Abstract
In 1985 Tulving introduced the remember–know procedure, whereby subjects are asked to distinguish between memories that involve retrieval of contextual details (remembering) and memories that do not (knowing). Several studies have been reported showing age-related declines in remember hits, which has typically been interpreted as supporting dual-process theories of cognitive aging that align remembering with a recollection process and knowing with a familiarity process. Less attention has been paid to remember false alarms, or their relation to age. We reviewed the literature examining aging and remember/know judgments and show that age-related increases in remember false alarms, i.e., false remembering, are as reliable as age-related decreases in remember hits, i.e., veridical remembering. Moreover, a meta-analysis showed that the age effect size for remember hits and false alarms are similar, and larger than age effects on know hits and false alarms. We also show that the neuropsychological correlates of remember hits and false alarms differ. Neuropsychological tests of medial-temporal lobe functioning were related to remember hits, but tests of frontal-lobe functioning and age were not. By contrast, age and frontal-lobe functioning predicted unique variance in remember false alarms, but MTL functioning did not. We discuss various explanations for these findings and conclude that any comprehensive explanation of recollective experience will need to account for the processes underlying both remember hits and false alarms.
Keywords: Human memory, Episodic memory, Cognitive aging, Remember–know, Meta-analysis, Frontal functioning, Medial-temporal functioning, Signal detection
Endel Tulving has made greater contributions to the study of retrieval processes than any other researcher. Tulving and Pearlstone (1966) distinguished between information available in memory (what is stored) and information that is accessible on a particular test (what is retrievable with certain cues). Although psychologists and neuroscientists would hope to determine all the information that is stored or available in memory, no techniques (psychological or physiological) can ever permit us to know for sure. Rather, our techniques only permit us to know what information is accessible on a particular test, with a certain set of cues, under specific encoding and retention conditions. The distinction between availability and accessibility seems widely (if not universally) accepted in writings of cognitive psychologists, but it still has not penetrated all scientific fields of memory. The primary method of examining variations in accessibility is by changing retrieval cues during the test, for example, comparing free and cued recall (as in Tulving & Pearlstone, 1966). Certain types of cues greatly increase accessibility relative to free recall (e.g., category names for lists composed of category members), whereas other types of cues that would seem to be valid ones (e.g., items from the list used to cue other list items) can actually decrease accessibility (Slamecka, 1968).
In the 1970s Tulving continued to pioneer the study of retrieval processes with a series of publications on the encoding specificity principle (e.g., Tulving & Thomson, 1973; among many others). Many experiments on encoding specificity involve the simultaneous manipulation of study conditions and test conditions. For example, one may have two encoding conditions (say A and B). Then test conditions are created such that one type of test is intended to match or recreate the encoding of the A condition (call it test condition A′) and another test condition is intended to re-arouse the encoding condition in B (test condition B′). In most experiments, when the test condition matches the encoding condition (A and A′; B and B′) performance is better than when the conditions mismatch (A and B′; B and A′). This outcome in many experiments caused Tulving and his colleagues to introduce the encoding specificity principle. Stated in brief, the idea is that encoding consists of certain features of an event being coded and represented in a memory trace; retrieval cues are effective to the extent that features extracted from the cue match or complement those in the trace (Tulving, 1983).
Another key contribution by Tulving to the study of retrieval processes occurred in the 1980s. Students of memory typically measure accessibility through a variety of methods (e.g., free recall, cued recall, recognition), with the implicit assumption seeming to be that the experience of retrieving a memory was much the same in all cases (or at least researchers did not make distinctions). However, in an important paper in 1985, Tulving argued that psychologists ought to study people's reported phenomenological experience while they retrieve events from memory. He argued that there could be at least two types of experience, remembering and knowing, and he developed a pioneering method to permit subjects to distinguish between the two states of conscious awareness during retrieval. Subjects reported that a retrieved experience was remembered if they could think back to the moment the experience occurred and recollect details of the event, or reported that the experience was one that they knew if it had occurred in the experiment but the precise moment of occurrence could not be recalled.
As in other introspective techniques, instructions to the subjects matter greatly. Despite some disbelief from the scientific public, researchers soon worked out good instructions that subjects could readily understand (Gardiner, 1988; Rajaram, 1993), and a large body of research has now been conducted with the remember/know procedure with generally consistent results (see Gardiner, 2008, for a review).
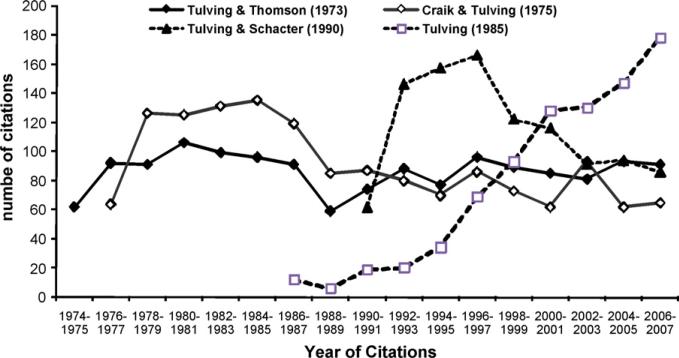
Tulving's (1985) remember/know distinction certainly qualifies as a seminal contribution in experimental psychology by any standard, with the paper garnering 838 citations through the end of 2007. This paper is the fourth most cited of Tulving's journal articles, with the three most cited papers being Tulving and Thomson (1973; 1470 total citations), Craik and Tulving (1975; 1463 total citations), and Tulving and Schacter (1990; 1040 total citations). The citation counts plotted by year (through 2007) for these three papers are shown in Fig. 1. The figure makes clear that the three most highly cited Tulving papers were very influential very soon after publication and have been fairly constant in their influence or have waned a bit. However, the 1985 paper took some time after publication to catch on. Part of the reason for this initial indifference to the paper may have been that it was published in Canadian Psychology, which has relatively few subscribers outside of Canada. It probably also took Gardiner's (1988) important replication and extension of Tulving's demonstration experiment to make the technique noticed. At any rate, the R/K procedure has had a steadily increasing number of citations since the mid-1990s as researchers became more interested in studying retrieval experience.
Fig. 1.
Number of citations for Tulving and Thomson (1973), Craik and Tulving (1975), Tulving and Schacter (1990) and Tulving (1985) plotted by year of citations..
Another major reason for the popularity of the remember–know procedure is the identification of remembering and knowing with distinct neural systems, or cognitive processes. Tulving (1985) suggested that remembering involved retrieval from the episodic memory system, whereas knowing reflected retrieval from the semantic memory system. More recently dual-process memory theorists have aligned remembering with a recollection process and knowing with a familiarity process1.
1. False remembering
The majority of published studies using the remember–know paradigm have primarily been concerned with recollection of studied events. One major exception to this generalization is the study of false memories for associatively related lures in the DRM paradigm (Deese, 1959; Roediger & McDermott, 1995). Certainly one of the most dramatic findings in the false memory literature is that lures such as sleep that are strongly related to studied items (bed, rest, awake, tired, etc.) receive remember responses on recognition tests at levels that are often comparable to the hit rates for studied items. Although many explanations have been proposed for these high rates of false remembering for associatively related items (e.g., Brainerd & Reyna, 2005; Lampinen, Meier, Arnal, & Leding, 2005; Roediger, Watson, Gallo, & McDermott, 2001), less attention has been paid to false remembering of ostensibly unrelated items on standard remember–know tests, which is typically low (e.g., ~3% according to Gardiner, Ramponi, & Richardson-Klavehn's, 2002 meta-analysis). Despite low levels of remember false alarms for unrelated lures, researchers have recently demonstrated systematic effects of false remember responses to unrelated distracters (e.g., response bias effects; Wixted & Stretch, 2004). Moreover, it has been noted that the very existence of remember false alarms is problematic for most dual-process theories of memory (Higham & Vokey, 2004; Wixted & Stretch, 2004), because dual-process theorists have argued that new items cannot truly be recollected because they were not actually studied (Eldridge, Sarfatti, & Knowlton, 2002; Yonelinas, Kroll, Dobbins, Lazzara, & Knight, 1998).
There are two possible solutions to the problem posed by remember false alarms for dual-process theories of memory (Higham & Vokey, 2004). One solution is to assume that either a recollection or familiarity process can give rise to the experience of subjective recollection, i.e., remembering. Thus, recollective experience as captured by remember judgments could be caused by a recollection process or by strong feelings of familiarity. This idea has recently been incorporated into several strength-based dual-process theories (e.g., Rotello, Macmillan, & Reeder, 2004; Wixted & Stretch, 2004). Another possible solution, based on the notion that memory judgments are attributional in nature (Jacoby, Kelley, & Dywan, 1989), is to assume that recollective experience can be cued by a test item regardless of whether it was studied, provided the item acts as an effective retrieval cue (cf., Tulving, 1974). For example, false remembering might be caused by confusions between test words and similar studied words (e.g., money was studied and cash was on the test), but false remembering may also result from source misattributions for items that are unrelated to studied items, e.g., when a new item cues recollection of an extra-experimental event that is erroneously misattributed to the study episode (McCabe & Geraci, in press). For example, imagine the word trial was a lure on a remember–know recognition test, and that trial was also the clue for an answer in a crossword puzzle a subject had completed that morning, prior to the experiment. The subject may recollect thinking of synonyms or words related to trial, and misat-tribute that recollection to the study episode. Thus, according to this source misattribution explanation of remember false alarms, these responses reflect source memory errors (cf., Roediger et al., 2001), and these source confusions would be expected to increase with age (Johnson, Hashtroudi, & Lindsay, 1993). This hypothesis is similar to a recent proposal by Dodson and colleagues (Dodson, Bawa, & Slotnick, 2007; Dodson, Bawa, & Krueger, 2007), who have shown that age-related source memory impairments can be explained by misrecollection of sources, rather than declines in veridical recollection of sources.
2. A meta-analysis of age-related changes in remember–know hits and false alarms
It is well established that aging is related to declines in recollection of studied items as measured by so-called objective measures, including source memory (Johnson et al., 1993) and process dissociation estimates (Jennings & Jacoby, 1997). These measures are corroborated by subjective experience measures, such as memory characteristic questionnaires (Gallo & Roediger, 2003; Mather, Henkel & Johnson, 1997) and remember responses using the remember–know paradigm (Bastin & Van der Linden, 2003; Parkin & Walter, 1992). In most previous remember–know studies including older adults, the study has been motivated by dual-process memory explanations; therefore, the studies have focused on age difference in veridical remembering of studied items. However, because remember false alarms can be very informative with respect to understanding the memory processes related to recollection (Higham & Vokey, 2004), a systematic examination of age effects on false remembering could be informative with respect to understanding the nature of recollection. It is unclear whether aging typically influences remember false alarms in the standard remember/know paradigm, i.e., with unrelated lures, because these data typically have not been considered theoretically relevant by most authors, and thus, even when age differences in remember false alarms are reported, they are typically not discussed.
In order to discover whether aging influences false remembering (in addition to veridical remembering) in standard remember/know recognition studies, we compared remember responses for studied and new items, i.e., remember hits and false alarms, for all published studies employing a standard (i.e., studied items and unrelated lures) remember/know recognition test with healthy younger and older adults. The average level of remember responses for both the unrelated lures and studied items are reported in Table 1. In all cases the values reported are raw percentages of remember hits and false alarms out of the total number of studied items or lures (see Appendix A for more details regarding study identification and inclusion criteria). As expected, the average level of remembering for studied items for younger adults across the 27 studies (weighted for sample size) was greater for younger adults, .542, than for older adults, .396. The robustness of this finding is evinced by comparing the number of studies in which younger adults show more remembering than older adults for studied items, with 23 of the 27 studies conforming to this pattern (with the other four showing the opposite pattern; Wilcoxon Signed Rank Z = 3.83, p < .001). More germane for present purposes are the remember false alarm data. Averaged across the 27 studies, older adults had more than twice as many remember false alarms (.064) than did younger adults (.025). Although this mean difference is small compared to the mean difference in veridical remembering, this finding is quite reliable, with 25 of the 27 studies conforming to this pattern (the other two were ties; Wilcoxon Signed Rank Z = 4.03, p < .0001). A similar pattern was found for estimates of the proportion of remember responses out of the number of items called “old”, i.e., R/(R + K), with weighted means of .66 for younger adults and .57 for older adults for hits, and .25 for younger adults and .37 for older adults for false alarms.
Table 1.
Average percentage of remember and know hits and false alarms (FAs) in published studies using the remember–know procedure with younger (YA) and older adults (OA).
| Remember |
Know |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hits |
FAs |
Hits |
FAs |
|||||
| YA | OA | YA | OA | YA | OA | YA | OA | |
| Parkin and Walter (1992; Expt 1)a | .52 | .20 | .00 | .01 | .24 | .49 | .03 | .08 |
| Parkin and Walter (1992; Expt 2)a | .37 | .12 | .02 | .02 | .44 | .59 | .04 | .06 |
| Mantyla (1993; Expt 1) | .54 | .26 | .05 | .05 | .12 | .10 | .02 | .03 |
| Mantyla (1993; Expt 2) | .43 | .24 | .03 | .05 | .11 | .19 | .03 | .04 |
| Perfect et al. (1995; Expt 1) | .53 | .18 | .05 | .07 | .13 | .53 | .05 | .03 |
| Perfect et al. (1995; Expt 2a; Shallow LOP) | .40 | .34 | .00 | .03 | .09 | .20 | .05 | .05 |
| Perfect et al. (1995; Expt 2a; Deep LOP) | .68 | .69 | .01 | .02 | .15 | .08 | .01 | .01 |
| Perfect et al. (1995; Expt 2b) | .76 | .25 | .01 | .03 | .10 | .39 | .02 | .08 |
| Norman and Schacter (1997; Expt 1; Explanation) | .54 | .55 | .01 | .10 | .05 | .22 | .07 | .10 |
| Norman and Schacter (1997; Expt 1; No Explanation) | .53 | .51 | .01 | .05 | .04 | .21 | .06 | .06 |
| Perfect and Dasgupta (1997) | .74 | .48 | .02 | .08 | .22 | .22 | .05 | .09 |
| Schacter, Koutstaal, Johnson, Gross, and Angell (1997; Expt 1; 3 repetitions) | .63 | .47 | .02 | .03 | .14 | .15 | .06 | .06 |
| Schacter et al. (1997; Expt 2) | .55 | .35 | .05 | .11 | .16 | .14 | .17 | .17 |
| Friedman and Trott (2000) a | .50 | .43 | .02 | .06 | .22 | .23 | .04 | .06 |
| Lovden, Ronnlund, and Nilsson (2002)b | .77 | .53 | .02 | .06 | .15 | .13 | .05 | .08 |
| Clarysetal. (2002)b | .72 | .52 | .07 | .10 | .10 | .33 | .05 | .09 |
| Bastin and Van der Linden (2003) | .40 | .30 | .04 | .10 | .14 | .13 | .11 | .17 |
| Bunce (2003) c | .49 | .38 | .02 | .02 | .21 | .42 | .03 | .04 |
| Comblain, D'Argembeau, Van der Linden, and Aldenhoff (2004) | .57 | .33 | .01 | .03 | .09 | .24 | .04 | .08 |
| D'Argembeau and Van der Linden (2004) | .49 | .40 | .08 | .15 | .10 | .22 | .11 | .17 |
| Bunce and Macready (2005) | .55 | .49 | .00 | .02 | .22 | .27 | .01 | .05 |
| Duarte, Ranganath, and Trujillo (2006) | .59 | .53 | .04 | .10 | .12 | .17 | .15 | .17 |
| Prulletal. (2006) | .58 | .36 | .02 | .08 | .14 | .33 | .07 | .14 |
| Bugaiska et al. (2007) | .30 | .18 | .02 | .03 | .16 | .29 | .06 | .07 |
| Parks (2007) | .41 | .31 | .01 | .04 | .15 | .17 | .10 | .13 |
| Duarte et al. (2008) | .54 | .59 | .02 | .08 | .19 | .26 | .11 | .17 |
| Skinner and Fernandes (in press) | .35 | .40 | .03 | .15 | .21 | .30 | .11 | .15 |
| Mean | .536 | .385 | .025 | .062 | .245 | .267 | .063 | .090 |
| Mean weighted by sample size | .542 | .396 | .025 | .064 | .260 | .258 | .064 | .096 |
This study was not included in the meta-analysis reported in Table 2 because effect sizes could not be calculated for remember hits or false alarms.
Weighted average of the two oldest groups.
Note that in OA Mean, combining high- and low-frontal groups (.023), was greater than the YA Mean (.016), though both round to .02.
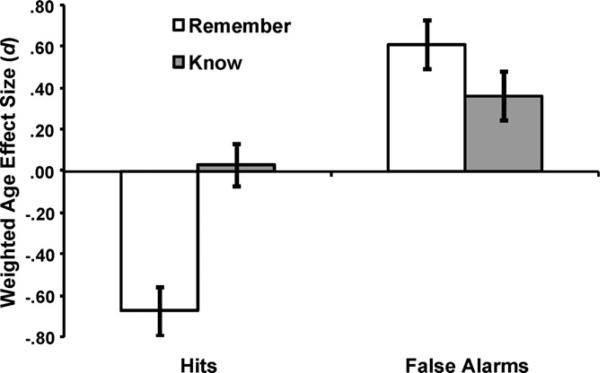
In order to better assess the overall magnitude of age differences in veridical and false remembering in published studies, we conducted a meta-analysis of the studies that were included in Table 1.2 As shown in Fig. 2, the absolute magnitude of the effect size comparing older and younger adults remember response rates for veridical remembering (d = –.68) and false remembering (d = .61) were similar, i.e., they were both in the medium-large range based on Cohen's (1988) criteria, and the magnitude of the 95% confidence intervals overlapped (indicating that the effect size did not differ; see Table 2). These data can be contrasted with the smaller age effects for Know hits (d = .03) and false alarms (d = .36). Thus, based on our review of the extant remember–know literature, we can conclude that age-related effects on knowing are small, but age-related increases in false remembering are just as common, and just as large, as age-related decreases in veridical remembering. This remember mirror effect must be explained by any comprehensive account of age-related changes in retrieval experience.
Fig. 2.
Weighted mean effect size for age group (older adult minus younger adult) for remember and know hits and false alarms. Note that the effect size for remember hits is negative, representing less remembering for older adults. Error bars represent 95% confidence intervals.
Table 2.
Effect sizes for remember and know hits and false alarms in published studies comparing younger adults (YA) and older adults (OA) using the remember–know paradigm.
| Item and response type | k | YA, N | OA, N | d | 95% CI |
Qw | |
|---|---|---|---|---|---|---|---|
| LL | UL | ||||||
| Remember hits | 24 | 674 | 711 | –.68 | –.57 | –.78 | 100.17 |
| Remember false alarms | 24 | 674 | 711 | .61 | .50 | .72 | 32.52 |
| Know hits | 24 | 674 | 711 | .03 | –.08 | .14 | 147.18 |
| Know false alarms | 24 | 674 | 711 | .36 | .26 | .47 | 30.34 |
k: # of studies; N: number of subjects; d: Cohen's d (effect size); CI: confidence interval; LL: lower limit; UL: upper limit; QW: Cochran's Q.
2.1. Neuropsychological correlates of age-related changes in memory performance
Age-related declines in different indices of recollection, including source memory, process-dissociation estimates, and veridical remembering, have been linked to age-related declines in medial-temporal lobe and frontal-lobe functioning (Glisky, Polster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001; Henkel, Johnson, & DeLeonardis, 1998; Prull, Dawes, Martin, Rosenberg, & Light, 2006). Recent neuroanatomical and functional research indicate that frontal and medial-temporal systems that support memory function are affected by aging more than many other brain areas (see Buckner, Head, & Lustig, 2006, for a review). Moreover, a review of neuroimaging and patient data indicates that both medial-temporal lobes and frontal lobes are related to remembering (Skinner & Fernandes, 2007). These findings are consistent with the component-process model of episodic memory (Moscovitch, 1992; Moscovitch & Winocur, 1992), which proposes that the medial-temporal lobes are responsible for binding during encoding and cue-dependent retrieval of episodic memories, whereas the frontal lobes are involved in strategic aspects of both encoding and retrieval (Wheeler, Stuss, & Tulving, 1997), which includes specification of retrieval cues and post-retrieval monitoring, i.e., “working with memory” (Moscovitch & Winocur, 1992).
Many researchers have adopted a neuropsychological test approach to examining the relative contributions of medial-temporal and frontal brain functioning to age-related changes in episodic memory (Butler, McDaniel, Dornburg, Price, & Roediger, 2004; Glisky et al., 1995, 2001; Henkel et al., 1998; Roediger & McDaniel, 2007). The general approach has been to examine whether there are different patterns of correlations between tests of frontal-lobe and medial-temporal lobe functioning, and different memory tasks or indices. For example, tests of medial-temporal lobe functioning have been found to be related to item recognition but not source recognition, whereas the opposite was true for frontal-lobe functioning (Glisky et al., 1995, 2001). More recently, age-related increases in false recall and false recognition have been shown to be related to frontal-lobe functioning, even when medial-temporal functioning was equivalent in the sample (Butler et al., 2004; Dornburg & McDaniel, 2006; Roediger & McDaniel, 2007; but see Balota et al., 1999).
The present study addresses the issue of age-related increase in false remembering on standard remember/know tests, and attempts to shed light on the neuropsychological test correlates of veridical and false remembering. Two different remember/know tests – one in which subjects saw or heard words at study and the other on which subjects read or generated words – were completed by each of over 200 subjects ranging in age from 18 to 90 years. Additionally, several neuropsychological tests of FL and MTL functioning were administered. Our primary interest was in examining whether the neuropsychological correlates of veridical and false memory differed.
3. Method
3.1. Subjects
Subjects were 202 adults (120 females) aged 18–90 from the Saint Louis metropolitan area who participated in this study as part of a larger study on aging and cognition (see McCabe, Roediger, McDaniel, Balota, & Hambrick, submitted for publication, for more details). Subjects were recruited from the Volunteers for Health participant pool which is maintained at the Washington University in St. Louis School of Medicine for purposes of screening and matching potential research participants with appropriate studies. Although age was a continuous variable, for purposes of presentation age was grouped in to three roughly equal numbers of subjects for data presentation purposes. Demographic characteristics for each of these groups are presented in Table 3. There were no significant differences for age groups for percentage of female subjects (59%), self-reported health (4.2), or number of years of education (15.2; all F's < 1.09). Age was positively correlated with the number of medications participants took on a regular basis (r = .46), and with Shipley vocabulary scores (r = .20; p's < .01). All subjects who were included in the analysis had a minimum of high school education and scored 26 or greater on the Mini Mental Status Exam (Folstein, Folstein, & McHugh, 1975; see Section 4 for exclusion criteria).
Table 3 Demographic characteristics and neuropsychological test performance.
| Variable | Age group |
Age, r | ||
|---|---|---|---|---|
| Younger | Middle-age | Older | ||
| N | 67 | 67 | 68 | |
| Age | 30.0 (7.5) | 53.6 (6.4) | 77.0 (6.8) | |
| Age range | 18–42 | 43–64 | 65–90 | |
| % females | 61 | 58 | 57 | |
| Education (high school = 12) | 15.3 (2.3) | 15.4 (2.5) | 15.0 (2.9) | –.01 |
| Self-reported health (max = 5) | 4.3 (0.9) | 4.2 (0.8) | 4.1 (0.6) | –.09 |
| No. of medications | 0.9 (l.3) | 1.6 (1.7) | 3.0 (2.4) | .46 |
| Shipley vocabulary (max = 40) | 33.0 (4.1) | 34.0 (3.6) | 34.5 (3.4) | .20 |
| Frontal-lobe functioning (FLF) tests | ||||
| Backward digit span | 7.9 (2.5) | 8.0 (2.6) | 6.9 (2.2) | –.18 |
| Mental arithmetic | 13.3 (2.8) | 14.0 (3.0) | 12.4 (3.0) | – .11 |
| Mental control | 30.7 (4.2) | 29.0 (5.1) | 26.0 (5.2) | –.35 |
| Verbal fluency (FAS) | 46.3 (11.3) | 42.3 (12.4) | 39.2 (10.5) | –.18 |
| Wisconsin card sorting | 18.0 (7.3) | 24.9 (13.6) | 37.8 (16.1) | .54 |
| FLF composite | .39 (0.78) | .23 (0.96) | –.62 (–0.95) | –.39 |
| Medial-temporal lobe functioning (MTLF) tests | ||||
| Logical memory | 32.6 (7.3) | 31.8 (6.6) | 28.9 (7.3) | –.22 |
| Verbal paired associates | 23.4 (6.7) | 19.7 (9.2) | 16.5 (7.9) | –.35 |
| California verbal learning test | 8.2 (2.3) | 7.8 (1.8) | 6.4 (2.0) | –.22 |
| MTLF composite | .36 (1.03) | .17 (0.88) | –.52 (–0.87) | –.38 |
3.2. General procedure
Participants were tested in two sessions, each lasting approximately 2.5 h. The first session included the medial-temporal lobe and frontal-lobe tests, while the second session included the remember–know recognition tests, vocabulary test, and demographic questionnaire. The sessions were at least one week apart, but never more than three weeks apart. Other tests were included that are not reported as part of this study (see McCabe et al., submitted for publication for details).
3.2.1. Frontal-lobe functioning
The frontal lobe (FL) functioning factor was based on five measures (Glisky et al., 1995), each of which taxed working memory, and has been shown to be related to the frontal lobes (e.g., see Glisky & Kong, 2008, p. 818, for a review of evidence for the relation between these tests and functioning of the frontal lobes). These tests included (1) the Wisconsin Card Sorting Test (WCST; Heaton, 1993; number of perseverative errors), (2) the verbal fluency task (using the letters F, A, and S; Thurstone, 1938), (3) Mental Arithmetic from the WAIS (Wechsler, 1997a), (4) Mental Control from the WAIS (Wechsler, 1997a), and (5) Backward Digit Span from the WMS-3 (Wechsler, 1997b).
3.2.2. Medial-temporal lobe functioning
The medial-temporal lobe (MTL) functioning factor was based on three measures (Glisky et al., 1995), each of which requires cued recall or free recall, which engage medial-temporal brain areas (see Yonelinas et al., 2002). These included logical memory I (WMS-3; Wechsler, 1997b), verbal paired associates I (WMS-3; Wechsler, 1997b), and the California verbal learning test (CVLT; list 1 recall; Delis, Kramer, Kaplan, and Ober, 2000).
3.2.3. Remember–know recognition tests
Two remember–know recognition tests were completed by each subject. The stimuli for the tests included a pool of 280 medium-frequency concrete nouns. Log word frequency based on the Lund and Burgess's HAL (1996) 131 million word database varied between 6.3 and 12.3 (M = 8.7, S.D. = 1.0) according to the English Lexicon Project database (Balota et al., 2007). Words ranged in length from four to eight letters (M = 5.2, S.D. = 1.2), and were high in concreteness (over 550 according to the MRC Psycholinguistic Database; Wilson, 1988). All words were presented in 72-point Arial font at the center of a 19-in. computer screen.
Half of the 280 words were randomly assigned to be used for the read–generate study-test condition (hereafter read–generate test) and the other half for see–hear study-test condition (hereafter see–hear test). The read–generate test was completed about an hour into the second session. For the read–generate test subjects studied 80 words at a rate of one word every five seconds. Half of the words were read intact, and the other half were generated from an anagram, randomly intermixed. In order to identify a word presented as an anagram subjects had to transpose two letters in the word that were underlined (e.g., SLIA). A practice phase involved having subjects study and complete 10 anagrams by writing down the corresponding intact words on an answer sheet that was provided. All subjects were able to do this perfectly.
After completing the study episode subjects completed two tests, each of which included 20 words that had been read intact, 20 that had been generated from anagrams, and 20 new words. The first test was a source memory test in which subjects were asked to determine, for each word, whether it had been read, generated, or was new (results from that test were reported in Lyle, McCabe, & Roediger, 2008, and will not be considered further here). For the second test, the remember–know test, subjects were asked to decide whether they had previously seen the word during the study period. If they had not seen the word, they were instructed to press a key marked “N”, to indicate the word was new. If they had seen the word in the study list, they were instructed to judge whether the word was recollected, in which case they pressed a key on the keyboard marked with an “R”, or if the word was studied but did not include recollective details, they should press a key marked with a “K”, to indicate that they “know” the word was presented. The term recollect was used instead of remember because the word recollect better describes the subjective experience of conscious recollection. The difference between recollect and know responses was closely based on the instructions by Rajaram (1993), and included examples of each dimension that could be used to provide a recollect response, and detailed examples of recollect and know experiences associated with normal daily activities.
The see–hear study and test phase was the first task completed in the second session and was identical to the read–generate test with respect to materials and study and test procedures, except that half the studied items were viewed on a computer screen, and half were heard over headphones. Headphone volume was adjusted by each subject while listening to a practice list prior to study. For the see–hear study list one word was presented every three seconds. Subjects first completed a source test determining whether they heard or saw 40 studied and 20 new words, followed by the RK test that included 40 different studied and new words.
4. Results
Results of statistical tests were significant at p < .01, unless otherwise noted. Because age was a continuous variable, age correlations are reported in addition to ANOVAs.
4.1. Neuropsychological test performance
There were age-related changes in performance on all of the FL functioning tests except for mental arithmetic, though that difference was also in the expected direction (see Table 3). The FL functioning composite score was computed by entering the five frontal tests into a principal component analysis, which created a factor accounting for 48% of the variance in performance. The factor loadings for each test were as follows: WCST: –.63, verbal fluency: .60, mental arithmetic: .76, mental control: .76, backward digit span: .70. There were also age-related changes in performance on all of the MTL functioning tests. The MTL functioning composite score was computed in the same manner as the FL functioning composite, which created a single factor accounting for 63% of the variance in performance. The factor loadings for each test were as follows: logical memory: .80, verbal paired associates: .81, CVLT: .78. Each composite score is a z-score based on the tests comprising that factor. The contribution of each test to the z-score is weighted by the factor loading in the principal components analysis.
4.2. Recognition test performance
Table 4 displays the average percentage of remember and know responses for studied and new items for each age group for the read–generate and see–hear tests. We began our analyses by examining whether there was a generation effect for remember and know responses (i.e., generated hits minus intact hits for each response type). There was a generation effect for remember responses, such that generated items received more remember responses (M = .45) than read items (M = .32), t(201) = 8.62, but not for know responses (M = .38 for generated and .35 for read), t(201) = 1.90. However, the generation effect for remember responses was unrelated to age (r = –.10). Because the generation effect was not our primary interest in the current study, these data will not be considered further.
Table 4.
Remembering and knowing.
| Item and response types | Age group |
|||
|---|---|---|---|---|
| Younger | Middle | Older | age r | |
| Read–generate test | ||||
| Studied | ||||
| Remember | .39 (.02) | .40 (.03) | .37 (.02) | –.02 |
| Know | .36 (.02) | .36 (.03) | .39 (.03) | .02 |
| Overall | .75 (.02) | .76 (.02) | .75 (.02) | .00 |
| New | ||||
| Remember | .04 (.01) | .09 (.01) | .11 (.01) | .32* |
| Know | .21 (.02) | .24 (.02) | .26 (.02) | .09 |
| Overall | .25 (.02) | .33 (.03) | .36 (.02) | .23* |
| Recollection and familiarity estimates | ||||
| Recollection: Remember d′ | 1.62 (.08) | 1.28 (.08) | .99 (.08) | .37* |
| Familiarity: IRK | .60 (.03) | .59 (.03) | .58 (.03) | .05 |
| See–hear test | ||||
| Studied | ||||
| Remember | .31 (.02) | .31 (.02) | .29 (.02) | –.04 |
| Know | .35 (.02) | .34 (.02) | .37 (.03) | .05 |
| Overall | .66 (.02) | .65 (.02) | .66 (.02) | .01 |
| New | ||||
| Remember | .02 (.01) | .05 (.01) | .09 (.02) | .27* |
| Know | .17 (.02) | .23 (.02) | .19 (.02) | .06 |
| Overall | .20 (.02) | .28 (.03) | .28 (.02) | .19* |
| Recollection and familiarity estimates | ||||
| Recollection: remember d′ | 1.49 (.07) | 1.29 (.08) | .92 (.09) | .35* |
| Familiarity: IRK | .52 (.02) | .50 (.03) | .53 (.02) | .05 |
Indicates the correlation was significant at p < .05.
As shown in Table 4, there was no age effect for know responses for studied items or new items on the read–generate test, but the pattern differed for remember responses. As shown in Fig. 3 (Panel A), collapsed across read and generated items, remember hits did not differ as a function of age; by contrast, there was an age effect for remember false alarms for new items (r = .32), indicating an age-related increase in false remembering. Performance for remember and know responses followed the same pattern for the see–hear condition, also shown in Table 4 and Fig. 3 (Panel B), with only the remember false alarms for new items showing a relation to age (r = .27).
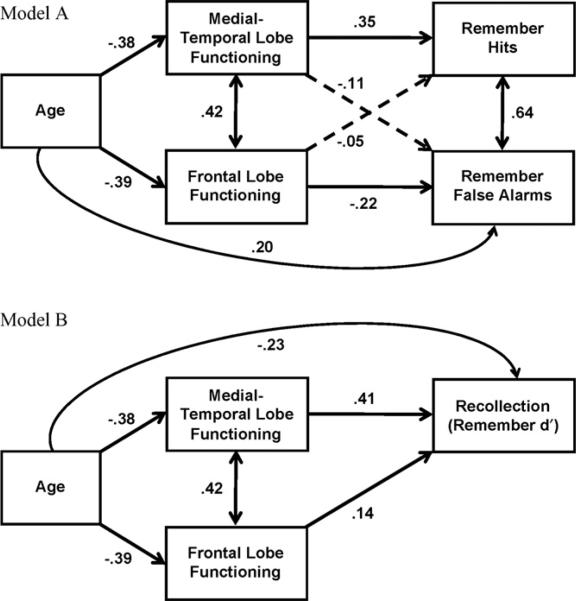
Fig. 3.
Path analysis models examining the relation between (A) age, medial-temporal lobe functioning, frontal-lobe functioning, and remember hits and remember false alarms, and (B) age, medial-temporal lobe functioning, frontal-lobe functioning, and recollection. Note that solid lines indicate a significant correlation, dotted lines indicate a non-significant correlation.
4.3. Recollection and familiarity estimates
Recollection was estimated using remember d′, i.e., by calculating the distance between the average of the remember response distributions for studied and new items. Because data indicate that remember responses are normally distributed (at least under some circumstances; e.g., Rotello, Macmillan, Reeder, & Wong, 2005), this estimate is likely more appropriate than simply subtracting remember false alarms from remember hits (Yonelinas, 2002) or using A′ (Bunce, 2003), each of which assumes a rectangular distribution. Age was negatively correlated with Recollection for the read–generate test (r = –.37) and for the see–hear test (r = –.35). Familiarity was estimated using the independence remember–know (IRK) procedure (i.e., know/(1 – remember); see Yonelinas, 2002, for details). The IRK estimates were uncorrelated with age for the read–generate and see–hear tests. Thus, age was related to Recollection but not Familiarity for both analyses, and this was obviously driven by the age effects on false remembering because there was no age-related difference in hit remember responses.
4.4. Correlations between neuropsychological test performance and memory indices
Because the patterns of performance on the two memory measures were the same (see Table 4), and correlations did not differ, we converted each measure to standardized scores and combined them to create a single factor score for each subject. This composite measure allowed better precision in computing regression models and simplified presentation of the data.
Table 5 shows the correlations between age, FL functioning and MTL functioning and each of the memory indices. For studied items the only significant correlation was between MTL functioning and remember hits (r = .29), with better MTL functioning performance associated with more remember hits, whereas for new items all three predictor variables were correlated with remember false alarms. FL functioning and MTL functioning were both associated with decreases in remember false alarms, whereas age was associated with an increase. FL functioning was associated with increases in know false alarms as well. In terms of the estimates of recollection and familiarity, age, FL functioning, and MTL functioning were all correlated with recollection, following the opposite pattern of that described for remember false alarms, but none of the predictors was correlated with the IRK estimate.
Table 5.
Correlation between age, frontal-lobe functioning (FLF) and medial-temporal lobe functioning (MTLF) composite scores, and memory indices.
| Measure | Age | FLF | MTLF |
|---|---|---|---|
| Remember hits | –.03 | .07 | .29* |
| Know hit | .04 | –.12 | –.12 |
| Remember false alarms | .34* | –.32* | –.24* |
| Know false alarms | .08 | –.20* | .10 |
| Recollection (remember d′) | –.44* | .43* | .57* |
| Familiarity (IRK) | .00 | –.08 | .10 |
Indicates the correlation was significant at p < .05.
4.5. Path model showing the effects of age, MTL, and FL functioning on remember hits and false alarms
We conducted path analyses in order to examine whether Age, MTL functioning, and FL functioning accounted for unique variance in performance on remember hits, false alarms, or recollection (remember d′). Model A, predicting remember hits and false alarms, is shown in the top panel of Fig. 3. Age was negatively related to both MTL and FL functioning, which were correlated with one another. Of primary interest was the relation between MTL and FL functioning and remembering. MTL functioning was related to remember hits, FL functioning was not. By contrast, FL functioning was related to remember false alarms, but MTL functioning was not. Remember hits and false alarms were also correlated with one another, which is consistent with the notion that both of these measures share variance related to response criteria (see Wixted & Stretch, 2004). Age also accounted for unique variance in remember false alarms, even after controlling for MTL and FL functioning. Age was not related to remember hits. This path was not included in the model because there was no relation between these variables in the bivariate correlation (see Table 4) and inclusion of the path would have led to model saturation (i.e., there would be zero degrees of freedom in the model if that path were included).
Using a minimum criterion for acceptability of fit as a CFI of .90 (Hu & Bentler, 1995), and a RMSEA of <.10 (Browne & Cudeck, 1993), the fit of Model A was good, χ2(1, N = 202) = 1.03, CFI = 1.00, RMSEA = .013, p = .31). The overall age effect on remember hits was –.09, with a direct effect of .00 and an indirect effect of –.09 (through MTL and FL functioning). The overall age effect on remember false alarms was .31, with a direct effect of .20 and an indirect effect of .10. The finding that remember false alarms were related to FL functioning and age, even after controlling for remember hits, indicates that false remember responses were not simply the result of changes in the response criterion. If remember false alarms were simply a measure of response bias, as signal detection models of remembering and knowing suggest (Butler et al., 2004; Wixted & Stretch, 2004), then there should have been no unique variance in false remember responses associated with other measures after accounting for remember hits.
Model B, shown in the bottom panel of Fig. 3, is essentially identical to Model A, except that recollection (remember d′) was the outcome measure, rather than remember hits and false alarms. This model showed that both MTL and FL functioning were related to Recollection. However, because the model was saturated, model fit statistics could not be computed. Nonetheless, the model offers a graphical depiction of the intercorrelations between the variables, and shows that age, MTL functioning, and FL functioning, all make significant unique contributions to recollection. The overall age effect on recollection in the model was –.43, with a direct effect of –.23, and an indirect effect of –.21 (through MTL and FL functioning).
5. General discussion
In the current study, we reported data from two remember–know tests administered to a life span sample of adults (aged 18–90). The results from both tests, using similar materials but different encoding tasks, were consistent in showing age-related increases in false remembering (i.e., remember responses to new, unstudied items) despite the fact that no age differences existed in veridical remembering (i.e., remember responses for studied items). Despite the lack of age differences for veridical remembering, performance on a battery of tasks related to medial-temporal lobe functioning was positively correlated with veridical remembering. By contrast, false remembering was uniquely related to age and frontal-lobe functioning, in addition to being related to medial-temporal lobe functioning. Recollection (estimated using d′) declined with age, and increased with medial-temporal lobe functioning and frontal-lobe functioning, but Familiarity (estimated using the IRK procedure; Yonelinas, 2002) was not related to age or neuropsychological functioning.
In our review of aging studies employing the remember–know paradigm, we found that both veridical and false remembering were affected by age, such that aging decreases veridical remembering and increases false remembering. In a sense, these data are not surprising, but the finding that the age effects are of similar magnitude for veridical and false remembering is noteworthy, and perhaps unexpected. Another noteworthy finding from the meta-analysis is that the age effect on false remembering is larger than the age effect on false knowing responses. This is unexpected because it is commonly assumed that age-related increases in false alarms are driven by an over-reliance on familiarity in older adults resulting from declines in recollection (Buchler & Reder, 2007; Multhaup, 1995). However, the large age-related increase in false remembering coupled with the smaller age-related increase in false knowing suggests that the typical age-related increase in false alarms is due to older adults’ increased likelihood of experiencing recollection for new, unstudied items (Jacoby, Bishara, Hessels, & Toth, 2005; Meade & Roediger, 2006). This finding is consistent with recent suggestions that age-related declines in memory performance are often related to misrecollection of features of studied events, rather than familiarity in the absence of recollection of studied events (Dodson, Bawa, & Krueger, 2007).
6. Neuropsychological correlates of veridical and false remembering
The finding that remember hits and false alarms had different neuropsychological test correlates indicates that different processes are involved in each type of response. Specifically, medial-temporal lobe functioning was the only variable included in this study that was correlated with remember hits. This is consistent with the idea that remember hits are dependent on an associative, cue-dependent memory system (Moscovitch, Fernandes, & Troyer, 2001). Interestingly, despite reasons to believe that remember hits should also be related to frontal-lobe functioning (e.g., Wheeler et al., 1997), this relationship failed to materialize in the present dataset (note though, that this lack of relation is not anomalous; Bastin & Van der Linden, 2003; Bunce & Macready, 2005; Perfect, Williams, & Anderton-Brown, 1995; Perfect & Dasgupta, 1997; Prull et al., 2006). Based on this finding, we believe that there is an increased reliance on FL functioning under conditions of uncertainty at retrieval. For example, if a new item cues partial recollection of information from a context other than the study episode, FL functioning is important to determine whether that partial recollection arose from the study episode or some other non-experimental context. In this respect, remember false alarms appear to be particularly reliant on decision processes engaged at retrieval, whereas remember hits are much more likely to be based on information that is well bound to the study context, perhaps overshadowing individual differences related to decision processes, and therefore FL functioning, that might be involved.
Several related explanations exist for how the frontal lobes are involved in retrieval. For example, some have argued that frontal functioning is important for specifying retrieval cues and/or monitoring the output of the medial-temporal lobe system (Moscovitch & Winocur, 1992). With respect to understanding the role of frontal-lobe functioning in false remembering, it is important that retrieval cues match the study context if subjective recollection (i.e., remembering) is to be accurate. If retrieval is not appropriately constrained to the study episode (Jacoby et al., 2005; Tulving, 1983), new items can elicit recollective responses that may inappropriately be attributed to the study episode. This interpretation of the role of FL functioning in remember false alarms is consistent with the idea that these responses are source misattributions (McCabe & Geraci, in press), such that they reflect recollection of events from an episode other than the study session, in addition to recollection of items from the study session (cf., Roediger & McDermott, 1995). The finding that false remembering was uniquely related to frontal-lobe functioning and age underscores the importance of false remembering, even for unrelated lures, as a target of theoretical investigation. Indeed, false remembering will need to be explained by any comprehensive account of subjective recollection.
We also estimated recollection and familiarity processes from the remember–know paradigm based on the methods proposed by Yonelinas (2002). The finding that neither age, nor neuropsychological functioning, was related to the IRK estimate of familiarity (or to know hits for that matter) is consistent with the notion that knowing is related to familiarity-based responding, rather than reflecting controlled processing. Alternatively, recollection was related to medial-temporal lobe functioning, frontal-lobe functioning, and age, supporting the notion that control processes underlie remembering. We should note too that the same pattern of relations with age and neuropsychological functioning was found for both recollection and false remembering, owing to the fact that the estimate of recollection incorporates remember false alarms. Thus, in estimating recollection, it appears that remembering for studied items does not give as accurate an estimate of the recollection process as does an estimate based on remember hits and remember false alarms.
Because the lack of age differences in remember hits in the current study is an unusual finding, we next consider possible reasons for this result. First, it is important to note that the levels of veridical remembering were low in this study, as compared to other studies of aging and remember–know judgments. Averaged across the read–generate and see–hear tests the level of remember hits was .35 for younger adults, and .33 for older adults. This level of remember hits is lower than remember hit rates for younger adults in 24 of the 27 studies reviewed in Table 1. Also note that the level of remembering for older adults in the current study is not unusual (.39, on average in the meta-analysis). Thus, our null age effect is probably related to a suppression of conscious recollection in young adults, rather than an enhancement of recollective experience for older adults. Support for this idea comes from a consideration of the relation between remember hits and age effect sizes from the meta-analysis. There was a clear relation between the level of younger adults’ remember hits and the age effect size, indicating that for studies in which the level of younger adults’ remember hits was lower, the magnitude of the age effect was smaller (r = –.43, p < .05; note that larger age effects are larger negative numbers). By contrast, there was also a clear relation between older adults’ remember hits and the magnitude of the age effect, but in the opposite direction (r = .49, p < .05). That is, for studies in which older adults’ remember hits were lower, the magnitude of the age effect was greater.
One possible reason that younger adults might have had lower than average levels of remember hits in the current study is that we used study lists that were longer than average (i.e., 80 words in our experiments), and longer list lengths are associated with lower levels of remembering in younger adults (cf., Cary & Reder, 2003). Another possibility is that having the source memory test prior to the remember–know test somehow biased responding or changed retrieval strategies on the remember–know test, though this seems unlikely based on research with younger adults showing the order of these tests has no effect on remembering and knowing (Wais, Mickes, & Wixted, 2008). A third possible reason is that the younger adults in this study were not college students, and thus, they may not have been as well practiced at remembering large amounts of information as younger adults in typical cognitive aging studies. Regardless of the exact reason for our lack of age differences in remember hits, a target for future research is to replicate the current findings using paradigms that reveal prototypical patterns of age differences in both remember hits and false alarms.
7. Conclusions
Tulving's (1985) introduction of the remember–know paradigm has sparked considerable interest and debate among psychologists and neuroscientists for over 20 years. The original theory proposed remembering and knowing as states of consciousness associated with distinct memory systems. We found some support for this idea, at least with regard to remembering, because recollection was correlated with medial-temporal lobe and frontal-lobe functioning. However, familiarity (as derived from knowing) was uncorrelated with neuropsychological test performance and age. The most noteworthy finding from the current study is that false remembering was most closely associated with neuropsychological functioning and age. Additionally, the meta-analysis of remember–know judgments shows that age affects veridical and false remembering to a similar degree, and more than it affects knowing. Indeed, the current study adds to the growing consensus that both veridical and false recollection must be explained by any comprehensive theory of memory.
Appendix A. Methodology for meta-analysis of remember data from published studies
Published studies using the remember–know procedure to compare healthy younger and older adults were identified by reviewing citations in existing papers, and by searching the PsycINFO database. PsycINFO searches included the term ‘aging’ with ‘remember’ and ‘know’, and ‘aging’ with ‘recollective’, and ‘aging’ with ‘recollection’. The matches returned for these searches were then inspected to determine whether the study included a standard remember–know recognition test and at least one group of younger adults (under 45 years of age), and a group of older adults (age 60 or older). The study was included in Table 1 if the percentage of remember responses was reported for both studied items and new items (or could be computed), and the remember false alarm rate was greater than zero for at least one age group. When necessary, levels of remembering were estimated by visual inspection of figures. Remember response levels were averaged across within-subject manipulations when necessary, and only responses for unrelated lures were included.
Studies were included in the meta-analysis if they allowed an estimation of effect sizes for remember responses for studied or new items, either from standard deviations, standard errors, or F or t values. In some cases these data were obtained by contacting the authors directly if the necessary statistics were not reported. We were able to obtain enough data to compute effect sizes for 24 of the 27 studies. Statistics for the effect size analysis are included in Table 2. There was substantial heterogeneity across the studies for remember hits (p < .00001) as evidenced by a significant Q statistic (the Q statistic is similar to a one-way ANOVA in terms of assessing variability across studies). However, we do not consider this finding to be problematic in the present analysis because we were interested only in estimating the overall effect size for age, and because all the studies included estimates of remember hits and false alarms for both age groups, all influences on performance are the same across hits and false alarms and response types. The Q statistic did not reach conventional significance levels for remember false alarms (p = .07). There was significant heterogeneity for know hits (p < .00001), but not for know false alarms as well.
Because the variance measured by studies with larger sample sizes is more precise and can introduce bias in effect size analysis (Hedges & Olkin, 1985) we examined the correlations between sample size and effect size across studies as well. The correlations were not significant for remember hits (r = –.05) or false alarms (r = –.21), or for know hits (r = –.39) or false alarms (r = .36). Because false remember responses were near floor in many of the studies, and could potentially be biased by restriction of range (the range averaged across age groups was .02–.11), we also examined whether the effect sizes were related to the overall level of false remembering across studies. The correlation between effect size for false remembering and level of false remembering was not significant (r = .09). Thus, the effect sizes did not appear to be influenced greatly by the number of subjects in each study or the overall level of false remembering, indicating that the effect size equivalence for studied and new items was not an artifact of these factors.
Footnotes
Because the distinction between systems and processes have blurred in recent years, we will refer to systems and process theories collectively as dual-process theories.
Because variability could not be assessed for four of the published studies these were not included in the meta-analysis (see Appendix A for details).
References
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, et al. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KI, Kessler B, Loftis B, et al. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: A study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. The science of false memory. Oxford University Press; New York: 2005. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage; Beverly Hills, CA: 1993. pp. 136–162. [Google Scholar]
- Buckner RL, Head D, Lustig C. Brain changes in aging: A lifespan perspective. In: Craik FIM, Bialystok E, editors. Lifespan cognition: Mechanisms of change. Oxford University Press; New York: 2006. [Google Scholar]
- Buchler NEG, Reder LM. Modeling age-related memory deficits: A two-parameter solution. Psychology and Aging. 2007;22:104–121. doi: 10.1037/0882-7974.22.1.104. [DOI] [PubMed] [Google Scholar]
- Bugaiska A, Clarys D, Jarry C, Taconnat L, Tapia G, Vanneste S, et al. The effect of aging in recollective experience: The processing speed and executive functioning hypothesis. Consciousness and Cognition. 2007;16:797–808. doi: 10.1016/j.concog.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bunce D. Cognitive support at encoding attenuates age differences in recollective experience among adults of lower frontal lobe function. Neuropsychology. 2003;17:353–361. doi: 10.1037/0894-4105.17.3.353. [DOI] [PubMed] [Google Scholar]
- Bunce D, Macready A. Processing speed, executive function, and age differences in remembering and knowing. The Quarterly Journal of Experimental Psychology. 2005;58A:155–168. doi: 10.1080/02724980443000197. [DOI] [PubMed] [Google Scholar]
- Butler KM, McDaniel MA, Dornburg CC, Price AL, Roediger HL. Age differences in veridical and false recall are not inevitable: The role of frontal lobe function. Psychonomic Bulletin & Review. 2004;11:921–925. doi: 10.3758/bf03196722. [DOI] [PubMed] [Google Scholar]
- Cary M, Reder LM. A dual-process account of the list-length and strength-based mirror effects in recognition. Journal of Memory and Language. 2003;49:231–248. [Google Scholar]
- Clarys D, Isingrini M, Gana K. Mediators of age-related differences in recollective experience in recognition memory. Acta Psychologica. 2002;109:315–329. doi: 10.1016/s0001-6918(01)00064-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Comblain C, D'Argembeau A, Van der Linden M, Aldenhoff L. Impact of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12:673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- D'Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Consciousness and Cognition. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal instructions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. 2nd ed. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Dodson CS, Bawa S, Krueger LE. Aging, metamemory, and high-confidence errors: A misrecollection account. Psychology and Aging. 2007;22:122–133. doi: 10.1037/0882-7974.22.1.122. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Slotnick SD. Aging, source memory, and misrecollections. Psychology and Aging. 2007;33:169–181. doi: 10.1037/0278-7393.33.1.169. [DOI] [PubMed] [Google Scholar]
- Dornburg CC, McDaniel MA. The cognitive interview enhances long-term free recall of older adults. Psychology and Aging. 2006;21:196–200. doi: 10.1037/0882-7974.21.1.196. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Sarfatti S, Knowlton BJ. The effect of testing procedure on remember–know judgments. Psychonomic Bulletin & Review. 2002;9:139–145. doi: 10.3758/bf03196270. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Trott C. An event-related potential study of encoding in young and older adults. Neuropsycholgia. 2000;38:542–557. doi: 10.1016/s0028-3932(99)00122-0. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Roediger HL. The effects of associations and aging on illusory recollection. Memory & Cognition. 2003;31:1036–1044. doi: 10.3758/bf03196124. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Remembering and knowing. In: Roediger HL III, Byrne J, editors. Cognitive psychology of memory, Vol. 2 of learning and memory: A comprehensive reference. Vol. 4. Elsevier; Oxford: 2008. pp. 285–305. [Google Scholar]
- Gardiner JM. Functional aspects of recollective experience. Memory & Cognition. 1988;16:309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Ramponi C, Richardson-Klavehn A. Recognition memory and decision processes: A meta-analysis of remember, know, and guess responses. Memory. 2002;10:83–98. doi: 10.1080/09658210143000281. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin card sorting test: Computer version. 2nd research ed. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; Orlando: 1985. [Google Scholar]
- Henkel LA, Johnson MK, De Leonardis DM. Aging and source monitoring: Cognitive processes and neuropsychological correlates. Journal of Experimental Psychology: General. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Higham PA, Vokey JR. Illusory recollection and dual-process models of recognition memory. Quarterly Journal of Experimental Psychology. 2004;57A:714–744. doi: 10.1080/02724980343000468. [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler P. Evaluating model fit. In: Hoyle RH, editor. Structural equation modeling. Concepts, issues, and applications. Sage; London: 1995. pp. 76–99. [Google Scholar]
- Jacoby LL, Bishara AJ, Hessels S, Toth JP. Aging, subjective experience, and cognitive control: Dramatic false remembering by older adults. Journal of Experimental Psychology: General. 2005;134:131–148. doi: 10.1037/0096-3445.134.2.131. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Kelley CM, Dywan J. Memory attributions. In: Roediger HL, Craik FIM, editors. Varieties of memory and consciousness: Essays in honour of Endel Tulving. Erlbaum; Hillsdale, NJ: 1989. pp. 391–422. [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychology and Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Lampinen JM, Meier C, Arnal JA, Leding JK. Compelling untruths: Content borrowing and vivid false memories. Journal of Experimental Psychology: Learning, Memory and Cognition. 2005;31:954–963. doi: 10.1037/0278-7393.31.5.954. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Rönnlund M, Nilsson L-G. Remembering and knowing in adulthood: Effects of enacted encoding and relations to processing speed. Aging, Neuropsychology and Cognition. 2002;9:184–200. [Google Scholar]
- Lyle KB, McCabe DP, Roediger HL. Handedness is related to memory via hemispheric interaction: Evidence from paired associate recall and source memory tasks in an adult lifespan sample. Neuropsychology. 2008;22:523–530. doi: 10.1037/0894-4105.22.4.523. [DOI] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments, & Computers. 1996;28:203–208. [Google Scholar]
- Mantyla T. Knowing but not remembering: Adult age differences in recollective experience. Memory & Cognition. 1993;21:379–388. doi: 10.3758/bf03208271. [DOI] [PubMed] [Google Scholar]
- Mather M, Henkel LA, Johnson MK. Evaluating the characteristics of false memories: Remember/know judgments and memory characteristics questionnaire compared. Memory & Cognition. 1997;25:826–837. doi: 10.3758/bf03211327. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Geraci L. The role of extra-list associations in false remembering: A source misattribution account. Memory & Cognition. doi: 10.3758/MC.37.2.130. in press. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: Converging evidence for a common executive attention construct. submitted for publication. [DOI] [PMC free article] [PubMed]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Fernandes MA, Troyer A. Working-with memory and cognitive resources: A component-process account of divided attention and memory. In: Naveh-Benjamin M, Moscovitch M, Roediger HL, editors. Perspectives on human memory and cognitive aging. Psychology Press; New York: 2001. pp. 171–192. [Google Scholar]
- Meade ML, Roediger HL. The effect of forced recall on illusory recollection in younger and older adults. American Journal of Psychology. 2006;119:433–462. [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Erlbaum; Hillsdale, NJ: 1992. pp. 315–372. [Google Scholar]
- Multhaup KS. Aging, source, and decision criteria: When false fame errors do and do not occur. Psychology & Aging. 1995;10:492–497. doi: 10.1037//0882-7974.10.3.492. [DOI] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in young and older adults: Exploring the characteristics of illusory memories. Memory & Cognition. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Dasgupta ZRR. What underlies the deficit in reported recollective experience in old age? Memory & Cognition. 1997;25:849–858. doi: 10.3758/bf03211329. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Williams RB, Anderton-Brown C. Age differences in reported recollective experience are due to encoding effects, not response bias? Memory. 1995;3:169–186. doi: 10.1080/09658219508258964. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: Adult age differences and neuropsychological test correlates. Psychology and Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: Two means of access to the personal past. Memory & Cognition. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDaniel MA. Illusory recollections in older adults: Testing Mark Twain's conjecture. In: Garry M, Hayne H, editors. Do justice and let the sky fall: Elizabeth F. Loftus and her contributions to science, law, and academic freedom. Erlbaum; Hillsdale, NJ: 2007. pp. 105–136. [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1995;21:803–814. [Google Scholar]
- Roediger HL, Watson JM, McDermott KB, Gallo DA. Factors that determine false recall: A multiple regression analysis. Psychonomic Bulletin & Review. 2001;8:385–407. doi: 10.3758/bf03196177. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA. Sum-difference theory of remembering and knowing: A two-dimensional signal detection model. Psychological Review. 2004;111:588–616. doi: 10.1037/0033-295X.111.3.588. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA, Wong M. The remember response: Subject to bias, graded, and not a process-pure indicator of recollection. Psychonomic Bulletin & Review. 2005;12:865–873. doi: 10.3758/bf03196778. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Johnson MK, Gross M, Angell KA. False recollection induced via photographs: A comparison of older and younger adults. Psychology and Aging. 1997;12:203–215. doi: 10.1037//0882-7974.12.2.203. [DOI] [PubMed] [Google Scholar]
- Skinner E, Fernandes MA. Neural correlates of Remembering and Knowing at retrieval: a review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Slamecka NJ. An examination of trace storage in free recall. Journal of Experimental Psychology. 1968;76:504–513. doi: 10.1037/h0025695. [DOI] [PubMed] [Google Scholar]
- Thurstone LL. Psychometric Monographs, No. 1. University of Chicago Press; Chicago: 1938. Primary mental abilities. [Google Scholar]
- Tulving E. Cue-dependent forgetting. American Scientist. 1974;62:74–82. [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford University Press; New York: 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. Journal of Verbal Learning and Verbal Behavior. 1966;5:381–391. [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Wais PE, Mickes L, Wixted JT. Remember/know judgments probe degrees of recollection. Journal of Cognitive Neuroscience. 2008;20:400–405. doi: 10.1162/jocn.2008.20041. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. WAIS-III/WMS-III technical manual. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC psycholinguistic database: Machine readable dictionary, version 2. Behavioral research methods. Instruments and Computers. 1988;20:6–11. [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal-detection interpretation of remember/know judgments. Psychonomic Bulletin & Review. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins IG, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember/know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:1–17. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]