Abstract
Objective
To examine the effect of hormone therapy and calcitriol on depression in elderly postmenopausal women and also to determine whether the response was associated with polymorphisms of estrogen receptor-alpha and vitamin D receptor.
Methods
In a double-blinded placebo controlled prospective trial involving 489 postmenopausal elderly women, a secondary analysis of depression was done. Geriatric Depression Scale was used to screen for depression. We used binary logistic regression to examine the effect of treatment on depression and one-way ANOVA to find relationship between gene polymorphisms and depression.
Results
There was no effect of hormone therapy (OR 1.65; 95% CI 0.66–4.12; p = 0.277), calcitriol (OR 1.15; 95% CI 0.43–3.11; p = 0.772) or hormone therapy with calcitriol (OR 1.01; 95% CI 0.36–2.80; p = 0.979) on depression. Neither polymorphisms of estrogen receptor-alpha (XbaI-beta=0.093, CI −0.337–1.350, p = 0.239 and PvuII-beta=−0.064, CI −1.171-0.491, p = 0.421) nor vitamin D receptor (BsmI-beta=0.044, CI −2.546–3.030, p = 0.865 and TaqI-beta=−0.015, CI −2.900-2.738, p = 0.955) were associated with depression.
Conclusion
In elderly post-menopausal women there was no effect of hormone therapy and calcitriol either individually or in combination with depression. Estrogen receptor-alpha and vitamin D receptor polymorphisms are not associated with depression or the response to intervention in elderly postmenopausal women. Additional trials are required to confirm these findings.
Keywords: hormone therapy, vitamin D, depression, estrogen receptor polymorphism, vitamin D receptor polymorphism and postmenopausal women
INTRODUCTION
Late-life Depression is common cause of disability in people over 65 years of age because of biological, psychological and social risk factors that come with age. The prevalence is approximately between 8–39% and it varied in different settings1,2. This has become a public health burden because it leads to decreased quality of life and increased hospitalization3 because it is often under diagnosed and undertreated4 in primary care setting as many of them may not present with typical symptoms of depression such as sadness5 or they may consider depressed mood as normal part of aging though it is not6.
Older women were known to have higher risk of developing depression when compared to men7. This could be partly due to loss of estrogen with menopause because estrogen is known to have neuroprotective effects on brain8 but when progestins are added to estrogens this effect may diminish9. Hormone therapy (HT) may improve the symptoms of depression in depressed people10 or decrease the risk of developing depressive symptoms in older women11, but this is unclear because in some studies HT does not stop the development of depressive symptoms in elderly postmenopausal women12,13,14. The exact role of estrogen still needs to be defined. The genomic actions of estrogen are exerted through two kinds of receptors-estrogen receptor-alpha (ER- α) and beta (ER- β). Two commonly known single nucleotide polymorphisms (SNP) of ER- α receptor are PvuII (rs2234693) and XbaI (rs9340799). Polymorphisms of the ER- α have been associated with psychiatric disorders like Alzheimer’s disease16 and one study has shown an association between ER- α genotype polymorphisms and depression in women15 but their exact role in depression has not been clearly defined.
Elderly are also at higher risk of developing vitamin D deficiency due to decreased dietary intake, less sun exposure, restricted outdoor activity and a decreased capacity to produce enough calcitriol as a result of an age related decline in hydroxylation by kidneys. Besides playing a role in calcium homeostasis, vitamin D also has the potential for a neuroactive effect on brain function17 through the vitamin D receptor (VDR). Low vitamin D levels may be associated with depression in elderly18,19 and taking supplements may improve the symptoms of depression20. Despite these findings the associations are not conclusive because some studies did not find any association21,22 and most of the studies that found an association between vitamin D and depression are cross sectional, observational or small sample studies with methodological problems. Although VDR genotype polymorphisms were shown to be associated with bone mineral density and risk of fractures23, their role in the pathology of depression has not been clearly established.
There are few prospective studies that have evaluated the relationship between either HT and depression or vitamin D and depression. However to our knowledge there were no studies that evaluated the effect of HT given with vitamin D along with their individual effects on depression in elderly postmenopausal women. Also few studies examined the relation between ER-α gene polymorphisms and depression24,25 and one study examined the association of VDR gene polymorphisms with depression26. In this 3- year double-blinded placebo controlled intervention study, the primary outcome was the effect of HT and calcitriol either individually or in combination on age-related bone loss in elderly postmenopausal women. However we also collected Geriatric Depression Scores (GDS) at baseline and at the end of 36 months (final visit) and the purpose of this paper is a secondary analysis of the longitudinal effect of hormone therapy and Vitamin D on depression along with the role of ER- α, VDR genotype polymorphisms and serum levels of estradiol, sex hormone binding globulin (SHBG) and testosterone.
METHODS
Participants in this study (STOP IT - Sites Testing Osteoporosis Prevention or Intervention Treatment) are 489 community dwelling elderly post-menopausal women aged between 65–77 years who were tested for the effect of HT and calcitriol either individually or in combination on age-related bone loss. Participants were randomly assigned to one of the four groups by computer generated randomization list-conjugated equine estrogens 0.625 mg/daily (ET) in hysterectomized women (or combined with medroxyprogesterone acetate 2.5 mg/daily (EPT) in women with intact uterus); Calcitriol 0.25 g twice a day; combination group (HT plus calcitriol) and matching placebos. All the women were given two pills (placebo and intervention pill) and placebo pills were made to appear identical to the treatment pills. Compliers in this study were defined as those who were 80 percent or more compliant in taking the study medication. It was estimated by pill counts. Compliance at 36 months was 92% in the group taking conjugated estrogens, 94% in the group taking medroxyprogesterone acetate, 93% in the group taking calcitriol and 92% in placebos.
Women who had chronic illness, primary hyperparathyroidism or active renal stone disease, and who were taking certain medications, such as bisphosphonates, anticonvulsants, estrogen, fluoride, or thiazide diuretics in the last 6 months were excluded from the study. The protocol was reviewed and approved by IRB and all participants were randomized only after signing an informed consent form. NIH established a Data Safety and Monitoring Board to monitor the study. Full details of the methodology can be obtained from the previously published analysis27.
GDS score measurement
Geriatric Depression Scale-Long Form 30(GDS-LF30) was used to collect data on depressive symptoms, consisting of 30 depression related self-rating questions with simple ‘yes’ or ‘no’ options and a score ranging from 0 to 30. This scale was exclusively developed for screening and rating severity of depression in elderly and has shown very good criterion validity. When a cutoff score of 11 is used, it has a sensitivity of 84% and specificity is 95%28. We used the same cutoff value to differentiate between non-depressed and depressed (0–10=Not depressed, 11–30=Depressed). GDS scores were collected at baseline and at the end of the study. At baseline we also collected information about other associated variables such as level of education, previous history of depression, history of hypothyroidism, living status, smoking habits and alcohol consumption.
Genotyping
Genotyping was performed on DNA extracted from the subject’s white blood cells using Puregene kit (Gentra systems) for ER- α SNP’s and standard phenol/chloroform extraction procedure for VDR SNP’s (BsmI- rs1544410 and TaqI- rs1544410). Genotype Coding for ER-alpha restriction sites was Pp for PvuII and Xx for XbaI. For VDR SNP’s, Bb denotes BsmI and Tt denote TaqI. Uppercase and lowercase letters denoted the presence and absence of a restriction sites respectively. Polymerase chain reaction (PCR) was used to extract DNA and the products were digested using appropriate restriction endonucleases. The fragments were processed using gel electrophoresis, stained with ethidium bromide for clear visualization under UV light and photographed.
Biochemical measurements
Serum 25-hydroxy vitamin D (25 (OH) D) was assayed with a competitive protein-binding assay. Serum total estradiol, SHBG and serum total testosterone were measured in fasting baseline serum samples using RIA kits (Diagnostic Systems Laboratories, Webster, TX). The intra-assay co-efficient of variation for the assays were as follows: serum 25OHD 5.1 percent, estradiol 4.1 percent, SHBG 8.2 percent and testosterone 7.4 percent.
Statistical Methods and analyses
All analyses were performed using SPSS Version 19 (IBM SPSS Inc). Baseline characteristics were compared among four treatment groups by means of one-way ANOVA for continuous variables and by chi-square test for categorical variables.
For the baseline cross sectional analysis we categorized 25OHD level into vitamin D insufficient or sufficient based on a cut-off of 20 ng/ml and used chi-square test to find correlation with depression (yes/no). To compare genotype polymorphisms among depressed and non-depressed people we used chi-square tests and, to compare genotype polymorphisms and response to treatment (defined as difference between final GDS and baseline GDS score) we used linear regression. We used one-way ANOVA to find association between baseline depression and serum total estradiol, SHBG and total testosterone.
For the longitudinal analyses we used binary logistic regression analysis to determine the effect of HT and calcitriol on depression. The final GDS score was categorized into depressed or non depressed and was used as the dependant variable and the treatment group (placebo as reference) as the categorical variable in binary logistic regression. We also did a subgroup analysis on depressed people at baseline using one-way ANOVA to find the effect of treatment. The confidence intervals (CI) estimated for odds ratios of depression were calculated with 95% probability. The significance level was set to 0.05 for all analyses in our study. We performed the modified intent-to-treat analyses on 412 participants who came in for both baseline and final visit. Also we performed the longitudinal analysis on compliers (n=337).
RESULTS
A total of 489 participants were enrolled in this study. One subject was excluded because of new onset Paget’s disease. All the participants completed the GDS questionnaire at baseline (n=488). At the end of the study 412 participants (85%) completed the GDS questionnaire and 76 women (15%) did not complete it (non-response) and 337 finished on active drug (compliers). Our study population was successfully randomized and the groups did not differ significantly from each other. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the study subjects (n=488)
| Variable | Value (All subjects n=488) | Placebo (n=1(n=123) | HTa+Calcitriolb (n=122) | HT (n=120) | Calcitriol (n=123) | P valuec |
|---|---|---|---|---|---|---|
| Age in years:mean (SDd) | 71.4(±3.5) | 71.1(±3.7) | 71.4(±3.6) | 71.4(±3.6) | 71.8(±3.4) | 0.536 |
| BMI (kg/m2):mean (SD) | 27.0(±4.8) | 27.22 (±5.0) | 26.7(±4.3) | 27.5 (±4.6) | 26.7(±5.4) | 0.541 |
| Education (n): | ||||||
| Minimum | 57 | 14 | 12 | 13 | 18 | 0.674 |
| More than minimum | 431 | 109 | 110 | 107 | 105 | |
| Living (n): | ||||||
| Living alone | 189 | 43 | 47 | 50 | 49 | 0.744 |
| Lining with others | 299 | 80 | 75 | 70 | 74 | |
| Hypothyroidism (n): | ||||||
| No | 399 | 100 | 98 | 99 | 102 | 0.952 |
| Yes | 89 | 23 | 24 | 21 | 21 | |
| Antidepressant use (n): | ||||||
| No | 469 | 117 | 119 | 115 | 118 | 0.796 |
| Yes | 19 | 06 | 03 | 05 | 05 | |
| Smoking (n): | ||||||
| No | 430 | 108 | 108 | 103 | 111 | 0.763 |
| Yes | 58 | 15 | 14 | 17 | 12 | |
| Alcohol (n): | ||||||
| No | 325 | 83 | 84 | 81 | 77 | 0.741 |
| Yes | 163 | 40 | 38 | 39 | 46 | |
| Baseline Depression (n) | 57 | 17 | 10 | 18 | 12 | 0.295 |
| Baseline GDSe Score: mean (SD) | 4.8 (±4.6) | 4.6(±4.5) | 4.3(±4.2) | 6.0(±5.0) | 4.5(±4.5) | 0.022 |
| Final GDS Score: mean (SD) | 4.2(±4.2) | 4.0(±4.0) | 4.0(±4.4) | 5.1(±4.4) | 3.9(±3.9) | 0.100 |
| Baseline serum 25-hydroxyvitamin -D, mean (SD), ng/ml | 31.3(±10.5) | 31.7(±11.0) | 32.0(±11.6) | 30.9(±9.9) | 30.6(±9.4) | 0.694 |
| Serum total estradiol (pg/ml): mean (SD) | 11.95(±5.07) | 12.23(±5.23) | 11.27(±4.55) | 11.54(±4.72) | 12.76(±5.63) | 0.155 |
| Serum SHBG (nmol/liter): mean (SD) | 147.47(±67.7) | 142.78(±66.3) | 159.53(±74.5) | 142.27(±65.5) | 145.74(±63.7) | 0.235 |
| Serum total testosterone, mean (SD), ng/ml | 0.23(±0.11) | 0.23(±0.11) | 0.23(±0.11) | 0.24(±0.10) | 0.24(±0.12) | 0.688 |
| XbaIf(n=337) | ||||||
| xx | 132 | 33 | 29 | 36 | 34 | 0.212 |
| Xx | 157 | 41 | 43 | 40 | 33 | |
| XX | 48 | 20 | 7 | 9 | 12 | |
| PvuIIg(n=338) | ||||||
| pp | 96 | 26 | 25 | 20 | 25 | 0.675 |
| Pp | 171 | 45 | 40 | 49 | 37 | |
| PP | 71 | 24 | 14 | 16 | 17 | |
| BsmIh(n=362) | ||||||
| bb | 141 | 44 | 33 | 32 | 32 | 0.898 |
| Bb | 169 | 46 | 40 | 42 | 41 | |
| BB | 52 | 12 | 11 | 16 | 13 | |
| TaqIi(n=362) | ||||||
| tt | 51 | 12 | 10 | 16 | 13 | 0.774 |
| Tt | 171 | 45 | 41 | 43 | 42 | |
| TT | 140 | 45 | 33 | 31 | 31 | |
Hormone Therapy with conjugated equine estrogens 0.625 mg/daily in hysterectomized women or combined with medroxyprogesterone acetate 2.5 mg/daily in women with intact uterus
Calcitriol 0.25g twice a day
One-way ANOVA was used for comparing continuous variables and Chi-square test for categorical variables
Standard deviation
Geriatric Depression Screening score
Estrogen receptor -alpha single nucleotide polymorphisms-numbers indicate frequencies
Vitamin D receptor single nucleotide polymorphisms- numbers indicate frequencies
The mean age was 71.4(SD±3.6). Nineteen (4%) women were taking antidepressants at baseline but we found 57(12%) women had depression based on baseline GDS scores. There were 89(18%) women who were hypothyroid and 189(39%) were living alone. There were 199(41%) hysterectomized women and 289(59%) non-hysterectomized women. There were 57(12%) participants who were minimally educated.
Mean GDS score at baseline was 4.8(SD±4.6) and at 36 months it was 4.2(SD±4.2). Mean serum 25(OH) D was 31.3 ng/ml (SD±10.5). Mean serum total estrogen was 11.95 pg/ml (SD±5.07), mean SHBG was 147.47 nmol/liter (SD±67.7) and mean total testosterone was 0.23 nmol/liter (SD±0.11). There were 57 women with depression at baseline: Placebo 17 women, HT with calcitriol 10 women, HT 18 women, and Calcitriol 12 women. At final visit the number of women with depression decreased in all the groups regardless of intervention given: Placebo 11 women, HT with calcitriol 8 women, HT 14 women, and Calcitriol 9 women.
The distribution of ER- α (XbaI, PvuII) and VDR (BsmI, TaqI) genotypes in our study population followed the Hardy–Weinberg Equilibrium.
Intent-to-treat Cross sectional Analysis at baseline
Serum 25OHD levels and Depression: At baseline chi-square analysis between serum 25(OH) D and depression showed no significant correlation (X2=0.944, p=0.331).
ER- α polymorphisms and depression: The chi square analysis showed no significant association between depression and ER- α polymorphisms XbaI and PvuII (X2 =0.894, p=0.640, and X2 =0.215, p=0.898 respectively)
VDR polymorphisms and depression: At baseline depression did not show any association with VDR polymorphisms BsmI and TaqI (X2 =0.771, p=0.680, and X2=0.901, p=0.637 respectively).
Total Estradiol, SHBG and Total Testosterone and depression: Serum total estradiol, SHBG and total testosterone level did not show any significant difference between depressed and non-depressed people at baseline (F=0.014, p=0.904; F=0.014, p=0.953 and F=1.561, p=0.212 respectively).
Intent-to-treat Longitudinal analysis
In the modified ITT analysis (n=412), we used binary logistic regression to find effect of intervention on depression and there was no effect of HT (OR 1.65; 95% CI 0.66–4.12; p = 0.277), calcitriol (OR 1.15; 95% CI 0.43–3.11; p = 0.772) or HT+ Calcitriol (OR 1.01; 95% CI 0.36–2.80; p = 0.979) on depression. (Table 2)
Table 2.
Factors affecting depression: Binary Logistic Regression Model
| Modified ITTa analysis (n=412) | |||||
|---|---|---|---|---|---|
| Variable | Standard Error | p (sig.) | OR | 95% Confidence Interval | |
| Lower | Upper | ||||
| Placebo (reference) | - | - | - | - | - |
| HTc+Calcitriol | .519 | .979 | 1.014 | .367 | 2.805 |
| HT | .465 | .277 | 1.657 | .666 | 4.120 |
| Calcitriol | .505 | .772 | 1.158 | .430 | 3.117 |
| Baseline GDSb score | .398 | .000 | 10.699 | 4.902 | 23.352 |
| Compliers analysis (n=337) | |||||
| Placebo (reference) | - | - | - | - | - |
| HTc+Calcitriol | .666 | .776 | 1.199 | .343 | 4.183 |
| HT | .588 | .140 | 2.224 | .769 | 6.428 |
| Calcitriol | .605 | .305 | 1.774 | .594 | 5.303 |
| Baseline GDS score | .513 | .000 | 12.368 | 5.061 | 30.225 |
Intent to treat
Geriatric Depression Screening score
Hormone therapy with conjugated equine estrogens 0.625 mg/daily in hysterectomized women or combined with medroxyprogesterone acetate 2.5 mg/daily in women with intact uterus
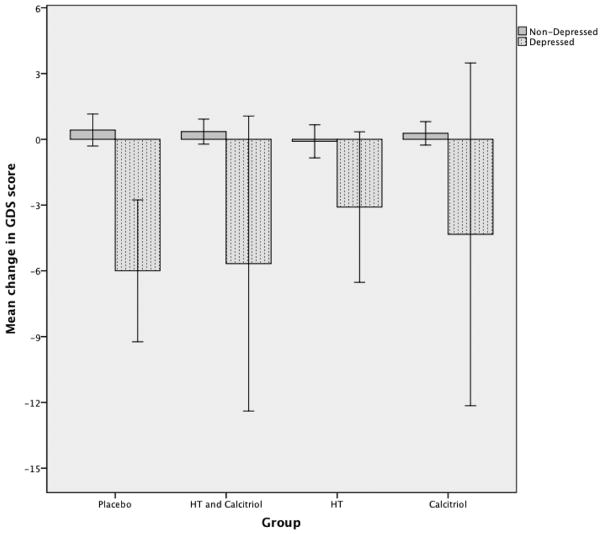
In sub-group analysis using one-way ANOVA, all the participants who were depressed at baseline shopwed a significant improvement in depression (final GDS-baseline GDS) when compared with non-depressed people (F=68.82; p=0.000). This improvement was seen in placebo and all treatment groups (figure 1). The response to treatment (defined as difference in final and baseline GDS scores) was not associated with ER- α and VDR polymorphisms. (beta=0.093, CI −0.337–1.350, p = 0.239 for XbaI; beta=−0.064, CI −1.171-0.491, p = 0.421 for PvuII; beta=0.044, CI −2.546–3.030, p = 0.865 for BsmI and beta=−0.015, CI −2.900-2.738, p = 0.955 for TaqI).
Fig. 1.
Comparison of mean change in GDS scores in depressed and non-depressed people.
Error bars represent 95% C.I.
HT-Hormone Therapy with conjugated equine estrogens 0.625 mg/daily in hysterectomized women or combined with medroxyprogesterone acetate 2.5 mg/daily in women with intact uterus. Calcitriol - 0.25g twice daily.
Y-axis represents mean of difference between final GDS and baseline GDS.
Complier Longitudinal Analysis
In compliers (n=337) the results remain unchanged and there was no effect of HT (OR 2.22; 95% CI 0.77–6.42; p = 0.140), calcitriol (OR 1.77; 95% CI 0.59–5.30; p = 0.305) or HT+ Calcitriol (OR 1.19; 95% CI 0.34–4.18; p = 0.776) (See Table 2).
DISCUSSION
The purpose of this secondary analysis was to see if HT and calcitriol either combined or individually had an effect depression in elderly postmenopausal women and to see if there was any relationship between depression and polymorphisms of ER- α and VDR. We believe that this is the first study to compare the combined and individual effects of HT/ET and calcitriol on depression longitudinally. The evaluation of association between ER- α, VDR genotype polymorphisms, estradiol, SHBG, testosterone and depression provides an insight for the future researchers.
It has been shown in several studies that estrogen has either a weak or no association with depression. Two large trials WHI (Women’s Health Initiative) and WISDOM (The Women’s international study of long-duration oestrogen after menopause) failed to demonstrate an improving effect of HT on depression. The Women’s Health Initiative trial was unable to demonstrate a benefit of estrogen on depressive symptoms and effect29. In a recent double-blinded placebo controlled trial involving 3721 women between 50–69 ages, it was shown that combined HT had no effect on depression30. Also in a study from three French cities (Three City Study), after evaluation of 4,069 postmenopausal elderly women by giving HT and following for four years authors could not find a protective effect of estrogen against the emergence of depressive symptoms31. Our results support these findings, since HT was showed no influence on depression. In contrast, a meta-analysis of the effect of HT on depressive mood by Zweifel et al suggested a moderate to large effect of HT on depressed mood32. However estrogen alone (ET) had effect but not when combined with medroxyprogesterone acetate (HT). In our analysis, women in estrogen only group were not influenced by treatment.
Depression is thought to be due to an alteration in serotonergic system although other neurotransmitters are also involved. Estrogen is known to have a modulating effect on all of these neurotransmitter pathways especially the serotonergic pathway33. In one study conjugated equine estrogen therapy improved mood in depressed postmenopausal women34. There is some data suggesting that progestins can antagonize the neuroprotective effects of estrogen9. However we saw no significant difference in the response to estrogen alone or combined with progestin.
The effect of vitamin D on depression in elderly population has not been studied extensively. Although few prospective studies demonstrated a relationship between vitamin D and depression but there exists a controversy. In a cohort study of 1155 participants older than 65yrs of age, low levels of serum 25OHD (<50 nmol/liter) were associated with depressive symptoms and the association was higher in women than men35. However in our study the baseline analysis did not show a relationship between baseline serum 25 (OH)D insufficiency (<20 ng/ml) and depression. At the end of our study there was no effect of vitamin D supplementation on depression.
Recently in a large randomized placebo controlled trial conducted by Sanders et al in Australia involving 2317 elderly women, annual high dose of vitamin D3 (500 000 IU) with three-year follow-up failed to prevent depressive symptoms22. In a cross sectional study by Jorde et al involving 441 obese men and women, it was shown that participants with serum 25(OH) D levels more than 40 nmol/liter were associated with low depression scores on the BDI scale but this study was unadjusted for the common confounders20. In another study on a small group of 21 elderly participants with secondary hyperparathyroidism not due to renal failure, they found that low serum 25-hydroxyvitamin D (50 nmol/liter) was associated with a lower depression score36. Recently evidence from WHI analysis of relationship between vitamin D intake from foods and supplements and depressive symptoms supports our finding that supplementation of vitamin D was not associated with depressive symptoms37. We could not find any significant relationship between serum 25 hydroxy vitamin D levels and depression.
Although several other studies demonstrated an association between low levels of serum 25OHD and major depressive disorder38 and also between vitamin deficiency and low mood19, long-term treatment in this study did not show an effect of calcitriol in postmenopausal women. No previous study has evaluated the combined effect of HT with calcitriol but no significant improvement in depression was observed in this study.
Genotypes can affect the response to treatment as found in the studies with regard to changes in lipids and bone density39,23. In one study by Malacara et al in 177 postmenopausal women, authors did not find a significant association between ER- α genotypes (PvuII and XbaI) and depression24. Another study reported increased risk of anxiety with ER- α polymorphisms but not depressive symptoms25. However in a case control study by Kim et al, they found a strong association between ER polymorphism and depression in postmenopausal women40. Similarly in the Three City Study, Ryan et al found two polymorphisms of ER- α and one polymorphism of ER- β were associated with depression in women15. In our study we did not find an association between either ER- α or VDR genotype polymorphisms and depression.
Vitamin D responsive elements are present in the promoter regions of serotonin receptors and tryptophan hydroxylase receptors, both of which are known to be associated with depression41. In one study it was shown that VDR polymorphisms influence the susceptibility to depression in old age26, but vitamin D levels were not measured which might have influenced the association. Our study showed no association between VDR polymorphisms and depression or the response to treatment. We recommend larger studies to confirm these genotype findings.
The strengths of this study are large postmenopausal population with 3 year follow-up, excellent validity of GDS LF 30 scale which is a powerful screening tool covering wide range of depressive symptoms, baseline adjustment to the known potential confounders. The unique strength of this study was combination effect of HRT and Calcitriol on depression. However all the data collected on depression were self-reported instead of clinical diagnosis. Recall bias may had an effect because of the age of the population. We had no data on socio-economic status, which is one of the known confounders. The finding that all the women showed improvement irrespective of intervention given might be due to the regression to mean (floor effect) as they have more chance for improvement compared to non-depressed people. In retrospect our study did not have sufficient numbers of depressed people with blood levels representative of moderate to severe vitamin D deficiency. Moreover larger studies will be required to confirm genotype association with depression because of low number of depressed people in our study.
CONCLUSION
In summary, neither HT nor calcitriol either individually or in combination showed a statistically significant effect on depression in elderly post-menopausal women. There was no evidence that ER- α and VDR polymorphisms were associated with depression or the response to treatment in elderly postmenopausal women.
Acknowledgments
The authors thank Dr. Paul D. Turner, PhD and Ryan Walters, MS (Division of Clinical Research & Evaluative Sciences, Creighton University Medical Center) for their advice on statistical analysis.
Financial Support – This study was supported by NIH funds (Grants UO1-AG10373 and AG10358).
Footnotes
Disclosures: VY has nothing to disclose. JCG has received consulting fees and honoraria from Wyeth-Pfizer and Roche. He has also served on the Advisory Council and as a speaker for Pfizer and Roche.
References
- 1.Gottfries CG. Late life depression. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II57–61. doi: 10.1007/BF03035129. [DOI] [PubMed] [Google Scholar]
- 2.Ina K, Hayashi T, Nomura H, Ishitsuka A, Hirai H, Iguchi A. Depression, quality of life (QoL) and will to live of community-dwelling postmenopausal women in three Asian countries: Korea, China and Japan. Arch Gerontol Geriatr. 2011 Jul-Aug;53(1):8–12. doi: 10.1016/j.archger.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Borowiak E, Kostka T. Predictors of quality of life in older people living at home and in institutions. Aging Clin Exp Res. 2004;16(3):212–220. doi: 10.1007/BF03327386. [DOI] [PubMed] [Google Scholar]
- 4.Klap R, Unroe KT, Unutzer J. Caring for mental illness in the United States: a focus on older adults. Am J Geriatr Psychiatry. 2003;11(5):517–524. [PubMed] [Google Scholar]
- 5.Gallo JJ, Rabins PV. Depression without sadness: alternative presentations of depression in late life. Am Fam Physician. 1999;60(3):820–826. [PubMed] [Google Scholar]
- 6.Sarkisian CA, Lee-Henderson MH, Mangione CM. Do depressed older adults who attribute depression to “old age” believe it is important to seek care? J Gen Intern Med. 2003;18(12):1001–1005. doi: 10.1111/j.1525-1497.2003.30215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry LC, Allore HG, Guo Z, Bruce ML, Gill TM. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psychiatry. 2008;65(2):172–178. doi: 10.1001/archgenpsychiatry.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz Brinton R, Chen S, Montoya M, et al. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol Aging. 2000;21(3):475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 9.Jayaraman A, Pike CJ. Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol. 2009;21(1):77–81. doi: 10.1111/j.1365-2826.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph I, Palombo-Kinne E, Kirsch B, Mellinger U, Breitbarth H, Graser T. Influence of a continuous combined HRT (2 mg estradiol valerate and 2 mg dienogest) on postmenopausal depression. Climacteric. 2004 Sep;7(3):301–11. doi: 10.1080/13697130400001802. [DOI] [PubMed] [Google Scholar]
- 11.Whooley MA, Grady D, Cauley JA. Postmenopausal estrogen therapy and depressive symptoms in older women. J Gen Intern Med. 2000;15(8):535–541. doi: 10.1046/j.1525-1497.2000.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein KM, Harpole LH, Stechuchak KM, Coffman CJ, Bosworth HB, Steffens DC, Bastian LA. Hormone therapy does not affect depression severity in older women. Am J Geriatr Psychiatry. 2005 Jul;13(7):616–23. doi: 10.1176/appi.ajgp.13.7.616. [DOI] [PubMed] [Google Scholar]
- 13.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27(1):141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Morrison MF, Kallan MJ, Ten Have T, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry. 2004;55(4):406–412. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Ryan J, Scali J, Carriere I, et al. Oestrogen receptor polymorphisms and late-life depression. Br J Psychiatry. 2011;199:126–131. doi: 10.1192/bjp.bp.111.091751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer’s disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 17.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends in Endocrinology & Metabolism. 2002;13(3):100– 105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 18.Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010;95(7):3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 20.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599– 609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 21.Pan A, Lu L, Franco OH, Yu Z, Li H, Lin X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1–3):240–243. doi: 10.1016/j.jad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial [PAPERS] The British Journal of Psychiatry. 2011;198(5):357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 23.Rapuri PB, Gallagher JC, Knezetic JA, Kinyamu HK, Ryschon KL. Association between Vitamin D receptor polymorphisms and the rate of bone loss in elderly women—importance of adjusting for dietary and lifestyle factors. J Steroid Biochem Mol Biol. 2004 May;89–90(1–5):503–6. doi: 10.1016/j.jsbmb.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 24.Malacara JM, Perez-Luque EL, Martinez-Garza S, Sanchez-Marin FJ. The relationship of estrogen receptor-alpha polymorphism with symptoms and other characteristics in post-menopausal women. Maturitas. 2004;49(2):163–169. doi: 10.1016/j.maturitas.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Tiemeier H, Schuit SC, den Heijer T, van Meurs JB, van Tuijl HR, Hofman A, Breteler MM, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene polymorphisms and anxiety disorder in an elderly population. Mol Psychiatry. 2005 Sep;10(9):806–7. doi: 10.1038/sj.mp.4001697. [DOI] [PubMed] [Google Scholar]
- 26.Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RG, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466–473. doi: 10.1016/j.neurobiolaging.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86(8):3618–3628. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 28.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 29.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 30.Welton AJ, Vickers MR, Kim J, et al. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ. 2008;337:a1190. doi: 10.1136/bmj.a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scali J, Ryan J, Carriere I, et al. A prospective study of hormone therapy and depression in community-dwelling elderly women: the Three City Study. J Clin Psychiatry. 2010;71(12):1673–1679. doi: 10.4088/JCP.09m05188blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22(3):189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]
- 33.Archer JS. NAMS/Solvay Resident Essay Award. Relationship between estrogen, serotonin, and depression. Menopause. 1999;6(1):71–78. [PubMed] [Google Scholar]
- 34.Carranza-Lira S. Estrogen therapy for depression in postmenopausal women. International Journal of Gynecology & Obstetrics. 1999;65(1):35–38. doi: 10.1016/s0020-7292(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 35.Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-Hydroxyvitamin D and Depressive Symptoms in Older Women and Men. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. J Neurol. 2006;253(4):464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 37.Bertone-Johnson ER, Powers SI, Spangler L, et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am J Clin Nutr. 2011;94(4):1104–1112. doi: 10.3945/ajcn.111.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider B, Weber B, Frensch A, Stein J, Fritz J. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm. 2000;107(7):839–842. doi: 10.1007/s007020070063. [DOI] [PubMed] [Google Scholar]
- 39.Sai AJ, Gallagher JC, Fang X. Effect of hormone therapy and calcitriol on serum lipid profile in postmenopausal older women: association with estrogen receptor-α genotypes. Menopause. 2011 Jun 24; doi: 10.1097/gme.0b013e318217d41d. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JJ, Pae CU, Kim MR, et al. Association between Estrogen Receptor Gene Polymorphisms and Depression in Post-Menopausal Women: A Preliminary Study. Psychiatry Investig. 2010;7(3):224–227. doi: 10.4306/pi.2010.7.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes de Abreu DA, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009 Dec;34(Suppl 1):S265–77. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]