Abstract
Our knowledge of how the body absorbs triacylglycerols (TAG) from the diet and how this process is regulated has increased at a rapid rate in recent years. Dietary TAG are hydrolyzed in the intestinal lumen to free fatty acids (FFA) and monoacylglycerols (MAG), which are taken up by enterocytes from their apical side, transported to the endoplasmic reticulum (ER) and resynthesized into TAG. TAG are assembled into chylomicrons (CM) in the ER, transported to the Golgi via pre-chylomicron transport vesicles and secreted towards the basolateral side. In this review, we mainly focus on the roles of key proteins involved in uptake and intracellular transport of fatty acids, their conversion to TAG and packaging into CM. We will also discuss intracellular transport and secretion of CM. Moreover, we will bring to light few factors that regulate gut triglyceride production. Furthermore, we briefly summarize pathways involved in cholesterol absorption.
Keywords: Intestine, Lipid Metabolism, fat absorption, fatty acids, triacylglycerols, acylglycerols, incretins, MTP, apoB, chylomicrons
2. Introduction
Triacylglycerols (TAG) are the major storage and transport forms of energy that provide 9 kcals/g. Under normal conditions, humans consume about 90–120 g of fat per day and more than 95% of it is absorbed. Absorption of dietary TAG is defined as transport from the intestinal lumen to the blood circulation and is essential for maintaining growth and development. Over several decades, enzymes and proteins involved in intestinal TAG hydrolysis, synthesis, transport and metabolism have been identified and focus has now turned to their regulation.
Enterocytes in the duodenum and proximal jejunum are the primary cells involved in lipid absorption. TAG absorption involves several steps; lumenal hydrolysis of dietary lipids, uptake of hydrolyzed products by the apical side of enterocytes; intracellular transport to endoplasmic reticulum (ER), synthesis of TAG, packaging into chylomicrons (CM) and their intracellular transport for secretion from the basolateral side. The lumenal hydrolysis of TAG in the intestinal lumen involves emulsification, hydrolysis and solubilization in bile salt micelles (Fig 1). The uptake process is facilitated by different transport proteins, such as FABPpm, FATP4, and FAT/CD36. Fatty acids are then transported to the ER by cytoplasmic fatty acid binding proteins (FABPs). In the ER, TAG are synthesized via two different pathways (monoacylglycerol acyltransferase (MAG) and glycerol phosphate pathway (Fig 1). Newly synthesized TAG are packaged into CMs. The assembly of CMs is critically dependent on two proteins; apolipoprotein B and microsomal triglyceride transfer protein (MTP). The newly assembled particles are transported from the ER in prechylomicron transport vesicles (PCTVs) and delivered to the Golgi via fusion. Ultimately, these particles are transported from the trans Golgi to the plasma membrane for secretion. In what follows, we shall describe a number of key proteins involved in different steps of intestinal TAG transport and their regulation (Table).
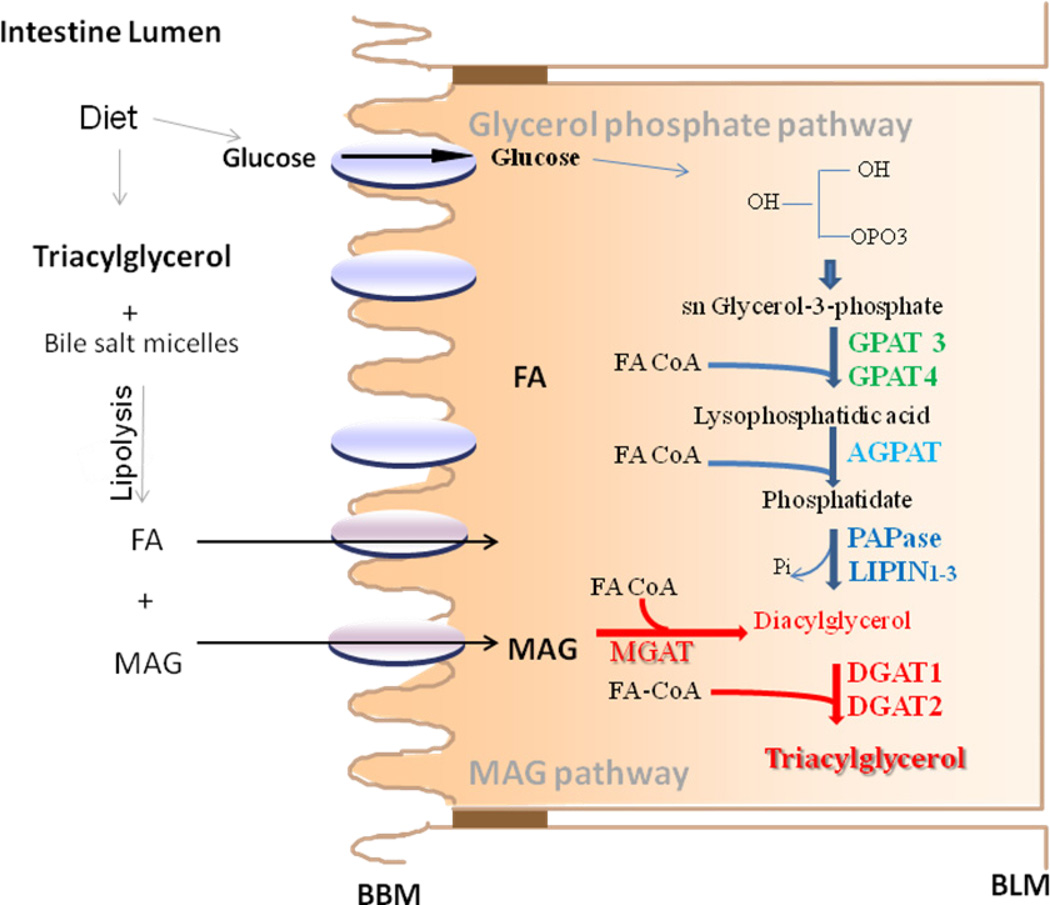
Fig 1. Biosynthesis of triacylglycerols in enterocytes.
Dietary TAG are solubilized in bile salt micelles and lipolysed in the intestinal lumen to free fatty acid and monoacylglycerol (MAG) that are taken up by enterocytes involving diffusion and protein meditated uptake, transported to the ER by carrier proteins and used for the synthesis of TAG by MAG pathway (red line, red). In this pathway, MAG and free fatty acids are first converted to DAG by monoacylglycerol:acylCoA acyltransferase (MGAT) enzymes. DAG is then converted to TAG by diacylglyerol:acylcoA acyltarnsfrease 1 and 2 (DGAT1 and DGAT2). TAG are also synthesized from glucose by the glycerol-3-phosphate pathway. In this pathway, GPAT3/GPAT4 catalyze the esterification of glycerol-3-phosphate to form lysophosphatidic acid. 1-acyl-sn-glycerol-3-phosphate acyltransferases (AGPAT1-5; also called LPAAT) esterify lysophosphatidic acid to form phosphatidate. Three isoforms of phosphatidic acid phosphohydrolase (PAPase, also known as lipins 1–3), hydrolyze the phosphate to form diacylglycerol (DAG). DAG is then converted to TAG by DGATs as in the MAG pathway.
Table.
Proteins that play a role in TAG absorption
| Protein type |
Major Physiological roles |
Tissue distribution |
Phenotype of KO mice |
|---|---|---|---|
| L-FABP |
|
small intestine, liver, kidney, pancreas |
|
| I-FABP |
|
small intestine, liver |
|
| FATP4 |
|
intestine brown/white adipose, skeletal muscle, heart, brain, liver, kidney, lung |
|
| CD36/FAT |
|
small intestine, kidney, lung etc. |
|
| MGAT2 |
|
small intestine |
|
| DGAT1 |
|
small intestine, skin, mammary gland, adipose tissue |
|
| DGAT2 |
|
small intestine, liver |
|
| MTP |
|
small intestine, liver |
|
| ApoB48, ApoB100 |
|
small intestine, liver |
|
| Sar1 |
|
small intestine |
|
| VAMP7 |
|
small intestine |
|
3. Hydrolysis of dietary triacylglycerols in the lumen of the intestine
Before absorption, dietary TAG are hydrolyzed in the intestinal lumen in a two step process. First, large aggregates of dietary TAG, which are virtually insoluble in an aqueous environment, are broken down physically in the mouth and stomach and solubilized in bile acid micelles in the duodenum and jejunum (Fig 1). Second, TAG molecules are digested by various lipases/colipases to yield monoacylglycerols (MAG) and fatty acids [32,82]. TAG hydrolysis starts in the mouth with lingual lipase and this has been shown to be important for sensing dietary fat in rodents [52]. However, lingual lipase dependent hydrolysis of TAG may not be important for food sensing in humans because their diet contains significant amounts of free fatty acids. A significant hydrolysis of dietary TAG occurs in the stomach by the action of lingual and gastric lipases [64] and is completed in the duodenum/jejunum by several pancreatic lipases that include pancreatic triglyceride lipase, pancreatic triglyceride lipase related protein 2 and carboxyl ester lipase [42,56]. More information about these enzymes and lumenal hydrolysis can be found in the reviews cited and references therein.
4. Uptake of MAG and fatty acids by the enterocytes
When concentrations are high, both these products can diffuse across the enterocyte membranes. However, these molecules can also be transported with the help of proteins into enterocytes against their concentration gradient. Although no specific protein(s) for MAG transport have yet been described, several proteins that facilitate long chain fatty acid uptake have been identified (Fig 2). Fatty acid uptake essentially involves three steps; interaction of extracellular fatty acids with the outer monolayer of the plasma membrane, flip-flop within the membrane bilayer, and intracellular delivery from the inner monolayer of the plasma membrane. Studies suggest that flip-flop may be the rate limiting step and membrane proteins can facilitate this process. Several proteins that interact and facilitate fatty acid uptake have been identified. They can be broadly classified into three categories: peripheral plasma membrane fatty acid-binding protein (FABPpm), fatty acid transport proteins (FATPs), and fatty acid translocase/cluster determinant 36 (FAT/CD36) [29,90,97]. Apart from these three types of fatty acid transporters, fatty acid uptake is also facilitated by other plasma membrane and intracellular proteins. A membrane protein that has been suggested to facilitate fatty acid uptake is caveolin. This protein is an important structural component of membrane domains called caveolae. Recruitment of CD36 into caveolae might enhance uptake of fatty acids through CD36. Another protein that is known to facilitate fatty acid uptake is acyl-CoA synthase 1; this membrane enzyme acylates fatty acids with Co-A on the cytosolic side of the plasma membrane essentially trapping fatty acids into the cytosolic compartment for different metabolic pathways. Intracellular proteins that facilitate fatty acid uptake are cytosolic fatty acid binding proteins. These proteins bind fatty acids delivered to the cytosolic side and transport them to other intracellular compartments for different metabolic usages and therefore assist in fatty acid uptake.
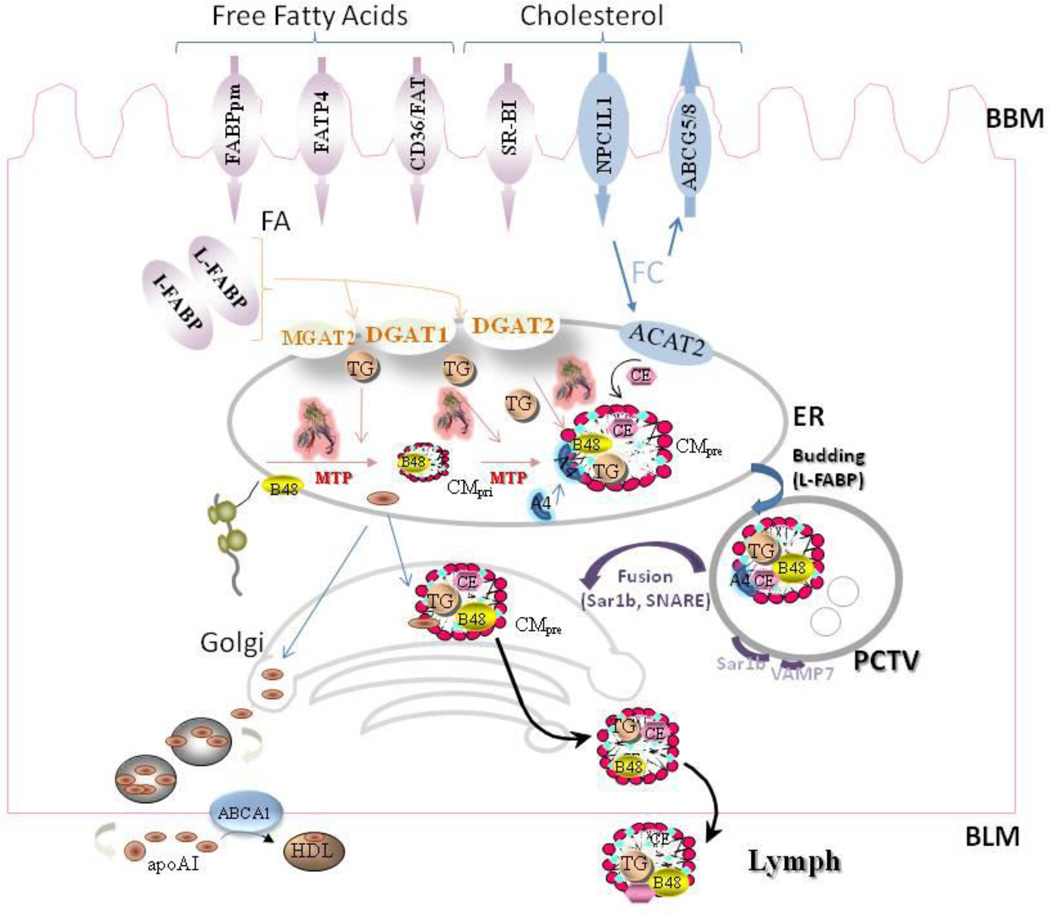
Fig 2. Cellular mechanisms involved in intestinal TAG absorption.
Apart from diffusion, the transport of free fatty acids (FA) across the brush border membrane (BBM) is facilitated by different proteins, such as plasma membrane fatty acid binding protein (FABPpm), fatty acid transport protein 4 (FATP4), and fatty acid translocase (FAT/CD36). It is then translocated to the endoplasmic reticulum (ER) by cytosolic fatty acid binding proteins (L-FABP and I-FABP). MGAT and DGAT enzymes then convert MAG and FA to TAG with an intermediate synthesis of DAG.
Cholesterol uptake is facilitated by Niemann-Pick C-1-like 1 (NPC1L1). There is evidence for the involvement of other proteins in this pathway such as SR-B1. In the intracellular compartment, free cholesterol has two fates. It can be exported back to the intestinal lumen by ATP-binding cassette transporters G5 and G8 (ABCG5/8) or converted to cholesterol esters by acyl CoA cholesterol acyltransferases 1& 2 (ACAT1 & ACAT2).
Newly translated apolipoprotein B (apoB-48) is lipidated by microsomal triglyceride transport protein (MTP) to form primordial chylomicrons (CM) that is further expanded in size by the addition of TAG in the core by MTP to form a pre-CM. These particles also acquire apoAIV at this time. Prechylomicrons are concentrated and exported from the ER in PCTVs. L-FABP facilitates the budding of PCTV from the ER. These vesicles then fuse with the cis-Golgi in a process that requires VAMP7. In the Golgi, apoA1 is added to prechylomicrons. Later, these particles are transported to plasma membrane using different transport vesicles and are ultimately released towards the basolateral side by enterocytes.
TAG are exclusively transported via the CM pathway. By contrast, cholesterol is transported across the intestinal epithelial cells by two pathways. One pathway is the same as the CM pathway. In this pathway free cholesterol and esterified cholesterol are added onto CM by MTP. In the second apoB-independent pathway, cholesterol is secreted involving ABCA1 and apoAI.
ApoAI is synthesized in the ER and transported independent of CMs to the Golgi where some of it associates with CMs and the rest is secreted independent of CMs. The free apoAI might play a role in cholesterol transport.
4.1. Plasma membrane fatty acid transport protein (FABPpm)
This protein of ~40-kDa was isolated from rat jejunal microvillous membrane using oleate-affinity chromatography [95] and was found to be identical to mitochondrial aspartate aminotransferase [9]. FABPpm protein peripherally attaches to the outer monolayer of the plasma membranes and antibodies against the protein have been shown to inhibit (25–75%) fatty acid binding to microvillous membranes. Because of its dual location it has been suggested that this protein might couple fatty acid uptake to cellular redox potential [97]. More needs to be learned about its role in fatty acid metabolism.
4.2. CD36/FAT
CD36 is a cell-surface glycosylated membrane protein of the type B scavenger receptor family. The rodent homolog of CD36 is called fatty acid translocase (FAT). It is ~53-kDa protein that migrates as an 88 kDa protein due to several posttranslational modifications and is expressed in a variety of cell types [1]. This protein is anchored in membranes by two N- and C-termini and contains a large extracellular domain. CD36 is mainly present in caveolae; microdomains of the plasma membrane enriched in sphingomyelin, cholesterol and caveolin. The recruitment of CD36 to the lipid rafts requires caveolin-1 as its deficiency mislocates CD36 to other regions and reduces fatty acid uptake.
In the apical brush border membrane of the duodenum and jejunum of rats, mice and humans, CD36 is mainly expressed in the intestinal epithelial enterocytes [1,19,77]. Its expression is increased in the small intestine by high-fat diets and long-chain fatty acids [6,34,77,78]. CD36 is thought to play an import role in the absorption of fatty acid by the proximal small intestine by acting as a high-affinity membrane receptor/transporter for long-chain fatty acids. Its expression decreases from proximal to distal axes of the small intestine. CD36 deficiency lowers secretion of apoB48 and CMs [68]. Although earlier studies indicated that intestinal lipid absorption is unaffected in CD36 deficient mice [31], Nassir et al reported that CD36 is important for fatty acid and cholesterol uptake in the proximal small intestine but not in the distal intestine [67]; therefore, CD36 deficiency delays, but does not disturb, fat absorption. Drover et al showed that CD36 is also important for the clearance of intestinal lipoproteins [24,31]. In fact, deficiency of CD36 in mice and humans leads to delayed clearance of triglyceride-rich lipoproteins. This has been attributed to accumulation of free fatty acids due to their reduced uptake by tissues in the absence of CD36 and consequent inhibition of lipoprotein lipase activity by these free fatty acids.
Apart from its role in fatty acid uptake, CD36 is known to act as a scavenger receptor for native and modified lipoproteins [33]. It has also been shown to be involved in several other physiological as well as pathological processes such as atherosclerosis, angiogenesis, phagocytosis. Recently, CD36 was found in mouse circumvallate papillae and shown to sense and impart preference for fat containing diets in rodents [52]. Further, it has been reported to be a non-redundant sensor for microbial diacylglycerides [37]. Hence, CD36 is truly a multifunctional protein.
4.3. Fatty acid transport proteins (FATPs)
FATP1 was identified by expression cloning that enhanced the uptake of fluorescently labeled fatty acids into cells [84]. Subsequently, five homologs, FATP2-6, have been identified. These integral proteins span membranes several times and have an ATP binding site that is important in fatty acid transport [96].
FATP4 (Fatty acid transport protein 4) is expressed in a variety of organs [36,84], but is abundantly expressed in the small intestine. FATP4 is found on the apical microvillus border of mature enterocytes [91]. Stahl et al have shown that knockdown of FATP4 in isolated enterocytes decreases uptake of radiolabeled long-chain fatty acids [91]. By contrast, Shim et al. did not observe any differences in intestinal TAG absorption or fecal losses in the absence of intestinal FATP4 deficiency compared with WT mice [87]. Similarly, FATP4 inhibitors failed to have a significant effect on fat absorption under either basal or high fat bolus stimulation [12].
The above discussion of several proteins involved in fatty acid uptake highlights the redundancy of the system. Moreover, as discussed before fatty acid uptake can occur via diffusion. Furthermore, small intestine has a large capacity to absorb fat. Another compensatory response in the absence of individual candidate fatty transporters in animals is the upregulation of glucose uptake and consumption mechanisms. Therefore, it is difficult to see significant effects on fat absorption by the inhibition or ablation of single gene and these manipulations usually do not reveal a significant phenotype. However, in several studies, importance of individual proteins has been obtained under conditions of stress such a fasting, exercise, insulin resistance. In short, several complementary and compensatory mechanisms exist to ensure complete uptake of fatty acids as an important energy source for biological functions.
5. Intracellular transport of long chain fatty acids to the ER
Intracellular transport of long chain fatty acids is carried out by fatty acid binding proteins (FABPs) mainly present in the cytoplasm of different tissues; sometimes they are referred to as FABPc to emphasize their cytosolic location and to differentiate with FABPpm. The FABP family includes nine, FABP1-9, members and cellular retinoid binding protein [93,94]. They show unique expression pattern indicative of adaptations to tissue-specific functions, for example FABP2 is only expressed in the intestine. But, other FABPs are expressed in more than one tissue. These proteins share very little primary sequence homology, but have very similar tertiary structure consisting of 10-antiparallel β-barrels and one helix-turn-helix motif. They contain one high affinity binding site for free fatty acids except for FABP1 that binds two molecules of fatty acids. Most of the FABPs interact with membranes to extract and deliver fatty acids. By contrast, FABP1 extracts and delivers fatty acids to membranes via aqueous diffusion that does not involve physical contact with membranes. Therefore, it has been suggested that FABP1 could act as a reservoir and deliver fatty acids via protein-protein interactions.
FABP1 (L-FABP) and FABP2 (I-FABP) are highly expressed in enterocytes of the proximal small intestine [30,99]. Based on a number of in vitro studies, it has been proposed that these proteins may carry out different functions. L-FABP may interact with specific membrane proteins, act as a cytosolic reservoir to assimilate long chain free fatty acids, operate as a regulator of intracellular lipid trafficking, and participate in CM trafficking [5,53,63,69]. L-Fabp−/− mice show decrease intestinal lipid secretion [70] perhaps secondary to its role in the budding of prechylomicron transport vesicles (PCTVs, see below) that transport nascent CMs from the ER to Golgi.
I-FABP might be involved in uptake and/or intracellular targeting of fatty acids toward TAG synthesis [4]. Two naturally occurring isoforms of I-FABP have been described. Using stably transfected Caco-2 cells, it has been suggested that the I-FABP Thr-54 isoform increases fatty acid transport and triglyceride secretion compared with the I-FABP Ala-54 isoform [7]. Vassileva et al shown that I- Fabp−/− male mice have higher plasma TAG concentration compared to wild-type mice and show no defect in the absorption of dietary fat, suggesting that I-FABP is not essential for dietary fat absorption in mice [3].
6. Synthesis of TAG
In enterocytes, MAG and FA are utilized to synthesize TAG in the ER via the sn-2-monoacylglycerol (MAG) pathway (Fig 1). In this pathway, fatty acids and MAG are covalently joined to synthesize diacylglycerol (DAG) by acyl coenzyme A:monoacylglycerol acyltransferase (MGAT). Then, DAG is further acylated by acyl coenzyme A:dicylglycerol acyltransferase 1 and 2 (DGAT1 and 2) to form TAG [14,60,102]. Enterocytes can also synthesize DAG from glucose via the glycerol phosphate or Kennedy pathway. This pathway involves conversion of glycerol-3-phosphate to DAG by several enzymes (Fig 1). DAG is then converted to TAG by DGATs as in the MAG pathway. Therefore, both these pathways share the last step of converting DAG into TAG. It is believed that 75–80% of the TAG synthesis occurs via the MAG pathway and the rest is synthesized by the glycerol phosphate pathway in the intestine [21,22,109]. In contrast to enterocytes, glycerol phosphate pathway is the major pathway for TAG synthesis in the liver. TAG synthesized via the MAG pathway is utilized rapidly for CM formation. However, TAG formed via the glycerol phosphate pathway is primarily stored in the cytosol and is subsequently hydrolyzed and re-esterified to produce TAG in the ER and then incorporated into CM.
6.1. Monoacylglycerol pathway
6.1.1. MGATs
Three monoacylglycerol acyltransferase (MGAT) enzymes are present in the ER. These enzymes use MAG and acyl-CoAs to form DAG. MGAT1 is expressed in most tissues but not in the intestine [113]. MGAT2 and MGAT3 are expressed predominantly in the small intestine [112,113]. However, MGAT3 is only expressed in humans but not in rodents [61,62]. Moreover, MGAT3 levels are higher in ileum than in jejunum. As mentioned above, MGAT3 has significant DGAT activity. Hence, MGAT2 is the major enzyme in the intestine contributing to the MGAT activity. MGAT2 catalyzes the acylation of sn-1-MAG, sn-2-MAG and sn-3-MAG [16] and is likely the key contributor in TAG absorption. This is supported by the observation that Mgat2−/− mice fail to develop hypertriglyceridemia following triglyceride ingestion and do not develop obesity on high fat diet compared with wild type mice [111]. MGAT2 is also very responsive to a high fat diet and is posttranslationally regulated [16]. It is likely that it could be a good target to reduce fat absorption.
6.1.2. DGATs
The final step in TAG synthesis is the acylation of DAG to TAG and is performed by acyl-CoA:diacylglycerol acyltransferase (DGAT). Three enzymes, DGAT1, DGAT2 and MGAT3, with DGAT activity have been identified. DGAT1 was the first enzyme to be sequenced and was shown to be related to the acyl CoA:cholesterol acyltransferase (ACAT) gene family [14,27,114]. In fact, using Dgat1−/− mice, Farese’s laboratory reported that DGAT1 deletion caused a substantial decrease, but not a total loss of CM production. It delayed fat absorption and the onset of postprandial hypertriglyceridemia [14,15,27]. It has been reported that about 76 and 89% of the TAG synthesis from MAG is mediated by DGAT1 in Caco-2 cells and rat intestinal mucosal membranes, respectively. Oral gavage of XP621, a specific inhibitor for DGAT1, in rats decreased lipid absorption by 50% and also decreased TAG and DAG synthesis in primary enterocytes [20]. Taken together, these studies suggest that acylation of acylglycerols by DGAT1 is important for dietary fat absorption in the intestine.
In 2001, Farese and associates cloned and expressed DGAT2. DGAT2 also plays a significant role in TAG metabolism in several tissues. DGAT2 has no sequence homology with DGAT1 but performs similar function as the DGAT1. In fact DGAT2 belongs to the MGAT family of acyltransferases [17]. Stone et al reported that knockdown of DGAT2 in mice reduced plasma TAG, free FA and glucose by 70%, induced lethal neonatal lipopenia, and resulted in skin barrier abnormalities [92], suggesting that DGAT2 plays a fundamental role in mammalian TAG synthesis and is required for survival too. These findings indicate that DGAT2 is likely the primary DGAT activity responsible for the majority of TAG synthesis in various tissues.
6.2. Glycerol phosphate pathway
In this pathway, glycerol-3-phosphate is first acylated at the sn-1 position to form lysophosphatidic acid by glycerol-3-phosphate acyltransferases 3 and 4 (GPAT3 and 4). Next, the sn-2 position is acylated with another fatty acid by sn-1-acylglycerol-3-phosphate-O-acyltranferase (AGPAT) resulting in the synthesis of phosphatidic acid. Finally, phosphate group of the phosphatidic acid is hydrolyzed by phosphatidic acid phosphohydrolases or lipins 1–3 generating DAG. DAG is then acylated by DGATs to form TAG as in the MAG pathway. More details about these enzymes can be found in several reviews [21,22,100].
7. Packaging of TAG into chylomicrons
Chylomicrons (CMs) are typically synthesized during postprandial periods to transport dietary fat by the small intestine. They are TAG-rich lipoproteins consisting of ~ 80–95% TAG. During interprandial and fasting periods, intestine synthesizes smaller very low-density lipoproteins (VLDLs) that consist of ~ 60–89% TAG. Chylomicron assembly begins in the ER by scaffolding a large hydrophobic apolipoprotein B48 (apoB48) with lipids by the microsomal triglyceride transfer protein (MTP). In this process, newly synthesized apoB48 interacts with the inner leaflet of the ER (Fig 2). MTP can physically interact with apoB and also add lipids onto apoB. As a result of these processes, apoB gets associated with lipids and is detached from the ER membrane forming a “primordial particle” that contains neutral lipids at its core. These primordial particles are further enlarged by the addition of lipids by MTP or by the fusion of preformed lipid droplets in a process termed “core expansion” [43–45]. Besides lipids, these particles also acquire various apolipoproteins. An important apolipoprotein that is known to play a role in intestinal lipid absorption is apoAIV. This apolipoprotein may assist in the stabilization of the surface of expanding CM particles. These prechylomicrons are transported from the ER to Golgi by prechylomicron transport vesicles (PCTVs). In these Golgi, these CM are further modified by the addition of apoA1 and other lipids, and the mature CM are secreted via secretory vesicles that bud off from the trans Golgi and fuse with the basolateral membrane releasing them into the lamina propria [28,43–45,60,62,71,72,98].
7.1. Apolipoprotein B
Apolipoprotein B (apoB) is required for the synthesis and secretion of triglyceride-rich lipoproteins and serves as a major structural protein for their assembly. It is highly expressed in mammalian liver and intestine. When apoB is virtually absent, as in some cases of homozygous familial hypobetalipoproteinemia [76,86], affected individuals have extremely low plasma levels of TAG and cholesterol and show impaired intestinal lipid absorption. ApoB exists in two forms, apoB100 and apoB48; they are encoded by the same gene but arise due to differential tissue-specific posttranscriptional editing of the mRNA. In the human liver, apoB mRNA does not undergo posttranscriptional editing and is translated into a single polypeptide of 4536 amino acids. In the intestine, apoB mRNA is posttranscriptionally edited by Apobec-1 resulting in the introduction of a stop codon and the edited mRNA is translated into a polypeptide of 2152 amino acids called apoB48. ApoB 100 has a pentapartite structure, consisting of 5 structural domains (βα1, β1, α2, β2 and α3) and is mainly present on liver derived VLDL, IDL and LDL. ApoB48 is the N-terminal 48% of apoB100 and consists of the βα1 and β1 domains and part of the α2 domains [85]. This peptide is used for the assembly of chylomicrons in the intestine.
Nascent apoB48 interacts with inner leaflet of the ER membrane. It is either lipidated by MTP or degraded by proteasomes. MTP mediated lipidation results in the formation of primordial particles that are secretion competent. There is evidence that particles can exist for long time towards the apical side in the enterocytes probably within the ER [18,65]. During postprandial conditions, these particles are further enriched with TAG and enlarged into prechylomicron particles that are larger in size. They are then transported to Golgi for further maturation and secretion as outlined below.
7.2. MTP
MTP is a heterodimer of a 97 kDa catalytic subunit and protein disulfide isomerase (PDI), predominantly found in the ER of hepatocytes and intestinal epithelial cells, where it loads TAG, cholesterol esters, and phospholipids onto apoB. In the absence of MTP-mediated lipid transfer, apoB is degraded and chylomicrons are not secreted from the intestines.
MTP knockout mice are not viable as embryos die at midgestation. There was a substantial accumulation of cytosolic fat in the Mttp−/− visceral yolk sac endoderm and very rare lipid-staining particles were observed within the Golgi compartments suggesting for defect in lipoprotein assembly [81]. However, the role of MTP in intestinal lipid absorption has been studied using intestine-specific Mttp KO (I-Mttp−/−) mice. Using I-Mttp−/− mice, Davidson's lab showed that CM secretion was reduced dramatically in vivo as well as there was 80% decrease in apoB48 secretion from primary enterocytes [107,108]. Additionally, using I-Mttp−/fl partially deficient mice, Iqbal et al showed that intestinal absorption of radiolabeled triolein was decreased by 63% compared with Mttpfl/fl mice [50]. Thus, partial and complete intestine-specific MTP deficiency leads to substantial decrease in triglyceride absorption.
7.3. Apolipoprotein A-IV
ApoAIV is an exchangeable apolipoprotein mainly synthesized by enterocytes. It is incorporated into nascent chylomicrons at an early stage of biogenesis in the ER and is secreted on the surface of chylomicrons at the basolateral membrane [26]. ApoAIV synthesis by the enterocytes is stimulated by active lipid absorption [11,32,101]. Recent studies have shown that apoAIV levels affect intestinal lipoprotein assembly. For instance, Black and colleagues have shown that increased apoAIV expression is associated with increased formation of CM and TAG [57,58]. Most recently, they have shown that overexpression of ApoAIV increases packaging of TAG into nascent CMs in the ER lumen as well as increases MTP expression [110]. In agreement with these data, we also observed that ApoAIV−/− mice have lower intestinal MTP levels (data not shown). At this time, it is not clear how apoAIV regulates MTP expression.
8. Intracellular transport and secretion of chylomicrons
Intracellular transport of chylomicrons has been explained by the excellent work of Mansbach and associates [60,62,69,88]. They showed that TAG carrying chylomicrons are exported from the ER in distinct vesicles called the prechylomicron transport vesicles (PCTVs) (Fig 2), which are different from the vesicles that transport proteins. These vesicles of about 250 nm in diameter are membrane enclosed structures that carry nascent prechylomicrons to the Golgi. Apart from chylomicron specific apolipoproteins B48 and apoAIV, these PCTVs contain COPII proteins, e.g. sar1, sec23, sec24, sec13, sec31, and unique proteins such as vesicle-associated membrane protein 7 (VAMP7), CD36 and L-FABP. The formation of these PCTVs is perhaps initiated by L-FABP (FABP1) as recombinant FABP1 can generate PCTVs on its own. PCTV biogenesis also requires ATP dependent phosphorylation of an unknown protein by PKCδ. FABP1 generated PCTVs lack COPII proteins and do not fuse with the Golgi [60,69,88];. Therefore, formation of PCTVs involves two types of proteins; first those involved in the formation of PCTV and cargo selection and second necessary for their fusion with Golgi. Proteomic analysis identified several more proteins in these vesicles, but their role in prechylomicron transport has not been determined [105,106].
After exiting from the ER, the PCTVs fuse with the cis-Golgi. Fusion of PCTVs with the cis-Golgi involves interaction of VAMP7 with syntaxin 5, rbet1, and vit1a present on the Golgi membrane. In the Golgi, chylomicrons acquire apoAI and apoB48 undergoes glycosylation [89] [10]. Although it is known that chylomicrons are transported from the trans Golgi to the basolateral membrane, very little is known about the exit of these particles from the Golgi and their fusion with the basolateral membrane. After secretion, chylomicrons are concentrated in lymphatics by unknown mechanisms and are delivered to blood circulation at the thoracic duct.
9. Cholesterol absorption
Cholesterol absorption largely follows the same pathway as TAG. Diet contains free and esterified cholesterol. Esterified cholesterol is hydrolyzed to free cholesterol and fatty acids in the intestinal lumen similar to TAG. Free cholesterol and FA are taken up by enterocytes. FA is taken up by diffusion and protein facilitated active processes discussed above. Niemann Pick C 1 like 1 protein (NPC1L1) is known to play a critical role in the uptake and delivery of free cholesterol to the ER where it is acylated with fatty acids by Acyl coA:cholesterol acyl transferases 1 and 2 (ACAT1 and ACAT2). Cholesterol ester synthesis is facilitated by the availability of free cholesterol and inhibited by the accumulation of cholesterol esters [51]. MTP can transfer both free and esterified cholesterol from the ER membrane to the nascent apoB-lipoproteins and regulate cholesterol esterification. Once incorporated, cholesterol follows the path of chylomicron processing and secretion.
Besides the secretion of free and esterified cholesterol by the apoB-dependent chylomicron pathway, free cholesterol is also secreted by the HDL pathway. In this pathway apolipoprotein A1 and a membrane transport protein ABCA1 have been shown to play an important role [13,48,49].
10. Regulation of TAG secretion
A number of factors are involved in the regulation of intestinal TAG absorption. They can affect various steps in TAG absorption. This is an emerging field and advances are being made in various directions. We point out few developments that impact on the regulation of intestinal TAG transport.
10.1. Insulin, Insulin Resistance, and Diabetes
Dyslipidemias in insulin resistant and diabetic animals and humans have been well documented. Earlier studies tended to explain the origin of these dyslipidemias on defects in lipoprotein clearance. However, recent studies point to the possibility that lipoprotein over production could also contribute to this dyslipidemia. First, it was shown hepatic lipoprotein biogenesis is enhanced. Now, recent studies point to the possibility that overproduction of intestinal lipoproteins might also contribute to dyslipidemia observed in insulin resistance and diabetes [2]. Adeli and coworker have shown that the insulin-resistant, sucrose-fed hamsters exhibit increased de novo lipogenesis, have higher amounts of MTP and over produce apoB48 [35]. This can be avoided by treating these hamsters with rosiglitazone, a PPARγ agonist, or a cinnamon extract [79]. Zoltowska et al have shown that Psammomys obesus insulin-resistant and diabetic sand rats contain increased amounts of L-FABP, higher MGAT and DGAT activities and show elevated apoB48 biogenesis [116,117]. Vine et al showed that obese, insulin resistant JCR:LA-cp rats absorb more triglyceride and accumulate more apoB48 in postprandial state [103]. Lally et al showed that streptozotocin induced diabetic rats [54] and Zucker fatty diabetic rats absorb more triglyceride and assemble more chylomicrons [55]. Sasase et al showed that spontaneously diabetic Torii rats absorb more fat and have increased chylomicron production [83]. Moreover, insulin resistant and diabetic humans also show increased intestinal lipoprotein production [25,38]. Therefore, insulin resistance and diabetes might enhance intestinal lipoprotein production involving increased de novo lipogenesis and chylomicron production.
10.2. Glucagon like peptides
New studies indicate that incretins, hormones elaborated by intestine in response to diet, regulate fat absorption. Glucagon like peptide 1 and 2 (GLP-1 and GLP-2) are secreted simultaneously in 1:1 molar ratio from L-cells predominantly present in the ileum in response to meals rich in carbohydrates and fats. GLP-1 has been shown to decrease triglyceride absorption and lymphatic apoB and apoAIV output in rats and dogs [80,104]. Moreover, pharmacologic inhibition as well as genetic ablation of GLP-1 receptor has been shown to increase CM production [40]. By contrast, GLP-2 stimulates CM secretion through enhanced intestinal lipid absorption, in chow-fed Syrian Golden hamsters [39]. Additionally, GLP-2 was shown to stimulate apoB48 secretion in jejunal fragments cultured ex vivo in C57BL/6 mice, however, this effect was lost in CD36 knockout mice [41]. It remains to be determined whether inhibition of GLP-2 receptor might be useful in lowering fat absorption.
10.3. Leptin
Leptin, the product of the obese gene (ob), is a hormone primarily produced in adipose tissue and stomach in response to nutrient ingestion [115]. Administration of leptin decreases food intake and increases energy expenditure. Leptin receptors are expressed at the apical and basolateral membranes of enterocytes [8]. Leptin-deficient insulin-resistant JCR:LA-cp rats secrete higher amounts of apoB48 [59]. Morton et al showed that enterocytes in the jejunum express leptin receptors and administration of leptin reduces apoAIV mRNA levels [66]. Doi et al have shown that leptin antagonizes lipid induced induction of apoAIV secretion [23]. Iqbal et al have shown that LEPR deficiency significantly decreases intestinal MTP expression and lipid absorption, but does not affect hepatocytes MTP and triglyceride secretion in mice [50]. Therefore, it is possible that leptin may regulate apoAIV as well as MTP gene expression and control TAG absorption.
10. 4. Effect of IRE1β
IRE1β, a protein closely related to the ubiquitously expressed ER stress-response protein IRE1α, is mainly expressed in the intestine. Ire1b−/− mice have been shown to enhance intestinal MTP expression and increases lipid absorption and chylomicron secretion in response to high-cholesterol and high-fat diets [47]. In addition, cell culture experiments suggest that IRE1β reduces MTP mRNA levels by augmenting posttranscriptional degradation [47]. These studies provide evidence for the presence of intestine-specific mechanisms controlling the assembly and secretion of apoB lipoproteins, wherein IRE1β regulates chylomicron production by degrading MTP mRNA.
10. 5. Effect of diurnal rhythm
Plasma TAG shows diurnal rhythms in ad libitum fed wild type (WT) rats and mice maintained in a 12-h photoperiod [46,73]; plasma TAG are high in the night due to changes in apoB-lipoproteins [74]. We first observed that absorption of 3H-triolein was higher at 24:00 h than at 12:00 h, indicating that intestinal lipoprotein production shows diurnal variations. Furthermore, intestinal MTP activity, protein, mRNA, and gene transcription also showed diurnal variations and were high at 24:00 h suggesting that diurnal modulation of MTP transcription might be a major determinant of daily changes in plasma lipids.
To understand the role of clock genes in the diurnal regulation of plasma lipids and MTP, we studied Clock mutant (Clkmt/mt) mice because they show defects in diurnal regulations. We showed that these mice absorb significantly more TAG and cholesterol at night compared with their WT siblings [74]. Additionally, isolated primary enterocytes from Clkmt/mt mice took up more [3H]oleic acid and [14C]cholesterol and secreted more lipids [74]. These studies indicate that Clock might be involved in the regulation of MTP and TAG absorption.
We have recently delineated mechanisms involved in the diurnal regulation of MTP by Clock [75]. We showed that knockdown of Clock increases MTP expression. We further showed that Clock regulates MTP involving a clock-controlled gene, small heterodimer partner (SHP, NROB2). Clock knockdown decreases SHP expression involving transcriptional regulation; Clock binds to the E-box in the SHP promoter and increases its expression in the daytime. We further showed that SHP interacts with HNF-4α/LRH-1/HNF-1α and reduces MTP expression (Fig 3). The role of SHP in MTP regulation was substantiated further by over expression and knockdown of SHP in hepatoma cell lines; these treatments decreased and increased MTP levels, respectively [75]. Further studies in Shp−/− mice revealed that plasma TAG do not show diurnal variations in these mice. But, these mice absorbed more TAG. To provide evidence for the role of SHP in Clock mediated regulation, we studied the expression of SHP, MTP and plasma lipids in Clkmt/mt mice. These mice have low levels of SHP, high MTP levels and exhibit hypertriglyceridemia. Over expression of SHP reduced MTP expression and hyperlipidemia. Thus, these studies indicated that Clock uses SHP to regulate MTP and plasma TAG.
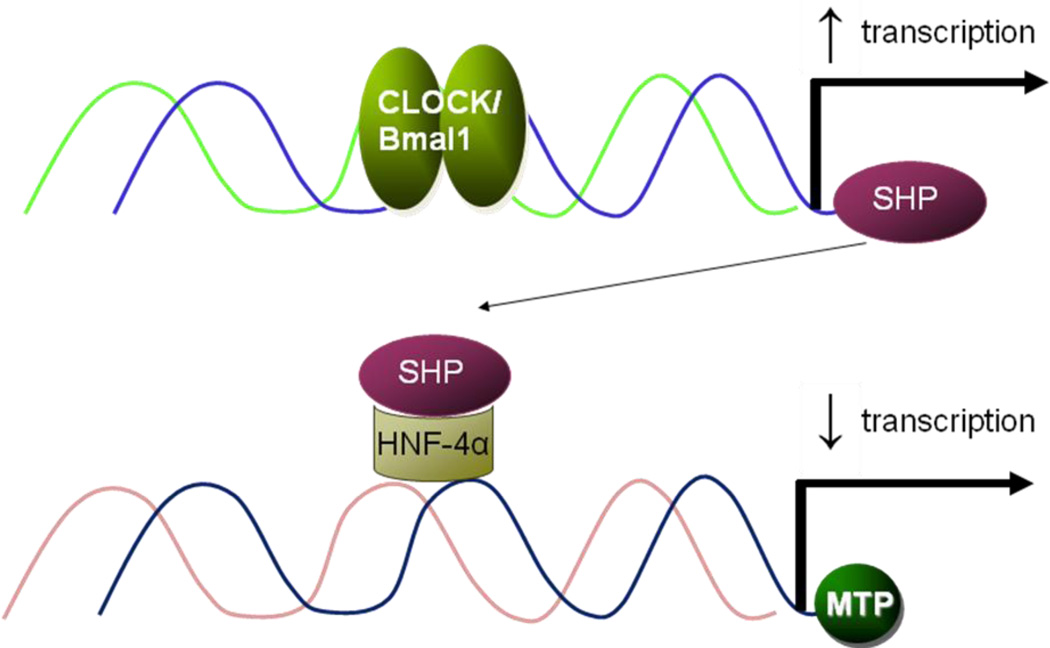
Fig 3. Regulation of MTP by Clock via SHP.
Clock represses MTP expression through an indirect mechanism. Clock/Bmal1 binds to the E-Box in the small heterodimer partner (SHP) promoter and increases its expression in the daytime. SHP, in turns, interacts with transcription factors/enhancer present on the MTP promoter and represses its activity leading to decreases in day time.
11. Future perspectives
As summarized above, significant information has been gathered about different steps involved in TAG transport by enterocytes. However, there are several gaps in this understanding. Although several transporters have been identified for long chain fatty acid uptake, there is still ambiguity as to their individual and collective roles in fatty acid uptake. It is not certain whether, we are aware of all the proteins involved in fatty acid uptake. We know the importance of apoB and MTP in the assembly of CMs, but very little is known about how these particles are put together. Particularly knowledge about the presumed second step of “core expansion” is poorly defined. Very little is known about the transport of CMs from the Golgi to the plasma membrane and their ultimate secretion. Similar to PCTVs, the transport of CM from the Golgi might involve specific vesicles different from those involved in proteins secretion. More importantly, how different proteins involved in various step of TAG transport are regulated is poorly understood. An area that was not discussed here relates to identification of inhibitors for various protein and enzymes involved in TAG production. Several proteins in this pathway are good candidates for the management, control, and treatment of obesity. Undoubtedly, we will learn more about this in the near future.
Highlights.
-
12
Triglyceride absorption involves many discrete steps
-
13
Dietary triacylglycerols are hydrolyzed in the lumen of the intestine
-
14
Enterocytes take up hydrolyzed products, free fatty acids and monoacylglycerols, from their apical side
Triacylglycerols are re-synthesized and packaged into chylomicrons in the endoplasmic reticulum
Chylomicrons are then transported to the Golgi compartment by prechylomicron transport vesicles
Transport of chylomicrons from the Golgi to the plasma membrane and their secretion toward the basolateral side is poorly understood
Acknowledgments
This work was supported in part by National Institutes of Health grants DK46900 and DK-81879 to MMH and American Heart Association Scientist Development Grant (2300158) to XP.
Abbreviations used
- ApoB
Apolipoprotein B
- ApoB48
Apolipoprotein B-48
- CM
chylomicron
- DAG
diacylglycerols
- DGAT
Diacylglycerol O-Acyltransferase
- ER
endoplasmic reticulum
- FA
fatty acids
- FABP
Fatty acid binding proteins
- MAG
monoacylglycerols
- MTP
microsomal triglyceride transfer protein
- TAG
triacylglycerols
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993;268:17665. [PubMed] [Google Scholar]
- 2.Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Curr. Opin. Lipidol. 2008;19:221. doi: 10.1097/MOL.0b013e3282ffaf82. [DOI] [PubMed] [Google Scholar]
- 3.Agellon LB, Drozdowski L, Li L, Iordache C, Luong L, Clandinin MT, Uwiera RR, Toth MJ, Thomson AB. Loss of intestinal fatty acid binding protein increases the susceptibility of male mice to high fat diet-induced fatty liver. Biochim. Biophys. Acta. 2007;1771:1283. doi: 10.1016/j.bbalip.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Alpers DH, Bass NM, Engle MJ, Schryver-Kecskemeti K. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim. Biophys. Acta. 2000;1483:352. doi: 10.1016/s1388-1981(99)00200-0. [DOI] [PubMed] [Google Scholar]
- 5.Alpers DH, Strauss AW, Ockner RK, Bass NM, Gordon JI. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc. Natl. Acad. Sci. U. S. A. 1984;81:313. doi: 10.1073/pnas.81.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J. Biol. Chem. 1995;270:2367. doi: 10.1074/jbc.270.5.2367. [DOI] [PubMed] [Google Scholar]
- 7.Baier LJ, Bogardus C, Sacchettini JC. A polymorphism in the human intestinal fatty acid binding protein alters fatty acid transport across Caco-2 cells. J. Biol. Chem. 1996;271:10892. doi: 10.1074/jbc.271.18.10892. [DOI] [PubMed] [Google Scholar]
- 8.Barrenetxe J, Villaro AC, Guembe L, Pascual I, Munoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, cytoplasm of enterocytes. Gut. 2002;50:797. doi: 10.1136/gut.50.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berk PD, Wada H, Horio Y, Potter BJ, Sorrentino D, Zhou SL, Isola LM, Stump D, Kiang CL, hung ST. Plasma membrane fatty acid-binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3484. doi: 10.1073/pnas.87.9.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berriot-Varoqueaux N, Dannoura AH, Moreau A, Verthier N, Sassolas A, Cadiot G, Lachaux A, Munck A, Schmitz J, Aggerbeck LP, Samson-Bouma ME. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson's disease. Gastroenterology. 2001;121:1101. doi: 10.1053/gast.2001.29331. [DOI] [PubMed] [Google Scholar]
- 11.Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am. J. Physiol Gastrointest. Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn C, Guan B, Brown J, Cullis C, Condon SM, Jenkins TJ, Peluso S, Ye Y, Gimeno RE, Punreddy S, Sun Y, Wu H, Hubbard B, Kaushik V, Tummino P, Sanchetti P, Yu SD, Daniels T, Tozzo E, Balani SK, Raman P. Identification and characterization of 4-aryl-3,4-dihydropyrimidin–2(1H)-ones as inhibitors of the fatty acid transporter FATP4. Bioorg. Med. Chem. Lett. 2006;16:3504. doi: 10.1016/j.bmcl.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 13.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JA, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhman KF, Accad M, Farese RV. Mammalian acyl-CoA:cholesterol acyltransferases. Biochim. Biophys. Acta. 2000;1529:142. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 15.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 2002;277:25474. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Lockwood J, Burn P, Shi Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 2003;278:13860. doi: 10.1074/jbc.M300139200. [DOI] [PubMed] [Google Scholar]
- 17.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, related family members. J. Biol. Chem. 2001;276:38870. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 18.Chateau D, Pauquai T, Delers F, Rousset M, Chambaz J, emignot SD. Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J. Cell Physiol. 2005;202:767. doi: 10.1002/jcp.20173. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am. J. Physiol Endocrinol. Metab. 2001;281:E916–E923. doi: 10.1152/ajpendo.2001.281.5.E916. [DOI] [PubMed] [Google Scholar]
- 20.Cheng D, Iqbal J, Devenny J, Chu C-H, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM, Pelleymounter MA, Hussain MM. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1): Fubctional importance of DGAT1 in the intestinal fat absorption. J. Biol. Chem. 2008;283:29802. doi: 10.1074/jbc.M800494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004;43:134. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RA, Lewin TM, Muoio DM. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu. Rev. Nutr. 2000;20:77. doi: 10.1146/annurev.nutr.20.1.77. [DOI] [PubMed] [Google Scholar]
- 23.Doi T, Liu M, Seeley RJ, Woods SC, Tso P. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am. J. Physiol Regul. Integr. Comp Physiol. 2001;281:R753–R759. doi: 10.1152/ajpregu.2001.281.3.R753. [DOI] [PubMed] [Google Scholar]
- 24.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 2005;115:1290. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 2006;26:1357. doi: 10.1161/01.ATV.0000222015.76038.14. [DOI] [PubMed] [Google Scholar]
- 26.Dvorin E, Mantulin WW, Rohde MF, Gotto AM, Jr, Pownall HJ, Sherrill BC. Conformational properties of human and rat apolipoprotein A-IV. J. Lipid Res. 1985;26:38. [PubMed] [Google Scholar]
- 27.Farese RV., Jr Acyl CoA:cholesterol acyltransferase genes and knockout mice. Curr. Opin. Lipidol. 1998;9:119. doi: 10.1097/00041433-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 2002;277:17377. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 29.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 30.Gordon JI, Alpers DH, Ockner RK, Strauss AW. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J. Biol. Chem. 1983;258:3356. [PubMed] [Google Scholar]
- 31.Goudriaan JR, Dahlmans VE, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol. Cell Biochem. 2002;239:199. [PubMed] [Google Scholar]
- 32.Green PH, Riley JW. Lipid absorption and intestinal lipoprotein formation. Australian & New Zealand Journal of Medicine. 1981;11:84. doi: 10.1111/j.1445-5994.1981.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 33.Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, transfusion medicine. Blood. 1992;80:1105. [PubMed] [Google Scholar]
- 34.Greenwalt DE, Scheck SH, hinehart-Jones TR. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J. Clin. Invest. 1995;96:1382. doi: 10.1172/JCI118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 2002;277:31646. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8625. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 38.Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J. Lipid Res. 2007;48:1336. doi: 10.1194/jlr.M600548-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh J, Hayashi AA, Webb J, Adeli K. Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008;9:7. doi: 10.1016/j.atherosclerosissup.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53:552. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 42.Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J. Lipid Res. 2002;43:2017. doi: 10.1194/jlr.r200013-jlr200. [DOI] [PubMed] [Google Scholar]
- 43.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 44.Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr. Opin. Lipidol. 2005;16:281. doi: 10.1097/01.mol.0000169347.53568.5a. [DOI] [PubMed] [Google Scholar]
- 45.Hussain MM, Kancha RK, Zhou Z, Luchoomun J, Zu H, Bakillah A. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1996;1300:151. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 46.Hussain MM, Pan X. Clock genes, intestinal transport and plasma lipid homeostasis. Trends Endocrinol. Metab. 2009;20:177. doi: 10.1016/j.tem.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1β inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 2005;46:1491. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J, Hussain MM. Intestinal lipid absorption. Am. J. Physiol Endocrinol. Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr, Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J. Lipid Res. 2010;51:1929. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iqbal J, Rudel LL, Hussain MM. Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. J. Biol. Chem. 2008;283:19967. doi: 10.1074/jbc.M800398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim. Biophys. Acta. 2009;1791:149. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Lagakos WS, Gajda AM, Agellon L, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol Gastrointest. Liver Physiol. 2011;300:G803–G814. doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism. 2007;56:430. doi: 10.1016/j.metabol.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 55.Lally S, Owens D, Tomkin GH. The different effect of pioglitazone as compared to insulin on expression of hepatic and intestinal genes regulating post-prandial lipoproteins in diabetes. Atherosclerosis. 2007;193:343. doi: 10.1016/j.atherosclerosis.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 56.Lowe ME. The triglyceride lipases of the pancreas. J. Lipid Res. 2002;43:2007. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 57.Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 2005 doi: 10.1074/jbc.M502501200. [DOI] [PubMed] [Google Scholar]
- 58.Lu S, Yao Y, Meng S, Cheng X, Black DD. Overexpression of apolipoprotein A-IV enhances lipid transport in newborn swine intestinal epithelial cells. J. Biol. Chem. 2002;277:31929. doi: 10.1074/jbc.M201418200. [DOI] [PubMed] [Google Scholar]
- 59.Mangat R, Su J, Scott PG, Russell JC, Vine DF, Proctor SD. Chylomicron and apoB48 metabolism in the JCR:LA corpulent rat, a model for the metabolic syndrome. Biochem. Soc. Trans. 2007;35:477. doi: 10.1042/BST0350477. [DOI] [PubMed] [Google Scholar]
- 60.Mansbach CM, Gorelick F. Development and Physiological Regulation of Intestinal Lipid Absorption. II. Dietary lipid absorption, complex lipid synthesis, the intracellular packaging and secretion of chylomicrons. Am. J. Physiol Gastrointest. Liver Physiol. 2007;293:G645–G650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 61.Mansbach CM, II, Parthasarathy S. A re-examination of the fate of glycerideglycerol in neutral lipid absorption and transport. J. Lipid Res. 1982;23:1009. [PubMed] [Google Scholar]
- 62.Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu. Rev. Physiol. 2010;72:315. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meydani M, Martin KR. Intestinal Absorption of Fat-Soluble Vitamins. In: Mansbach CM II, Tso P, Kuksis A, editors. Intestinal lipid metabolism. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 367–381. [Google Scholar]
- 64.Miled N, Canaan S, Dupuis L, Roussel A, Riviere M, Carriere F, de CA, Cambillau C, Verger R. Digestive lipases: from three-dimensional structure to physiology. Biochimie. 2000;82:973. doi: 10.1016/s0300-9084(00)01179-2. [DOI] [PubMed] [Google Scholar]
- 65.Morel E, Demignot S, Chateau D, Chambaz J, Rousset M, Delers F. Lipid-dependent Bidirectional Traffic of Apolipoprotein B in Polarized Enterocytes. Mol. Biol. Cell. 2004;15:132. doi: 10.1091/mbc.E03-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J. Biol. Chem. 1998;273:26194. doi: 10.1074/jbc.273.40.26194. [DOI] [PubMed] [Google Scholar]
- 67.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 2007;282:19493. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 68.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J. Biol. Chem. 2007;282:17974. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 70.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 2003;278:51664. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 71.Olofsson SO, Asp L, Borén J. The assembly and secretion of apolipoprotein Bcontaining lipoproteins. Curr. Opin. Lipidol. 1999;10:341. doi: 10.1097/00041433-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Olofsson SO, Bostrom P, Andersson L, Rutberg M, Levin M, Perman J, Boren J. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr. Opin. Lipidol. 2008;19:441. doi: 10.1097/MOL.0b013e32830dd09b. [DOI] [PubMed] [Google Scholar]
- 73.Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J. Biol. Chem. 2007;282:24707. doi: 10.1074/jbc.M701305200. [DOI] [PubMed] [Google Scholar]
- 74.Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J. Lipid Res. 2009;50:1800. doi: 10.1194/jlr.M900085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel S, Pessah M, Beucler I, Navarro J, Infante R. Chylomicron retention disease: Exclusion of apolipoprotein B gene defects and detection of mRNA editing in an affected family. Atherosclerosis. 1994;108:201. doi: 10.1016/0021-9150(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 77.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine - Comparison with fatty acid- binding proteins (FABP) Eur. J. Biochem. 1996;238:368. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 78.Poirier H, Niot I, Degrace P, Monnot MC, Bernard A, Besnard P. Fatty acid regulation of fatty acid-binding protein expression in the small intestine. Am. J. Physiol. 1997;273:G289–G295. doi: 10.1152/ajpgi.1997.273.2.G289. [DOI] [PubMed] [Google Scholar]
- 79.Qin B, Polansky MM, Sato Y, Adeli K, Anderson RA. Cinnamon extract inhibits the postprandial overproduction of apolipoprotein B48-containing lipoproteins in fructose-fed animals. J. Nutr. Biochem. 2009;20:901. doi: 10.1016/j.jnutbio.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, D'Alessio DA, Tso P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, apolipoprotein production in rats. Am. J. Physiol Gastrointest. Liver Physiol. 2005;288:G943–G949. doi: 10.1152/ajpgi.00303.2004. [DOI] [PubMed] [Google Scholar]
- 81.Raabe M, Flynn LM, Zlot CH, Wong JS, Véniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: Reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8686. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley JW, Glickman RM. Fat malabsorption--advances in our understanding. Am. J. Med. 1979;67:980. doi: 10.1016/0002-9343(79)90639-9. [DOI] [PubMed] [Google Scholar]
- 83.Sasase T, Morinaga H, Yamamoto H, Ogawa N, Matsui K, Miyajima K, Kawai T, Mera Y, Masuyama T, Shinohara M, Ohta T, Matsushita M. Increased fat absorption and impaired fat clearance cause postprandial hypertriglyceridemia in Spontaneously Diabetic Torii rat. Diabetes Res. Clin. Pract. 2007;78:8. doi: 10.1016/j.diabres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 84.Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 85.Segrest JP, Jones MK, De Loof H, ashti ND. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 2001;42:1346. [PubMed] [Google Scholar]
- 86.Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 2005;16:325. doi: 10.1097/01.mol.0000169353.12772.eb. [DOI] [PubMed] [Google Scholar]
- 87.Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J. Lipid Res. 2009;50:491. doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siddiqi SA, Mansbach CM. PKC zeta-mediated phosphorylation controls budding of the pre-chylomicron transport vesicle. J. Cell Sci. 2008;121:2327. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 89.Siddiqi SA, Siddiqi S, Mahan J, Peggs K, Gorelick FS, Mansbach CM. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J. Biol. Chem. 2006;281:20974. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 91.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol. Cell. 1999;4:299. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 92.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004;279:11767. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 93.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 2008;28:73. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 94.Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009;50 Suppl:S126–S131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stremmel W, Lotz G, Strohmeyer G, Berk PD. Identification, isolation, partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J. Clin. Invest. 1985;75:1068. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stuhlsatz-Krouper SM, Bennett NE, Schaffer JE. Substitution of alanine for serine 250 in the murine fatty acid transport protein inhibits long chain fatty acid transport. J. Biol. Chem. 1998;273:28642. doi: 10.1074/jbc.273.44.28642. [DOI] [PubMed] [Google Scholar]
- 97.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 2009;20:72. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi K, Odani S, Ono T. A close structural relationship of rat liver Z-protein to cellular retinoid binding proteins and peripheral nerve myelin P2 protein. Biochem. Biophys. Res. Commun. 1982;106:1099. doi: 10.1016/0006-291x(82)91225-6. [DOI] [PubMed] [Google Scholar]
- 100.Takeuchi K, Reue K. Biochemistry, physiology, genetics of GPAT, AGPAT, lipin enzymes in triglyceride synthesis. Am. J. Physiol Endocrinol. Metab. 2009;296:E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tso P, Liu M. Apolipoprotein A-IV, food intake, obesity. Physiol Behav. 2004;83:631. doi: 10.1016/j.physbeh.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 102.Turkish AR, Sturley SL. The genetics of neutral lipid biosynthesis: an evolutionary perspective. Am. J. Physiol Endocrinol. Metab. 2009;297:E19–E27. doi: 10.1152/ajpendo.90898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190:282. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 104.Wasada T, McCorkle K, Harris V, Kawai K, Howard B, Unger RH. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J. Clin. Invest. 1981;68:1106. doi: 10.1172/JCI110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong DM, Webb JP, Malinowski PM, Macri J, Adeli K. Proteomic profiling of the prechylomicron transport vesicle involved in the assembly and secretion of apoB-48-containing chylomicrons in the intestinal enterocytes. Proteomics. 2009;9:3698. doi: 10.1002/pmic.200800914. [DOI] [PubMed] [Google Scholar]
- 106.Wong DM, Webb JP, Malinowski PM, Xu E, Macri J, Adeli K. Proteomic profiling of intestinal prechylomicron transport vesicle (PCTV)-associated proteins in an animal model of insulin resistance (94 char) J. Proteomics. 2010;73:1291. doi: 10.1016/j.jprot.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 107.Xie Y, Luo J, Kennedy S, Davidson NO. Conditional intestinal lipotoxicity in Apobec-1−/− Mttp-IKO mice: a survival advantage for mammalian intestinal apolipoprotein B mRNA editing. J. Biol. Chem. 2007;282:33043. doi: 10.1074/jbc.M705386200. [DOI] [PubMed] [Google Scholar]
- 108.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 2006;281:4075. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 109.Yang LY, Kuksis A. Apparent convergence (at 2-monoacylglycerol level) of phosphatidic acid and 2-monoacylglycerol pathways of synthesis of chylomicron triacylglycerols. J. Lipid Res. 1991;32:1173. [PubMed] [Google Scholar]
- 110.Yao Y, Lu S, Huang Y, Beeman-Black CC, Lu R, Pan X, Hussain MM, Black DD. Regulation of microsomal triglyceride transfer protein by apolipoprotein A-IV in newborn swine intestinal epithelial cells. Am. J. Physiol Gastrointest. Liver Physiol. 2011;300:G357–G363. doi: 10.1152/ajpgi.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 2009;15:442. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yen CL, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003;278:18532. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 113.Yen CL, Stone SJ, Cases S, Zhou P, Farese RV., Jr Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8512. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008;49:2283. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 116.Zoltowska M, Ziv E, Delvin E, Lambert M, Seidman E, Levy E. Both insulin resistance and diabetes in Psammomys obesus upregulate the hepatic machinery involved in intracellular VLDL assembly. Arterioscler. Thromb. Vasc. Biol. 2004;24:118. doi: 10.1161/01.ATV.0000105901.18785.99. [DOI] [PubMed] [Google Scholar]
- 117.Zoltowska M, Ziv E, Delvin E, Sinnett D, Kalman R, Garofalo C, Seidman E, Levy E. Cellular aspects of intestinal lipoprotein assembly in Psammomys obesus: a model of insulin resistance and type 2 diabetes. Diabetes. 2003;52:2539. doi: 10.2337/diabetes.52.10.2539. [DOI] [PubMed] [Google Scholar]