Abstract
Growth cones, found at the tip of axonal projections, are the sensory and motile organelles of developing neurons that enable axon pathfinding and target recognition for precise wiring of neural circuitry. To date, many families of conserved guidance molecules and their corresponding receptors have been identified that work in space and time to ensure billions of axons to their targets. Research in the past two decades has also gained significant insight into the mechanisms by which growth cones translate extracellular signals into directional migration. This review aims to examine new progress towards understanding the cellular mechanisms underlying directional motility of the growth cone and to discuss questions that remain to be addressed. Specifically we will focus on the cellular ensemble of cytoskeleton, adhesion, and membrane and examine how the intricate interplay between these processes orchestrates the directed movement of growth cones.
Keywords: growth cone, axon guidance, cytoskeleton, membrane recycling, adhesion, signaling, motility, actin, microtubule
As vividly described by Santiago Ramón y Cajal (Ramón y Cajal, 1909), “the growth cone may be regarded as a sort of club or battering ram, endowed with exquisite chemical sensitivity, with rapid ameboid movements, and with certain impulsive force, thanks to which it is able to proceed forward and overcome obstacles met in its way, forcing cellular interstices until it arrives at its destination” Cajal, who first indentified the structures in 1890 (Ramón y Cajal, 1890), further postulated that growth cones exhibit and depend on chemotropism to cues presented in the developing brain to reach specific targets. However, direct support of chemotropic guidance of growth cones was not obtained until nearly a century later, highlighted by the identification of the netrin family of chemoattractants in the floor plate of the spinal cord that guide the axons of commissural interneurons (Kennedy et al., 1994; Tessier-Lavigne et al., 1988) and the correlate discovery of unc-6/netrin and its receptors unc-5 and unc-40 in C. elegans (Hedgecock et al., 1990; Hedgecock et al., 1987; Ishii et al., 1992). The molecular identities of many factors involved in axon guidance have since been revealed, largely fueled by astonishing growth in molecular biology and genetic techniques. We have now learned that a variety of evolutionarily conserved guidance molecules, either attractive or repulsive in nature, provide the spatiotemporal cues for growth cone navigation through a complex physical and chemical topology to reach it specific destination (Kolodkin and Tessier-Lavigne, 2011).
While Cajal provided the vivid description of nerve growth cones from the static images of histological staining, it wasn’t until the invention of modern tissue culture by Ross Harrison that allowed the first live microscopy of growth cones (Harrison, 1910). Subsequent studies have taken great advantage of cultured growth cones to gain a fairly detailed picture on their structure and motile properties. In particular, this “early” phase of growth cone research has advanced our understanding of how extracellular and intracellular signals influence growth cone extension and the cytoskeletal architecture underlying growth cone motility. For example, Ca2+ was established as a key second messenger that profoundly influences growth cone motility. The discovery that an optimal range of intracellular Ca2+ concentration is required for growth cone advancement provided the foundation for a wealth of research geared towards understanding the complex role of Ca2+ signaling in growth cone guidance (Gomez and Zheng, 2006; Kater et al., 1988). Moreover, this phase of research yielded detailed imaging results on the cytoskeletal architecture of the growth cone, establishing distinct roles for actin and microtubules in controlling the protrusive machinery and net migration (Bentley and O’Connor, 1994; Lin et al., 1994; Smith, 1988).
The identification of in vivo guidance cues fueled the “second” phase of growth cone research. We now know that the cytoskeleton and focal adhesion are the major targets of intricate signaling cascades to generate specific motile behaviors (Dickson, 2001; Huber et al., 2003; Kalil and Dent, 2005; Korey and Van Vactor, 2000; Myers et al., 2011; Wen and Zheng, 2006). Recent studies have also shown the involvement of membrane recycling in growth cone responses (Tojima et al., 2011). It is conceivable that different signaling cascades elicited by extracellular factors could target a distinct component of growth cone motility, but the specific response likely involves concerted actions of multiple motility apparatuses (Lowery and Van Vactor, 2009). The next challenge is to fully elucidate the intricacies of these mechanisms and how they are orchestrated to enable the agile and adaptive motile behaviors of the growth cone. In this review, we will discuss three major mechanisms of growth cone motility: cytoskeleton, adhesion, and membrane turnover. Each topic, rather than providing an extensive overview, will be highlighted with specific examples of molecules that play a pivotal role in axon growth and guidance yet whose exact functions in these processes remains to be fully elucidated. We set out to reveal the complexity of cellular behavior underlying growth cone directional motility and to postulate important unknown questions. At the end, we will discuss the intricate interplay amongst these components and how multiple networks coordinate to enable the growth cone to respond and navigate through complex terrains in order to reach its specific target.
Nerve growth cone: a primer
The growth cone is a dilated terminal of axonal and dendritic processes. Under light microscopy, the growth cone can be seen to have two distinct compartments: the peripheral and central regions (P- and C-region) (see Figure 1). The P-region is a broad and flat area that is highlighted by lamellipodia and filopodia, two types of membrane protrusions containing a meshwork of branched actin filaments and long parallel bundles of actin filaments, respectively. The C-region, located behind the P-region and connected to the axonal shaft, is enriched in cellular organelles such as mitochondria and exocytotic vesicles. A predominant feature of the C-region is a dense microtubule array that extends from the axonal shaft to support growth cone movement and to serve as the track for transport of membraneous organelles. While the majority of microtubules terminate at the C-region, single microtubules do venture into the P-region where their interactions with actin and cell signaling components are of importance for growth cone motility. High resolution imaging studies of the growth cone’s cytoskeleton have revealed a third functionally distinct region, the transitional zone (T-zone) (Lowery and Van Vactor, 2009; Rodriguez et al., 2003). The T-zone is located between the P- and C-regions and is believed to contain the actomyosin contractile structures that play a strong role in the regulation of both the actin and microtubules in the growth cone, including regulating the rearward flow of actin in the P-region and maintaining the C-region localization of the microtubule lattice (Burnette et al., 2008; Medeiros et al., 2006; Zhang et al., 2003).
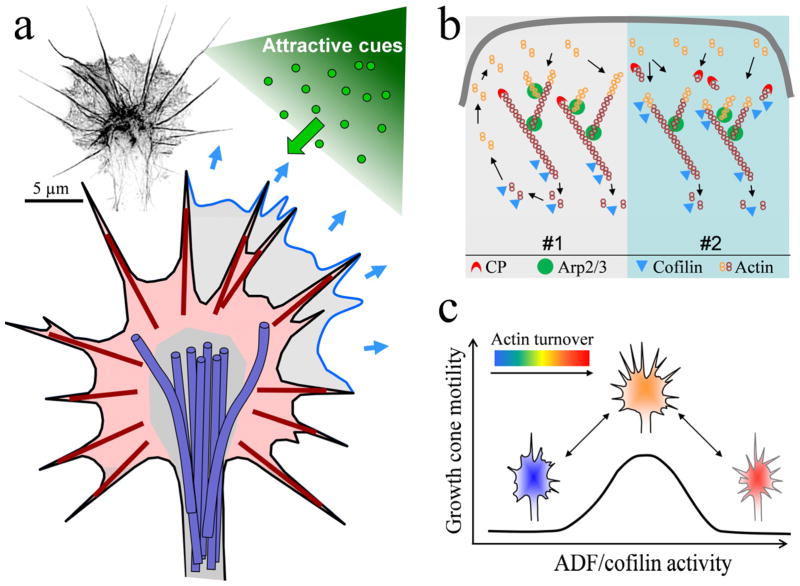
Figure 1.
Actin cytoskeleton of the growth cone. (a) Schematic of the actin cytoskeleton of a growth cone undergoing an attractive guidance response. The growth cone’s periphery contains actin-rich lamellipodia (light red shaded) and filopodia (dark red lines. The lamellipodia consists of a network of short, branched actin filaments that serves as the protrusion machinery of the growth cone. Newly formed lamellipodia on the side undergoing a positive turning response is shown in blue. Filopodia are composed of long, bundles actin filaments. They participate in environment sensing and guidance. Microtubules in the growth cone (shown in purple) are largely restricted to the central region (gray shaded) by the actin cytoskeleton. The inset on the top left shows a fluorescent image of the actual F-actin architecture within the growth cone. This image was obtained using the Nikon N-SIM Super Resolution microscope and inverted in grayscale for display. Scale bar: 5 μm. (b) Two models of ADF/cofilin-mediated regulation of actin dynamics underlying lamellipodial protrusion. The #1 is the classical model in which ADF/cofilin functions mainly in depolymerization at the rear of the actin meshwork and recycling the actin monomers to the leading front for assembly. In the #2 model, ADF/cofilin severing creates new barbed ends to promote actin assembly and membrane protrusion. (c) A hypothesized model in which an optimal range of ADF/cofilin activity may be required for growth cone motility. Above and below this range of ADF/cofilin activity inhibits growth cone motility. Depending on upon the amount of active ADF/cofilin and the dynamic state of the actin network, a gradient of guidance cues could asymmetrically target the ADF/cofilin activity to generate either attractive (into the optimal range) or repulsive (out of the optimal range) responses.
Growth cones represent the major site of attachment to the outside environment in both axons and dendrites. Actin-based protrusions are coupled with selective adhesion to extracellular components to provide the force necessary to drive the growth cone forward, leading to the elongation of axonal and dendritic processes. The growth cone is also the major site of membrane recycling in the form of exocytosis and endocytosis. Imaging work has shown that membraneous organelles are largely concentrated in the C-region (Bunge, 1973), though vesicular components can be found in the lamella and lamellipodia (Tojima et al., 2011), and even more rarely in filopodia (Sabo and McAllister, 2003). Membrane recycling at the growth cone can serve many purposes, ranging from the regulation of available membrane surface area to receptor trafficking. While the cytoskeleton, adhesion to the extracellular environment, and membrane turnover are often studied separately with respect to growth cone motility and guidance, work done in recent years has shown that there is an elaborate cross-talk between these components and that they must be carefully balanced to productively steer a neuronal process to its specified target.
Actin in the driver seat
Actin plays a pivotal role in growth cone motility and guidance responses. A combination of actin polymerization near the plasma membrane, myosin-based actin retrograde flow, and selective engagement of the “clutch” to the adhesion substrate is believed to drive the growth cone forward (Lowery and Van Vactor, 2009; Suter and Forscher, 1998). The actin cytoskeleton is targeted by many signaling cascades, of which the Rho-family GTPases represent a key node for connecting extracellular signals to regulated actin dynamics (Burridge and Wennerberg, 2004; Hall and Nobes, 2000). Rho GTPases have been shown to play a fundamental role in axonal growth and guidance (Dickson, 2001; Ng et al., 2002). However, the downstream effectors and precise actin mechanisms that control the directional motility of growth cones remain to be fully determined. Actin filaments are built through a balancing act of filament assembly at the barbed ends and disassembly at the pointed ends, but their rates are influenced by a wide range of regulatory proteins. Moreover, an even larger number of accessory proteins are present in cells to organize actin filaments into distinct networks in specific subcellular locations (Chhabra and Higgs, 2007; Pollard et al., 2000; Pollard and Borisy, 2003). For example, lamellipodia and filopodia, two membrane protrusions that function in growth cone movement and environmental sensing, respectively, are based on distinct F-actin structures. The former contains a meshwork of short, branched actin filaments that depends on the Arp2/3 nucleation complex, whereas the later is supported by long unbranched actin filaments involving formin family of molecules and regulated by Ena/Vasp proteins. A number of excellent reviews are available that have provided comprehensive coverage on the actin structures and dynamics of lamellipodia and filopodia in both non-neuronal cells and nerve growth cones (Dent et al., 2011; Lowery and Van Vactor, 2009; Pollard and Cooper, 2009; Rodriguez et al., 2003). Here, we will only discuss a few of the actin regulatory molecules whose function in growth cone motility is complex and remains to be fully understood.
In vertebrate cells, a large array of regulatory proteins control the actin network and its dynamics through a diverse set of actions, including filament nucleation, severing, crosslinking, and end capping, as well as monomer sequestering. Many of these proteins have not been well studied in neuronal growth cones, and whether and how they function in growth cone migration and guidance remains to be seen (Dent et al., 2011). In a minimal model proposed for the actin assembly and disassembly underlying lamellipodial protrusion, just five families of actin binding proteins were thought to be needed: WASp, Arp2/3, capping protein, ADF/cofilin, and profilin/β-thymosin (Pollard et al., 2000). Of them, WASp, Arp2/3, and ADF/cofilin have been investigated in nerve growth cones (Dent et al., 2011; Lowery and Van Vactor, 2009), whereas thymosin/profilin and capping protein have received less attention.
Capping barbed ends of actin filaments represents an important mechanism to regulate filament elongation (Pollard and Borisy, 2003). Capping proteins bind to free barbed ends and prevent addition or loss of actin subunits. Of the known actin capping proteins, the predominant species in most non-muscle cell types is CapZ (commonly abbreviated as CP). CP is an obligate heterodimer consisting of α and β subunits (Cooper and Sept, 2008; Schafer, 2004). While both α1 and α2 isoforms are abundant in most tissues (Hart et al., 1997), β2 is the primary isoform in the mammalian brain (Schafer et al., 1994). Barbed end capping is believed to promote lamellipodial protrusion by increasing the local availability of polymerization competent G-actin for Arp2/3-mediated nucleation (Akin and Mullins, 2008). A loss of CP leads to the formation of actin bundles and filopodia, which in part mediated by the anti-capping activity of Ena/Vasp proteins (Kapustina et al., 2010; Mejillano et al., 2004; Vitriol et al., 2007). It remains to be determined if a similar interplay of CP and Arp2/3 operates in nerve growth cones and if so, whether it plays a role in axon guidance. Specifically, it has not been determined if growth cone steering in response to guidance cues depends on spatiotemporally restricted capping activity (see Figure 1). This question is confounded by our lack of knowledge as to how CP is regulated in living cells. We know that modulation of CP plays a major role in actin physiology, as its off-rate to actin filaments in vivo is three orders of magnitude faster than that in vitro (Miyoshi et al., 2006). CP is known to bind Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and this interaction inhibits its ability to bind actin barbed end (Schafer, 2004). It was shown that asymmetric PI(4,5)P2 phosphorylation by Phosphoinositide 3-kinase mediates growth cone chemotaxis (Henle et al., 2011), which could potentially lead to asymmetric capping and lamellipodial protrusion leading to growth cone steering. Moreover, the Ena/VASP family of actin regulatory proteins exhibit anti-capping activity and could play a role in antagonizing actin capping during growth cone steering (Bear et al., 2002), though they are not essential for retinal axon pathfinding in Xenopus (Dwivedy et al., 2007). Interestingly, a recent study shows that CP interacts with β–tubulin to regulate the extension of MT in the growth cone (Davis et al., 2009), thus providing a potential point of crosstalk among the actin and microtubule cytoskeletal systems. However, whether the CP-MT interaction plays a role in the growth cone directional response to guidance cues remains to be examined.
Besides a long list of actin regulatory proteins whose function in growth cone guidance remains unclear (Dent et al., 2011), several well-studied actin factors have complex ramifications on the actin physiology, even to the point of appearing to cause opposite effects on growth cone motile responses. One example is ADF/cofilin, which represents a highly conserved family of actin-associated proteins from different genes (cofilin1, 2, and ADF) but with similar functions on actin dynamics (thus referred to as AC hereafter for simplicity) (Bernstein and Bamburg, 2010; Van Troys et al., 2008). AC was initially identified for its ability to increase the rate of ADP-actin dissociation from the pointed end of actin filaments to promote depolymerization (Carlier et al., 1997), as well as to sever actin filaments into small fragments for disassembly (Maciver, 1998). In the classic model of lamellipodial protrusion, ADF/cofilin functions at the rear of the lamellipodial actin meshwork to breakdown actin filaments and recycle the actin monomers for further leading edge assembly (Figure 1b). However, AC severing of actin filaments also creates new barbed ends, which can synergize with actin polymerization factors to promote filament assembly and membrane protrusion (Kuhn et al., 2000; Pollard et al., 2000). The opposite functions of AC on actin filaments likely depend on its local concentration of AC and the ratio of AC against actin monomers: severing and disassembly are more favorable when AC is at a lower concentration, whereas nucleating occurs at higher AC concentrations (Andrianantoandro and Pollard, 2006).
The precise function of AC in nerve growth cones remains to be fully understood. AC is expressed at high levels and colocalizes with F-actin in neuronal growth cone (Bamburg and Bray, 1987). Overexpression of AC in neurons leads to increased neurite outgrowth (Meberg et al., 1998), indicating that actin turnover may promote motility (Bradke and Dotti, 1999). However, AC activation has also been associated with growth cone collapse (Aizawa et al., 2001; Hsieh et al., 2006; Piper et al., 2006), demonstrating a negative impact of AC on growth cone motility. In growth cone steering, asymmetric AC inhibition was shown to mediate attractive turning of the growth cone, whereas local AC activation elicited repulsion (Wen et al., 2007). These findings are consistent with the classic depolymerizing/severing functions of AC on the actin cytoskeleton. However, AC activation was shown in some cases to promote actin-based membrane protrusion in non-neuronal cells (DesMarais et al., 2005; Ghosh et al., 2004) and to mediate growth cone attraction in cultured dorsal root ganglion neurons (Marsick et al., 2010). It is plausible that different types of cells exploit specific end results of AC activity, and their unique cytosolic environment may contribute to the opposite outcomes of increased AC activity on motility. It is also possible that the same neurons may have varying levels of basal actin dynamics, upon which AC may generate different effects. For example, growth cones from young neurons tend to be very motile and have a high level of actin turnover, whereas those from more mature neurons have relatively stable F-actin and reduced motility. AC activation could in principle impact the motility of these growth cones in an opposite manner. We propose that an optimal range of AC activity is required to generate the dynamic turnover of the actin cytoskeleton underlying high growth cone motility, and that this range is dependent on the kinetic state of the actin network at that time. In this instance, modulation of AC activity in either direction could either accelerate or decrease motility (Figure 1c), or if done assymetrically within the growth cone, cause a positive or negative turning response. As a result, attractive steering may involve local elevated AC activity in growth cones with more stable actin cytoskeleton, but require local AC inhibition for growth cones with a high actin turnover rate. Clearly, this model and the precise functions of AC in growth cone actin dynamics and guidance responses require further studies, which may benefit from the emerging super resolution imaging techniques (Toomre and Bewersdorf, 2010).
Microtubules: TIPs are welcome
Microtubules (MTs), the cylindrical filaments each consisting of 13 protofilaments, are a major cytoskeletal system within the axonal and dendritic projections. MTs are intrinsically polarized due to their head-to-tail assembly from α/β tubulin heterodimers. While the plus- and minus-ends of MTs favor polymerization and depolymerization, respectively, the minus-ends of MTs are often capped and stabilized inside cells (Dammermann et al., 2003). Instead, MT plus-ends exhibit “dynamic instability”, in which their polymerization-based growth is interrupted by “catastrophe” phases of rapid depolymerization and shrinkage (Cassimeris et al., 1987). It is believed that dynamic instability provides MTs with the ability to quickly remodel their organization and selectively grow in response to extracellular signals. In neurons, most of MTs are believed to be polymerized from the centrosome, but are severed, released, and transported into long axons and dendritic arbors where they form dense arrays (or bundles). These condensed MT arrangements are the structural foundation for the extension and maintenance of highly elongated and elaborated nerve processes. In addition, MT arrays serve as the railway tracks for long-range transport of cellular organelles and cargos, which is essential for the survival and function of the neuron (Hirokawa et al., 2010). Finally, spatiotemporally regulated dynamics of these MTs may play an important role in specifying axonal and dendritic polarization (Witte et al., 2008).
How MTs are involved in the directional responses of the growth cone has only begun to be elucidated (Dent et al., 2011; Gordon-Weeks, 2004; Lowery and Van Vactor, 2009). The dense MT arrays in the neurite shaft typically terminate in the growth cone C-region, with a small number of MTs splaying out into the actin rich P-region (Figure 2). These individual MTs appear to exhibit a high degree of dynamics and track along the actin filaments (Schaefer et al., 2002). It should be noted that MTs in axons are organized in uniform polarity such that individual MTs in the growth cone are pioneered by their plus-ends. Therefore, the behavior of MTs exploring the growth cone P-region is largely dictated by how their dynamic instability is regulated. It is believed that actin-based growth cone movement requires the local stabilization of dynamic MTs exploring the P-region, followed by site-directed MT polymerization and delivery of cellular cargos to consolidate the space created by the forward movement of the growth cone. Conversely, dynamic MTs in the growth cone could play a more direct role in migration, as local modification of MT polymerization and depolymerization can impact actin-based membrane protrusion to elicit a growth cone steering response (Buck and Zheng, 2002; Mack et al., 2000; Rochlin et al., 1999). Therefore, dynamic MTs and the actin cytoskeleton appear to engage in bidirectional interactions that each can trigger the motile responses involving the other cytoskeletal component for coordinated cell movement (Goode et al., 2000; Lowery and Van Vactor, 2009; Rodriguez et al., 2003).
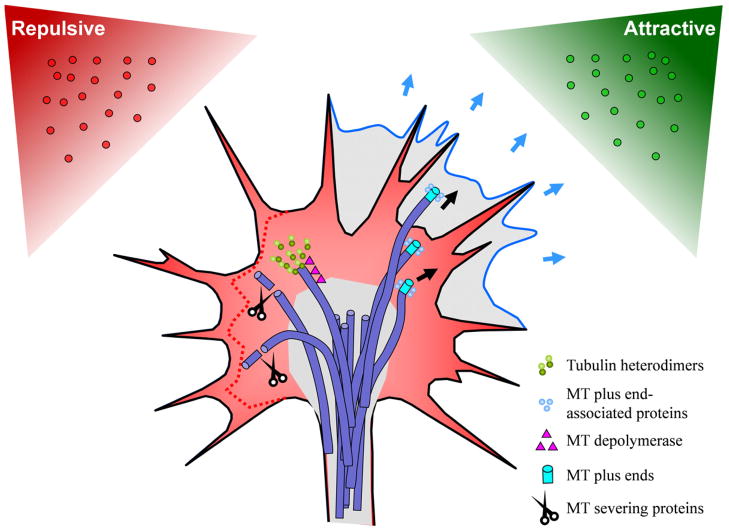
Figure 2.
Microtubules in growth cone steering. This schematic shows the hypothesized model involving asymmetric modification of MT dynamics during growth cone attraction and repulsion. Newly formed lamellipodia is shown in blue, retracting lamellipodia is indicated by the dotted red line. Microtubules in the growth cone (shown in purple) are largely restricted to the Central region by the actin cytoskeleton, but some enter into the P-region, where they play an important role in axon guidance. MT localization is controlled by the actin cytoskeleton and a host of MT regulatory proteins. During retraction, MTs are removed from the periphery through selective targeting by depolymerases and severing proteins. During attraction, proteins bind to MT plus-ends, stabilizing them or enhancing polymerization and further growth.
Similar to the actin cytoskeleton, a wide range of MT-binding proteins (MAPs) exist to regulate MT polymerization and depolymerization, stability, crosslinking, motor interaction, severing, and transport (Hirokawa et al., 2010; Maccioni and Cambiazo, 1995). Recent studies have revealed the importance of proteins associated with and localized to the plus-ends of MTs in growth cone motility and responses to extracellular signals (Lowery and Van Vactor, 2009). In particular, the plus-end tracking proteins (+TIPs), such as the end binding protein (EB) and the cytoplasmic linker protein (CLIP) molecules, have been shown to especially relevant. Many of these +TIPs can be targeted by a wide range of signaling cascades. For example, the CLIP-associated protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Able to mediate axon guidance (Lee et al., 2004). The CLIP family of +TIPs interact with Adenomatous Polyposis Coli (APC) to regulate Glycogen synthesis kinase 3β activity, which has been shown to regulate MT dynamics and growth cone guidance by Wnt molecules (Ciani et al., 2004; Lucas et al., 1998; Zhou et al., 2004). It has also been shown that CLIPs interact with IQGAP, which targets Rac1/Cdc42 GTPases to regulate the actin dynamics in growth cones (Fukata et al., 2002; Kholmanskikh et al., 2006). Moreover, several +TIPs interact with the dynactin complex, helping to localize to MT plus-ends. Although plus-end localization of dynactin is not required for intracellular membrane traffic (Watson and Stephens, 2006), it could be involved in local membrane turnover and recycling in the growth cone, leading to the modification of growth cone locomotion (Tojima et al., 2011) or the generation of “signaling endosomes” for retrograde neurotrophin signaling (Zweifel et al., 2005). It is also possible, though not exclusively, that plus-end localization of the dynactin complex may function to regulate MT polymerization (Ligon et al., 2003) and/or to work with dynein and Lis1 to regulate MT advance during growth cone remodeling and extension in response to extracellular signals (Grabham et al., 2007).
Distinct from +TIPs, the mitotic centromere-associated kinesin (MCAK)/KIF2c belongs to the kinesin-13 family of the middle motor domain KIFs (M-KIFs) that bind to MT plus-ends to promote MT depolymerization (Hirokawa et al., 2010; Howard and Hyman, 2007). Both KIF2a and KIF2c exhibit ATP-dependent depolymerizing activity, presumably by removing the GTP-cap at the plus-end to induce catastrophe (Howard and Hyman, 2007). KIF2a is highly expressed in postmitotic neurons of developing brains, KIF2a knockout caused brain defects of disrupted migration and excessive axonal branching (Homma et al., 2003). It is believed that the branching phenotype is caused by the lack of KIF2a-mediated MT depolymerization in the growth cones of collateral branches. While it is not known if KIF2 is involved in growth cone guidance, directional movement of the growth cone requires polarized membrane extension on one side and retraction on the other. KIF2 mediated MT depolymerization could in principle be involved in the disassembly of the MTs on the retracting side of the growth cone (see Figure 2). For KIF2 to function in asymmetric modification of MTs during growth cone guidance, its localization or depolymerizing activity needs to be regulated in a spatiotemporal manner. Currently, there is little information in regards to how KIF2 is regulated in cells and whether or not there is a spatiotemporal component to restrict its MT depolymerizing activity. However, the fact that KIF2a-induced MT depolymerization appears to be restricted only in the collateral growth cones suggests the existence of a local control mechanism. Finally, although KIF2s are not thought to move directionally along the MT lattice (Helenius et al., 2006), KIF2a has been shown to function in transport of membraneous organelles involved in growth cone membrane expansion (Morfini et al., 1997; Noda et al., 1995; Pfenninger et al., 2003). The depolymerizing and trafficking activities of KIF2s appear to be counterintuitive with regards to growth cone locomotion. Therefore, it would be of interest to determine if KIF2s may selectively engage in either MT depolymerization or vesicle transport at a specific time and/or location.
KIF2s are not only the molecules that can negatively affect the MT structure and dynamics to potentially function in polarized growth cone extension. Recent studies have identified katanin and spastin as proteins that sever MTs to create shorter fragents that are more prone to depolymerization if not protected or stabilized (Roll-Mecak and McNally, 2010). In migrating non-neuronal cells, short MT fragments have been seen within the lamellipodial region of the leading edge. Live cell imaging studies have yielded data indicating that MTs are severed within the lamellipodia due to physical stress caused by actin retrograde flow (Gupton et al., 2002; Schaefer et al., 2002; Waterman-Storer and Salmon, 1997), though a possible involvement of enzymatic MT severing has not been excluded. Interestingly, a recent study has shown that katanin can function both as a MT severing enzyme and plus-end depolymerase to regulate the MT dynamics at the cell cortex (Zhang et al., 2011). Importantly, katanin was found to localize to the leading edge of polarized cells to negatively regulate their migration, demonstrating a negative role for MT severing enzymes in cell motility. It would be interesting to see if spatiotemporal katanin-mediated MT severing and depolymerization are employed to regulate growth cone migration and directional responses to guidance cues (Figure 2).
Like many of the actin regulatory proteins, the exact effects of MT severing on neurons can be complex and may depend on a number of factors such as how the MT is posttranslationaly modified and what other MT-binding proteins are present. For example, severing of stable MTs enables the release of short MTs from the centrosomal region for their transport down the axon and organized into the dense MT array in the axonal shaft (Yu et al., 2005). Local severing of MT arrays has been shown to be involved in the formation of collateral braches, a process that may involve the local creation of dynamic MT plus-ends (Yu et al., 2008). In nerve growth cones, MT severing has been observed to break down the looped MTs that are often associated with stalled growth cones (Dent et al., 1999; Schaefer et al., 2002). Finally, very limited attention has been given to the minus-ends of MTs. Both axons and dendrites contain microtubule fragments with exposed minus-ends. Surprisingly, these minus-ends undergo little depolymerization, indicating the existence of a mechanism that caps and stabilizes them (Dammermann et al., 2003). It is of interest to know that KIF2 localizes to both plus- and minus-ends for depolymerization. Therefore, protecting the minus-ends could have a larger impact on axonal growth and guidance than one might think.
Adhesion: it’s all about turnover
Cell-cell and cell-matrix adhesions are macromolecular protein complexes that provide a direct linkage between the cell and its external environment. They are essential for tissue morphogenesis and cell migration. During brain development adhesion molecules provide an important roadmap, and together with secreted cues, guide axonal and dendritic growth to form the neural circuitry (Kamiguchi, 2007; Kolodkin and Tessier-Lavigne, 2011; Maness and Schachner, 2007; Myers et al., 2011). In growth cones, adhesions can be derived from several different receptors including integrins, cadherins, and the immunoglobin superfamily (IgSF) members. In neurons adhesions often appear as small, punctate structures and are referred to as point contacts. The ligands for integrins are found in the extracellular matrix, while cadherins and IgSF proteins interact homophillically with molecules expressed on the surface of adjacent cells (Kolodkin and Tessier-Lavigne, 2011). Following receptor activation at the plasma membrane, intracellular adhesion components are recruited to the nascent contact, providing the platform needed for chemical and force-based adhesive signaling events (Huttenlocher and Horwitz, 2011).
A popular model to describe how adhesions modulate motility is the “molecular clutch.” The clutch hypothesis predicts that cell adhesions couple with actin undergoing retrograde flow. Clutch engagement provides the mechanical resistance that is needed for the actin network to overcome the rearward current of retrograde flow, which in turn allows the plasma membrane to translocate forward. Forces generated by clutch resistance of the F-actin network are transmitted back to the adhesions, resulting in increased surface traction (Aratyn-Schaus and Gardel, 2010; Brown et al., 2006; Giannone et al., 2009; Hu et al., 2007). Compelling evidence of the clutch model has been demonstrated in neurons (Bard et al., 2008; Chan and Odde, 2008). In nascent protrusions that result from clutch engagement, newly polymerized actin primes and positions integrins for activation (Chan and Odde, 2008). Adhesions experiencing increased force undergo higher component turnover (Wolfenson et al., 2011). Together, these create a cycle promoting dynamic fluctuation and positive growth. It is clear that mechanical signaling plays an important role in outgrowth and guidance, but its exact mechanism of controlling overall movement has not yet been determined.
Adhesions are dynamic complexes whose turnover is critical for cell movement (Huttenlocher and Horwitz, 2011). Stable adhesions immobilize the cell while lack of adhesion makes it incapable of crawling on a substrate. In a motile growth cone, adhesions assemble and disassemble to change in number, size, and position (Myers and Gomez, 2011; Thoumine, 2008). Additionally, the individual adhesion components, including the surface receptors, undergo turnover within the point contacts (Dequidt et al., 2007). Adhesions are protein structures in constant flux, ready to immediately respond to internal and external signals. Modification of adhesion dynamics can effect overall migration or, if done locally within the growth cone, cause a directional guidance response (Myers and Gomez, 2011; Myers et al., 2011; Woo and Gomez, 2006). Several traditional signaling pathways that work through focal adhesions have been shown to mediate both attractive and repulsive guidance responses. There are numerous reviews that are specifically dedicated to the vast signaling networks that work through adhesions (Kamiguchi, 2007; Kolodkin and Tessier-Lavigne, 2011; Maness and Schachner, 2007; Myers et al., 2011). In this section, we would like to focus on focal adhesion kinase (FAK), a single, well-studied adhesion protein, to highlight how its complex regulation can induce polar effects in the migrating growth cone. We hope to demonstrate the need to understand the spatiotemporal regulation of adhesive contacts.
Focal Adhesion Kinase (FAK) is a protein-tyrosine kinase that provides a direct link between adhesions and intracellular signaling pathways (Mitra et al., 2005; Parsons, 2003). FAK is downstream of both extracellular matrix and intracellular signaling components, therefore it is in a position to transduce signals to and from adhesions. FAK contains multiple tyrosine and serine phosphorylation sites that are crucial for its ability to regulate adhesions and the cytoskeleton (Chacon and Fazzari, 2011; Grigera et al., 2005; Ma et al., 2001; Mitra et al., 2005). FAK is upstream of numerous signaling pathways inside the cell, including regulation of Src-family kinases, Rho-family GTPases, actin regulatory molecules, adhesion components, and microtubules (Chacon and Fazzari, 2011; Mitra et al., 2005). In neuronal adhesions, FAK is activated downstream of netrins and integrins, where it has been shown to be essential for regulating outgrowth and guidance in response to adhesion receptor activation (Bechara et al., 2008; Chacon and Fazzari, 2011; Li et al., 2004; Liu et al., 2004; Myers and Gomez, 2011; Ren et al., 2004; Robles and Gomez, 2006). FAK mediates these effects in part by altering the dynamics of point contacts. In fact, FAK activity in neurons is necessary to assemble, stabilize, and break down adhesions (Bechara et al., 2008; Robles and Gomez, 2006). Ultimately (and purportedly through its ability to modulate adhesion dynamics), FAK is needed for proper organismal development as FAK activity is implicated in ventral midline crossing, outgrowth of Rohan-beard neurons from the neural tube, and retinotopic mapping (Chacon and Fazzari, 2011; Myers et al., 2011). In growth cones, localized regulation of FAK has been implicated in both attractive and repulsive signaling (Bechara et al., 2008; Chacon and Fazzari, 2011; Myers and Gomez, 2011).
How can a single molecule be involved in adhesion assembly and disassembly, outgrowth and inhibition, attraction and repulsion? The answer may be that it is highly spatiotemporally regulated, and that it can exhibit diverse effects within the growth cone depending on where, when, and how much it is activated. In addition to FAK’s specific localization to adhesive contacts, FAK activity is also controlled in time through its complex signaling interactions, autoinhibition, self phosphorylation, and instigation of feedback loop pathways. Furthermore, the fact that FAK is a mechanosensor indicates that it is asymmetrically activated amongst adhesions experiencing varying mechanical loads. As the tools necessary for elucidating the dynamics of FAK activation within subcellular structures (Cai et al., 2008; Seong et al., 2011) and determining the functional outcome of its localized activation (Karginov et al., 2010; Slack-Davis et al., 2007) become available, we will be able to resolve the complexities of FAK signaling during neuritogenesis, axon pathfinding, and regeneration. Finally, FAK is but a single component of the 100+ member adhesome (Geiger and Yamada, 2011). We must understand the role it plays in the larger picture of adhesion based signaling.
Membrane recycling: create and destroy
Membrane trafficking that occurs at the growing axon tip involves both membrane addition and internalization in the forms of exocytosis and endocytosis, respectively. These processes may be constitutive or evoked. In addition to delivering or removing plasma membrane, trafficking in the growth cone can involve the transport and internalization of cell adhesion molecules, signaling proteins such as Rho-Family GTPases and Src-family kinases, lipid mediators, and guidance receptors (Bloom and Morgan, 2011). Localized delivery of these cargos ensures the spatial organization of signaling networks within the growth cone that is needed for directed movement. Further, the removal or addition of plasma membrane may serve as an important physical constraint that regulates movement (Meldolesi, 2011). As a neurite continues to extend away from the cell body, it increases in autonomy and the trafficking/recycling pathways are one way in which it can maintain a level of independence from the cell body. Though these processes were discovered in the growth cone nearly 40 years ago, a number of recent advances have shown how localized vesicle traffic regulates axon growth and guidance.
The plasma membrane (or plasmalemma) is the neuron’s largest organelle and during axon growth it must be expanded to accommodate the neuron’s rapidly increasing surface area (Meldolesi, 2011). Although lipid and protein synthesis do occur in the distal regions of the axon, the majority of plasmalemma expansion occurs through exocytosis within the growth cone. Bulk exocytic vesicles such as Plasmalemma Precursor Vesicles (PPVs) and enlargeosomes, derived in the cell body and actively transported to the axon tip via microtubules, are constitutively inserted into the C-domain where they promote axon growth (Pfenninger et al., 2003; Racchetti et al., 2010). Though fusion of this type of exosome with the plasma membrane can be induced downstream of guidance cues (Pfenninger et al., 2003), there have been no studies that have linked this process to directional steering of the growth cone.
A separate class of exocytic structure, VAMP2 positive synaptic precursor vesicles, has been shown to be involved in growth cone guidance responses. Tojima et al. demonstrated that VAMP2 exocytic vesicles are trafficked from the C domain of the growth cone to the periphery in response to attractive intracellular Ca2+ signals and that this type of exocytosis exclusively functions in attractive turning and not repulsion or overall outgrowth (Tojima et al., 2007). Partial colocalizion of VAMP2 vesicles with an endocytic marker and internalized cell surface receptors implies that this localized delivery of components to the plasmalemma is involved in the recycling pathway, thought the specific cargo of these vesicles has not been identified. It also remains to be determined if local exocytosis functions to cause an asymmetric expansion of the plasma membrane, to deliver and recycle important cell surface molecules, or both.
As with membrane addition, the growth cone is the primary location for membrane internalization in the developing axon. Endocytosis in the growth cone can be constitutive or evoked; these are distinct processes carried out by different types of vesicles (Diefenbach et al., 1999). Bulk (or constitutive) endocytosis occurs in growing axons (Bonanomi et al., 2008). It represents a fluid-phase type of endocytosis and its vesicles are free of any markers that would implicate them in the recycling pathway, such as clatherin or caveolin. Though, the fact that relevant cell surface proteins are excluded indicates there is some selectivity in bulk endosome vesicle content. Consistent with its role in positive outgrowth, the rate of bulk endocytosis positively correlates with neurite extension speed and occurs more prominently in the early developmental stages of outgrowth (Bonanomi et al., 2008). Both bulk endocytosis and exocytosis occur downstream of the activation of the small GTPase Rac (Bonanomi et al., 2008; Racchetti et al., 2010), begging the question if they are coordinated by the same intracellular signaling pathways. It is still unclear why this type of rapid, non-specific back and forth membrane transport needed for efficient neurite elongation. It is plausible that bulk membrane recycling is involved in dynamic renewal and modification of membrane lipid composition. Alternatively, it could simply function in reshaping the membranous distribution associated with the rapid changing cell geometry.
Despite its linkage to rapid outgrowth, there is little evidence showing that constitutive endocytosis occurs asymmetrically in the growth cone during guidance. Though recently, Kolpak and colleagues documented a functional role for asymmetric fluid-phase endocytosis during repulsive signaling (Kolpak et al., 2009). This was counterintuitive to previous studies showing positive correlations between bulk endocytosis and axon growth (Bonanomi et al., 2008). In addition to demonstrating that bulk fluid-phase uptake occurs during growth cone collapse and determining some of the regulatory molecules involved, direct evidence was provided that locally applied Sonic Hedgehog, at a repulsive concentration, caused a macropinocytic-like uptake of dextran on the side of growth cone receiving the negative cue. The endocytic response was immediate and preceded growth cone turning. Interestingly, and true to form of bulk endocytosis, the internalized vesicles did not contain the Sonic Hedgehog receptor. What this type of membrane internalization’s role is in establishing repulsive asymmetry and why it can be utilized for both positive and negative migration of the growth cone remains to be determined.
Evoked endocytosis is a stimulus dependent means of membrane internalization and recycling. It is relevant for both positive and negative regulation of axon growth (Tojima et al., 2011). A hallmark of this process is that following membrane depolymerization, evoked endosomes are released from the growth cone (Diefenbach et al., 1999). Though some studies have revealed a role for evoked fluid-phase endocytosis in growth cone collapse downstream of Ca2+ elevation and Semaphorin 3A (Kabayama et al., 2009; Kabayama et al., 2011), stimulus dependent endocytosis in the growth cone has mostly been shown to occur through clatherin mediated endocytosis (CME). CME is a known regulator of the surface expression of receptors involved in outgrowth and clatherin activity is necessary for both guidance and desensitization to guidance cues (Tojima et al., 2011). CME occurs downstream of Ca2+ elevation and is an essential mediator of Ca2+ induced chemorepulsion (Tojima et al., 2010). It is highly likely that CME is one of the first downstream events following Ca2+ elevation as it precedes any cytoskeletal remodeling associated with the turning response (Tojima et al., 2010).
Recently, it has become evident that asymmetric CME is essential for mediating guidance responses within the growth cone during repulsion. During myelin-associated glycoprotein (MAG) induced repulsion, there is a rapid spatial remodeling of cell adhesion components, including the surface receptor β1-integrin, with their distribution shifting toward the side that is opposite to the one stimulated by MAG (Hines et al., 2010). This is achieved through CME surface removal of the β1-integrin on the side of the growth cone undergoing repulsion. CME also occurs following local application of Semaphorin 3A (Tojima et al., 2010). Furthermore, local inhibition of CME through application of the clatherin inhibitor MDC was sufficient to cause an attractive guidance response (Tojima et al., 2010). While these data support the notion that asymmetric alteration of the balance of exo- and endocytosis can elicit growth cone steering, they do not directly demonstrate that endocytosis is sufficient to induce growth cone repulsion. An ultimate test for a sufficient role of local endocytosis in growth cone repulsion would require techniques that can directly and specifically elicit local endocytosis to examine the growth cone’s response.
Final thoughts: a myriad network of crosstalk and the need for new tools
It has become increasingly clear that directional growth cone motility is controlled by a combination of mechanisms. While each of these processes regulates distinct sets of cellular activities, they must work in concert to enable the growth cone to respond to environmental signals. Remarkably, there is a substantial amount of crosstalk amongst different pathways (Figure 3). For example, while the actin cytoskeleton plays a predominant role in motility by providing the major force behind cell protrusions, it also has been shown to spatially regulate microtubule dynamics and membrane recycling. This in turn would affect the delivery and retrieval of migration-relevant molecules, whose downstream targets are ultimately the actin cytoskeleton. Similarly, adhesions are both upstream and downstream of signals from the actin cytoskeleton, microtubules, and membrane recycling pathways (Kolodkin and Tessier-Lavigne, 2011; Myers et al., 2011). Moreover, both actin and microtubule cytoskeleton have been shown to be involved in regulating surface receptor trafficking. For example, ADF/cofilin dynamics regulates the insertion of neurotransmitter receptors (Gu et al., 2010; Lee et al., 2009). Microtubule plus-ends have also been shown to be involved in targeting of neuronal ion channels (Gu et al., 2006; Shaw et al., 2007). Therefore, guidance signaling cascades could also target the distribution of the guidance receptors for asymmetric signaling or the adaptation process (Ming et al., 2002). Finally, recent studies have also provided evidence that regulated local translation of receptors, signaling components, and cytoskeletal proteins plays a role in growth cone migration and guidance (Hengst and Jaffrey, 2007; Lin and Holt, 2008). Given that translation is regulated by distinct sets of signaling pathways, these results further expand the intricate network of signaling pathways that can affect the growth cone motility in space and time.
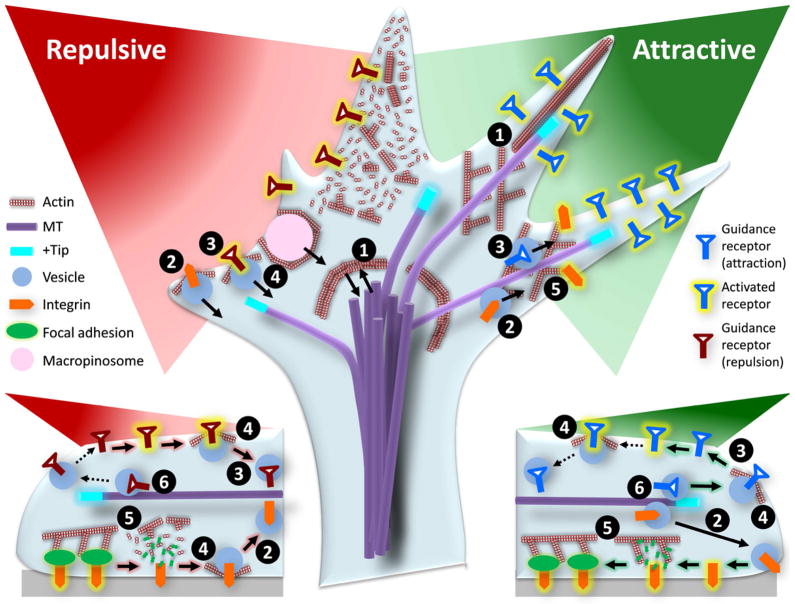
Figure 3.
Schematic showing the complexity of cross-talk among different cellular machineries. The directional movement of the growth cone involves membrane protrusion at the front and retraction at the rear, which are controlled by bi-directional interactions between the actin and microtubules (1). Successful locomotion also requires the formation of new adhesion at the front and the destruction of adhesion at the rear, which are mediated by membrane recycling (2). Vesicular trafficking and recycling also control and regulate the number and distribution of guidance receptors on the surface, which will impact the spatiotemporal signal transduction (3). While the actin cytoskeleton is the driving force for membrane protrusion, it also regulates endocytosis and exocytosis in a spatiotemporal fashion, which could impact both the adhesion and receptor recycling (4). The actin cytoskeleton is also a part of the integrin-adhesion complex and regulates the stability and turnover of adhesion (5). Finally, microtubules and their plus-ends play a role in trafficking membrane channels and receptors (6).
It is conceivable that the elaborate network of signaling cascades that regulates distinct aspects of cellular activities is “purposely” built, such that changes affecting one pathway will be transduced to and integrated with the other pathways to generate a particular growth cone behavior. Such an integrative mechanism could have two important advantages for growth cones. First, it empowers the growth cone with a much higher ability to adapt to the diverse array of environmental cues that it will encounter along its journey to a specific target. Given that a growth cone is likely to be exposed to more than one extracellular cue at a given time, the integration of multiple signaling pathways could be essential for the decision making process that underlies guidance responses. Second, this mechanism can also ensure that a more subtle alteration of growth cone behavior, rather than a binary switch effect, could be generated from a single input in vivo. This may be one of the reasons that targeting a specific signaling pathway (such as RhoA) has failed regeneration therapy (Tonges et al., 2011). Therefore, the challenge for future growth cone research is that we must consider that seemingly separate aspects of cell biology are actually seamlessly integrated, that a loss of function in one process may have multiple outputs that alter the fate of the growth cone. For example, endocytic vesicles are found in regions undergoing repulsion and local inhibition of clatherin-mediated endocytosis is sufficient to cause an attractive turning response (Tojima et al., 2010). The immediate conclusion is that upregulation of local CME, in and of itself, is sufficient to cause repulsion and that downregulation of CME will have the opposite effect. But what remain unclear are the downstream ramifications of altering locally CME. One consequence is that cell surface receptors are no longer internalized, numbing the cell to guidance cue gradients. Another is that the balance between endo- and exocytosis is simply upset (assuming exocytosis remains at the same level), causing an asymetric distribution of protein and lipid in the cell membrane. Furthermore, what are the downstream ramifications of locally altering CME and how is the guidance signal transduced? Are adhesion dynamics affected because internalization of integrins is changed? If so, what are the ramifications on the actin network that is directly coupled to these adhesions? What happens after this? Is the actin and actin regulatory proteins that are normally dedicated to CME being redirected to leading edge structures? Surely microtubule dynamics are being altered as well, since they are intimately dependent upon actin regulation. Finally, when do these events occur relative to the physical guidance response? The time has come for us to connect all of the dots between the initial signaling event and the final downstream consequences. Understanding such a complex network of regulation on growth cone motility could provide the important ground for better identifying targets of pharmaceutical interventions for axon regeneration after nerve injury. For example, RhoA, a small GTPase that is a master regulator of the cytoskeleton, has been highly implicated in growth cone collapse, axon retraction, and inhibition of growth (Tonges et al., 2011). It is a logical target of pharmaceutical inhibition for nerve injury. However, some studies have reported that RhoA actually contributes to positive axon growth (Arakawa et al., 2003; Woo and Gomez, 2006). While RhoA inhibition does aid regeneration somewhat, it’s effects on nerve injury in living organisms are not as potent as once hoped (Tonges et al., 2011). Perhaps if we focused on inhibiting RhoA in particular subcellular locations and at times where it has an inhibitory effect on axon growth and not in other instances where it promotes neuritogenesis, using knowledge acquired from an understanding of the complete spatiotemporal picture of RhoA signaling in growth cones, that the in vivo effect of RhoA inhibition on nerve regeneration would be more pronounced.
A technical challenge in teasing out the exact functions of a particular player in growth cone motility and guidance is that these signals are often transient by nature and occur in small subcellular compartments. Additionally, these specific pathways are often a part of larger regulatory networks that involve substantial crosstalk and compensatory mechanisms. Current studies predominantly depend on the long term alterations of a protein level or activity (e.g. knockdown or overexpression). Since most of these proteins are involved in the fundamental structure and function of the cell, long-term manipulations may reveal their general importance but not their specific cellular functions. For example, knockout of some of the key actin regulatory proteins (e.g. Arp2/3 complex) is embryonic lethal. Moreover, compensatory mechanisms by homologous proteins or other molecules could make it difficult to accurately interpret results from long-term manipulation. While biochemical activities can help the understanding of a protein’s functions, they can not reveal the spatiotemporal dynamics of the protein and its activities which are often associated with its specific function in polarized cells, especially neurons. Therefore, the future challenge will be to develop tools that enable one to inactivate or activate a specific protein instantly and with subcellular precision, giving no chance for the cell to compensate for the change.
One promising approach is optical manipulation of signaling networks using genetically engineered probes. For example, Chromophore Assisted Laser Inactivation (CALI), a process in which proteins are inactivated with light by irradiating an attached photosensitizer chromophore, has been successfully in cells to knockdown target molecules in a spatiotemporal manner (Jacobson et al., 2008). CALI occurs because highly reactive photoproducts are generated when the photosensitizer chromophore is excited. However, the short-lived nature of these reactive species limits the damage radius to only proteins that are immediately adjacent to the chromophore from which they arose, ensuring a measure of specificity. CALI has been successfully used in neurons and growth cones (Diefenbach et al., 2002; Marek and Davis, 2002; Poskanzer et al., 2003; Sydor et al., 1996; Wang et al., 1996), though the technique never reached widespread appeal. This was in part due to cumbersome methodologies used to label target proteins with a CALI chromophore, which included microinjection of non-function blocking, labeled antibodies and the use of the biarsenical dyes FlAsH and ReAsH. Recent advances in CALI have made the technique much more feasible for studies in neurons. First, it has been shown that fluorescent proteins (FPs) can be successfully used as CALI chromophores (Rajfur et al., 2002; Tanabe et al., 2005; Vitriol et al., 2007). FP-CALI obviates the need to add exogenous labeling reagents, because the chromophore is covalently attached to its target during translation. Furthermore, FP-fusion protein expression can be combined with knockdown of the endogenous homologue so that the only version of the target expressed is susceptible to light inactivation, enhancing the CALI effect (Vitriol et al., 2007). EGFP has been primarily used for FP-CALI, but an exciting candidate, Killer Red, has been developed that increases the efficiency of CALI so that it can be performed with minimal light irradiation (Bulina et al., 2006). Killer Red is more than an order of magnitude more efficient at ROS production than EGFP. Additionally, its 585nm excitation peak allows the usage of yellow- orange light, rather than cyan, for CALI, minimizing non-specific absorption by off-target molecules. Another potential new genetically encoded CALI reagent is miniSOG (miniature Singlet Oxygen Generator), a 106-amino acid monomeric fluorescent flavoprotein that is less than half the size of conventional FPs (Shu et al., 2011).
Moreover, genetically-engineered proteins that allow light-induced activation will enable one to fully appreciate the functions of a specific molecule at the subcellular level (Toettcher et al., 2011; Wu et al., 2009). While light induced photoactivation has been in the cell biologists toolkit for decades, these methods have required chemical modification of molecules or proteins, which must then be somehow introduced to the intracellular environment and uncaged with UV light. It is only in recent times that the technique has been combined with the ease of genetically encoded expression and the use of visible light wavelengths, making the experiments very amenable to live neurons in culture or even in vivo. This can be attributed to exploitation of light sensitive domains isolated from plant proteins. These can be used to either allosterically block an active protein from interacting with its effectors (Wu et al., 2009), or to artificially dimerize two targets (Kennedy et al., 2010; Levskaya et al., 2009). In the latter case, artificial interaction can be used to either anchor a target protein to a specific subcellular compartment or to cause an association between two targets. In both cases, light absorption activates a synthetic signaling cascade that is both reversible and dose dependent. Like CALI, light-induced photoactivation is instantaneous and can be performed at the subcellular level. Overall, this type of manipulation is a better approximation of actual signaling events and an invaluable tool for deducing the true function of a protein in a specific cellular process. Together with the development and deployment of super resolution imaging techniques (Toomre and Bewersdorf, 2010), we might be closer to a better understanding of the full orchestra of the players that power the growth cone.
Acknowledgments
Research in authors’ lab is supported in part by grants from the National Institutes of Health to JQZ and a Ruth L. Kirschstein National Research Service Award to EAV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Akin O, Mullins RD. Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell. 2008;133:841–851. doi: 10.1016/j.cell.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratyn-Schaus Y, Gardel ML. Transient frictional slip between integrin and the ECM in focal adhesions under myosin II tension. Curr Biol. 2010;20:1145–1153. doi: 10.1016/j.cub.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bray D. Distribution and cellular localization of actin depolymerizing factor. Journal of Cell Biology. 1987;105:2817–2825. doi: 10.1083/jcb.105.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard L, Boscher C, Lambert M, Mege RM, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, O’Connor T. Cytoskeletal events in growth cone steering. Current Opinion in Neurobiology. 1994;4:43–48. doi: 10.1016/0959-4388(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends in Cell Biology. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. %U http://www.ncbi.nlm.nih.gov/pubmed/20133134. [DOI] [PMC free article] [PubMed]
- Bloom OE, Morgan JR. Membrane trafficking events underlying axon repair, growth, and regeneration. Mol Cell Neurosci. 2011;48:339–348. doi: 10.1016/j.mcn.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Bonanomi D, Fornasiero EF, Valdez G, Halegoua S, Benfenati F, Menegon A, Valtorta F. Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones. J Cell Sci. 2008;121:3757–3769. doi: 10.1242/jcs.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci. 2006;119:5204–5214. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. Journal of Neuroscience. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. Journal of Cell Biology. 1973;56:713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Cai X, Lietha D, Ceccarelli DF, Karginov AV, Rajfur Z, Jacobson K, Hahn KM, Eck MJ, Schaller MD. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28:201–214. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. Journal of Cell Biology. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris LU, Walker RA, Pryer NK, Salmon ED. Dynamic instability of microtubules. BioEssays: news and reviews in molecular, cellular and developmental biology. 1987;7:149–154. doi: 10.1002/bies.950070403. [DOI] [PubMed] [Google Scholar]
- Chacon MR, Fazzari P. FAK: dynamic integration of guidance signals at the growth cone. Cell Adh Migr. 2011;5:52–55. doi: 10.4161/cam.5.1.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. The Journal of Cell Biology. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol. 2008;267:183–206. doi: 10.1016/S1937-6448(08)00604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A, Desai A, Oegema K. The minus end in sight. Current Biology. 2003;13:R614–624. doi: 10.1016/s0960-9822(03)00530-x. [DOI] [PubMed] [Google Scholar]
- Davis DA, Wilson MH, Giraud J, Xie Z, Tseng HC, England C, Herscovitz H, Tsai LH, Delalle I. Capzb2 interacts with beta-tubulin to regulate growth cone morphology and neurite outgrowth. PLoS Biol. 2009;7:e1000208. doi: 10.1371/journal.pbio.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. Journal of neuroscience. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harbor perspectives in biology. 2011:3. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequidt C, Danglot L, Alberts P, Galli T, Choquet D, Thoumine O. Fast turnover of L1 adhesions in neuronal growth cones involving both surface diffusion and exo/endocytosis of L1 molecules. Mol Biol Cell. 2007;18:3131–3143. doi: 10.1091/mbc.E06-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. Journal of Cell Science. 2005;118:19–26. doi: 10.1242/jcs.01631. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Diefenbach TJ, Guthrie PB, Stier H, Billups B, Kater SB. Membrane recycling in the neuronal growth cone revealed by FM1–43 labeling. Journal of Neuroscience. 1999;19:9436–9444. doi: 10.1523/JNEUROSCI.19-21-09436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach TJ, Latham VM, Yimlamai D, Liu CA, Herman IM, Jay DG. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–2146. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 Capture Microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Current Opinion in Cell Biology. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks PR. Microtubules and growth cone function. Journal of Neurobiology. 2004;58:70–83. doi: 10.1002/neu.10266. [DOI] [PubMed] [Google Scholar]
- Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigera PR, Jeffery ED, Martin KH, Shabanowitz J, Hunt DF, Parsons JT. FAK phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4931–4935. doi: 10.1242/jcs.02696. [DOI] [PubMed] [Google Scholar]
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Salmon WC, Waterman-Storer CM. Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells. Current biology: CB. 2002;12:1891–1899. doi: 10.1016/s0960-9822(02)01276-9. [DOI] [PubMed] [Google Scholar]
- Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. Journal of Experimental Zoology. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- Hart MC, Korshunova YO, Cooper JA. Vertebrates have conserved capping protein alpha isoforms with specific expression patterns. Cell Motil Cytoskeleton. 1997;38:120–132. doi: 10.1002/(SICI)1097-0169(1997)38:2<120::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in Caenorhabditis elegans. Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 2007;18:209–215. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle SJ, Wang G, Liang E, Wu M, Poo MM, Henley JR. Asymmetric PI(3,4,5)P3 and Akt signaling mediates chemotaxis of axonal growth cones. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:7016–7027. doi: 10.1523/JNEUROSCI.0216-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Abu-Rub M, Henley JR. Asymmetric endocytosis and remodeling of beta1-integrin adhesions during growth cone chemorepulsion by MAG. Nat Neurosci. 2010;13:829–837. doi: 10.1038/nn.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman AA. Microtubule polymerases and depolymerases. Current Opinion in Cell Biology. 2007;19:31–35. doi: 10.1016/j.ceb.2006.12.009. %U http://www.ncbi.nlm.nih.gov/pubmed/17184986. [DOI] [PubMed]
- Hsieh SH, Ferraro GB, Fournier AE. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. Journal of Neuroscience. 2006;26:1006–1015. doi: 10.1523/JNEUROSCI.2806-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the Growth Cone: Ligand-Receptor Complexes and the Control of Axon Growth and Guidance. Annual Review of Neuroscience. 2003;28:28. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabayama H, Nakamura T, Takeuchi M, Iwasaki H, Taniguchi M, Tokushige N, Mikoshiba K. Ca2+ induces macropinocytosis via F-actin depolymerization during growth cone collapse. Mol Cell Neurosci. 2009;40:27–38. doi: 10.1016/j.mcn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kabayama H, Takeuchi M, Taniguchi M, Tokushige N, Kozaki S, Mizutani A, Nakamura T, Mikoshiba K. Syntaxin 1B suppresses macropinocytosis and semaphorin 3A-induced growth cone collapse. J Neurosci. 2011;31:7357–7364. doi: 10.1523/JNEUROSCI.2718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Dent EW. Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr Opin Neurobiol. 2005;15:521–526. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. The role of cell adhesion molecules in axon growth and guidance. Adv Exp Med Biol. 2007;621:95–103. doi: 10.1007/978-0-387-76715-4_7. [DOI] [PubMed] [Google Scholar]
- Kapustina M, Vitriol E, Elston TC, Loew LM, Jacobson K. Modeling capping protein FRAP and CALI experiments reveals in vivo regulation of actin dynamics. Cytoskeleton (Hoboken) 2010;67:519–534. doi: 10.1002/cm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol. 2010;28:743–747. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater SB, Mattson MP, Cohan C, Connor J. Calcium regulation of the neuronal growth cone. Trends in Neurosciences. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpak AL, Jiang J, Guo D, Standley C, Bellve K, Fogarty K, Bao ZZ. Negative guidance factor-induced macropinocytosis in the growth cone plays a critical role in repulsive axon turning. J Neurosci. 2009;29:10488–10498. doi: 10.1523/JNEUROSCI.2355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korey CA, Van Vactor D. From the growth cone surface to the cytoskeleton: one journey, many paths. Journal of Neurobiology. 2000;44:184–193. [PubMed] [Google Scholar]
- Kuhn TB, Meberg PJ, Brown MD, Bernstein BW, Minamide LS, Jensen JR, Okada K, Soda EA, Bamburg JR. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. Journal of Neurobiology. 2000;44:126–144. [PubMed] [Google Scholar]
- Lee CW, Han J, Bamburg JR, Han L, Lynn R, Zheng JQ. Regulation of acetylcholine receptor clustering by ADF/cofilin-directed vesicular trafficking. Nat Neurosci. 2009;12:848–856. doi: 10.1038/nn.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004;42:913–926. doi: 10.1016/j.neuron.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. Epub 2004 Oct 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Shelly SS, Tokito M, Holzbaur EL. The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization. Molecular Biology of the Cell. 2003;14:1405–1417. doi: 10.1091/mbc.E02-03-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Current Opinion in Neurobiology. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Thompson CA, Forscher P. Cytoskeletal reorganization underlying growth cone motility. Current Opinion in Neurobiology. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. Epub 2004 Oct 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. Journal of Cell Science. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- Ma A, Richardson A, Schaefer EM, Parsons JT. Serine phosphorylation of focal adhesion kinase in interphase and mitosis: a possible role in modulating binding to p130(Cas) Molecular Biology of the Cell. 2001;12:1–12. doi: 10.1091/mbc.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiological Reviews. 1995;75:835–864. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- Maciver SK. How ADF/cofilin depolymerizes actin filaments. Current Opinion in Cell Biology. 1998;10:140–144. doi: 10.1016/s0955-0674(98)80097-5. [DOI] [PubMed] [Google Scholar]
- Mack TG, Koester MP, Pollerberg GE. The microtubule-associated protein MAP1B is involved in local stabilization of turning growth cones. Molecular & Cellular Neurosciences. 2000;15:51–65. doi: 10.1006/mcne.1999.0802. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): acute inactivation of synaptotagmin I. Neuron. 2002;36:805–813. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- Marsick BM, Flynn KC, Santiago-Medina M, Bamburg JR, Letourneau PC. Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev Neurobiol. 2010;70:565–588. doi: 10.1002/dneu.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Meldolesi J. Neurite outgrowth: this process, first discovered by Santiago Ramon y Cajal, is sustained by the exocytosis of two distinct types of vesicles. Brain Res Rev. 2011;66:246–255. doi: 10.1016/j.brainresrev.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Tsuji T, Higashida C, Hertzog M, Fujita A, Narumiya S, Scita G, Watanabe N. Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J Cell Biol. 2006;175:947–955. doi: 10.1083/jcb.200604176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Quiroga S, Rosa A, Kosik K, Caceres A. Suppression of Kif2 in Pc12 Cells Alters the Distribution of a Growth Cone Nonsynaptic Membrane Receptor and Inhibits Neurite Extension. Journal of Cell Biology. 1997;138:657–669. doi: 10.1083/jcb.138.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Gomez TM. Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. J Neurosci. 2011;31:13585–13595. doi: 10.1523/JNEUROSCI.2381-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev Neurobiol. 2011;71:901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Noda Y, Sato-Yoshitake R, Kondo S, Nangaku M, Hirokawa N. KIF2 is a new microtubule-based anterograde motor that transports membranous organelles distinct from those carried by kinesin heavy chain or KIF3A/B. Journal of Cell Biology. 1995;129:157–167. doi: 10.1083/jcb.129.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. Journal of Cell Science. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH, Laurino L, Peretti D, Wang X, Rosso S, Morfini G, Caceres A, Quiroga S. Regulation of membrane expansion at the nerve growth cone. Journal of Cell Science. 2003;116:1209–1217. doi: 10.1242/jcs.00285. [DOI] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annual Review of Biophysics and Biomolecular Structure. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. %U http://www.ncbi.nlm.nih.gov/pubmed/10940259. [DOI] [PubMed]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Marek KW, Sweeney ST, Davis GW. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–563. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- Racchetti G, Lorusso A, Schulte C, Gavello D, Carabelli V, D’Alessandro R, Meldolesi J. Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargeosome. J Cell Sci. 2010;123:165–170. doi: 10.1242/jcs.059634. [DOI] [PubMed] [Google Scholar]
- Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol. 2002;4:286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Notas anatómicas. I. Sobre la aparición de las expansiones celulares en la médula embrionaria. Gac Sanit Barc. 1890;12:413–419. [Google Scholar]
- Ramón y Cajal S. Histologie du System Nerveux de l’Homme et des Vertebres. Paris: Malonine; 1909. [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, et al. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. Epub 2004 Oct 1217. [DOI] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Dailey ME, Bridgman PC. Polymerizing microtubules activate site-directed F-actin assembly in nerve growth cones. Molecular Biology of the Cell. 1999;10:2309–2327. doi: 10.1091/mbc.10.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Current Opinion in Cell Biology. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, McAllister AK. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nature Neuroscience. 2003;6:1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. Journal of Cell Biology. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA. Cell biology: Barbed ends rule. Nature. 2004;430:734–735. doi: 10.1038/430734a. %U http://dx.doi.org/710.1038/430734a. [DOI] [PubMed]
- Schafer DA, Korshunova YO, Schroer TA, Cooper JA. Differential localization and sequence analysis of capping protein beta-subunit isoforms of vertebrates. J Cell Biol. 1994;127:453–465. doi: 10.1083/jcb.127.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong J, Ouyang M, Kim T, Sun J, Wen PC, Lu S, Zhuo Y, Llewellyn NM, Schlaepfer DD, Guan JL, et al. Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat Commun. 2011;2:406. doi: 10.1038/ncomms1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- Smith SJ. Neuronal cytomechanics: the actin-based motility of growth cones. Science. 1988;242:708–715. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- Suter DM, Forscher P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr Opin Neurobiol. 1998;8:106–116. doi: 10.1016/s0959-4388(98)80014-7. [DOI] [PubMed] [Google Scholar]
- Sydor AM, Su AL, Wang FS, Xu A, Jay DG. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. Journal of Cell Biology. 1996;134:1197–1207. doi: 10.1083/jcb.134.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Oyamada M, Fujita K, Dai P, Tanaka H, Takamatsu T. Multiphoton excitation-evoked chromophore-assisted laser inactivation using green fluorescent protein. Nat Methods. 2005;2:503–505. doi: 10.1038/nmeth770. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Thoumine O. Interplay between adhesion turnover and cytoskeleton dynamics in the control of growth cone migration. Cell Adh Migr. 2008;2:263–267. doi: 10.4161/cam.2.4.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nature methods. 2011;8:35–38. doi: 10.1038/nmeth.f.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011:1–13. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Tonges L, Koch JC, Bahr M, Lingor P. ROCKing Regeneration: Rho Kinase Inhibition as Molecular Target for Neurorestoration. Front Mol Neurosci. 2011;4:39. doi: 10.3389/fnmol.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomre D, Bewersdorf J. A new wave of cellular imaging. Annual review of cell and developmental biology. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Vitriol EA, Uetrecht AC, Shen F, Jacobson K, Bear JE. Enhanced EGFP-chromophore-assisted laser inactivation using deficient cells rescued with functional EGFP-fusion proteins. Proc Natl Acad Sci U S A. 2007;104:6702–6707. doi: 10.1073/pnas.0701801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FS, Wolenski JS, Cheney RE, Mooseker MS, Jay DG. Function of myosin-V in filopodial extension of neuronal growth cones. Science. 1996;273:660–663. doi: 10.1126/science.273.5275.660. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. Journal of Cell Biology. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Stephens DJ. Microtubule plus-end loading of p150(Glued) is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. Journal of Cell Science. 2006;119:2758–2767. doi: 10.1242/jcs.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. Journal of Cell Biology. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. The Journal of Cell Biology. 2008;180:619–632. doi: 10.1083/jcb.200707042. %U http://www.ncbi.nlm.nih.gov/pubmed/18268107. [DOI] [PMC free article] [PubMed]
- Wolfenson H, Bershadsky A, Henis YI, Geiger B. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci. 2011;124:1425–1432. doi: 10.1242/jcs.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. Journal of Neuroscience. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Molecular Biology of the Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Solowska JM, Qiang L, Karabay A, Baird D, Baas PW. Regulation of microtubule severing by katanin subunits during neuronal development. Journal of neuroscience. 2005;25:5573–5583. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Grode KD, Stewman SF, Diaz-Valencia JD, Liebling E, Rath U, Riera T, Currie JD, Buster DW, Asenjo AB, et al. Drosophila katanin is a microtubule depolymerase that regulates cortical-microtubule plus-end interactions and cell migration. Nature cell biology. 2011;13:361–370. doi: 10.1038/ncb2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nature Reviews. Neuroscience. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]