Abstract
Biliary tract infection with the Group I carcinogenic liver fluke Opisthorchis viverrini is associated with severe inflammation leading to cholangiocarcinoma – a major biliary cancer in Southeast Asia. However, mechanism(s) by which the liver fluke induces host mucosal immune/inflammatory responses are unclear. In the present study we address whether a normal immortalized human cholangiocyte cell line (H69 cells) recognizes and responds to O. viverrini excretory/secretory products (OVES). Expression of multiple TLRs, activation of NF-κB, and expression of proinflammatory cytokines were monitored in the presence and absence of OVES. Our results showed that OVES induced increased cholangiocyte TLR4 mRNA expression, induced IκB-α degradation in a MyD88-dependent manner, and activated NF-κB nuclear translocation. Moreover, OVES induced expression and secretion of the strong chemoattractant chemokine interleukin 8 (IL-8) and pro-inflammatory cytokine IL-6. These results demonstrate that secreted/excreted products of O. viverrini are recognized by human cholangiocytes and initiate innate mucosal immunity/inflammatory cascades, a primary event in the pathogenesis of opisthorchiasis and cholangiocarcinoma.
Keywords: Opisthorchis viverrini excretory/secretory products, Cholangiocyte, Toll-like receptors, NF-κB, Cytokines
Introduction
Liver fluke infection caused by Opisthorchis viverrini remains a major public health problem in Southeast Asia, particularly Thailand, Lao PDR, central Vietnam and Cambodia. It is estimated that 10 million people are infected with O. viverrini [1, 2] and more than 80 million people are at risk of infection [3]. Infection is acquired by ingestion of raw or undercooked fresh water cyprinoid fish harboring infective metacercariae. The infection is associated with a number of hepatobiliary diseases, including cholangitis, obstructive jaundice, hepatomegaly, cholecystitis and cholelithiasis [4]. Moreover, both experimental and epidemiological evidence strongly implicate liver fluke infection in the etiology of cholangiocarcinoma (CCA) [5–7]. According to the International Agency for Research on Cancer (IARC), World Health Organization, O. viverrini is classified as a Group I carcinogen – a metazoan parasite that is carcinogenic to humans [8, 9]. O. viverrini endemic areas in Northeast Thailand report higher incidence of CCA than other parts of the country as well as the world [10, 11]. Chronic inflammation of the infected bile ducts is a known predisposing factor in the pathogenesis of this cancer [6]. However, the mechanism(s) of O. viverrini-induced immune/inflammatory responses remain unclear.
O. viverrini is a mucosal trematode inhabiting both intrahepatic and extrahepatic bile ducts. Strikingly, however, the parasite induces a severe inflammatory response with neutrophils and mononuclear cell infiltration to the portal tracts, particularly during the early stages of infection [4, 12]. Host-parasite interaction at the mucosal interface of the infected bile ducts may play a crucial role in the initiation of inflammation. Indeed, Sripa and Kaewkes [13] observed intense inflammatory cell infiltration in areas where Opisthorchis antigens were observed at the apical surface and in the cytoplasm of biliary epithelium, suggesting that a mucosal immune response to parasite antigens may play a significant role in the inflammatory response. Toll-like receptors (TLRs) and the innate mucosal immune response have been implicated in the recognition and regulation in helminth infection [14, 15]. Human biliary epithelial cells, cholangiocytes, express multiple TLRs, and are known to play an important role in mucosal immunology and immunopathogenesis of several biliary diseases including parasitic infection [16–18].
In the present study we report for the first time that OVES (i) induces the upregulation of cholangiocyte TLR4 mRNA; (ii) promotes the degradation of IκB-αin a MyD88-dependent manner, (iii) induces the nuclear translocation of NF-κB; and, (iv) induces the upregulation and secretion of Interleukin-8 (IL-8) and IL-6. Therefore, the results of our study demonstrate a novel mechanism of O. viverrini-induced inflammatory signaling cascades involving cholangiocyte recognition of OVES, which induces MyD88-dependent signaling events leading to the expression of the potent chemoattractant, IL-8 and pro-inflammatory cytokine IL-6.
2. Materials and methods
2.1. Animals
Male golden Syrian hamsters (from the Animal Unit, Faculty of Medicine, Khon Kaen University), aged 6 to 8 weeks at the commencement of the study, are used throughout the experiments. The hamsters were kept 5 to a cage, housed under conventional conditions, and fed a stock diet and water ad libitum. The maintenance and care of the animals are complied according to the guidelines of the National Laboratory Animal Center.
2.2. O. viverrini metacercariae and adult worms
The metacercariae of O. viverrini are obtained from naturally infected cyprinoid fish captured from endemic area in Khon Kaen province, Northeast Thailand. The fish were washed with tap water, minced by electric blender and digested with artificial gastric juice, a solution of 0.25% pepsin A and 1.5% HCl, at 37°C in a shaking water bath for one hour. The digested mixture was strained through a set of three sieves with the mesh size of 1000, 300 and 106 µm, respectively. Then the pellet on the last sieve (106 µm) was sedimented in normal saline solution (0.85% NaCl, NSS) in a sedimentation jar until the supernatant was clear. O. viverrini metacercariae were identified, collected under a dissecting microscope, and stored in NSS at 4°C until used for animal infection. Fifty viable active cysts were fed to the hamsters by intragastic intubation. Adult worms were obtained by linear teasing after 2–3 months post-infection. The fresh worms were washed several times in cold NSS containing penicillin (200 U/ml) and streptomycin (200 µg/ml) to remove any debris and residual blood. After washing thoroughly the viable worms were used for preparation of excretory/secretory products (OVES).
2.3. O. viverrini excretory/secretory products
O. viverrini ES products were prepared as previously described with slight modification [19]. Briefly, the fresh worms were cultured in RPMI-1640, penicillin (100 U/ml) and streptomycin (100 µg /ml) and protease inhibitors (0.1 mM Phenylmethanesulfonyl fluoride (PMSF), 1 mM leupeptin and 0.1 mM N-[N-(L-3-trans-carboxyoxiran-2-carbonyl)-L-leucine]-agmatine, E-64). The worms were maintained in vitro at 37°C up to 7 days. Dead worms were periodically removed. The culture fluid was collected every 12 h and centrifuged at 3,000 rpm for 10 min to remove the eggs. No sign of bacterial contamination in the cultures from each collection was observed. The clear supernatant was pooled, dialyzed in PBS, concentrated, absorbed with Triton-X114 to remove residual lipopolysaccharide (LPS) [20], followed by Bio-Beads SM2 (Bio-Rad) to remove Triton-X114, filtered through a 0.2 micrometer membrane and then aliquoted at −80°C for in vitro assays. Protein concentration was measured by Bradford method (Bio-Rad).
2.4. human biliary cell lines
H69 cholangiocyte cell line (a gift from Dr. D. Jefferson, Tufts University, Boston, MA) is a SV40-transformed human bile duct epithelial cell line originally derived from a normal liver harvested for transplantation. These cells have been extensively characterized. For experiments, H69 cells were used between passage 27 and 33. H69 cells were grown in DMEM and DMEM/Ham F-12 supplemented with 10% FBS, adenine, insulin, epinephrine, T3-T, epidermal growth factor (EGF), hydrocortisone and antibiotics.
H69 cells were seeded at a density of 2×105 cells per T75 flask and incubated with OVES at 5–20 µg/ml of media cells for up to 48 hrs depending on specific experiments. The cells were then harvested for investigation of mRNA or protein expression level. H69-MyD88 dominant negative studies were performed in H69 cells stably express the MyD88-DN (dominant negative) [18]. The MyD88-DN dominant negative plasmid was a gift from Prof. J. Tschopp (University of Lausanne, Lausanne, Switzerland).
2.5. RT-PCR
Total cellular RNA was extracted using TriZol® Reagent (Invitrogen, Life technologies). Total RNA (5 µg) was reverse transcribed to cDNA by using a SuperScript™ III First-Strand Synthesis System for RT-PCR Kit (Invitrogen, Life Technologies). Following reverse transcription, cDNA was amplified using PCR with gene-specific primers designed to amplify a portion of the coding sequences. cDNA was amplified using Amplitaq Gold PCR Master Mix (Roche Diagnostic Systems). The PCR consisted of one cycle of 10 min denaturation at 95°C; 34–37 cycles of 1 min at 94°C, 1 min at 53–57°C, and 1 min at 72°C, and a final extension step at 72°C for 10 min. Ribosomal RNA (18s) was also amplified to confirm that an equal amount of total cDNA was used for each sample, and all experiments were done in triplicate. Detail of primer sequences for TLRs and cytokine genes, and PCR conditions is shown in Table 1 [18].
Table 1.
Toll like receptor gene primers and PCR conditions [18]. Positive control is 18S RNA (product size 315 bp) using 18S primer (Ambion).
| Gene | Forward primer (5’---3’) | Reverse primer (5’---3’) | Product Size (bp) |
Tm | Cycle |
|---|---|---|---|---|---|
| TLR1 | TGCCAAATGGAACAGACAAG | GTGCCCAATATGCCTTTGTT | 189 | 53 | 37 |
| TLR2 | TGATGCTGCCATTCTCATTC | CGCAGCTCTCAGATTTACCC | 157 | 53 | 37 |
| TLR3 | AGGCGGGTGTTTTTGAACTA | TCTTCGCAAACAGAGTGCAT | 224 | 53 | 37 |
| TLR4 | GCGTGGAGGTGGTTCCTAAT | CACCTGCAGTTCTGGGAAAC | 161 | 57 | 34 |
| TLR6 | AGGGCTGGCCTGATTCTTAT | TGGCACACCATCCTGAGATA | 202 | 55 | 37 |
A quantitative RT-PCR approach using a LightCycler (Roche Diagnostic Systems) was established. Briefly, total RNA was harvested from the cells after incubation with OVES and reverse transcribed to cDNA and amplified using Light cycler®FastStart DNA Master SYBR Green I (Roche Diagnostic Systems). Data were expressed as copies of target gene vs total 18s.
2.6. Western blotting
H69 cells were seed at 500,000 cells per T75 flask for 48 hrs, serum starved overnight, then incubated with OVES at 10 µg/ml of media/20,000 cells for 48 hrs. Cells were then lysed with the M-PER mammalian protein extraction reagent (Pierce), and protein concentrations were determined using Bradford reagent according to the instructions of the supplier (Sigma-Aldrich). Ten micrograms of lysate protein per lane were separated by SDS-PAGE under reducing conditions and blotted onto nitrocellulose membranes. Membranes were incubated with the primary antibody to TLR4 at 3 µg/ml (Imgenex), IκB-alpha at 3 µg/ml (Santa Cruz Biotechnology) or beta-actin (Sigma-Aldrich), washed, and then incubated with HRP-conjugated secondary antibody. Bands were visualized with ECL light substrate (ECL; Amersham Biosciences) and band intensity was analyzed using densitometry (Bio-Rad, GS-700 imaging densitometer).
2.7. Immunofluorescent microscopy
H69 cells were treated with OVES as described above. After an 8 h incubation, the cells were fixed with ice cold 100% methanol at 37°C for 20 min and permeabilized with 0.2% (v/v) Triton X-100 in PBS. For immunofluorescent labeling, fixed cells were incubated with mouse anti- NF-κB p65 mAb (Santa Cruz Biotechnology) followed by rhodamine-labeled anti-mouse Ab (Molecular Probes). In some experiments, 4,6-diamidino-2-phenylindole (DAPI; 5 µM) was used to stain cell nuclei. Labeled cells were rinsed three times with PBS and once with distilled water, then mounted with mounting medium (H-1000; Vector Laboratories), and assessed by confocal laser scanning microscopy. Images obtained from the Zeiss 510 confocal microscope (Zeiss) were manipulated uniformly for contrast and intensity using the Zeiss 510 confocal software (Zeiss).
2.8. Cytokine assay
IL-6 and IL-8 cytokine levels in the H69-OVES cultured supernatant were measured by a bead based Analyte Detection System (FlowCytomix Simplex kit, eBioscience) according to the manufacturer’s instruction. A standard curve for each cytokine was developed by mixing known quantities of recombinant human cytokines IL-6 and IL-8 in RPMI-1640 media for culture supernatant assays. Level of cytokine was determined by flow cytometry (Beckman Coulter Cytomics™ FC500) equipped with CXP software, and data were analyzed using FlowCytomix Pro 2.2 software. The sensitivity of the assays was from 0.5 and 1.2 pg/mL for IL-8 and IL-6, respectively.
2.9. Luciferase assay
H69 cells were seeded at 50,000 cells/well (6 well-plate) for 24 hrs, then co-transfected with the IL-6-promoter/pGL3 vector or IL8-promoter/pGL3 vector and the TK renilla plasmid (transfection control) for 24 hrs. Transfections were performed using Fugene HD (Roche) transfection reagent. The cells were then treated with a total OVES protein concentration of 20 µg/ml or 100 ng/ml lipopolysaccharide (LPS, positive control) for 4 hrs. LPS inhibition assays were performed by preincubating the OVES or LPS with 2 µg/ml polymyxin B. Luciferase activity (dual-luciferase reporter assay, Promega) was then performed. Data is presented as IL-6 or IL-8 promoter driven firefly luciferase normalized to TK-Renilla luciferase fold-change compared to H69 uninfected cells.
2.10. Statistical analysis
All values are given as mean ±SE. Means of groups were compared with the Student’s t test (unpaired) or ANOVA test when appropriate. P values <0.05 were considered statistically significant.
3. Results
3.1. Screening of TLRs by RT-PCR and validation of TLR4 by qRT-PCR and Western blot
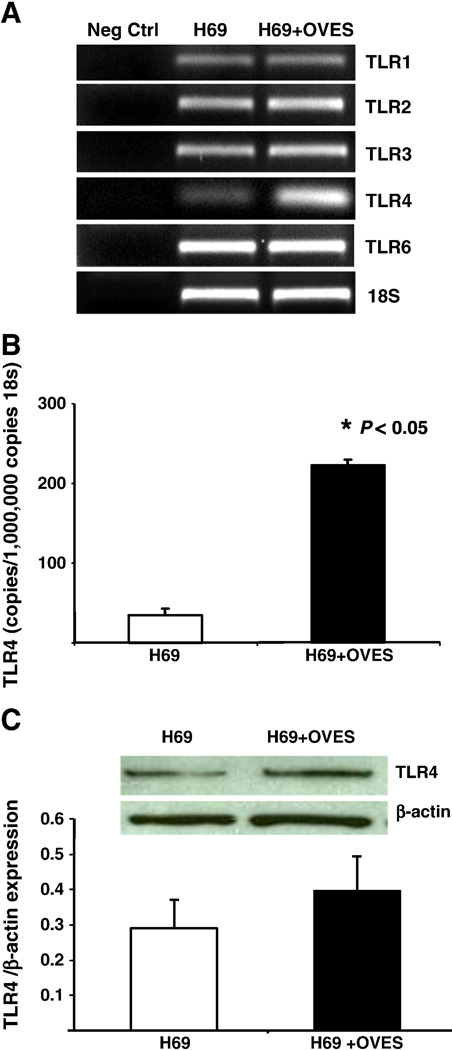
TLR1, TLR2, TLR3, TLR4 and TLR6 expression in cholangiocytes (H69) co-cultured in the presence or absence of OVES products were investigated by RT-PCR. As demonstrated previously [18], all cell surface TLRs were expressed in cultured normal human cholangiocytes (Fig. 1A). Densitometric analysis of our ethidium bromide stained PCR products suggested that TLR4 message increased following exposure to OVES. We therefore performed realtime quantitative PCR to validate this observation. Indeed, we found that the TLR4 message increased over five-fold following treatment with OVES (Fig. 1B). Furthermore, we observed an increase in TLR4 protein expression as assessed by Western blot (Fig. 1C).
Fig. 1.
Expression of TLR 1, TLR2, TLR3, TLR4 and TLR 6 in normal cholangiocytes (H69) and cholangiocytes cocultured with OVES. Semi-quantitative RT-PCR shows increased TLR4 mRNA (A). Significant increases in copy numbers of TLR4 transcript and protein are shown in cholangiocytes following exposure to OVES by qRT-PCR (B) and Western blot (C). Each value represents the mean ± SE of three independent experiments.
3.2. IκB-α degradation
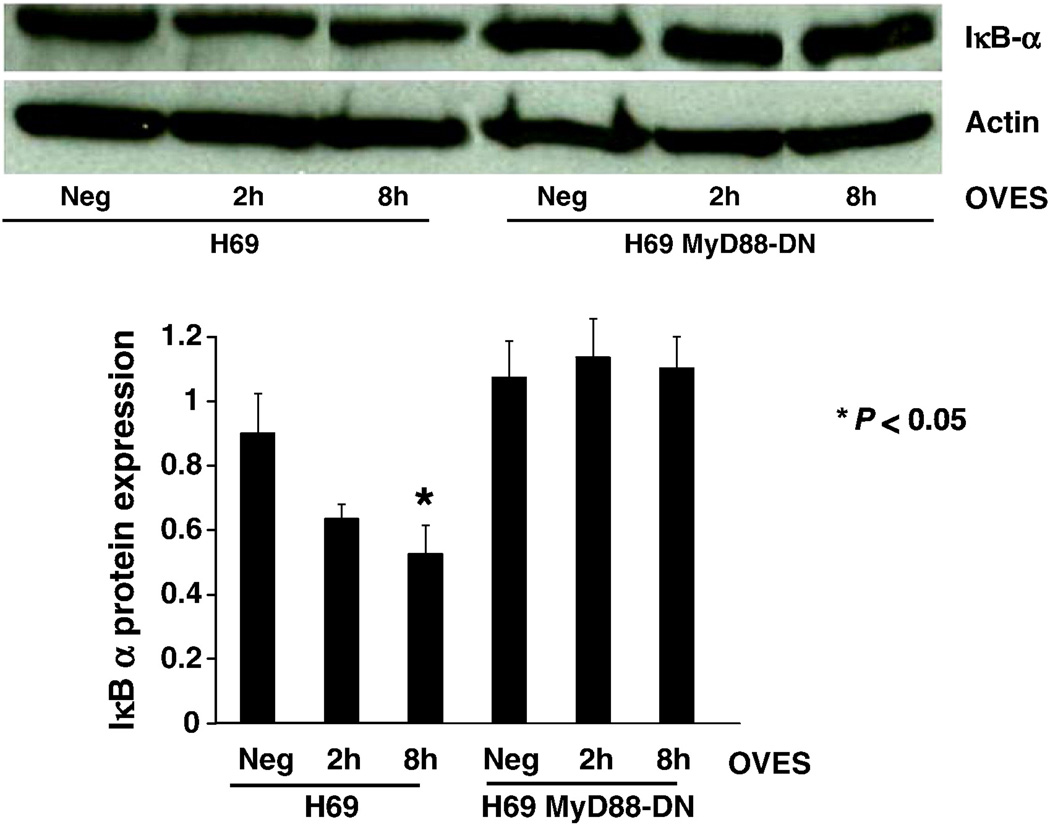
The cell surface TLRs signal through the adaptor molecule MyD88, while TLR4 can also signal through a MyD88-independent pathway leading to activation of Interferon Regulatory Factors. We therefore asked whether OVES treated H69 cells exhibited decreased IκB-α expression, which is required for NF-κB activation, and whether this occurred in a MyD88-dependent manner. We found that OVES induced significant degradation of IκB-α within 8 h in the H69 cells, while those cells force expressing a MyD88 dominant negative exhibited no change of the IκB-α level (Fig. 2). This result demonstrates that OVES induces the degradation of IκB-α in a MyD88-dependent manner in wild type H69 cells. Having demonstrated the reduced expression of IκB-α, we next assessed NF-κB nuclear translocation using immunofluorescent confocal microscopy. In the absence of OVES, the p65 subunit of NF-κB localized primarily to the cytoplasm. However, significant nuclear translocation of p65 occurs following treatment with the OVES (Fig. 3).
Fig. 2.
Western blot analysis of IκB-α in control (neg), O. viverrini ES cocultured for 2 h and 8 h in H69 cells (H69) or H69-MyD88 dominant negative (H69-MyD88-DN). Densitometric analysis normalized to actin is shown in the lower panel. Significant (P < 0.05) degradation of IκB-α, compared to control, is shown at 8 h following incubation with the OVES. Each value represents the mean ± SE of three independent experiments.
Fig. 3.
Nuclear translocation of NF-κB p65 in OVES cocultured cholangiocytes (H69+OVES) and control (H69) using imunofluorescence confocal microscopy. Average intensity of nuclear fluorescence analyzed from 200 cells is shown in the lower panel. Significant increase (P < 0.05) of fluorescence intensity is seen in the nucleus of OVES cocultured H69 cells. Each value represents the mean ± SE of three independent experiments.
3.3. Cytokine expression
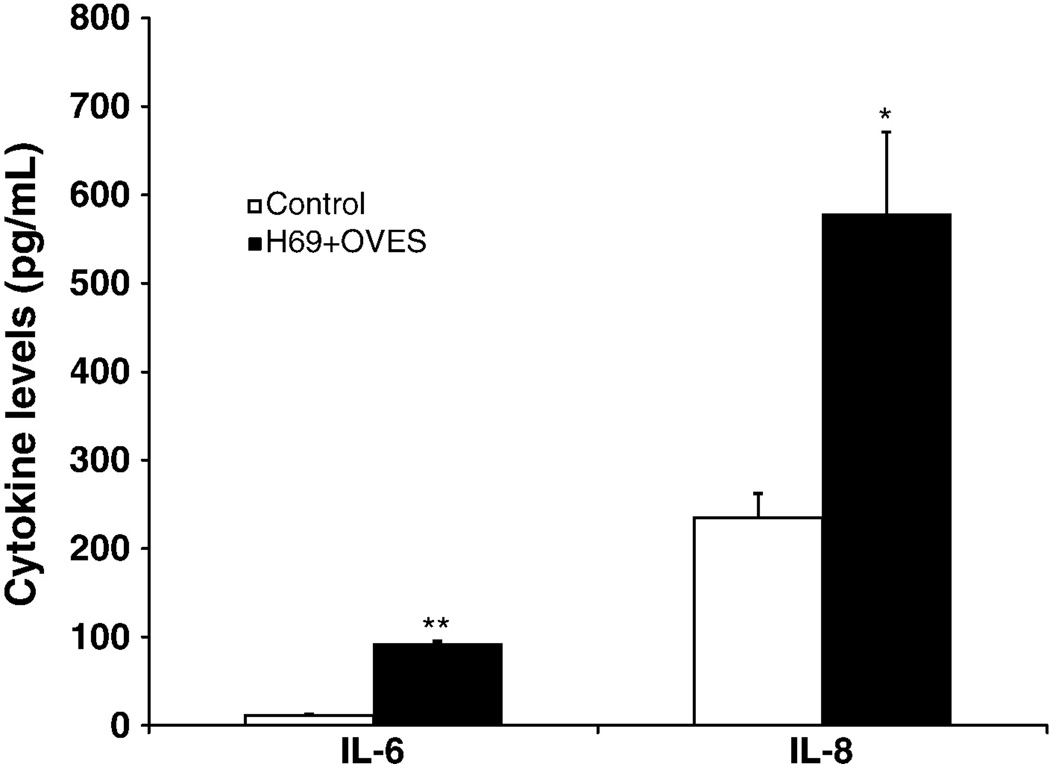
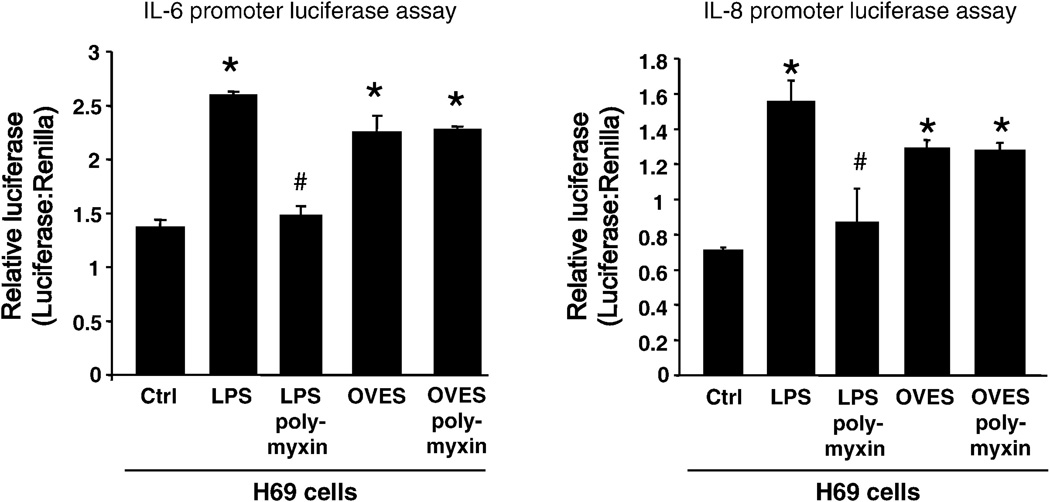
MyD88-dependent activation of NF-κB, in response to pathogen recognition, is known to drive the expression of multiple chemokines/cytokines. Furthermore, it has become increasingly clear that cholangiocytes not only respond to these factors, but actively participate in the induction of a proinflammatory response through the secretion of multiple immune-associated factors including antimicrobial peptides and chemokines/cytokines. We therefore asked whether treatment of H69 cells with OVES, and subsequent NF-κB activation promotes the expression of cytokines. Here we focus on cholangiocyte expression of two proinflammatory factors, IL-6, a potent mitogen and monocyte chemoattractant associated with multiple hepatobiliary diseases, and IL-8 a strong neutrophil chemoattractant. Secreted cytokines were detected in culture media, following exposure to OVES, using a bead-based flow cytometric analysis. Significant increases in IL-6 (P<0.001) and IL-8 (P<0.05) levels were detected following OVES stimulation (Fig. 4). We next validated the expression of IL-6 and IL-8 and assessed transcription of these cytokines using an IL-6 or IL-8 promoter driven luciferase assay (Fig. 5). H69 cells transfected with the pGL3-IL-6 or pGL3-IL-8 construct demonstrated significantly increased luciferase expression following exposure to OVES and LPS (P<0.01), a TLR4 agonist derived from gram-negative bacteria. Importantly, the LPS inhibitor polymyxin B significantly inhibited both IL-6 and IL-8 transcription in H69 cells cocultured with LPS but not OVES. These results support our earlier findings that IL-6 and IL-8 are expressed following OVES treatment.
Fig. 4.
Cytokine levels of IL-6 and IL-8 in culture supernatants of H69 (control) and H69 cocultured with OVES (H69+OVES) by bead-based flow cytometery. Bar graph shows the levels of IL-6 or IL-8 in pg/mL. Note that OVES induces significant increased of IL-6 (**, P<0.001) and IL-8 (*, P<0.05) compared to control. Each value represents the mean ± SD of three independent experiments.
Fig. 5.
Relative luciferase activity in OVES induced IL-6 and IL-8 promoter activation in H69. LPS is used as positive control for IL-6 and IL-8 activation. The IL-6 or IL-8 promoter driven luciferase activity, relative to transfection control Renilla luciferase expression, is significantly increased in OVES cocultured H69 biliary cells. However, polymyxin B inhibits LPS stimulated H69 cells but not OVES. Each value represents the mean ± SE of three independent experiments.
* = P < 0.01 vs control (Ctrl), # = P<0.01 vs treatment without polymyxin B.
4. Discussion
Liver fluke infection caused by O. viverrini is a major health problem in Thailand, particularly in the Northeast. Pathological consequences of infection include inflammation, epithelial desquamation, epithelial and adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis and granuloma formation. However, detail of the cellular and molecular mechanisms, particularly host immune/inflammatory responses, is not well understood. Our study, for the first time, demonstrates in vitro the activation of TLRs and their downstream signaling pathway by OVES products. We demonstrate that (i) cultured human cholangiocytes express all cell surface TLRs, and one of these, TLR4 is upregulated following exposure to OVES; (ii) OVES induces cholangiocyte IκB-α degradation in a MyD88-dependent manner and induces NF-κB nuclear translocation; and, (iii) OVES induces the expression and secretion of both IL-6 and IL-8.
It has long been suspected that host immune responses and immunopathologic processes mediate hepatobiliary damage in opisthorchiasis [12, 13, 21, 22]. Sripa and Kaewkes [19] showed that inflammation around infected hamster bile ducts was a consequence of the host cellular response to Opisthorchis antigens. Marked infiltration of inflammatory cells at the periportal areas of infected hamster liver was associated with the presence of parasite antigens in the bile duct epithelium as detected by immunohistochemistry. This study has demonstrated the activation of the cholangiocyte host innate immune response, specifically TLRs and MyD88, following exposure to antigens derived from O. viverrini. We further demonstrate that cholangiocyte recognition of O. viverrini derived products results in the expression of both IL-6 and IL-8, two factors that are known to mediate hepatobiliary inflammation.
TLRs are highly conserved from Drosophila to humans and share structural and functional similarities. They recognize pathogen-associated molecular patterns (PAMPs) that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity. Nine TLRs (named simply TLR1 to TLR9) have been identified in humans. By using normal cholangiocytes (H69 cell) cocultured with O. viverrini ES products, we examined the expression of cell surface TLR (TLR1, TLR2, TLR4, and TLR6) expression in the cholangiocytes. The results revealed that all the TLRs studied are expressed in the normal cholangiocyte similar to those previously reported [18]. Only TLR4 showed upregulated expression in H69 cells exposed to OVES. The upregulation was confirmed by realtime RT-PCR. TLR4 protein expression as revealed by immunoblot was also increased. This is the first report, to our knowledge, demonstrating induced TLR4 upregulation in response to human liver fluke parasite in biliary cells. Upregulation of TLR4 has been reported in several conditions, such as in chronic hepatitis B virus infection [23], mechanical cyclic stretch in cultured cardiocytes [24] and certain growth factor stimulation [25]. Pinlaor et al. [26] recently reported that O. viverrini somatic extracts can induce upregulation of TLR2 but not TLR4 in Raw 264.7 macrophage cells and triggered NF-κB signaling, stimulate inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression. TLR expression is cell type and pathogen specific [27], inflammatory cells may express TLRs different from those of epithelium. Using a similar model, Chen et al. [18] reported that Cryptosporidium parvum stimulated both TLR2 and TLR4 upregulation in a normal biliary cell line (H69). For other trematode parasites, a lipid fraction from Schistosoma mansoni eggs containing lysophosphatidylserine has been shown, in a TLR2-dependent mechanism, to induce the activation of dendritic cells (DCs) that promote Th2 and regulatory T-cell development [28], and lacto-N-fucopentaose III (LNFPIII), a synthetic copy of a schistosome egg glycan, has been shown to promote Th2 differentiation by DCs via a TLR4-dependent pathway [29]. We now add that biliary epithelial cells recognize and respond to liver fluke excreted/secreted products. Interestingly, preincubation of our OVES preparation with 2 µg/ml polymyxin B, a potent antibiotic that binds the Lipid A component of LPS and thereby neutralizes the molecule, had no effect on OVES-induced expression of IL-6 and IL-8, but completely blocked LPS-induced expression. This confirms that a trace amount of LPS presented in our OVES preparation (after Triton-X114 removal method) as determined by Limulus assay (data not shown) did not affect the TLR activation. Therefore, LPS-independent activation of TLR4 may occur as described in other helminths. S. mansoni worm glycolipids can induce inflammatory cytokine production (IL-1, IL-6, IL-8, IL-12p40, and tumor necrosis factor-alpha, TNF-alpha) in human dendritic cells by cooperation of TLR4 and DC-SIGN [30]. We previously reported N-linked glycoconjugates in O. viverrini adults [31] and also glycolipids (Talabnin et al., unpublished). The liver fluke glycolipids may be responsible for the TLR4 activation and detailed study is in progress in our laboratory. However, recognition of OVES by TLRs other than TLR4 can be involved.
Downstream signaling from TLRs in response to OVES stimulation has been described in this study. These include MyD88-dependent IκB-α degradation, NF-κB activation (by nuclear translocation) and the production of IL-6 and IL-8 inflammatory cytokines. It was found that OVES induced the upregulation of IL-6 and IL-8 in this model of biliary epithelial cell response to liver fluke antigen. TLR activation has been noted as one of the earliest events leading to IL-6 production [32]. IL-6 is also a pro-proliferative, anti-apoptotic cytokine, the regulation of which is controlled by one of the master integrators of stress stimuli, NF-κB. Although important for the quick induction of an innate immune response, IL-6 dysregulation is central to states of chronic inflammation, which manifest clinically as diseases, including autoimmune disorders, such as inflammatory bowel disease and rheumatoid arthritis, as well as inflammation-associated malignancies [33]. IL-8 is a potent neutrophil chemotactic factor and a crucial mediator in neutrophil-dependent acute inflammation and has been described in biliary disease [34]. However, accumulated studies of IL-8-mediated signaling have revealed that IL-8 activates a wide range of signaling molecules in a coordinate manner. IL-8 has been proven to have diverse actions on various types of leukocytic and nonleukocytic cells besides neutrophils including T lymphocytes, monocytes, and hematopoietic progenitor cells [35].
In conclusion, the current study provides mechanisms of O. viverrini induced cholangiocyte proinflammatory response in vitro. Our results demonstrate the upregulation of TLR4, along with other TLRs, and their downstream signal transduction including MyD88-dependent IκB-α degradation, NF-κB activation and increased expression of IL-6 and IL-8. We propose that cholangiocyte TLR recognition of O. viverrini products and subsequent activation of NF-κB and induction of cytokines/chemokines, may be a central component to the pathology observed during O. viverrini infection and contributes to the severe inflammation in opisthorchiasis which has been associated with cholangiocarcinogenesis.
Acknowledgments
This work was supported by National Research Council of Thailand (NRCT), Thailand Research Fund (TRF grant no. BRG4580016), the National Institutes of Health Grant DK57993 (N.F.L), and in part, by the NIH-NIAID (award number 1UO1 AI065871). Kantima Ninlawan is a Royal Golden Jubilee PhD Scholar through Dr. Banchob Sripa.
References
- 1.Sripa B, Kaewkes S, Intapa P, Maleewong W, Brindley P. Food-borne trematodiases in Southeast Asia: epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72C:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 2.Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Haswell-Elkins MRME, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Sithithaworn P, Elkins DB. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Parasitol. 1994;59:505–509. doi: 10.1002/ijc.2910590412. [DOI] [PubMed] [Google Scholar]
- 6.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 8.IARC. IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon 15–22 February 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;60:1–560. [Google Scholar]
- 9.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 10.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatanasapt V, Uttaravichien T, Mairiang EO, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- 12.Bhamarapravati N, Thammavit W, Vajrasthira S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am J Trop Med Hyg. 1978;27:787–794. doi: 10.4269/ajtmh.1978.27.787. [DOI] [PubMed] [Google Scholar]
- 13.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 14.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venugopal PG, Nutman TB, Semnani RT. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res. 2009;43:252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada K, Nakanuma Y. Biliary innate immunity in the pathogenesis of biliary diseases. Inflamm Allergy Drug Targets. 2010;9:83–90. doi: 10.2174/187152810791292809. [DOI] [PubMed] [Google Scholar]
- 17.Chen XM, O'Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86:497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen XM, O'Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175:7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 19.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 20.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-144. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 21.Flavell DJ, Flavell SU. Opisthorchis viverrini: pathogenesis of infection in immunodeprived hamsters. Parasite Immunol. 1986;8:455–466. doi: 10.1111/j.1365-3024.1986.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 22.Haswell-Elkins MR, Sithithaworn P, Mairiang E, Elkins DB, Wongratanacheewin S, Kaewkes S, et al. Immune responsiveness and parasite-specific antibody levels in human hepatobiliary disease associated with Opisthorchis viverrini infection. Clin Exp Immunol. 1991;84:213–218. doi: 10.1111/j.1365-2249.1991.tb08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Lian JQ, Huang CX, Wang JP, Wei X, Nan XP, et al. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Shyu KG, Wang BW, Lin CM, Chang H. Cyclic stretch enhances the expression of toll-like receptor 4 gene in cultured cardiomyocytes via p38 MAP kinase and NF-kappaB pathway. J Biomed Sci. 2010;17:15. doi: 10.1186/1423-0127-17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micera A, Stampachiacchiere B, Normando E, Lambiase A, Bonini S, Bonini S. Nerve growth factor modulates toll-like receptor (TLR) 4 and 9 expression in cultured primary VKC conjunctival epithelial cells. Mol Vis. 2009;15:2037–2044. [PMC free article] [PubMed] [Google Scholar]
- 26.Pinlaor S, Tada-Oikawa S, Hiraku Y, Pinlaor P, Ma N, Sithithaworn P, et al. Opisthorchis viverrini antigen induces the expression of Toll-like receptor 2 in macrophage RAW cell line. Int J Parasitol. 2005;35:591–596. doi: 10.1016/j.ijpara.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 28.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 29.Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–5841. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 30.van Stijn CM, Meyer S, van den Broek M, Bruijns SC, van Kooyk Y, Geyer R et al. Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC-SIGN. Mol Immunol. 2010;47:1544–1552. doi: 10.1016/j.molimm.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Talabnin K, Yagi H, Takahashi N, Suzuki T, Kato T, Uemura H et al. Glycobiological study of adult Opisthorchis viverrini: characterization of N-linked oligosaccharides. Mol Biochem Parasitol. 2006;147:230–233. doi: 10.1016/j.molbiopara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 34.Isse K, Harada K, Nakanuma Y. IL-8 expression by biliary epithelial cells is associated with neutrophilic infiltration and reactive bile ductules. Liver Int. 2007;27:672–680. doi: 10.1111/j.1478-3231.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 35.Mukaida N. Interleukin 8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–398. [PubMed] [Google Scholar]