| Female patients (n = 26) | ||||||||||
Male patients (n = 45) and female patients (n = 26).
All P values <.05.
Table III.
Multiple logistic regression models for the prediction of brown adipose tissue
| β | SE | P value | R2 | |
|---|---|---|---|---|
| BAT ± | ||||
| Age | −0.084 | 0.102 | .406 | |
| Sex | 0.487 | 0.604 | .420 | |
| BMI | −0.020 | 0.047 | .670 | 0.103 |
| Season | 0.123 | 0.238 | .607 | |
| Neck muscle | 0.001 | 0.001 | .018 | |
| BAT ± | ||||
| Age | −0.114 | 0.107 | .284 | |
| Sex | 0.639 | 0.635 | .314 | |
| BMI | −0.075 | 0.059 | .205 | 0.124 |
| Season | 0.123 | 0.243 | .612 | |
| Gluteus muscle | 0.003 | 0.026 | .010 |
Discussion
In this study, we found that young patients with functioning BAT on PET/CT examinations had significantly greater muscle volume than patients of the same age and BMI with no visibly identifiable BAT. These findings were present in the axial and appendicular skeletons of both male and female patients. On average, patients with pediatric cancer who had visualized BAT had 50% greater neck volume and 33% greater gluteal muscle volume than patients with no BAT. Additionally, we found significant relations between the presence of BAT and the volume of muscle in the axial and appendicular skeletons, which were independent of age, sex, body size, and season in which the study was done. Our results showing a link between BAT and muscle in patients undergoing PET/CT examinations represent the clinical correlate of basic studies indicating that brown fat and skeletal muscle share a common progenitor cell.11
In contrast, the results of this study show no significant differences in the age, weight, BMI, or measures of subcutaneous adiposity between patients with and patients without functioning BAT. Earlier studies on the relation among BAT and BMI and adiposity were generally done in adult populations and yielded conflicting results. Although some studies found inverse relations between BAT and BMI, between BAT and measures of adiposity, or both,1,6,24,25 other studies found no such relation.2,32–34 These discrepant results may be caused by differences in patient populations and in measurement instruments. Generally, the relations between BAT and adiposity were analyzed solely with anthropometric measurements and less frequently with bioelectric impedance2,34 or dual-energy absorptiometry24 in studies with small cohorts. The use of CT, an imaging modality that provides accurate and independent measurements of muscle and adipose tissue simultaneously in a relatively large number of patients, is a strength of this study.
Our study corroborates earlier investigations suggesting a higher prevalence of BAT in pediatric PET/CT studies35–37 when compared with that reported for adult patients.1 It also indicates that the presence of BAT is common in PET/CT examinations of children and adolescents, even when they live in warm climates like California. This was somewhat unexpected, because BAT activity is greater in colder climates, consistent with the need for non-shivering thermo-genesis.38 Moreover, earlier data suggest that nearly all PET/CT examinations in young men depict BAT when obtained at 16°C,24 half when measured at 19°C,2,34 but none when examinations were obtained during warmer conditions. However, we found that approximately 40% of PET/CT studies in young patients from our study cohort depicted BAT, even when obtained under thermoneutral conditions (22°C).
This study has several notable limitations. It is based on a retrospective, cross-sectional analysis of PET/CT examinations of children and adolescents with cancer. Further work is needed to investigate and better determine the strength of this association in the elderly and in healthy populations. Most important, information on the total amount of BAT is unavailable, and it is likely that depicted metabolically active BAT represents only a small, undetermined proportion of total BAT. Indeed, whether the association found between functional BAT in the upper body and gluteal musculature is a result of the strong correlation between nuchal and gluteal musculature, the surrogacy of functional BAT as a measure of brown fat at other anatomical sites, or a distant effect is unknown. However, finding an association between BAT and muscle in patients with significant disease and with a technique that does not allow for the complete characterization of BAT underscores the strength of the link between these two tissues. The recent development of sensitive and noninvasive magnetic resonance imaging techniques to characterize BAT could greatly aid in delineating the contributions of this tissue in regulating human physiology.39
Glossary
- BAT
Brown adipose tissue
- BMI
Body mass index
- HU
Hounsfield units
- PET/CT
Positron emission tomography/computed tomography
- WAT
White adipose tissue
Footnotes
The authors declare no conflicts of interest.
References
- 1.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 6.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 7.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol. 2009;218:444–449. doi: 10.1002/jcp.21621. [DOI] [PubMed] [Google Scholar]
- 15.Christian M, Parker MG. The engineering of brown fat. J Mol Cell Biol. 2010;2:23–25. doi: 10.1093/jmcb/mjp035. [DOI] [PubMed] [Google Scholar]
- 16.Farmer SR. Brown fat and skeletal muscle: unlikely cousins? Cell. 2008;134:726–727. doi: 10.1016/j.cell.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Clarke IJ, Henry BA. Targeting energy expenditure in muscle as a means of combating obesity. Clin Exp Pharmacol Physiol. 2010;37:121–124. doi: 10.1111/j.1440-1681.2009.05259.x. [DOI] [PubMed] [Google Scholar]
- 18.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 20.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- 21.Kim JK, Kim HJ, Park SY, Cederberg A, Westergren R, Nilsson D, et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes. 2005;54:1657–1663. doi: 10.2337/diabetes.54.6.1657. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 23.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 24.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 25.Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, et al. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. 2006;33:785–791. doi: 10.1007/s00259-006-0066-x. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc. 2009;68:401–407. doi: 10.1017/S0029665109990255. [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008;32 Suppl. 7:S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 32.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 33.Sturkenboom MG, Franssen EJ, Berkhof J, Hoekstra OS. Physiological uptake of [18F]fluorodeoxyglucose in the neck and upper chest region: are there predictive characteristics? Nucl Med Commun. 2004;25:1109–1111. doi: 10.1097/00006231-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.105. In publication. [DOI] [PubMed] [Google Scholar]
- 35.Bar-Sever Z, Keidar Z, Ben-Barak A, Bar-Shalom R, Postovsky S, Guralnik L, et al. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging. 2007;34:630–637. doi: 10.1007/s00259-006-0253-9. [DOI] [PubMed] [Google Scholar]
- 36.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, et al. Seasonal variation in the effect of constant ambient temperature of 24 degrees C in reducing FDG uptake by brown adipose tissue in children. Eur J Nucl Med Mol Imaging. 2010 doi: 10.1007/s00259-010-1485-2. In publication. [DOI] [PubMed] [Google Scholar]
- 37.Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 38.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 39.Hu HH, Smith DL, Jr, Nayak KS, Goran MI, Nagy TR. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging. 2010;31:1195–1202. doi: 10.1002/jmri.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]