Abstract
Malaria is a leading cause of morbidity and mortality in the tropics. Chemotherapeutic and vector control strategies have been applied for more than a century but have not been efficient in disease eradication. Increased resistance of malaria parasites to drug treatment and of mosquito vectors to insecticides requires the development of novel chemotherapeutic agents. Malaria parasites exhibit rapid nucleic acid synthesis during their intraerythrocytic growth phase. Plasmodium purine and pyrimidine metabolic pathways are distinct from those of their human hosts. Thus, targeting purine and pyrimidine metabolic pathways provides a promising route for novel drug development. Recent developments in enzymatic transition state analysis have provided an improved route to inhibitor design targeted to specific enzymes, including those of purine and pyrimidine metabolism. Modern transition state analogue drug discovery has resulted in transition state analogues capable of binding to target enzymes with unprecedented affinity and specificity. These agents can provide specific blocks in essential pathways. The combination of tight binding with the high specificity of these logically designed inhibitors, results in low toxicity and minor side effects. These features reduce two of the major problems with the current antimalarials. Transition state analogue design is being applied to generate new lead compounds to treat malaria by targeting purine and pyrimidine pathways.
Keywords: Antimalarials, malaria, purines, pyrimidines, transition state analogues, immucillins
INTRODUCTION
Malaria causes 300 to 500 million clinical cases and 1.5 to 2.7 million deaths per year in the broad tropical regions across all continents [1]. Malaria is caused by protozoan parasites of the Plasmodium genus, of which Plasmodium falciparum is responsible for most of the fatal cases. P. falciparum infection of the human host is initiated by injection of sporozoites into the bloodstream by an infected female Anopheles mosquito. The sporozoites gain access to the liver and invade hepatocytes, where asymptomatic asexual multiplication (exoerythrocytic schizogony) occurs, leading to the production of several thousand merozoites. These are released into the bloodstream and invade erythrocytes. Erythrocytes are the site of major parasite expansion resulting from several rounds of asexual multiplication, where each parasite produces 8 to 24 new merozoites. This erythrocytic schizogony phase of the infection is responsible for malaria pathogenesis and therefore is the target for most antimalarial compounds. The erythrocyte is the metabolic warehouse for all essential metabolites for Plasmodium parasites to cause clinical disease. During a mosquito-borne infection, the parasite expansion is many billion-fold, requiring extraordinary resources from the host to support parasite growth.
Malaria has been treated for many years through chemotherapeutic and vector control strategies. These approaches have not prevented widespread disease occurrence and resurgence from the ecologically devastating use of insecticides. Currently, an alarming increase in both the resistance of malaria parasites to drug treatment and in mosquito vectors to insecticides renders the development of novel chemotherapeutic agents an urgent task [2, 3]. Advances in the knowledge of the metabolic and nutritional needs of the parasite offer new potential routes for chemotherapy. The rapid rate of nucleic acid synthesis during the intraerythrocytic growth phase makes purine and pyrimidine metabolic pathways promising targets for novel drug development.
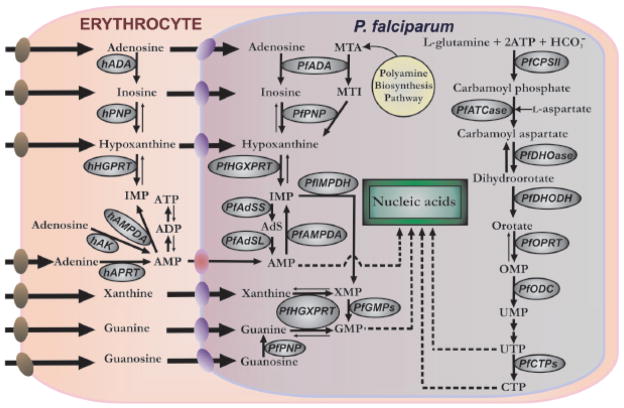
P. falciparum is a purine auxotroph, salvaging host cell purines for synthesis of cofactors and nucleic acids [4, 5]. Erythrocytes contain millimolar concentrations of ATP in equilibrium with ADP and AMP hence the parasite has had no evolutionary pressure to retain de novo purine synthetic pathways. Purine nucleosides and nucleobases can be transported across the parasite plasma membrane by the P. falciparum NT1 transporter Fig. (1). The mechanisms by which Plasmodium salvage purines during the intraerythrocytic cycle are diverse with regard to primary sources and to routes of interconversion. Hypoxanthine is the key precursor for all purine synthesis in Plasmodium metabolism and is commonly used as a nutritional supplement in malarial culture media. A key source of hypoxanthine in vivo is from the erythrocyte purine pool, where ATP is in dynamic metabolic exchange with hypoxanthine via ADP, AMP, IMP, inosine and adenosine [5]. Human erythrocytes also lack de novo purine biosynthesis and maintain their adenine nucleotide pools by adenosine salvage from plasma by the action of adenosine kinase (AK, EC 2.7.1.20). The catalytic efficiency of adenosine kinase keeps erythrocytic adenosine at low concentrations and Plasmodium has not retained an adenosine kinase activity. Instead, P. falciparum can salvage adenosine by conversion to hypoxanthine using the sequential activities of adenosine deaminase (ADA, EC 3.5.4.4) and purine nucleoside phosphorylase (PNP, EC 2.4.2.1). Transport of most purine bases and nucleosides into P. falciparum are facilitated by the NT1 Fig. (1). Hypoxanthine is then converted to IMP by hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRT, EC 2.4.2.8 and EC 2.4.2.22). IMP serves as the metabolic precursor for all purine nucleotides and deoxynucleotides needed for nucleic acid synthesis. ADA, PNP and HGXPRT are highly expressed proteins in the Plasmodium [6, 7]. Since no adenosine kinase (AK) gene has been found in the P. falciparum genome [8] and no adenosine kinase activity is detected by metabolic labeling [9], the parasite can not directly convert adenosine to AMP. However, metabolic analysis revealed that P. falciparum can use AMP synthesized in the erythrocyte cytosol when high concentrations of exogenous adenosine are used to force erythrocytic AMP production [9].
Fig. (1).
Purine and pyrimidine metabolism in P. falciparum infected-erythrocytes. Purine pathway: AMP, adenosine 5′-monophosphate; ADP, adenosine 5′-diphosphate; ATP, adenosine 5′-triphosphate; IMP, inosine 5′-monophosphate; XMP, xanthosine 5′-monophosphate; GMP, guanosine 5′-monophosphate; hADA, human adenosine deaminase; MTA, methylthioadenosine; MTI, methylthioinosine; AdS, adenoylsuccinate; hPNP, human purine nucleoside phosphorylase; hHGPRT, human hypoxanthine-guanine phosphoribosyl transferase; hAK, human adenosine kinase; hAMPDA, human adenosine 5′-monophosphate deaminase; hAPRT, human adenine phosphoribosyl transferase; PfADA, P. falciparum adenosine deaminase; PfPNP, P. falciparum purine nucleoside phosphorylase; PfHGXPRT, P. falciparum hypoxanthine-guanine-xanthine phosphoribosyl transferase; PfAMPDA, P. falciparum adenosine 5′-monophosphate deaminase; PfIMPDH, P. falciparum inosine 5′-monophosphate dehydrogenase; PfGMPs, P. falciparum guanosine 5′-monophosphate synthase; PfAdSS, adenylosuccinate synthetase; PfAdSL, adenylosuccinate lyase. Pyrimidine pathway: OMP, orotidine 5′-monophosphate; UMP, uridine 5′-monophosphate; UTP, uridine 5′-triphosphate; CTP, cytidine 5′-triphosphate; PfCPSII, P. falciparum carbamoyl phosphate synthetase II; PfATCase, P. falciparum aspartate carbamoyltransferase; PfDHOase, P. falciparum dihydroorotase; PfDHODH, P. falciparum dihydroorotate dehydrogenase; PfOPRT, P. falciparum orotate phosphoribosyltransferase; PfODC, P. falciparum orotidine 5′-monophosphate decarboxylase; PfCTPs, P. falciparum cytidine 5′-triphosphate synthase. Bold arrows on reversible steps, indicates the metabolically favored direction. Nucleoside/nucleobase transporters are indicated on each membrane: human erythrocyte nucleoside transporter (brown), P. falciparum NT1 transporter (purple) and yet-to-be characterized adenosine 5′-monophosphate transporter (red).
Unlike the abundance of purines in human erythrocytes, pyrimidines exist at only small concentrations. Therefore, P. falciparum has retained the capacity for de novo synthesis of pyrimidines and does not have active pathways for the salvage of pyrimidines from the host. De novo synthesis from carbamoyl phosphate and aspartic acid follows essentially the same metabolic steps found in other eukaryotes, including the human host. In this six-step process, orotic acid is formed by dihydroorotase (DHOase; EC 3.5.2.3) and dihydroorotate dehydrogenase (DHODH; EC 1.3.3.1) followed by addition to 5′-phospo-D-ribosyl-α-1-pyrophosphate to form orotidine 5′-monophosphate (OMP) by orotate phosphoribosyltransferase (OPRT; EC 2.4.2.10). OMP is subsequently decarboxylated to uridine 5′-monophosphate (UMP), the precursor of all other pyrimidine nucleotides and deoxynucleotides needed for nucleic acid synthesis Fig. (1). Following phosphorylation to UTP, CTP synthase (CTPs; EC 6.3.4.2) converts UTP to CTP in a rate-limiting step, the only known route for de novo synthesis of cytosine derivatives. Conversion of purine and pyrimidine nucleotides to deoxyribonucleotides, as in most eukaryotes, takes place at the level of nucleoside diphosphates, catalyzed by ribonucleotide diphosphate reductase (RNR; EC 1.17.4.1). Formation of thymine nucleotides requires methylation of dUMP to produce dTMP by thymidylate synthase (TS; EC 2.1.1.45), another step that parallels human metabolism and makes the parasite succeptible to dihydrofolate reductase inhibitors that disrupt the folate pathway.
P. falciparum cell growth and division demand robust purine and pyrimidine supplies, in particular dATP and dTTP, since the parasite contains the most (A+T)-rich genome sequenced to date (approximately 80%). Recent applications of enzymatic transition state theory to the practical problem of transition state analogue design has permitted the design and synthesis of unique and powerful inhibitors of ribosyltransferases, phosphoribosyltransferases and deaminases, enzymes essential to the pathways of nucleic acid production in P. falciparum. Inhibition of the purine salvage pathway with transition state analogue (TSA) inhibitors of both human and Plasmodium PNP, such as Immucillin-H (Imm-H) [10] and DADMeImm-G [9], is lethal for P. falciparum in vitro. Coformycin is a picomolar, transition state analogue inhibitor of both human and P. falciparum ADAs [11]; 2′-deoxycoformycin, a related ADA inhibitor, have been reported to have antimalarial potential in primates [12, 13]. Phosphorylated Immucillins including Immucillin-H 5′-phosphate and Immucillin-G 5′-phosphate bind tightly to P. falciparum HGXPRT [14]. Traditional drug discovery applied to malaria has relied on screening for biological activity, with many compounds being used in humans without knowledge of their mechanism of action. Quinine was the first such example and this discovery approach continues into the 21st century, where the artemisinins have gained wide use [15, 16]. Transition state analogue design is a departure by using transition state information for specific enzymes to provide powerful inhibitors as lead molecules with specific modes of action. Here we briefly review how TSA design is being applied to generate new lead compounds targeting purine and pyrimidine pathways related to the infective process of malaria parasites.
ENZYMATIC TRANSITION STATES AND ANALOG DESIGN
Enzymatic transition state analysis provides an understanding of catalysis from the perspective of bond changes at the catalytic site. Knowledge of the transition state permits the design of transition state inhibitors. All chemical transformations pass through the transition state, the point of equal probability for reactants on the reaction coordinate to be converted to product or to return to reactant. This structure is a characteristic of both the reactant and the catalyst; specifically for enzymes, involves the instantaneous geometry of the catalytic site at the transition state Fig. (2). Transition states have lifetimes similar to single bond vibrations, near 10−14 sec; methods are not available to directly observe transition state structures for enzymatic reactions. Transition state structure can be approached experimentally by measuring intrinsic kinetic isotope effects for the specific reaction. Computational chemistry is used to generate a quantum chemical structure of the transition state that matches the intrinsic isotope effects. This theoretical structure provides the blueprint for the transition state analogue design. Excellent accounts of this approach and its development are available [17–19].
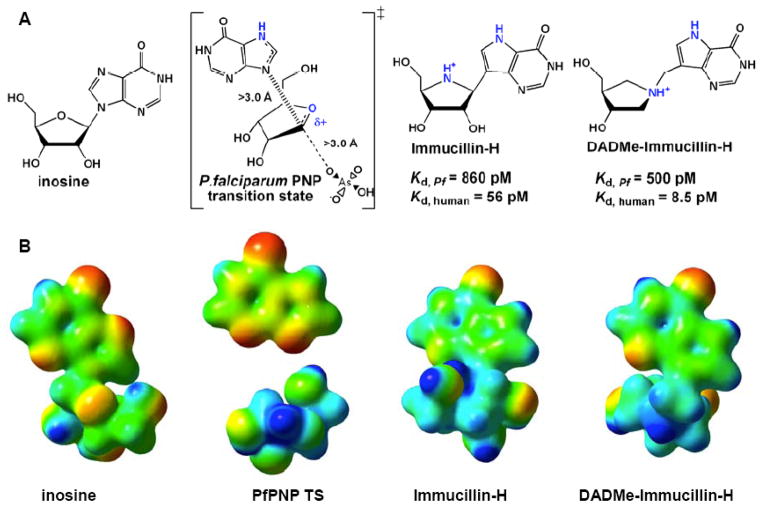
Fig. (2).
(A) Substrate, transition state and transition state analogue inhibitors for P. falciparum PNP. Atoms of critical importance in the reaction and in the transition state analogue design are colored in blue. (B) Molecular electrostatic potential surfaces of inosine, PfPNP transiton state, Immucillin-H and DADMe-Immucillin-H. Blue is electron deficient and red is electron rich in this diagram. Note that the stick figure of inosine in (A) is rotated 180° in the vertical axis for presentation in (B).
Transition state theory proposes that a perfectly designed, chemically stable mimic of the transition state will bind better than the substrate to the target enzyme by a factor equivalent to the catalytic rate enhancement imposed by the enzyme. Since enzymes typically accelerate solution reactions by factors of 1010 to 1015, it is theoretically possible to design inhibitors binding with unprecedented affinity [19]. In practice, dissociation constants in the range of 10−13 M have been achieved [20]. Transition state analogues capture the simultaneous dynamic molecular excursions used by enzymes to generate the transition state. This state is linked to the dynamic path of protein conformational change that the enzyme has evolved to favor catalysis. In the presence of a transition state analogue, the protein structure collapses around the analogue into a static, thermodynamically equilibrated potential energy well. Release of the analogue requires the slow and energetically unfavorable conformational expansion of the protein. Release rates of transition state analogue inhibitors are very slow, from minutes to days, resulting in tight binding [21]. The combination of tight binding with the high specificity of these “custom-made” inhibitors, results in low toxicity and minor side effects, two of the major problems with the current antimalarials. A more detailed explanation about enzymatic transition states and transition state analogues can be found in the literature [22, 23].
PURINE SALVAGE METABOLISM ENZYMES
Adenosine Deaminase
Mammalian adenosine deaminases (ADA, EC 3.5.4.4) catalyze the physiologically irreversible hydrolysis of adenosine or 2′-deoxyadenosine to the corresponding (deoxy)inosine and ammonia. P. falciparum ADA also deaminates 5′-methylthioadenosine (MTA) in addition to adenosine, Fig. (1) [24]. Thus, P. falciparum ADA serves the dual functions of adenosine salvage and recycling MTA formed from the synthesis of polyamines [24]. Mammalian ADAs do not deaminate MTA and instead express a specific MTA phosphorylase for recycling of MTA [24]. Mammalian erythrocytes do not synthesize polyamines. Thus, an intact polyamine synthetic pathway is required for the viability of malaria parasites [24, 25]. In P. falciparum, MTA is deaminated by malarial ADA to 5′-methylthioinosine (MTI), a metabolite that has not been reported in mammalian metabolism [26]. The Plasmodium PNP also serves a dual purpose by converting both inosine and MTI to hypoxanthine for conversion to IMP and incorporation into nucleic acids [24].
The unique substrate specificity of the P. falciparum ADA led us to synthesize 5′-methylthio-coformycin (MT-coformycin) as a specific transition state analogue inhibitor of plasmodial ADAs [11]. MT-Coformycin is a sub-nanomolar inhibitor of malarial ADA and demonstrates >20,000 fold selectivity for Plasmodium ADA relative to human ADA. This selectivity is remarkable since coformycin, a transition-state analogue, and 2′-deoxy-coformycin are powerful picomolar inhibitors of both human and P. falciparum ADAs [11]. Thus, coformycin, inhibits P. falciparum and human ADA with dissociation constants of 60 and 100 pM, respectively. The chemical structure of the P. falciparum and human ADA transition states resembles the sp3-hybridized Meisenheimer intermediate, rather than the reactant adenosine and the product inosine [27]. Although transition state features are similar for the reaction centers in human and malarial ADAs, distinct substrate specificity permits the design of parasite-specific transition state analogues. The tight binding of coformycin and 2′-deoxycoformycin with adenosine deaminases support a capture of transition state similarity as inhibitor binding energy.
The crystal structure of ADA from Plasmodium vivax in complex with MT-coformycin revealed an unprecedented binding geometry for 5′-methylthioribosyl groups in the malarial ADAs [13]. Compared to malarial ADA complexes with adenosine or 2′-deoxycoformycin, the 5′-methylthioribosyl groups are rotated 130°. A hydrogen bonding network between Asp172 and the 3′-hydroxyl of MT-coformycin is essential for recognition of the 5′-methylthioribosyl group. Water occupies the 5′-hydroxyl binding site when MT-coformycin is bound. Mutagenesis of Asp172 abrogates the substrate specificity for MTA and MT-coformycin. Coformycin alone does not inhibit parasite growth in cultured erythrocytes, a demonstration that host and parasite ADAs are not essential for growth in culture media [9]. However, when MTA was used as the sole purine source in cultured P. falciparum, we demonstrated that P. falciparum growth is inhibited by coformycin or MT-coformycin [13]. One indication that ADA may act as a target for drug design comes from a report that 2′-deoxycoformycin (Pentostatin) causes decreased parasitemia in P. knowlesi-infected primates [12]. Studies of MT-coformycin in Plasmodium-infected primates would test the hypothesis that the parasite ADA is important in establishing a viable infection.
Purine Nucleoside Phosphorylase
Mammalian purine nucleoside phosphorylases (PNP, EC 2.4.2.1) convert inosine, deoxyinosine, guanosine and deoxyguanosine into hypoxanthine and guanine, in each case releasing α-D-(deoxy)ribose 1′-phosphate. Daddona and coworkers first described the activity of P. falciparum PNP using protein extracts of parasites grown in erythrocytes from PNP-deficient donors. They were able to show that infection restores PNP-activity to high levels and that the parasite enzyme showed a approximately four-fold lower KM for both inosine and guanosine than human PNP [28]. Two decades later we expressed recombinant P. falciparum PNP and confirmed the high affinity of P. falciparum PNP for its substrates and also showed the decreased catalytic efficiency for 2′-deoxyinosine and 2′-deoxyguanosine. These findings support a metabolic role for P. falciparum PNP being hypoxanthine release from the host cell inosine obtained from the erythrocyte ATP pool [29]. In contrast, the role of human PNP is suppression of blood deoxyguanosine, which otherwise leads to a specific T-cell deficiency autoimmune disorder in humans [30].
The crystal structure of P. falciparum PNP bound to the transition state analogue Imm-H and a sulfate ion revealed a large solvent-filled cavity in the malarial PNP active site and is located near the 5′-hydroxyl group of Imm-H. This discovery distinguished the parasite enzyme from its human counterpart and indicated that P. falciparum PNP can accept bulky substitutions at the 5′-position. Kinetic investigations and structural analysis identified 5′-methylthioinosine as a good substrate for P. falciparum PNP [7]. Further metabolic investigation of the parasite identified a second role (see above) for malarial PNP and its partner, adenosine deaminase, in recycling of 5′-methylthiopurines formed in polyamine metabolism Fig. (1) [24].
Inhibitor design for PNP began with the transition state structure of PNP prepared from calf spleen. The transition state model showed a very early SN1-like transition state with an N-ribosyl bond distance of 1.8 Å, the ribosyl group exhibiting partial carbocation character, an increased pKa of the purine N7; no significant participation of the attacking anion nucleophile (arsenate was used to replace phosphate) [31]. Later, a comparative transition state analysis of the human and P. falciparum PNPs demonstrated these PNPs to have nearly identical transition states, even though their steady-state kinetic properties were distinct [32]. These transition states differed from bovine PNP in one key respect. The distance between ribose and hypoxanthine at the transition state was 1.8 Å in bovine PNP but was 3.0 Å for both human and P. falciparum PNPs. The chemically large difference in transition state structures distinguishes both the geometry and the charge of these transition states. Human and P. falciparum PNPs both generate a fully-developed ribocation at the transition state while bovine enzyme does not.
Using the electrostatic potential map of the transition state model of the bovine PNP transition state as a guide, we designed the transition state mimic Imm-H, which binds over 700,000 times tighter than substrate to the bovine enzyme [33]. Imm-H also shows strong, time-dependent inhibition of P. falciparum PNP to give a dissociation constant of 0.6 nM, 9,000-fold greater than the KM value for substrate [29]. The biological availability of Imm-H was demonstrated by the robust killing of P. falciparum in cell culture assays without excess hypoxanthine in the growth medium [10]. This killing was termed “purine-less death” since inhibition of PNP with Imm-H induced purine starvation. The Imm-H blocked both human and malarial PNPs and was rescued only by supraphysiological levels of hypoxanthine.
Design of Plasmodium-specific inhibitors was based on the dual substrate specificity found for P. falciparum PNP. Since P. falciparum PNP is active on inosine and 5′-methylthioinosine, 5′-methylthio-Immucillin-H (MT-Imm-H) was synthesized and tested. Disruption of the purine pathways with MT-Imm-H inhibited parasite growth in cell-culture assay with an IC50 of 50 nM similar to that of Imm-H. Parasite growth is slowed at low nanomolar concentrations; however, complete inhibition of growth was not achieved until MT-Imm-H reached micromolar concentrations [24]. The two-phase response curve to MT-Imm-H indicated that while P. falciparum PNP is important for normal growth of the parasite, human PNP must also be inhibited to completely block purine salvage in the cultured parasites.
Second-generation Immucillins, the DADMe-Immucillins, incorporate the specific features of the transition state structures specific for human and P. falciparum PNPs. A methylene linker between the iminoribitol and purine ring more accurately mimics the geometry, electrostatics and relative positions of the ribocation group and the hypoxanthine leaving group [34]. DADMe-Immucillin-H and DADMe-Immucillin-G inhibit human PNP with Ki values of 16 pM and 7 pM [21] and P. falciparum PNP with Ki values of 500 pM and 890 pM, respectively. As a proof of concept of the applicability of transition state analogues in vivo, a single oral dose of DADMe-Immucillin-H is able to completely inhibit erythrocyte PNP activity in mice for the entire duration of the erythrocyte life cycle [21].
Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase
Given the central role of hypoxanthine in the metabolism of P. falciparum, hypoxanthine/guanine (EC 2.4.2.8); xanthine phosphoribosyltransferase (EC 2.4.2.22) activities are critical for purine salvage. In P. falciparum, both activities are found in a single enzyme called HGXPRT. The malarial HGXPRT catalyzes the transfer of the phosphoribosyl moiety of PRPP to the 6-oxopurine nucleobases creating the respective monophosphorylated nucleotide and inorganic pyrophosphate. The human enzyme does not use xanthine as substrate, marking the divergence of the host and parasite purine salvage pathways. In humans, formation of xanthine constitutes irreversible loss from the purine pool since the only fate of xanthine in humans is oxidation to uric acid by xanthine oxidase. Purine metabolites earlier than xanthine in human and parasite metabolic pathways freely exchange between the host and parasite cytosols via the P. falciparum NT1 [35]. Other transporters are also likely to be involved since NT1 is the only characterized of four purine transports predicted to be present in the Plasmodium genome [36]. After the phosphoribosyl transfer, the purine nucleotides are sequestered in the parasite cytosol by the 5′-phosphate charges. Early studies of HGXPRT isolated from whole-cell lysates identified the unique ability of P. falciparum HGXPRT to use xanthine, hypoxanthine and guanine as substrates [37, 38]. Studies with recombinant HGXPRT were complicated by the low catalytic activity of P. falciparum enzyme expressed in Escherichia coli. Detailed analysis of preincubation conditions solved this problem of converting the inactive conformation to achieve an appreciable increase in catalytic activity [39]. While there are differing reports on the specific activity of P. falciparum HGXPRT, most agree that the KM of the enzyme for the nucleobase hypoxanthine is submicromolar, well below that of the human HGPRT [37–41]. This high affinity is indicative of the metabolic role for P. falciparum HGXPRT of binding free hypoxanthine, guanine and xanthine and preventing diffusional release until the enzyme can convert these bases to the nucleotide monophosphates, thus sequestering them into the parasite.
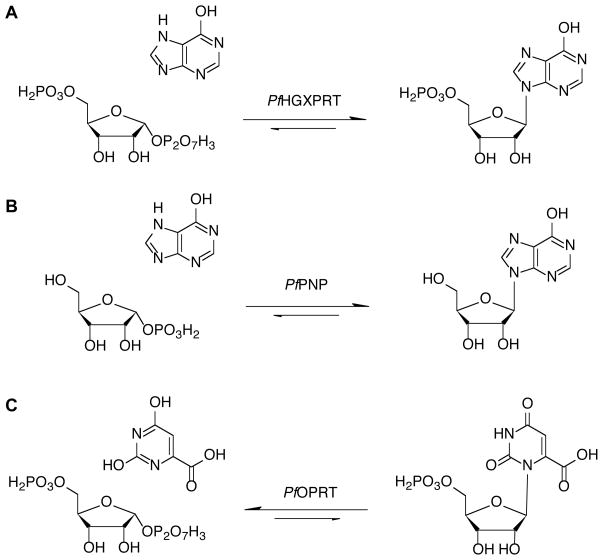
Tight binding of substrates to P. falciparum HGXPRT prevent the measurement of intrinsic kinetic isotope effects; hence, no transition state of a purine phosphoribosyltransferase has been solved. However, enzymes catalyzing similar reactions have had their transition states solved Fig. (3). Transition state structures have been solved for the orotate phosphoriboyltransferases from human, P. falciparum and Salmonella typhimurium sources, using a slow substrate analogue in place of pyrophosphate [42, 43]. These transition states show a highly-dissociative ribocation character with weak participation of the attacking nucleophiles. Other ribosyltransferases are similar to the reaction of HGXPRT, in that the transition state involves bond breaking between ribose and a 6-oxypurine leaving group. Specifically, PNP reactions have this type of transition state. As these ribosyltransferases all have ribocation character at their transition states, the transition state of HGXPRT may be similar.
Fig. (3).
Comparison of the HGXPRT reaction (A) to similar reactions in which the transition state structures have been solved by the combination of kinetic isotope effects and computational chemistry described in the text. The reaction in (B) is for purine nucleoside phosphorylase and in (C) for orotate phosphoribosyltransferase. The pyrophosphate products are not showed.
Transition state analogue design elements from the PNP and OPRT reactions were used to design inhibitors of P. falciparum HGXPRT. The 5′-phosphorylated Immucillins, Immucillin-H 5′-P (Imm-HP) and Immucillin-G 5′-P (Imm-GP) are low nanomolar inhibitors for both the human HGPRT and P. falciparum HGXPRT [14]. Crystal structures of the human and parasite enzymes bound to Imm-GP and Imm-HP, respectively, did not reveal significant differences in their active sites that can be readily exploited [44, 45]. However, recent work from John de Jersey’s group has reported a family of linear-phosphonate compounds linked to purine bases that are selective for the parasite relative to the human purine phosphoribosyltransferases [46]. A significant barrier for inhibitor design against HGXPRT is the apparent need for the 5′-phosphate group for tight binding to the enzyme. Nucleotides are not easily transported into cells and are readily hydrolyzed in the gut, making oral delivery problematic. Prodrug protecting group chemistries are possible, but these increase the cost of goods to beyond useful ranges for most tropical disease markets.
Downstream Enzymes in the Purine Salvage Metabolism
Although the discussion above has focused on early steps in purine salvage pathways, all downstream enzymes (IMP → nucleic acid precursors) are also viable targets. Of these, only the guanosine monophosphate synthase (GMPs, EC 6.3.5.20) [47] adenylosuccinate synthetase (AdSS, EC 6.3.4.4) [48, 49] and adenylosuccinate lyase (AdSL, EC 4.3.2.2) [50] had been characterized in P. falciparum. GMPs catalyses the last step in GMP formation in the purine salvage pathway Fig. (1). The irreversible conversion of XMP into GMP is a reaction that requires ATP and involves the transfer of an amino group from glutamine to the C-2 carbon of XMP via an adenyl-XMP intermediate. Adenylosuccinate lyase is known to catalyze two distinct but chemically similar reactions in purine biosynthesis. One reaction is exclusive to the de novo pathway and involves cleavage of 5-aminoimidazole-4-(N-succinylcarboxamide) ribonucleotide (SAICAR) to 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and fumarate but this pathway is absent in P. falciparum. The second reaction catalyzed by this enzyme is required downstream of IMP and involves the cleavage of succinyladenosine monophosphate (SAMP) to AMP and fumarate. P. falciparum AdSL catalyzes the cleavage of SAICAR with a KM of 40.0 ± 8 μM and binds AICAR with affinity similar to that of AMP [50]. Binding of AICAR has no function in P. falciparum metabolism, but this residual memory of its evolutionary history can be explored as a potential target for drug design.
DE NOVO PYRIMIDINE METABOLISM ENZYME
Carbamoyl Phosphate Synthetase II
Carbamoyl phosphate synthetase II (CPSII, EC 6.3.5.5) catalyzes the formation of carbamoyl phosphate in the cytosol from bicarbonate, glutamine and ATP. This is the first step of de novo pyrimidine biosynthesis. CPSII is activated by ATP and PRPP and inhibited by UTP [51]. P. falciparum CPSII is a monofunctional protein, while human CPSII activity is included in a trifunctional protein named CAD [5]. The mRNA transcript of P. falciparum CPSII possesses two inserted sequences that are absent in other homologues. These insertions are located between junctions of the glutamine aminotransferase and synthetase domains [52]. A synthetic ribozyme with specificity for the P. falciparum CPSII gene has been shown to have potent inhibitory effect on growth of cultured P. falciparum parasites and no toxicity to mammalian cells [53]. Encoded amino acid insertions are common in Plasmodium transcripts, are unique to the parasite and provide potential targets for nucleic acid therapy. Hendry and colleagues have redesigned and tested a series of miniribozymes with high specificities against P. falciparum CPSII gene in vitro [54]. These ribozyme derivatives with chemical modifications exhibit improved cleavage activities and metabolic stabilities. Although ribozyme approaches are technologically amusing, the barriers to application in tropical medicine are significant, including target accessibility, stability, specificity and delivery efficiency [55].
Dihydroorotase
Dihydroorotase (DHOase, EC 3.5.2.3), the third enzyme in the pyrimidine biosynthesis pathway, catalyzes the reversible cyclization of N-carbamoyl-L-aspartate (CA-asp) to L-dihydroorotate (DHO). P. falciparum DHOase is a mono-functional protein with sequence most similar to Escherichia coli. Mammalian DHOase is the central sequence of the CAD trifunctional protein [56]. 5′-Substituted orotate derivatives are weak competitive inhibitors of P. falciparum DHOase [57]. L-6-Thiodihydroorotate (TDHO) is a moderate inhibitor of malarial DHOase with a Ki of 6.5 μM. Inhibition of DHOase in P. falciparum by TDHO or its methyl ester results in accumulation of CA-asp in cultured parasites [58]. Several dihydropyrimidine analogues have been synthesized and exhibit inhibition against mammalian DHOase at nanomolar concentrations [59–61].
Dihydroorotate Dehydrogenase
Dihydroorotate dehydrogenase (DHODH, EC 1.3.5.2) is a flavin mononucleotide (FMN)-dependent catalyst for the conversion of dihydroorotate (DHO) to orotate. There are two types of DHODH. Type I is a cytosolic enzyme found in Saccharomyces and certain protozoa and requires fumarate or NAD+ as electron acceptor. P. falciparum and humans express type II DHODH, a membrane-bound protein linked to ubiquinone (CoQ) as the electron acceptor [62, 63]. Malarial and human DHODH reside in the outer side of the inner mitochondrial membrane. The first half of reaction catalyzed by P. falciparum and human DHODHs involves oxidation of DHO to orotate coupled with electron transfer to FMN. The electron pair is then transferred to CoQ in the second half of reaction, with concomitant recycling of FMNH2 to FMN.
Human DHODH is a validated drug target. Leflunomide has been used for the treatment of rheumatoid arthritis [64, 65]. Its active metabolite A77-1726 exhibits potency against human DHODH activity by acting as CoQ analogue [66]. Brequinar, a potent 4-quinolinecarboxylic acid inhibitor of human DHODH, has been used as an anti-tumor and immunosuppressive agent [67, 68].
By employing structure-based computational drug design, Davies and colleagues have synthesized a variety of A77-1726 derivatives with good inhibitory potential against P. falciparum DHODH [69]. From a library containing 220,000 drug-like molecules, Baldwin and colleagues identified a competitive inhibitor of P. falciparum DHODH with an IC50 value of 16 nM and 12,500-fold selectivity over the human enzyme [70]. Chemical library screening of >200,000 compounds by Patel and colleagues has identified potent inhibitors with different scaffolds for malarial DHODH [71]. These species-specific inhibitors display submicromolar IC50 values against cultured P. falciparum and have low toxicity for human cells. Phillips and colleagues have identified a triazolopyrimidine-based compound with potent antimalarial activity and high specificity for P. falciparum DHODH [72]. This P. falciparum DHODH-specific inhibitor arrests parasite growth in vitro with an IC50 of 0.14 μM for the Dd2 strain. However, this compound has no activity in vivo due to its metabolic instability. In contrast, phenyl-substituted triazolopyrimidines are metabolically stable and possess similar potent inhibitory and selectivity on P. falciparum DHODH. A p-trifluoromethylphenyl exhibits prolonged exposure in vivo and suppresses the parasitemia in P. berghei infected mice [73]. Tricyclic aromatic amines have also been identified as potent inhibitors against malarial DHODH with low nanomolar binding affinities [74]. In vitro studies show that these inhibitors block the growth of cultured parasites at submicromolar concentrations.
Atovaquone, a CoQ analogue, has an IC50 of 1.0 nM against cultured P. falciparum [75, 76]. By forming a covalent bond with a protein from Complex III in the parasite mitochondria, atovaquone collapses the membrane potential and causes cell death and the indirect inhibition of P. falciparum DHODH activity [77–79]. The selective action of atovaquone on the parasite electron transport chain results from the structural difference in the CoQ complexes of humans and parasites [5]. Malarone™, an antimalarial approved by the U.S. Food and Drug Administration (FDA), is a combination of atovaquone and proguanil. Proguanil is a prodrug of cycloguanil, a dihydrofolate reductase inhibitor and therefore brings a synergistic effect to atovaquone action [80].
Orotate Phosphoribosyltransferase
Orotate phosphoribosyltransferase (OPRT, EC 2.4.2.10) is the fifth enzyme in the de novo pyrimidine biosynthesis pathway. It catalyzes the formation of orotidine 5′-monophosphate (OMP) from α-D-phosphoribosylpyrophosphate (PRPP) and orotate. In most prokaryotes OPRT and OMP decarboxylase (ODC) activities are encoded in separate proteins. However, in eukaryotes, uridine 5′-monophosphate (UMP) synthase is bifunctional and contains both enzyme activities [62, 81, 82]. Transition state structures of P. falciparum and human OPRT were analyzed by KIEs, computational chemistry and substrate specificity [42]. Both P. falciparum and human OPRT form late associative transition states with complete orotate loss and partially associative nucleophile. p-Nitrophenyl β-D-ribose 5′-phosphate is a poor substrate of both enzymes, but is a nanomolar inhibitor. The use of these agents as antimalarials has not been reported.
5′-Fluoroorotate is an alternative substrate for P. falciparum OPRT with the same kinetic parameters as orotate [83]. It inhibits the in vitro growth of P. falciparum with an IC50 less than 10 nM [84, 85]. The potent antimalarial activity of 5′-fluoroorotate is related to the inactivation of malarial thymidylate synthase by 5′-fluoro-2′-deoxy-UMP metabolite through covalent binding to methylene tetrahydrofolate at the active site. 5′-Fluoroorotate clears parasitemia from mice infected with P. berghei [86]. Other 5′-substituted orotate analogues display competitive inhibitory effects against P. falciparum OPRT activity, but the antimalarial activities of those compounds are at least 100-fold less than 5′-fluoroorotate [83]. Uracil and 5′-fluorouracil are weak competitive inhibitors of P. falciparum OPRT and reduce P. falciparum in vitro growth with IC50 values of 5 to 10 μM [84, 87].
Pyrazofurin, a C-riboside antibiotic, is a modest inhibitor of P. falciparum OPRT. Its antimalarial activity exhibits IC50 values of 6 to 24 μM by blocking the maturation of trophozoites to schizonts [84, 88]. Since Plasmodium lack enzymes for pyrimidine salvage, the toxicity was not relieved by the addition of uracil or uridine to the culture. Pyrazofurin does not inhibit the OPRT activity in mammalian cells [89]. However, its 5′-monophosphate derivative is a potent inhibitor against both mammalian and malarial ODC [58, 90, 91].
Orotidine 5′-Monophosphate Decarboxylase
Orotidine 5′-monophosphate decarboxylase (ODC, EC 4.1.1.23), has received considerable attention as being one of the most proficient enzymes. It catalyzes the decarboxylation of orotidine 5′-monophosphate (OMP) to uridine 5′-monophosphate (UMP) with a rate enhancement in excess of 1017-fold [92]. Unlike most decarboxylases, ODC activity does not require the presence of a cofactor or metal ion [93]. ODC-catalyzed conversion of OMP to UMP in the final step of de novo pyrimidine biosynthesis pathway. The ODC and orotate phosphoribosyltransferase (OPRT) activities in most prokaryotes and lower eukaryotes are distinct proteins but are expressed as a bifunctional UMP synthase in higher eukaryotes [82].
Nucleotide 5′-monophosphate analogues of P. falciparum ODC, including 6-aza-UMP, allopurinol 5′-monophosphate, pyrazofurin 5′-monophosphate and xanthosine 5′-monophosphate (XMP) are competitive inhibitiors with tighter binding than OMP [94]. Of these, XMP is highly selective with a Ki of 4.4 nM for P. falciparum ODC and 670 nM for human ODC, a 150-fold preference for the malarial enzyme. 6-Aza-UMP has a Ki of 12 nM for P. falciparum ODC and 510 nM for human ODC, 43-fold selectivity P. falciparum ODC. Pyrazofurin 5′-monophosphate is a potent inhibitor against P. falciparum ODC with Ki of 3.6 nM.
6-Iodouridine 5′-monophosphate (6-iodo-UMP) and 6-azidouridine 5′-monophosphate (6-N3-UMP) are potent, covalent inhibitors of P. falciparum ODC [95, 96]. Mass spectral analyses and crystal structures of enzyme-inhibitor complexes reveal that both inhibitors form covalent adducts to Lys138. 6-Iodouridine, the nucleoside form of 6-iodo-UMP, shows antimalarial activities with an IC50 of 4.4 μM and 6.2 μM against P. falciparum ItG strain (chloroquine-resistant) and 3D7 strain (chloroquine-susceptible). Cytotoxicity against CHO cells gives an IC50 of 366 μM for 6-iodouridine, 60-fold lower than toxicity against parasites. Unlike 6-iodouridine, 6-azidouridine (nucleoside form of 6-N3-UMP) does not inhibit cultured P. falciparum growth, possibly from inefficient cellular phosphorylation.
Barbiturate 5′-monophosphate (BMP) is a powerful inhibitor of human and yeast ODC with Ki of 9 pM [97, 98] but its complex inhibitory effect on P. falciparum ODC activity has not been mechanistically resolved [94]. Other 6-substituted UMP derivatives are competitive inhibitors of P. falciparum ODC with submicromolar Ki values [96]. For the nucleoside forms of these 6-substituted derivatives, only 6-N-methylamino uridine and 6-N,N-dimethylamino uridine exhibit moderate antimalarial activities with IC50 of 28 μM and 32 μM, respectively. Other derivatives have no inhibitory effect against cultured P. falciparum. Despite the inhibitory potential for the pyrimidine nucleoside and nucleotide analogues listed above, concerns remain for side effects with those analogues capable of being incorporated into host nucleic acids.
Deoxyuridine 5′-Triphosphate Nucleotide Hydrolase
Deoxyuridine 5′-triphosphate nucleotidohydrolase (dUT-Pase, EC 3.6.1.23) catalyzes the hydrolysis of deoxyuridine 5′-triphosphate (dUTP) to deoxyuridine 5′-monophosphate (dUMP) and inorganic pyrophosphate. Because most DNA polymerases can not distinguish between dUTP and dTTP, misincorporation of dUTP into DNA depends on low levels of dUTP. Loss of this enzyme activity generates cells with high mutation rates [99]. The product dUMP is the precursor for dTMP synthesis. In many organisms dCMP deamination also generates dUMP, but Plasmodium only encode dCTP deaminases [99]. Thus, dUTPase appears to be essential for dUMP synthesis. The expression of P. falciparum dUTPase is associated with parasite proliferative stages [100]. P. falciparum dUTPase is strictly specific for dUTP with discrimination against ribosyl and base changes [101]. It functions as homotrimer with similar structural fold and subunit organization to human dUTPase [100].
Triphenylmethane derivatives of deoxyuridine are reported to be selective inhibitors against P. falciparum dUT-Pase and exhibit moderate inhibitory effects on in vitro growth with IC50 in the low micromolar range [100]. Nguyen and colleagues have identified a number of 5′-tritylated deoxyuridine analogues with antimalarial activities and more than 200-fold selectivities relative to human dUTPase [102]. Acyclic nucleoside derivatives also show inhibitory effects against P. falciparum dUTPase [103]. The most potent acyclic analogues (containing triphenylsilyloxy groups) arrest P. falciparum in vitro growth with an IC50 of 0.4 μM and display low toxicity to mammalian cells. Tritylated uracil acetamide derivatives exhibit selective action on P. falciparum dUTPase and moderate antimalarial activities [104].
Dihydrofolate Reductase
Dihydrofolate reductase (DHFR, EC 1.5.1.3) is indispensable for de novo folate biosynthesis, salvage of folate derivatives and recycling of dihydrofolate (DHF) formed in dTMP synthesis [105]. P. falciparum DHFR activity is the N-terminal portion of the bifunctional protein thymidylate synthase-dihydrofolate reductase (TS-DHFR) [106, 107]. Human DHFR and TS activities are present in two separate proteins. In P. falciparum the TS-DHFR catalyzes two essential reactions, dTMP synthesis and the conversion of DHF to methylenetetrahydrofolate (CH2H4folate). DHFR is one of the best established, multiply-targeted enzymes in malaria.
Pyrimethamine is a potent inhibitor of P. falciparum DHFR with Ki of 0.2 nM [108]. In combination with sulfadoxine, an inhibitor of dihydropteroate synthase (DHPS), pyrimethamine is marketed as Fansidar™. The combination of DHFR and DHPS inhibitors is synergistic since Plasmodium also synthesizes the dihydropteroate group needed for folate synthesis, a process not found in humans. Cycloguanil, the active metabolite of proguanil, shares the 2, 4-diamino scaffold with pyrimethamine and exhibits potency against P. falciparum DHFR (Ki = 0.3 nM) [108]. Lapdap™ is a combination of chloroproguanil (chlorcycloguanil prodrug, an inhibitor of DHFR) and dapsone (an inhibitor of DHPS) [109]. This antimalarial is effective against pyrimethamine-resistant P. falciparum strains [110]. The diamino triazine derivative WR99210 has a flexible trichlorophenoxypropyloxy side chain and is effective against the pyrimethamine-resistant parasites [111, 112]. The Ki value of WR99210 is in the subnanomolar range for native P. falciparum TS-DHFR as well as for double and quadruple mutant strains [108]. The cytotoxicity and poor oral bioavailability of WR99210 prevent its clinical use [113, 114]. PS-15, the phenoxypropoxybiguanide precursor of WR99210, shows improved bioavailability and tolerance by the host cells [115].
Analogues of pyrimethamine and cycloguanil have been developed against drug-resistant strains. Several of these are low nanomolar inhibitors of P. falciparum DHFR from mutant strains and show low toxicity to mammalian cells [116]. Trimethoprim (TMP) derivatives have also been developed and show antimalarial activities against parasites with mutant DHFR enzymes [117]. Through in silico screening, Dasgupta and colleagues identified three inhibitors of P. falciparum DHFR with IC50 of 20 μM for wild type or antifolate-resistant P. falciparum strains [118]. These biguanide compounds exhibit highly specific action on parasites. Antisense oligodeoxynucleotides with sequences targeting mRNA transcripts of P. falciparum DHFR also show selective growth inhibition on cultured parasites (IC50 ~ 0.05 μM) [119].
Thymidylate Monophosphate Kinase
Thymidylate monophosphate kinase (TMK, EC 2.7.4.9) catalyzes the reversible phosphorylation of dTMP to deoxythymidine diphosphate (dTDP), an essential step for cellular DNA synthesis. TMK from Mycobacterium tuberculosis is a target for antituberculosis agents with bicyclic thymidine derivatives providing micromolar binding affinities to M. tuberculosis TMK [120]. Thymidine analogues with 5′-thiourea-substituents inhibit M. tuberculosis TMK at nanomolar concentrations. These compounds also act on malarial enzyme TMK, display moderate antituberculosis activities, and exhibit 600-fold selectivity relative to human TMK [121]. P. falciparum TMK has broad substrate specificity including dTMP, dUMP and purine nucleotides [122]. This specificity distinguishes malarial TMK from other homologues including the human enzyme and makes the Plasmodium enzyme a viable target. A few nucleoside analogues display low micromolar inhibition activity against P. falciparum TMK [123].
Cytidine Triphosphate Synthetase
Cytidine triphosphate synthetase (CTPs, EC 6.3.4.2) catalyzes the conversion of UTP to CTP. The de novo pyrimidine biosynthesis pathway-catalyzed formation of CTP is the only route leading to cytosine derivatives [5]. CTPs is actively expressed in P. falciparum parasites [124]. P. falciparum CTPs protein contains two unique peptide segments, differing from human CTPs, suggesting a potential target for drug design [125]. Although there are no potent inhibitors against this enzyme, 3-deazauridine and cyclopentenyl cytosine are converted to triphosphate derivatives in mammalian cells and become potent inhibitors of mammalian CTPs [126, 127]. These nucleoside analogues have shown anti-tumor and anti-HIV activities [128–131].
SUMMARY
Targeting the catalytic machinery of Plasmodium species in the design of antimalarials has only begun. Differences in protein structure and specificity provide one approach. Differences in the host and parasite metabolism provide another. Finally, the trend toward drug combinations for malaria is expected to continue and broaden as powerful new inhibitors are brought to bear on novel targets.
Acknowledgments
SUPPORT
Work reported here and the production of this article was supported by NIH research grants AI049512 and GM41916.
We thank our laboratory members, Dr. Emilio F. Merino for making Fig. (1) and Dr. Jemy A. Gutierrez for helpful discussions. Chemical synthesis of the Immucillins is a product of our long and productive collaboration with Richard H. Furneaux, Peter C. Tyler and Gary B. Evans; others at the Carbohydrate Chemistry Division of Industrial Research Ltd., Lower Hutt, New Zealand. Their contributions are acknowledged in the appropriate references.
Footnotes
POTENTIAL CONFLICT OF INTEREST
Some of the Immucillin transition state analogues described here are in clinical development by BioCryst Pharmaceuticals Inc., under license from the Albert Einstein College of Medicine. Vern L. Schramm is a consultant to BioC-ryst Pharmaceuticals, Inc.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyde JE. Drug-resistant malaria. Trends Parasitol. 2005;21:494–498. doi: 10.1016/j.pt.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyde JE. Drug-resistant malaria - an insight. Febs J. 2007;274:4688–4698. doi: 10.1111/j.1742-4658.2007.05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HP, Bridges DJ, Burchmore RJS. Purine and pyrimidine transport in pathogenic protozoa: From biology to therapy. FEMS Microbiol Rev. 2005;29:987–1020. doi: 10.1016/j.femsre.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Hyde JE. Targeting purine and pyrimidine metabolism in human apicomplexan parasites. Curr Drug Targets. 2007;8:31–47. doi: 10.2174/138945007779315524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes P, Rathod PK, Sanchez DJ, Mrema JE, Rieckmann KH, Heidrich HG. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1982;5:275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- 7.Shi W, Ting LM, Kicska GA, Lewandowicz A, Tyler PC, Evans GB, Furneaux RH, Kim K, Almo SC, Schramm VL. Plasmodium falciparum purine nucleoside phosphorylase: crystal structures, immucillin inhibitors; dual catalytic function. J Biol Chem. 2004;279:18103–18106. doi: 10.1074/jbc.C400068200. [DOI] [PubMed] [Google Scholar]
- 8.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassera MB, Hazleton KZ, Riegelhaupt PM, Merino EF, Luo M, Akabas MH, Schramm VL. Erythrocytic adenosine monophosphate as an alternative purine source in Plasmodium falciparum. J Biol Chem. 2008;283:32889–32899. doi: 10.1074/jbc.M804497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kicska GA, Tyler PC, Evans GB, Furneaux RH, Schramm VL, Kim K. Purine-less death in Plasmodium falciparum induced by immucillin-H, a transition state analogue of purine nucleoside phosphorylase. J Biol Chem. 2002;277:3226–3231. doi: 10.1074/jbc.M105906200. [DOI] [PubMed] [Google Scholar]
- 11.Tyler PC, Taylor EA, Frohlich RF, Schramm VL. Synthesis of 5′-methylthio coformycins: specific inhibitors for malarial adenosine deaminase. J Am Chem Soc. 2007;129:6872–6879. doi: 10.1021/ja0708363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster HK, Wiesmann WP, Pavia CS. Adenosine deaminase in malaria infection: effect of 2′-deoxycoformycin in vivo. Adv Exp Med Biol. 1984;165(Pt A):225–229. doi: 10.1007/978-1-4684-4553-4_44. [DOI] [PubMed] [Google Scholar]
- 13.Ho MC, Cassera MB, Madrid DC, Ting LM, Tyler PC, Kim K, Almo SC, Schramm VL. Structural and metabolic specificity of methylthiocoformycin for malarial adenosine deaminases. Biochemistry. 2009;48:9618–9626. doi: 10.1021/bi9012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CM, Tyler PC, Furneaux RH, Kicska G, Xu Y, Grubmeyer C, Girvin ME, Schramm VL. Transition-state analogs as inhibitors of human and malarial hypoxanthine-guanine phosphoribosyltransferases. Nat Struct Biol. 1999;6:582–587. doi: 10.1038/9367. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhou B. Biological actions of artemisinin: insights from medicinal chemistry studies. Molecules. 2010;15:1378–1397. doi: 10.3390/molecules15031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin--the debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfenden R. Transition state analogues for enzyme catalysis. Nature. 1969;223:704–705. doi: 10.1038/223704a0. [DOI] [PubMed] [Google Scholar]
- 18.Schramm VL. Enzymatic transition state theory and transition state analogue design. J Biol Chem. 2007;282:28297–28300. doi: 10.1074/jbc.R700018200. [DOI] [PubMed] [Google Scholar]
- 19.Schramm VL. Enzymatic transition state poise and transition state analogues. Acc Chem Res. 2003;36:588–596. doi: 10.1021/ar0200495. [DOI] [PubMed] [Google Scholar]
- 20.Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, Howell PL, Schramm VL. Femtomolar transition state analogue inhibitors of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J Biol Chem. 2005;280:18265–18273. doi: 10.1074/jbc.M414472200. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowicz A, Tyler PC, Evans GB, Furneaux RH, Schramm VL. Achieving the ultimate physiological goal in transition state analogue inhibitors for purine nucleoside phosphorylase. J Biol Chem. 2003;278:31465–31468. doi: 10.1074/jbc.C300259200. [DOI] [PubMed] [Google Scholar]
- 22.Schramm VL. Enzymatic transition states and transition state analog design. Annu Rev Biochem. 1998;67:693–720. doi: 10.1146/annurev.biochem.67.1.693. [DOI] [PubMed] [Google Scholar]
- 23.Schramm VL. Enzymatic transition states and transition state analogues. Curr Opin Struct Biol. 2005;15:604–613. doi: 10.1016/j.sbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Ting LM, Shi W, Lewandowicz A, Singh V, Mwakingwe A, Birck MR, Ringia EA, Bench G, Madrid DC, Tyler PC, Evans GB, Furneaux RH, Schramm VL, Kim K. Targeting a novel Plasmodium falciparum purine recycling pathway with specific immucillins. J Biol Chem. 2005;280:9547–9554. doi: 10.1074/jbc.M412693200. [DOI] [PubMed] [Google Scholar]
- 25.Trackman PC, Abeles RH. Methionine synthesis from 5′-S-Methylthioadenosine. Resolution of enzyme activities and identification of 1-phospho-5-S methylthioribulose. J Biol Chem. 1983;258:6717–6720. [PubMed] [Google Scholar]
- 26.Olszewski KL, Morrisey JM, Wilinski D, Burns JM, Vaidya AB, Rabinowitz JD, Llinas M. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe. 2009;5:191–199. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo M, Singh V, Taylor EA, Schramm VL. Transition-state variation in human, bovine; Plasmodium falciparum adenosine deaminases. J Am Chem Soc. 2007;129:8008–8017. doi: 10.1021/ja072122y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daddona PE, Wiesmann WP, Milhouse W, Chern JW, Townsend LB, Hershfield MS, Webster HK. Expression of human malaria parasite purine nucleoside phosphorylase in host enzyme-deficient erythrocyte culture. Enzyme characterization and identification of novel inhibitors. J Biol Chem. 1986;261:11667–11673. [PubMed] [Google Scholar]
- 29.Kicska GA, Tyler PC, Evans GB, Furneaux RH, Kim K, Schramm VL. Transition state analogue inhibitors of purine nucleoside phosphorylase from Plasmodium falciparum. J Biol Chem. 2002;277:3219–3225. doi: 10.1074/jbc.M105905200. [DOI] [PubMed] [Google Scholar]
- 30.Markert ML. Purine nucleoside phosphorylase deficiency. Immunodefic Rev. 1991;3:45–81. [PubMed] [Google Scholar]
- 31.Kline PC, Schramm VL. Purine nucleoside phosphorylase. Catalytic mechanism and transition-state analysis of the arsenolysis reaction. Biochemistry. 1993;32:13212–13219. doi: 10.1021/bi00211a033. [DOI] [PubMed] [Google Scholar]
- 32.Lewandowicz A, Schramm VL. Transition state analysis for human and Plasmodium falciparum purine nucleoside phosphorylases. Biochemistry. 2004;43:1458–1468. doi: 10.1021/bi0359123. [DOI] [PubMed] [Google Scholar]
- 33.Miles RW, Tyler PC, Furneaux RH, Bagdassarian CK, Schramm VL. One-third-the-sites transition-state inhibitors for purine nucleoside phosphorylase. Biochemistry. 1998;37:8615–8621. doi: 10.1021/bi980658d. [DOI] [PubMed] [Google Scholar]
- 34.Lewandowicz A, Shi W, Evans GB, Tyler PC, Furneaux RH, Basso LA, Santos DS, Almo SC, Schramm VL. Over-the-barrier transition state analogues and crystal structure with Mycobacterium tuberculosis purine nucleoside phosphorylase. Biochemistry. 2003;42:6057–6066. doi: 10.1021/bi0343830. [DOI] [PubMed] [Google Scholar]
- 35.Riegelhaupt PM, Cassera MB, Frohlich RF, Hazleton KZ, Hefter JJ, Schramm VL, Akabas MH. Transport of purines and purine salvage pathway inhibitors by the Plasmodium falciparum equilibrative nucleoside transporter PfENT1. Mol Biochem Parasitol. 2010;169:40–49. doi: 10.1016/j.molbiopara.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RE, Henry RI, Abbey JL, Clements JD, Kirk K. The ‘permeome’ of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum. Genome Biol. 2005;6:R26. doi: 10.1186/gb-2005-6-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Queen SA, Vander Jagt D, Reyes P. Properties and substrate specificity of a purine phosphoribosyltransferase from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1988;30:123–133. doi: 10.1016/0166-6851(88)90105-3. [DOI] [PubMed] [Google Scholar]
- 38.Vasanthakumar G, Davis RL, Jr, Sullivan MA, Donahue JP. Cloning and expression in Escherichia coli of a hypoxanthine-guanine phosphoribosyltransferase-encoding cDNA from Plasmodium falciparum. Gene. 1990;91:63–69. doi: 10.1016/0378-1119(90)90163-l. [DOI] [PubMed] [Google Scholar]
- 39.Keough DT, Ng AL, Winzor DJ, Emmerson BT, de Jersey J. Purification and characterization of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase and comparison with the human enzyme. Mol Biochem Parasitol. 1999;98:29–41. doi: 10.1016/s0166-6851(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 40.Raman J, Ashok CS, Subbayya SI, Anand RP, Selvi ST, Balaram H. Plasmodium falciparum hypoxanthine guanine phosphoribosyltransferase. Stability studies on the product-activated enzyme. FEBS J. 2005;272:1900–1911. doi: 10.1111/j.1742-4658.2005.04620.x. [DOI] [PubMed] [Google Scholar]
- 41.Sujay Subbayya IN, Balaram H. Evidence for multiple active states of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase. Biochem Biophys Res Commun. 2000;279:433–437. doi: 10.1006/bbrc.2000.3962. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Luo M, Schramm VL. Transition states of Plasmodium falciparum and human orotate phosphoribosyltransferases. J Am Chem Soc. 2009;131:4685–4694. doi: 10.1021/ja808346y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao W, Grubmeyer C, Blanchard JS. Transition state structure of Salmonella typhimurium orotate phosphoribosyltransferase. Biochemistry. 1996;35:14–21. doi: 10.1021/bi951898l. [DOI] [PubMed] [Google Scholar]
- 44.Shi W, Li CM, Tyler PC, Furneaux RH, Grubmeyer C, Schramm VL, Almo SC. The 2.0 Å structure of human hypoxanthine-guanine phosphoribosyltransferase in complex with a transition-state analog inhibitor. Nat Struct Biol. 1999;6:588–593. doi: 10.1038/9376. [DOI] [PubMed] [Google Scholar]
- 45.Shi W, Li CM, Tyler PC, Furneaux RH, Cahill SM, Girvin ME, Grubmeyer C, Schramm VL, Almo SC. The 2.0 Å structure of malarial purine phosphoribosyltransferase in complex with a transition-state analogue inhibitor. Biochemistry. 1999;38:9872–9880. doi: 10.1021/bi990664p. [DOI] [PubMed] [Google Scholar]
- 46.Keough DT, Hockova D, Holy A, Naesens LM, Skinner-Adams TS, Jersey J, Guddat LW. Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics. J Med Chem. 2009;52:4391–4399. doi: 10.1021/jm900267n. [DOI] [PubMed] [Google Scholar]
- 47.Bhat JY, Shastri BG, Balaram H. Kinetic and biochemical characterization of Plasmodium falciparum GMP synthetase. Biochem J. 2008;409:263–273. doi: 10.1042/BJ20070996. [DOI] [PubMed] [Google Scholar]
- 48.Eaazhisai K, Jayalakshmi R, Gayathri P, Anand RP, Sumathy K, Balaram H, Murthy MR. Crystal structure of fully ligated adenylosuccinate synthetase from Plasmodium falciparum. J Mol Biol. 2004;335:1251–1264. doi: 10.1016/j.jmb.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 49.Raman J, Mehrotra S, Anand RP, Balaram H. Unique kinetic mechanism of Plasmodium falciparum adenylosuccinate synthetase. Mol Biochem Parasitol. 2004;138:1–8. doi: 10.1016/j.molbiopara.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Bulusu V, Srinivasan B, Bopanna MP, Balaram H. Elucidation of the substrate specificity, kinetic and catalytic mechanism of adenylosuccinate lyase from Plasmodium falciparum. Biochim Biophys Acta. 2009;1794:642–654. doi: 10.1016/j.bbapap.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Gero AM, Brown GV, O’Sullivan WJ. Pyrimidine de novo synthesis during the life cycle of the intraerythrocytic stage of Plasmodium falciparum. J Parasitol. 1984;70:536–541. [PubMed] [Google Scholar]
- 52.Flores MV, O’Sullivan WJ, Stewart TS. Characterisation of the carbamoyl phosphate synthetase gene from Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:315–318. doi: 10.1016/0166-6851(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 53.Flores MV, Atkins D, Wade D, O’Sullivan WJ, Stewart TS. Inhibition of Plasmodium falciparum proliferation in vitro by ribozymes. J Biol Chem. 1997;272:16940–16945. doi: 10.1074/jbc.272.27.16940. [DOI] [PubMed] [Google Scholar]
- 54.Hendry P, McCall MJ, Stewart TS, Lockett TJ. Redesigned and chemically-modified hammerhead ribozymes with improved activity and serum stability. BMC Chem Biol. 2004;4:1. doi: 10.1186/1472-6769-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez-Salas LM. Nucleic acids as therapeutic agents. Curr Top Med Chem. 2008;8:1379–1404. doi: 10.2174/156802608786141133. [DOI] [PubMed] [Google Scholar]
- 56.Simmer JP, Kelly RE, Rinker AG, Jr, Zimmermann BH, Scully JL, Kim H, Evans DR. Mammalian dihydroorotase: nucleotide sequence, peptide sequences; evolution of the dihydroorotase domain of the multifunctional protein CAD. Proc Natl Acad Sci USA. 1990;87:174–178. doi: 10.1073/pnas.87.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krungkrai SR, Wutipraditkul N, Krungkrai J. Dihydroorotase of human malarial parasite Plasmodium falciparum differs from host enzyme. Biochem Biophys Res Commun. 2008;366:821–826. doi: 10.1016/j.bbrc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 58.Seymour KK, Lyons SD, Phillips L, Rieckmann KH, Christopherson RI. Cytotoxic effects of inhibitors of de novo pyrimidine biosynthesis upon Plasmodium falciparum. Biochemistry. 1994;33:5268–5274. doi: 10.1021/bi00183a033. [DOI] [PubMed] [Google Scholar]
- 59.Manthey MK, Huang DT, Bubb WA, Christopherson RI. Synthesis and enzymic evaluation of 4-mercapto-6-oxo-1, 4-azaphosphinane-2-carboxylic acid 4-oxide as an inhibitor of mammalian dihydroorotase. J Med Chem. 1998;41:4550–4555. doi: 10.1021/jm970814z. [DOI] [PubMed] [Google Scholar]
- 60.Adams JL, Meek TD, Mong SM, Johnson RK, Metcalf BW. cis-4-Carboxy-6-(mercaptomethyl)-3,4,5,6-tetrahydropyrimidin-2(1 H)-one, a potent inhibitor of mammalian dihydroorotase. J Med Chem. 1988;31:1355–1359. doi: 10.1021/jm00402a018. [DOI] [PubMed] [Google Scholar]
- 61.Christopherson RI, Schmalzl KJ, Szabados E, Goodridge RJ, Harsanyi MC, Sant ME, Algar EM, Anderson JE, Armstrong A, Sharma SC, et al. Mercaptan and dicarboxylate inhibitors of hamster dihydroorotase. Biochemistry. 1989;28:463–470. doi: 10.1021/bi00428a009. [DOI] [PubMed] [Google Scholar]
- 62.Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes; regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- 63.Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249–267. doi: 10.1146/annurev.micro.091208.073424. [DOI] [PubMed] [Google Scholar]
- 64.Williamson RA, Yea CM, Robson PA, Curnock AP, Gadher S, Hambleton AB, Woodward K, Bruneau JM, Hambleton P, Spinella-Jaegle S, Morand P, Courtin O, Sautes C, Westwood R, Hercend T, Kuo EA, Ruuth E. Dihydroorotate dehydrogenase is a target for the biological effects of leflunomide. Transplant Proc. 1996;28:3088–3091. [PubMed] [Google Scholar]
- 65.Fox RI, Herrmann ML, Frangou CG, Wahl GM, Morris RE, Strand V, Kirschbaum BJ. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999;93:198–208. doi: 10.1006/clim.1999.4777. [DOI] [PubMed] [Google Scholar]
- 66.Williamson RA, Yea CM, Robson PA, Curnock AP, Gadher S, Hambleton AB, Woodward K, Bruneau JM, Hambleton P, Moss D, Thomson TA, Spinella-Jaegle S, Morand P, Courtin O, Sautes C, Westwood R, Hercend T, Kuo EA, Ruuth E. Dihydroorotate dehydrogenase is a high affinity binding protein for A77 1726 and mediator of a range of biological effects of the immunomodulatory compound. J Biol Chem. 1995;270:22467–22472. doi: 10.1074/jbc.270.38.22467. [DOI] [PubMed] [Google Scholar]
- 67.Chen SF, Ruben RL, Dexter DL. Mechanism of action of the novel anticancer agent 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarbo xylic acid sodium salt (NSC 368390): inhibition of de novo pyrimidine nucleotide biosynthesis. Cancer Res. 1986;46:5014–5019. [PubMed] [Google Scholar]
- 68.McLean JE, Neidhardt EA, Grossman TH, Hedstrom L. Multiple inhibitor analysis of the brequinar and leflunomide binding sites on human dihydroorotate dehydrogenase. Biochemistry. 2001;40:2194–2200. doi: 10.1021/bi001810q. [DOI] [PubMed] [Google Scholar]
- 69.Davies M, Heikkila T, McConkey GA, Fishwick CW, Parsons MR, Johnson AP. Structure-based design, synthesis; characterization of inhibitors of human and Plasmodium falciparum dihydroorotate dehydrogenases. J Med Chem. 2009;52:2683–2693. doi: 10.1021/jm800963t. [DOI] [PubMed] [Google Scholar]
- 70.Baldwin J, Michnoff CH, Malmquist NA, White J, Roth MG, Rathod PK, Phillips MA. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. 2005;280:21847–21853. doi: 10.1074/jbc.M501100200. [DOI] [PubMed] [Google Scholar]
- 71.Patel V, Booker M, Kramer M, Ross L, Celatka CA, Kennedy LM, Dvorin JD, Duraisingh MT, Sliz P, Wirth DF, Clardy J. Identification and characterization of small molecule inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. 2008;283:35078–35085. doi: 10.1074/jbc.M804990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips MA, Gujjar R, Malmquist NA, White J, El Mazouni F, Baldwin J, Rathod PK. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J Med Chem. 2008;51:3649–3653. doi: 10.1021/jm8001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gujjar R, Marwaha A, El Mazouni F, White J, White KL, Creason S, Shackleford DM, Baldwin J, Charman WN, Buckner FS, Charman S, Rathod PK, Phillips MA. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem. 2009;52:1864–1872. doi: 10.1021/jm801343r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heikkila T, Ramsey C, Davies M, Galtier C, Stead AM, Johnson AP, Fishwick CW, Boa AN, McConkey GA. Design and synthesis of potent inhibitors of the malaria parasite dihydroorotate dehydrogenase. J Med Chem. 2007;50:186–191. doi: 10.1021/jm060687j. [DOI] [PubMed] [Google Scholar]
- 75.Hudson AT. Atovaquone - a novel broad-spectrum anti-infective drug. Parasitol Today. 1993;9:66–68. doi: 10.1016/0169-4758(93)90040-m. [DOI] [PubMed] [Google Scholar]
- 76.Christopherson RI, Lyons SD, Wilson PK. Inhibitors of de novo nucleotide biosynthesis as drugs. Acc Chem Res. 2002;35:961–971. doi: 10.1021/ar0000509. [DOI] [PubMed] [Google Scholar]
- 77.Hammond DJ, Burchell JR, Pudney M. Inhibition of pyrimidine biosynthesis de novo in Plasmodium falciparum by 2-(4-t-butylcyclohexyl)-3-hydroxy-1,4-naphthoquinone in vitro. Mol Biochem Parasitol. 1985;14:97–109. doi: 10.1016/0166-6851(85)90109-4. [DOI] [PubMed] [Google Scholar]
- 78.Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J Biol Chem. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 79.Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 80.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 81.Suchi M, Mizuno H, Kawai Y, Tsuboi T, Sumi S, Okajima K, Hodgson ME, Ogawa H, Wada Y. Molecular cloning of the human UMP synthase gene and characterization of point mutations in two hereditary orotic aciduria families. Am J Hum Genet. 1997;60:525–539. [PMC free article] [PubMed] [Google Scholar]
- 82.Yablonski MJ, Pasek DA, Han BD, Jones ME, Traut TW. Intrinsic activity and stability of bifunctional human UMP synthase and its two separate catalytic domains, orotate phosphoribosyltransferase and orotidine-5′-phosphate decarboxylase. J Biol Chem. 1996;271:10704–10708. doi: 10.1074/jbc.271.18.10704. [DOI] [PubMed] [Google Scholar]
- 83.Krungkrai SR, Aoki S, Palacpac NM, Sato D, Mitamura T, Krungkrai J, Horii T. Human malaria parasite orotate phosphoribosyltransferase: functional expression, characterization of kinetic reaction mechanism and inhibition profile. Mol Biochem Parasitol. 2004;134:245–255. doi: 10.1016/j.molbiopara.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Queen SA, Jagt DL, Reyes P. In vitro susceptibilities of Plasmodium falciparum to compounds which inhibit nucleotide metabolism. Antimicrob Agents Chemother. 1990;34:1393–1398. doi: 10.1128/aac.34.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rathod PK, Khatri A, Hubbert T, Milhous WK. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1989;33:1090–1094. doi: 10.1128/aac.33.7.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krungkrai J, Krungkrai SR, Phakanont K. Antimalarial activity of orotate analogs that inhibit dihydroorotase and dihydroorotate dehydrogenase. Biochem Pharmacol. 1992;43:1295–1301. doi: 10.1016/0006-2952(92)90506-e. [DOI] [PubMed] [Google Scholar]
- 87.Rathod PK, Khosla M, Gassis S, Young RD, Lutz C. Selection and characterization of 5-fluoroorotate-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1994;38:2871–2876. doi: 10.1128/aac.38.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott HV, Gero AM, O’Sullivan WJ. In vitro inhibition of Plasmodium falciparum by pyrazofurin, an inhibitor of pyrimidine biosynthesis de novo. Mol Biochem Parasitol. 1986;18:3–15. doi: 10.1016/0166-6851(86)90045-9. [DOI] [PubMed] [Google Scholar]
- 89.Suttle DP, Stark GR. Coordinate overproduction of orotate phosphoribosyltransferase and orotidine-5′-phosphate decarboxylase in hamster cells resistant to pyrazofurin and 6-azauridine. J Biol Chem. 1979;254:4602–4607. [PubMed] [Google Scholar]
- 90.Dix DE, Lehman CP, Jakubowski A, Moyer JD, Handschumacher RE. Pyrazofurin metabolism, enzyme inhibition; resistance in L5178Y cells. Cancer Res. 1979;39:4485–4490. [PubMed] [Google Scholar]
- 91.Suttle DP. Increased levels of UMP synthase protein and mRNA in pyrazofurin-resistant rat hepatoma cells. J Biol Chem. 1983;258:7707–7713. [PubMed] [Google Scholar]
- 92.Radzicka A, Wolfenden R. A proficient enzyme. Science. 1995;267:90–93. doi: 10.1126/science.7809611. [DOI] [PubMed] [Google Scholar]
- 93.Miller BG, Wolfenden R. Catalytic proficiency: the unusual case of OMP decarboxylase. Annu Rev Biochem. 2002;71:847–885. doi: 10.1146/annurev.biochem.71.110601.135446. [DOI] [PubMed] [Google Scholar]
- 94.Langley DB, Shojaei M, Chan C, Lok HC, Mackay JP, Traut TW, Guss JM, Christopherson RI. Structure and inhibition of orotidine 5′-monophosphate decarboxylase from Plasmodium falciparum. Biochemistry. 2008;47:3842–3854. doi: 10.1021/bi702390k. [DOI] [PubMed] [Google Scholar]
- 95.Bello AM, Poduch E, Fujihashi M, Amani M, Li Y, Crandall I, Hui R, Lee PI, Kain KC, Pai EF, Kotra LP. A potent, covalent inhibitor of orotidine 5′-monophosphate decarboxylase with antimalarial activity. J Med Chem. 2007;50:915–921. doi: 10.1021/jm060827p. [DOI] [PubMed] [Google Scholar]
- 96.Bello AM, Poduch E, Liu Y, Wei L, Crandall I, Wang X, Dyanand C, Kain KC, Pai EF, Kotra LP. Structure-activity relationships of C6-uridine derivatives targeting plasmodia orotidine monophosphate decarboxylase. J Med Chem. 2008;51:439–448. doi: 10.1021/jm7010673. [DOI] [PubMed] [Google Scholar]
- 97.Levine HL, Brody RS, Westheimer FH. Inhibition of orotidine-5′-phosphate decarboxylase by 1-(5′-phospho-beta-d-ribofuranosyl)barbituric acid, 6-azauridine 5′-phosphate; uridine 5′-phosphate. Biochemistry. 1980;19:4993–4999. doi: 10.1021/bi00563a010. [DOI] [PubMed] [Google Scholar]
- 98.Miller BG, Traut TW, Wolfenden R. Effects of substrate binding determinants in the transition state for orotidine 5 ′-monophosphate decarboxylase. Bioorg Chem. 1998;26:283–288. [Google Scholar]
- 99.Vertessy BG, Toth J. Keeping uracil out of DNA: physiological role, structure and catalytic mechanism of dUTPases. Acc Chem Res. 2009;42:97–106. doi: 10.1021/ar800114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whittingham JL, Leal I, Nguyen C, Kasinathan G, Bell E, Jones AF, Berry C, Benito A, Turkenburg JP, Dodson EJ, Ruiz Perez LM, Wilkinson AJ, Johansson NG, Brun R, Gilbert IH, Gonzalez Pacanowska D, Wilson KS. dUTPase as a platform for antimalarial drug design: structural basis for the selectivity of a class of nucleoside inhibitors. Structure. 2005;13:329–338. doi: 10.1016/j.str.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 101.Quesada-Soriano I, Casas-Solvas JM, Recio E, Ruiz-Perez LM, Vargas-Berenguel A, Gonzalez-Pacanowska D, Garcia-Fuentes L. Kinetic properties and specificity of trimeric Plasmodium falciparum and human dUTPases. Biochimie. 92:178–186. doi: 10.1016/j.biochi.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 102.Nguyen C, Kasinathan G, Leal-Cortijo I, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Johansson NG, Gonzalez-Pacanowska D, Gilbert IH. Deoxyuridine triphosphate nucleotidohydrolase as a potential antiparasitic drug target. J Med Chem. 2005;48:5942–5954. doi: 10.1021/jm050111e. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen C, Ruda GF, Schipani A, Kasinathan G, Leal I, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Sahlberg BL, Johansson NG, Gonzalez-Pacanowska D, Gilbert IH. Acyclic nucleoside analogues as inhibitors of Plasmodium falciparum dUTPase. J Med Chem. 2006;49:4183–4195. doi: 10.1021/jm060126s. [DOI] [PubMed] [Google Scholar]
- 104.McCarthy O, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Johansson NG, Pacanowska DG, Gilbert IH. Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur J Med Chem. 2009;44:678–688. doi: 10.1016/j.ejmech.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 105.Nzila A. Inhibitors of de novo folate enzymes in Plasmodium falciparum. Drug Discov Today. 2006;11:939–944. doi: 10.1016/j.drudis.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Dasgupta T, Anderson KS. Probing the role of parasite-specific, distant structural regions on communication and catalysis in the bifunctional thymidylate synthase-dihydrofolate reductase from Plasmodium falciparum. Biochemistry. 2008;47:1336–1345. doi: 10.1021/bi701624u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ivanetich KM, Santi DV. Thymidylate synthase-dihydrofolate reductase in protozoa. Exp Parasitol. 1990;70:367–371. doi: 10.1016/0014-4894(90)90119-w. [DOI] [PubMed] [Google Scholar]
- 108.Yuvaniyama J, Chitnumsub P, Kamchonwongpaisan S, Vanichtanankul J, Sirawaraporn W, Taylor P, Walkinshaw MD, Yuthavong Y. Insights into antifolate resistance from malarial DHFR-TS structures. Nat Struct Biol. 2003;10:357–365. doi: 10.1038/nsb921. [DOI] [PubMed] [Google Scholar]
- 109.Anderson AC. Targeting DHFR in parasitic protozoa. Drug Discov Today. 2005;10:121–128. doi: 10.1016/S1359-6446(04)03308-2. [DOI] [PubMed] [Google Scholar]
- 110.Winstanley PA, Mberu EK, Szwandt IS, Breckenridge AM, Watkins WM. In vitro activities of novel antifolate drug combinations against Plasmodium falciparum and human granulocyte CFUs. Antimicrob Agents Chemother. 1995;39:948–952. doi: 10.1128/aac.39.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hankins EG, Warhurst DC, Sibley CH. Novel alleles of the Plasmodium falciparum dhfr highly resistant to pyrimethamine and chlorcycloguanil, but not WR99210. Mol Biochem Parasitol. 2001;117:91–102. doi: 10.1016/s0166-6851(01)00335-8. [DOI] [PubMed] [Google Scholar]
- 112.Hekmat-Nejad M, Rathod PK. Plasmodium falciparum: kinetic interactions of WR99210 with pyrimethamine-sensitive and pyrimethamine-resistant dihydrofolate reductase. Exp Parasitol. 1997;87:222–228. doi: 10.1006/expr.1997.4228. [DOI] [PubMed] [Google Scholar]
- 113.Warhurst DC. Resistance to antifolates in Plasmodium falciparum, the causative agent of tropical malaria. Sci Prog. 2002;85:89–111. doi: 10.3184/003685002783238906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Canfield CJ, Milhous WK, Ager AL, Rossan RN, Sweeney TR, Lewis NJ, Jacobus DP. PS-15: a potent, orally active antimalarial from a new class of folic acid antagonists. Am J Trop Med Hyg. 1993;49:121–126. doi: 10.4269/ajtmh.1993.49.121. [DOI] [PubMed] [Google Scholar]
- 115.Rieckmann KH, Yeo AE, Edstein MD. Activity of PS-15 and its metabolite, WR99210, against Plasmodium falciparum in an in vivo-in vitro model. Trans R Soc Trop Med Hyg. 1996;90:568–571. doi: 10.1016/s0035-9203(96)90326-0. [DOI] [PubMed] [Google Scholar]
- 116.Kamchonwongpaisan S, Quarrell R, Charoensetakul N, Ponsinet R, Vilaivan T, Vanichtanankul J, Tarnchompoo B, Sirawaraporn W, Lowe G, Yuthavong Y. Inhibitors of multiple mutants of Plasmodium falciparum dihydrofolate reductase and their antimalarial activities. J Med Chem. 2004;47:673–680. doi: 10.1021/jm030165t. [DOI] [PubMed] [Google Scholar]
- 117.Sirichaiwat C, Intaraudom C, Kamchonwongpaisan S, Vanichtanankul J, Thebtaranonth Y, Yuthavong Y. Target guided synthesis of 5-benzyl-2,4-diamonopyrimidines: their antimalarial activities and binding affinities to wild type and mutant dihydrofolate reductases from Plasmodium falciparum. J Med Chem. 2004;47:345–354. doi: 10.1021/jm0303352. [DOI] [PubMed] [Google Scholar]
- 118.Dasgupta T, Chitnumsub P, Kamchonwongpaisan S, Maneeruttanarungroj C, Nichols SE, Lyons TM, Tirado-Rives J, Jorgensen WL, Yuthavong Y, Anderson KS. Exploiting structural analysis, in silico screening; serendipity to identify novel inhibitors of drug-resistant falciparum malaria. ACS Chem Biol. 2009;4:29–40. doi: 10.1021/cb8002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barker RH, Jr, Metelev V, Rapaport E. Inhibition of Plasmodium falciparum malaria using antisense oligodeoxynucleotides. Proc Natl Acad Sci USA. 1996;93:514–518. doi: 10.1073/pnas.93.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Daele I, Munier-Lehmann H, Hendrickx PM, Marchal G, Chavarot P, Froeyen M, Qing L, Martins JC, Van Calenbergh S. Synthesis and biological evaluation of bicyclic nucleosides as inhibitors of M. tuberculosis thymidylate kinase. Chem Med Chem. 2006;1:1081–1090. doi: 10.1002/cmdc.200600028. [DOI] [PubMed] [Google Scholar]
- 121.Van Daele I, Munier-Lehmann H, Froeyen M, Balzarini J, Van Calenbergh S. Rational design of 5′-thiourea-substituted alpha-thymidine analogues as thymidine monophosphate kinase inhibitors capable of inhibiting mycobacterial growth. J Med Chem. 2007;50:5281–5292. doi: 10.1021/jm0706158. [DOI] [PubMed] [Google Scholar]
- 122.Kandeel M, Kitade Y. Molecular characterization, heterologous expression and kinetic analysis of recombinant Plasmodium falciparum thymidylate kinase. J Biochem. 2008;144:245–250. doi: 10.1093/jb/mvn062. [DOI] [PubMed] [Google Scholar]
- 123.Kandeel M, Ando T, Kitamura Y, Abdel-Aziz M, Kitade Y. Mutational, inhibitory and microcalorimetric analyses of Plasmodium falciparum TMP kinase. Implications for drug discovery. Parasitology. 2009;136:11–25. doi: 10.1017/S0031182008005301. [DOI] [PubMed] [Google Scholar]
- 124.Yuan P, Hendriks EF, Fernandez HR, O’Sullivan WJ, Stewart TS. Functional expression of the gene encoding cytidine triphosphate synthetase from Plasmodium falciparum which contains two novel sequences that are potential antimalarial targets. Mol Biochem Parasitol. 2005;143:200–208. doi: 10.1016/j.molbiopara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 125.Hendriks EF, O’Sullivan WJ, Stewart TS. Molecular cloning and characterization of the Plasmodium falciparum cytidine triphosphate synthetase gene. Biochim Biophys Acta. 1998;1399:213–218. doi: 10.1016/s0167-4781(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 126.Kang GJ, Cooney DA, Moyer JD, Kelley JA, Kim HY, Marquez VE, Johns DG. Cyclopentenylcytosine triphosphate. Formation and inhibition of CTP synthetase. J Biol Chem. 1989;264:713–718. [PubMed] [Google Scholar]
- 127.McPartland RP, Wang MC, Bloch A, Weinfeld H. Cytidine 5′-triphosphate synthetase as a target for inhibition by the antitumor agent 3-deazauridine. Cancer Res. 1974;34:3107–3111. [PubMed] [Google Scholar]
- 128.Viola JJ, Agbaria R, Walbridge S, Oshiro EM, Johns DG, Kelley JA, Oldfield EH, Ram Z. In situ cyclopentenyl cytosine infusion for the treatment of experimental brain tumors. Cancer Res. 1995;55:1306–1309. [PubMed] [Google Scholar]
- 129.Yee LK, Allegra CJ, Trepel JB, Grem JL. Metabolism and RNA incorporation of cyclopentenyl cytosine in human colorectal cancer cells. Biochem Pharmacol. 1992;43:1587–1599. doi: 10.1016/0006-2952(92)90218-8. [DOI] [PubMed] [Google Scholar]
- 130.Gao WY, Johns DG, Mitsuya H. Potentiation of the anti-HIV activity of zalcitabine and lamivudine by a CTP synthase inhibitor, 3-deazauridine. Nucleosides Nucleotides Nucleic Acids. 2000;19:371–377. doi: 10.1080/15257770008033015. [DOI] [PubMed] [Google Scholar]
- 131.Savickiene J, Gineitis A. 3-Deazauridine triggers dose-dependent apoptosis in myeloid leukemia cells and enhances retinoic acid-induced granulocytic differentiation of HL-60 cells. Int J Biochem Cell Biol. 2003;35:1482–1494. doi: 10.1016/s1357-2725(03)00130-4. [DOI] [PubMed] [Google Scholar]