Abstract
Background and Aims
Early growth response gene-1 (Egr-1) is an important inflammatory transcription factor. We hypothesize that leukocyte-derived Egr-1 plays a key inflammatory role in causing postoperative ileus.
Methods
Wild-type, Egr-1 knockout and chimera WT mice (constructed by irradiation followed by injection with Egr-1+/+ or Egr-1−/− bone marrow) were subjected to surgical manipulation of the gastrointestinal tract to induce ileus. RT-PCR, Western blot and immunohistochemistry quantified and localized Egr-1. Lumenal transit of non-absorbable FITC-labeled dextran and in vitro organ bath techniques measured functional gastrointestinal motility. Inflammatory mediator expressions were measured by Griess reaction, ELISA and multiplex Luminex assay.
Results
Intestinal manipulation rapidly and significantly induced Egr-1 mRNA and protein within the inflamed muscularis externa. Egr-1 was co-localized early to smooth muscle and enteric neurons and later in extravasated monocytes after surgery when postoperative ileus was functionally prominent. The functional severity of postoperative ileus was significantly ameliorated in mice deficient in Egr-1−/− and chimera WT mice transplanted with Egr-1−/− bone marrow, whereas KO mice with Egr-1+/+ bone marrow again displayed significant ileus. Motility was mechanistically associated in Egr-1−/− gene deficiency with a down regulation in the release of nitric oxide, prostanoids, MCP-1, MIP-1α, IL-6, IL-1, and GM-CSF, as well as a decrease in the recruitment of leukocytes into the manipulated muscle wall of the intestine compare to wild-type mice.
Conclusion
Leukocyte-derived Egr-1 plays an early critical inflammatory role in the initiation of the postoperative inflammatory response, which leads to a prolonged decreased in gastrointestinal motility after intestinal surgery.
Keywords: gastrointestinal motility, postoperative ileus, Egr-1, nitric oxide, prostaglandin, MCP-1, IL-6, inflammation
Introduction
The early growth response gene family (Egr) of the zinc finger transcription factors includes Egr-1, Egr-2, Egr-3 and Egr-4. Egr-1 is the prototypic member of this family that is induced by a variety of stimuli in numerous cell types. Egr-1 was first identified through its characteristic expression pattern in response to mediators associated with growth and differentiation1. Egr-1 has been termed an immediate-early response gene, based on the rapid kinetics of its expression and induction. Egr-1 is similar to Fos and Jun, which increase in the absence of de novo protein synthesis by transactivation2. Studies have demonstrated that Egr-1 is a master regulator that plays a key role in a variety of acute and chronic pathological processes such as inflammation, atherosclerosis, intimal thickening following vascular injury, ischemia-reperfusion injury, angiogenesis, allograft rejection, smooth muscle hyperplasia and hypertrophy3. Egr-1 is recognized as a pleiotropic inflammatory transactivator that plays a critical role in the regulation of over 300 target genes, including TNF-α, ICAM-1, MCP-1, MIP-1, IL-1β, IL-6, TGF- β, CD44, PAI-1 and tissue factor3, 4. In turn, Egr-1 expression is dependent on Ras-Raf-MEK-ERK1/2 pathway5.
Recent studies have demonstrated that a complex cascade of inflammatory responses can be attributed as the root cause of postoperative ileus after abdominal surgery6. Confirmed results demonstrate that simple surgical handling of the intestine activates the dense network of normally resident muscularis macrophages, which subsequently orchestrates a succession of molecular inflammatory responses. These responses include the triggering of the mitogen-activated protein kinase pathways (ERK1/2, p38 and JNK)7, the activation of transcription factors (NF-κB, NF-IL6, STAT3)8, the recruitment of leukocytes into the manipulated muscularis externa via MCP-1, MIP-1 and ICAM-1 up regulation, the release of inflammatory cytokines such as TNF-α, IL-1β and IL-68 within the intestinal wall that could alter enteric ganglionic and neuromuscular transmission9, and the secretion of the kinetically active inhibitory substances, such as nitric oxide (iNOS)10 and prostaglandins (COX-2)11.
We have previously observed that the anti-inflammatory mediator Hmox-1 is induced as part of the postoperative muscularis inflammatory milieu12. Furthermore, we have demonstrated that the pretreatment of mice, rats or pigs with the Hmox-1 end product carbon monoxide can substantially prevent postoperative ileus13. Recently, Mishra et al. have demonstrated that low dose carbon monoxide can also alleviate ischemic lung injury and furthermore that this effect is mediated by a suppression in ERK-dependent Egr-1 expression5. These observations led us to specifically investigate the inflammatory role of Egr-1 in postoperative ileus. Our data demonstrate that leukocyte-derived Egr-1 plays a critical role in the postoperative inflammatory response, which leads to a prolonged decreased in gastrointestinal motility after intestinal surgery.
Methods
Animals
Adult male and female mice of 2–4 months of age weighing 25–30 grams were used. Homozygous wild type (Egr-1+/+) and homozygous mutant Egr-1 knockout (Egr-1−/−) (KO) mice were obtained by crossing heterozygous mice bearing a targeted mutation of the Egr-1 gene, as female homozygous knockout mice are infertile. The breeding pairs were kindly provided by Dr. David Pinsky of Columbia University (originally generated in the laboratory of Dr. Jeffrey Milbrandt14–17). The University of Pittsburgh Institutional Animal Care and Use Committee approved all experimental animal protocols. Mouse genotypes were determined by DNA isolated from tail clippings.
Experimental groups and operative procedures
The small intestine of each Egr-1+/+ and Egr-1−/− animal was subjected to an easily standardized, surgical manipulation as described previously12. Additionally, Egr-1 wild-type or KO mice were irradiated with 10 Gy to extinguish host bone marrow cells. The animals were then rescued by donor bone marrow injection from either wild-type animals or isogenic Egr-1−/− mice. This procedure produced chimeras of a normal phenotype (WT-BmTx Egr-1+/+), Egr-1 expressing mice which selectively lacked Egr-1 in their hematopoietic cells (WT-BmTx Egr-1−/−) or KO mice which selectively expressed Egr-1 in their hematopoietic cells (KO-BmTx Egr-1+/+). Animals were studied 12 weeks after irradiation. We have used this technique previously10. Age-matched littermates served as corresponding controls. Animals were sacrificed at various time points between 0 and 24 hours after manipulation and the isolated intestinal muscularis externa was used for mRNA extraction, protein analysis, immunohistochemistry, histochemistry, nitric oxide and prostaglandin measurement, in vitro organ bath contractile recordings and in vivo intestinal transit studies.
RNA extraction and mRNA expression
Total RNA was extracted from the isolated intestinal muscularis externae of mice at a series of specific time points after intestinal manipulation (0, 3, 6, 12 and 24 hours, N=5 each). The isolated muscularis was immediately snap frozen in liquid nitrogen and stored at −80°C. Total RNA extraction was performed as previously described 18. SYBR Green two-step, real-time RT-PCR for the mouse Egr-1 primer sequences [sense: 5'-GTCCTTTTCTGACATCGCTCTGA 3'] [anti-sense: 3'-CGAGTCGTTTGGCTGGGATA-5'] and mouse GAPDH [sense: 5'-TGA AGG TCG GTG TGA ACG GAT TTG GC-3'] [antisense: 5'-CAT GTA GGC CAT GAG GTC CAC CAC-3'] (see supplemental methods).
Western Blot
Standard Western blotting techniques were used on the isolated jejunal muscularis externa. Egr-1 (C-19) and actin (C-11) antibodies were purchased from Santa Cruz Biotechnology. Secondary antibodies (Pierce, Rockville, IL) were diluted in blocking buffer and applied for one hour. SuperSignal West Dura (for Egr-1) and SuperSignal West Pico (for Actin) chemiluminescent substrates were applied to membranes for ten minutes. Membranes were developed and scanned followed by densitometry measurement (see supplemental methods).
Histo- and immunohistochemistry stainings
Whole-mounts of the jejunal intestinal muscularis were investigated for the presence of resident and recruited myeloperoxidase-positive neutrophils (Hander-Yates reaction) and monocytes (F4/80), as well as, the cellular localization of Egr-1 protein (C-19) as described previously12 (see supplemental methods).
Measurement of nitric oxide and prostaglandins in muscularis cultured supernatant
The release of nitric oxide (NO) (Griess reaction) and prostaglandins (ELISA) was measured in culture media supernatant of the isolated muscularis externa obtained from Egr-1+/+ and Egr-1−/− animals 24 hours after intestinal manipulation and from control unoperated genetically matched animals (see supplemental methods).
Luminex Multiplex Bead Immunoassays
The release of 20 inflammatory analytes into the tissue culture supernatant, obtained as above, was quantified with a Luminex 100™ using microsphere-based multiplexing technology. The mouse Twenty-Plex immune kit was comprised of analyte specific components for the simultaneous measurement of the following mouse cytokines: FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α and TNF-α.
Functional studies
Mechanical activity of individual jejunal circular muscle strips stretched to L0 exposed to bethanechol (0.3 – 300µM) was measured as previously described10. Integrated muscle contractions for each 10 minute period were analyzed using the ADI analysis system (ADI, Colorado Springs, CO). Contractile activity was calculated as grams/mm2/sec by converting weight and length of the strip to square millimeters of tissue.
Gastrointestinal transit was measured in Egr-1+/+, Egr-1−/− and bone marrow transplanted animals 24 hours postoperatively with and without intestinal surgical manipulation by evaluating the intestinal distribution of the aboral transit of the non-absorbable tracer, fluorescein isothiocyanate-labeled dextran with an average molecular mass of 70 kDa (FD70), as previously described7, 12, 19.
Solutions and Statistics
A standard Krebs Ringers buffer (KRB) was used as described previously 18. This physiologic solution was gassed with 97% O2 – 3% CO2 to establish a pH of 7.4. Muscle chamber temperatures were constantly monitored and maintained at 37 ± 0.5°C by the perfusion of the pre-warmed KRB solution. KRB constituents and bethanechol were obtained from Sigma Chemical Company (St. Louis, MO).
Results are presented as means ± standard error (SEM). The data were analyzed using student-t test or analysis of variance (ANOVA). EZAnalyze add-in for Microsoft Excel was used for F-test and Bonferroni post-hoc group comparisons where appropriate. p values < 0.05 were considered significant.
Results
Intestinal manipulation activates and up-regulates the expression of Egr-1
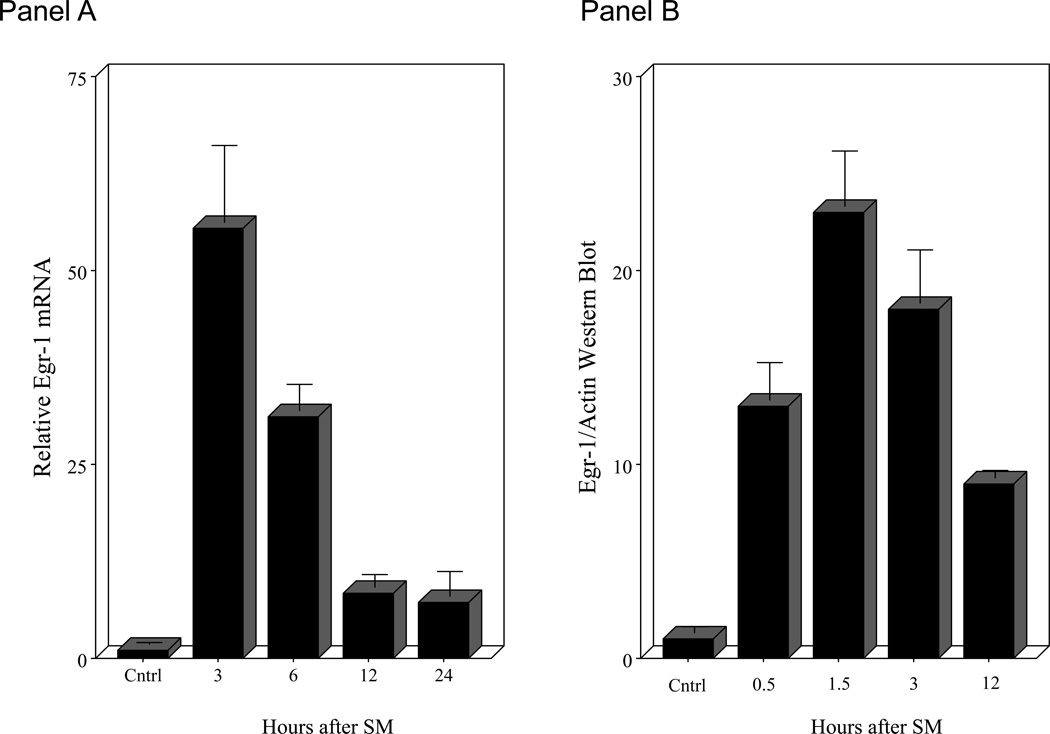
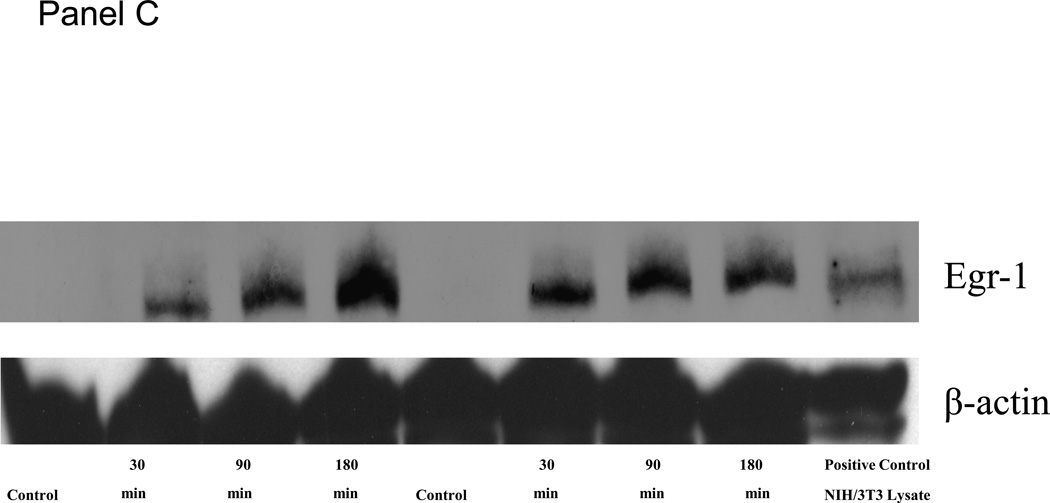
Intestinal manipulation initiates a complex molecular and cellular inflammatory response within the intestinal muscularis that leads to intestinal ileus. Here we show that manipulation induces a significant rapid 55-fold up-regulation in Egr-1 mRNA within 3 hours from extracts of the harvested jejunal muscularis using real time RT-PCR (Figure 1 - Panel A). This induction then subsided over a period of the first 12 hours but stayed significantly elevated for up to 24 hours (7-fold). We also measured Egr-1 protein using Western blot techniques to demonstrate that transcription factor protein is rapidly produced in the manipulated jejunal muscularis. Harvested muscle extracts blotted 30 minutes after manipulation already showed a significant increased presence of protein (Figure 1 - Panel C). A time profile for the production of Egr-1 protein is shown in the histogram of Figure 1 - Panel B. Temporal analysis shows that Egr-1 production is elevated for over 12 hours after surgery. Egr-1−/− mice did not express detectable Egr-1 mRNA or protein (data not shown).
Figure 1.
Panel A in the histogram shows the significant temporal induction of jejunal muscularis Egr-1 mRNA levels in wild-type animals at the time intervals of 0, 3, 6, 12 and 24 hours postoperatively. Panels B and C: Histogram of the densitometry analysis of the time-dependent increase in Egr-1 protein levels in muscularis extracts at increasing time points after surgical manipulation (control, 30, 90, 180 and 12 hours).
Effect of surgical manipulation on intestinal muscle function in Egr-1 knockout mice
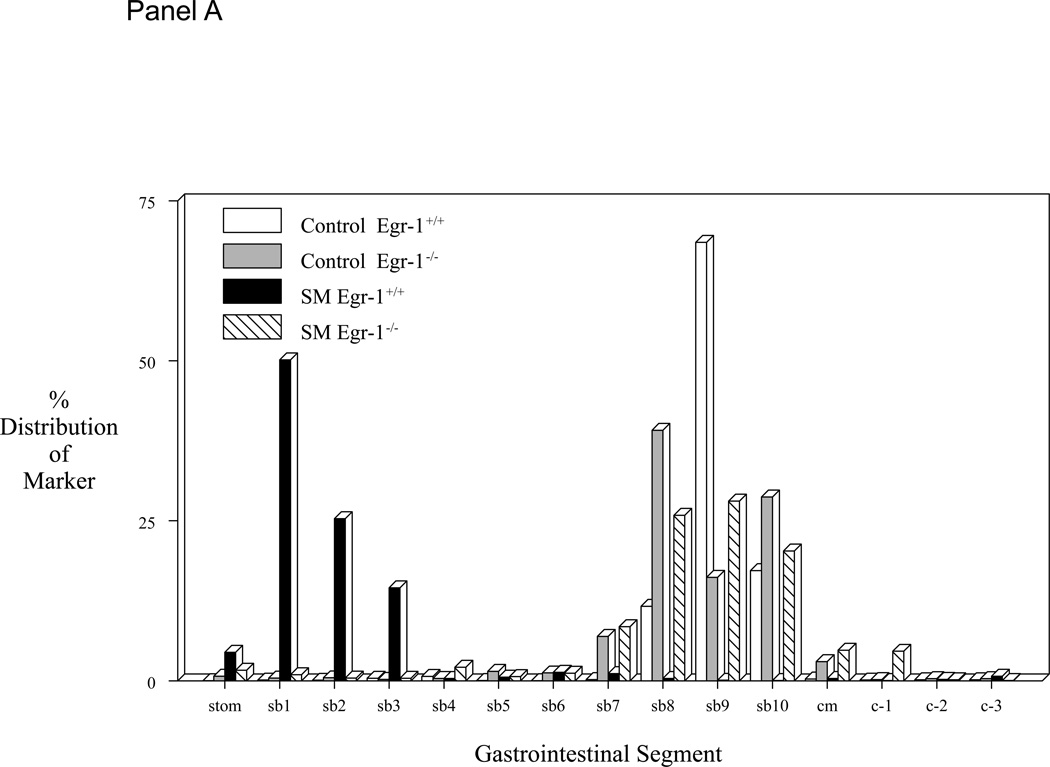
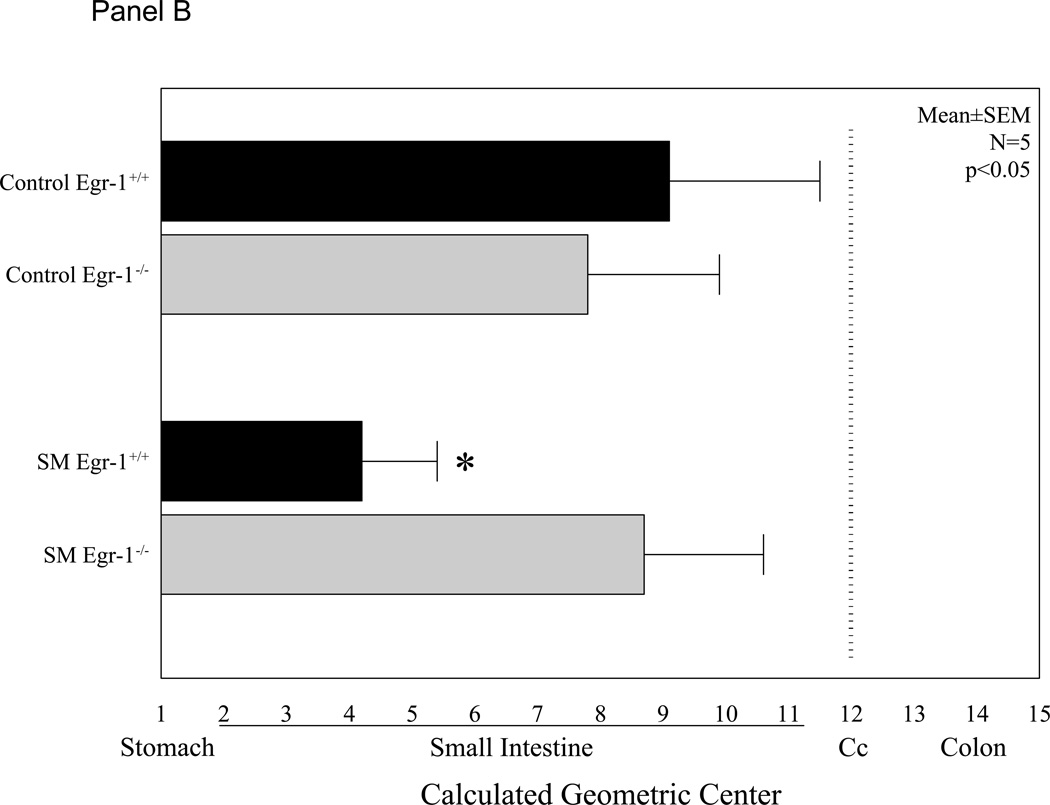
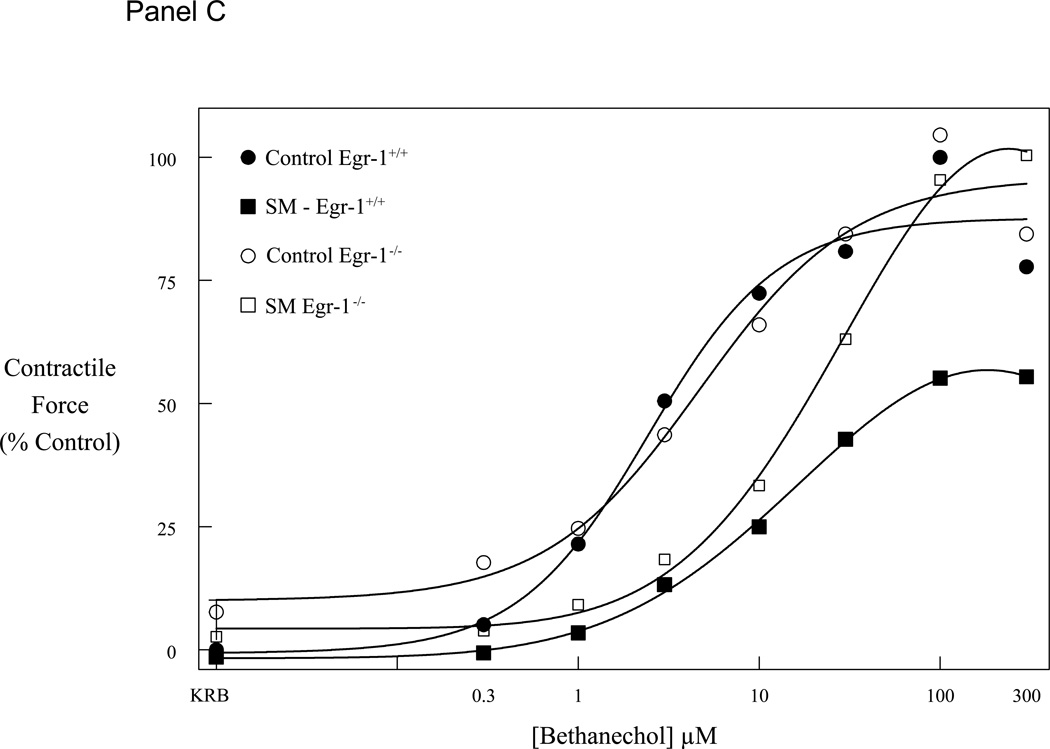
Mice selectively deficient in the Egr-1 gene were used to determine if this transcription factor plays a role in the observed decrease in gastrointestinal transit and suppression in jejunal circular muscle contractile activity following surgical manipulation of the gastrointestinal tract. Gastrointestinal transit was determined in two genetically different groups of mice without and with surgical manipulation of the intestine (control Egr-1+/+, control Egr-1−/−, SM Egr-1+/+ and SM Egr-1−/−). Twenty-four hours after surgery, animals were orally fed 10 µl of a non-absorbable FITC-labeled dextran and after a period of 90 minutes, the distribution of the florescence was determined within the lumen of the gastrointestinal tract. As shown in Figure 2 - Panel A, both wild-type and knockout control groups of mice transported the fluorescently labeled dextran down the intestine towards its distal end with the peak signal detected in the ileum. As predicted from previous observations, surgical manipulation of the intestine of wild-type mice caused a significant delay in gastrointestinal transit. The peak fluorescence in the manipulated animals only emptied from the stomach and accumulated in the duodenum. This manipulation-induced delay was significantly prevented in Egr-1 knock-out mice. These animals transported the fluorescent marker to the distal small bowel even after surgical manipulation. Gastrointestinal transit was quantified by calculating the geometric center from the transit distribution histograms (Figure 2 - Panel B). ANOVA statistical analysis of the geometric centers for each of the four groups of mice with Bonferroni post-hoc analysis demonstrated that manipulation statistically delay manipulation and that Egr-1−/− mice were protected from this delay.
Figure 2.
Panel A plots the averaged gastrointestinal transit distribution histograms using FITC-dextran measured in control Egr-1+/+ and Egr-1−/− mice (open and gray bars). Twenty-four hours after surgical manipulation (SM) a delay was observed in the gastrointestinal transit of Egr-+/+ mice (solid bars), but not in Egr-1−/− mice (cross-hatched bars) (st=stomach, sb=small bowel, cm=cecum, c=colon). Panel B: Calculated geometric centers from the transit distribution histograms for the four groups of mice. Panel C: Graph of bethanechol-stimulated dose-response curves for jejunal circular muscle strips obtained from control and manipulated, Egr-1+/+ and Egr-1−/− mice. Control dose-response curves to increasing concentrations of bethanechol were similar between Egr-1+/+ (● closed circles) and Egr-1−/− (○ open circles) mice. Twenty-four hours after surgical manipulation muscle strip contractions were diminished in Egr-1+/+ (■ closed square), but not in Egr-1−/− (□ open squares) mice. Data are expressed as mean ± SEM (N=5).
A second series of functional experiments were designed to determine if the apparent action of Egr-1 to promote ileus following surgery played a functionally active role specifically within the muscularis externa of the small intestine. For these studies, we compared the effect of surgical manipulation on in vitro jejunal circular muscle contractility harvested from homozygote wild type (Egr-1+/+) and knock-out mice (Egr-1−/−). Control jejunal muscle strips from both Egr-1+/+ and Egr-1−/− mice exhibited spontaneous regular monophasic contractions with a frequency of 39.6 ± 0.22 and 38.9 ± 0.14 events/minute, respectively (N=6 each). Both control jejunal muscles exposed to bethanechol demonstrated a similar concentration-dependent increase in contractile activity with large phasic contractions overlying a relatively small tonic contractile component. Again, as we have reported for wild type mice, intestinal manipulation caused a significant suppression in spontaneous and bethanechol stimulated contractility (Figure 2 – Panel C, an average 45% decrease at 100 µM bethanechol compared to control, p>0.05). We then compared the effect of surgical manipulation on jejunal circular muscle contractions recorded from age-matched sibling Egr-1−/− mice. Interestingly, low dose bethanechol stimulated contractions recorded from Egr-1 deficient animals tended to exhibit a surgically-induced suppression in muscle contractility, but at higher doses this inhibition in contraction was overcome and the muscles contracted with a vigor similar to the unmanipulated muscles demonstrating only an average 4% decrease in contractility at 100 µM bethanechol compared to control (Figure 2 – Panel C).
Egr-1 dependent inflammatory mediators
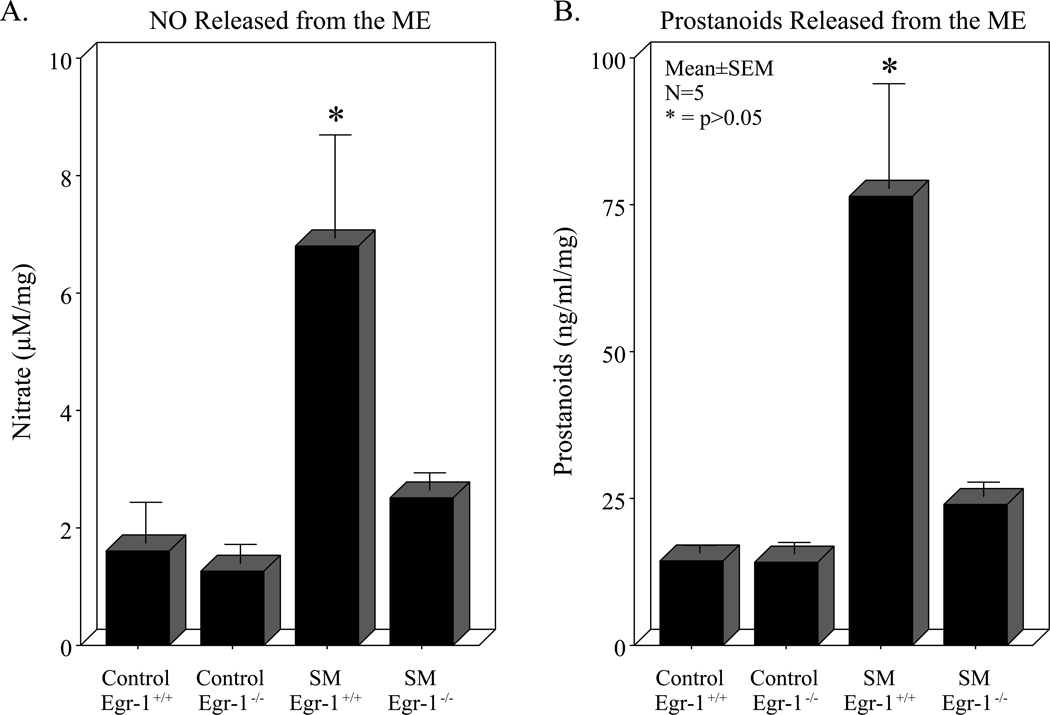
Results from our earlier work demonstrated the functional importance of endogenously released nitric oxide and prostaglandins from the inflamed postoperative bowel 11, 20. Therefore, we sought to determine if the generation of these two kinetically active smooth muscle inhibitors would also be altered in the absence of Egr-1. No difference was measured in the culture media from the isolated muscularis of control Egr-1+/+ and control Egr-1−/− animals, while surgical manipulation of wild-type animals resulted in a significant robust release of both nitric oxide and prostanoids (Panels A and B of Figure 3). In accordance with the improvement in motility observed in Egr-1−/− mice shown above, in the bowel manipulated genetic mutants levels of released nitric oxide and prostanoids were both significantly less compared to manipulated wild-type mice (p<0.05, N=5 each).
Figure 3.
Panel A: The first two bars of the histogram show no difference in basal nitric oxide levels in the culture media assayed by the Griess reaction from the 24 hour-incubated muscularis externa of control Egr-1+/+ and Egr-1−/− mice. The third bar illustrates a significant increase in nitric oxide produced in the culture media from manipulated Egr-1+/+ mice. The fourth bar demonstrates that postsurgical Egr-1−/− mice elaborated less nitric oxide compared to Egr-1+/+ mice. Panel B: The first two bars of the histogram show no basal difference in prostanoid levels in the culture media assayed by ELISA from the 24 hour-incubated muscularis externa of control Egr-1+/+ and Egr-1−/− mice. The third bar illustrates a significant increase in prostanoids secreted into the culture media from manipulated Egr-1+/+ mice. The fourth bar demonstrates that postsurgical Egr-1−/− mice elaborated less prostanoids compared to Egr-1+/+ mice (ANOVA with Bonferroni post-hoc comparison * = p-value < 0.05, N = 5 each).
Panel C. A murine Twenty-Plex Luminex assay was used to quantify the release of inflammatory proteins into the media of the 24 hour organ cultured muscularis externa (FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α and TNF-α). Quantification of the twenty analytes demonstrated no significant difference in basal release from control Egr-1+/+ and Egr-1−/− mice. The third bar for each of the selected analytes illustrates a significant increase in IL-10, FGF-β, IL-6, GM-CSF, MIP-1α, KC, IL-1β and MCP-1 secreted into the culture media from the 24 hour-incubated postsurgical inflamed muscularis externa of manipulated Egr-1+/+. The fourth bar of each selected analyte demonstrates that mice homozygous deficient in Egr-1 elaborated less GM-CSF, IL-1α, IL-6, MCP-1 and MIP-1α into the media from the 24 hour organ cultured postsurgical inflamed muscularis externa compared to Egr-1+/+ mice (ANOVA with Bonferroni post-hoc comparison * = p-value < 0.05, N = 5 each).
The regulation by Egr-1 of other downstream inflammatory mediators released from the isolated muscularis externa was also determined using the mouse Twenty-Plex Luminex assay. The muscularis was isolated from four groups of animals (Egr-1+/+ and Egr-1−/− mice with and without intestinal manipulation after 24 hours) and then incubated for a 24 hour period in DMEM in an oxygenated culture chamber (N=5 each). The tissue weight adjusted release of twenty mouse cytokines was determined in the supernatant of each incubated tissue sample (FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α and TNF-α). No significant difference in baseline release of these inflammatory mediators was measured in the twenty analytes of control Egr-1+/+ and Egr-1−/− mice, but a slight trend for a decrease in mediator release was observed for most of the proteins. Intestinal manipulation of Egr-1+/+ mice resulted in the significant increased detection of IL-10, FGF-β, IL-6, GM-CSF, MIP-1α, KC, IL-1β and MCP-1 of the 20 analytes obtained from the organ cultured wild-type muscularis. In the manipulated Egr-1−/− mice, the levels of GM-CSF, IL-1α, IL-6, MCP-1 and MIP-1α were all measured to be significantly less compared to the manipulated wild-type animals, demonstrating a general down-stream alteration in numerous cytokines in the mice lacking the Egr-1 transcription factor (Figure 3 – Panel C).
Leukocyte infiltration after surgical manipulation in Egr-1 deficient mice
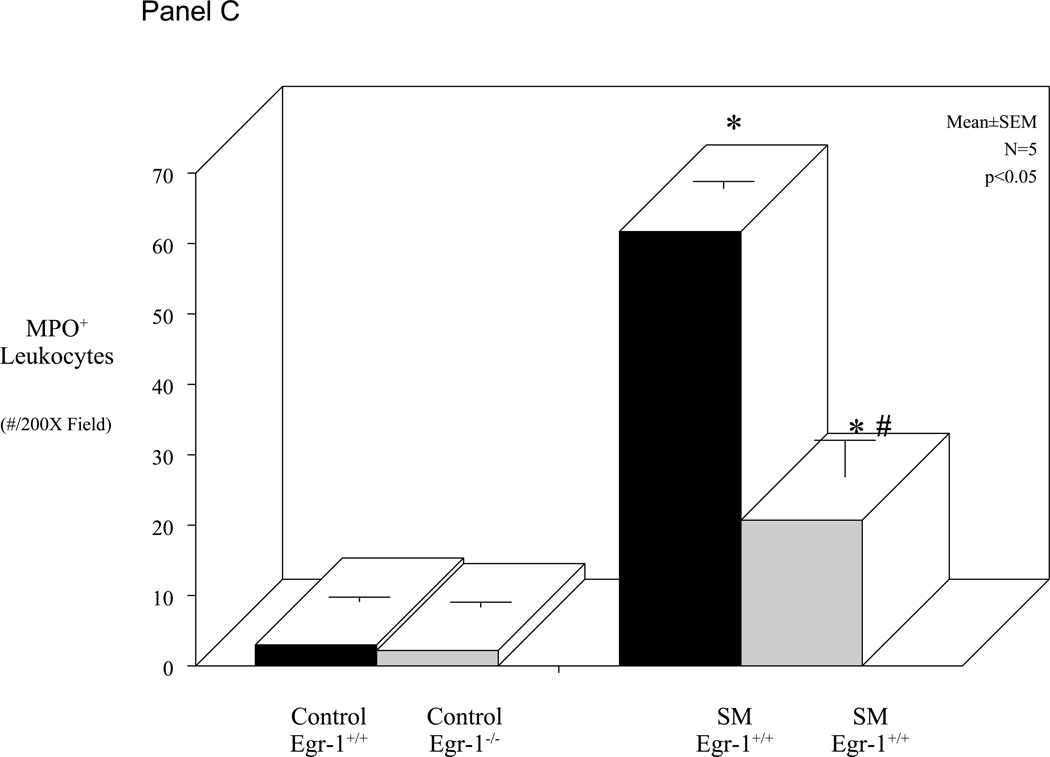
As reported above, Egr-1 deficient mice have a diminished molecular inflammatory response and improved gastrointestinal motility compared to control wild-type mice. Previously, we have shown a correlation between the post-surgical suppression in muscle function and leukocyte infiltration into the intestinal muscularis. Therefore, we sought to quantify the recruitment of neutrophils into the muscularis externa of both Egr-1+/+ and Egr-1−/− mice before and after surgical manipulation. Whole-mounts of the jejunal muscularis were prepared and stained for myeloperoxidase positive neutrophils from control and manipulated mice with normal and Egr-1 deficient genetic backgrounds. Control whole-mounts contain only a couple myeloperoxidase positive cells and surgical manipulation of the intestine of Egr-1+/+ resulted in the recruitment of a significant number of neutrophils into the intestinal muscularis (Figure 4 - Panel A), as we have also shown before in C57BL/6 genetically normal mice12. In correlation with the improved gastrointestinal motility measurements, the muscularis of Egr-1−/− mice recruited significantly fewer neutrophils compared to manipulated wild-type animals (Figure 4 - Panel B). The histogram in Figure 4 – Panel C shows the quantification of myeloperoxidase positive leukocytes in whole-mounts from the four groups of animals in our study. Statistical analysis of the mean data shows no difference between the number of neutrophils in Egr-1+/+ and Egr-1−/− control whole-mounts, a significant increase after surgical manipulation and a significant reduction in the myeloperoxidase positive cells in the manipulated Egr-1−/− whole-mounts compared to the manipulated wild-type preparations (ANOVA with Bonferroni post-hoc comparison, p<0.05).
Figure 4.
Panel A: Hanker-Yates myeloperoxidase histochemical reaction in full thickness whole-mount of the muscularis externa displaying the recruitment of myeloperoxidase laden neutrophils into the jejunal smooth muscle syncytium of a surgically manipulated Egr-1+/+ mouse. Panel B shows a relative decrease in the number of myeloperoxidase laden neutrophils extravasated into the muscularis of the Egr-1−/− mouse (original magnification 250X, 24 hours after surgery). Panel C: Histogram of myeloperoxidase positive neutrophils counted in muscularis whole-mounts showing in the third bar a significant recruitment of neutrophils into whole-mounts from the 24 hour postsurgical inflamed muscularis externa of manipulated Egr-1+/+. The fourth bar demonstrates that Egr-1−/− mice contained significantly less neutrophils than manipulated wild-types (ANOVA with Bonferroni post-hoc comparison * = p-value < 0.05, N = 5 each).
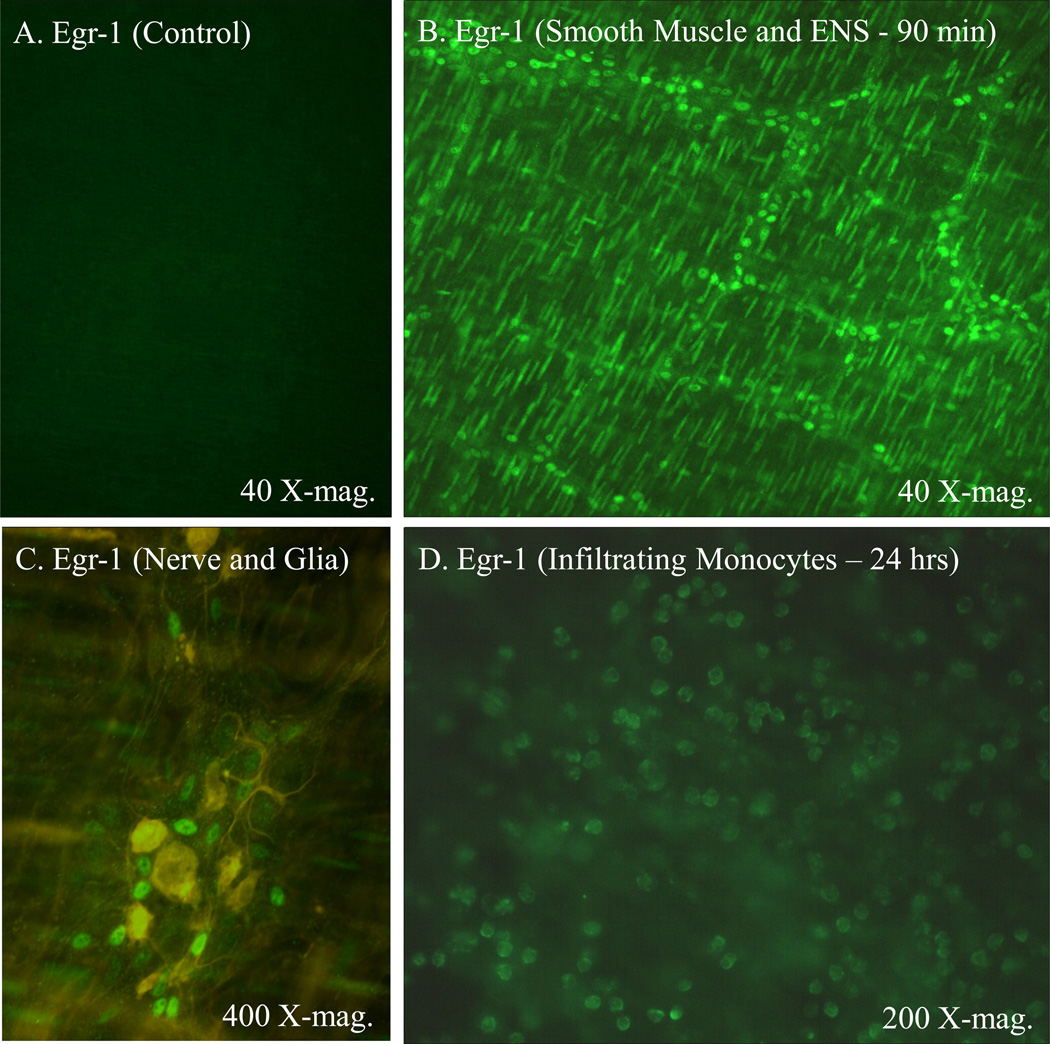
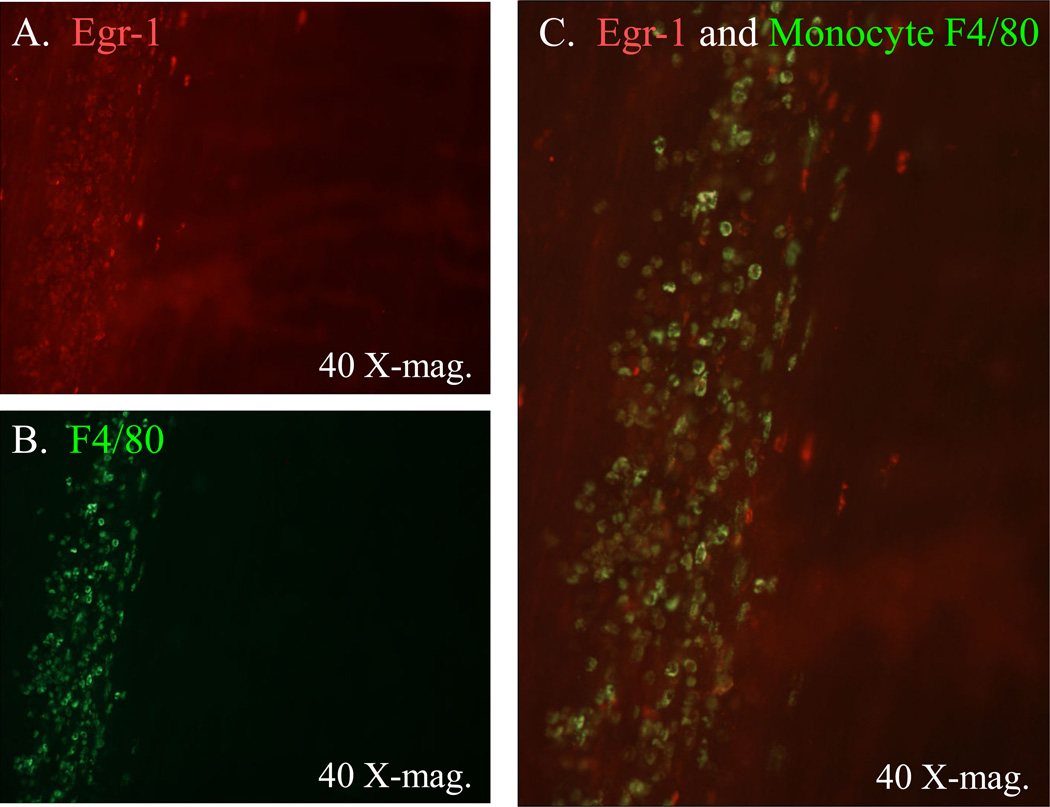
Egr-1 has been shown to be nearly ubiquitously expressed in response to numerous mediators and to be pluripotent 21. Hence, we next sought to localize the Egr-1 protein expression to specific cellular targets following surgery. Jejunal muscularis whole-mounts stained for Egr-1 immunoreactivity showed no staining in control tissues (Figure 5, Panel A). In marked contrast, 90 minutes after surgery intense immunoreativity was observed in the nuclei of smooth muscle and a population of enteric ganglionic cells (Figure 5, Panel B). The identity of the ganglionic cell type was determined by double staining ganglionic whole-mounts for GFAP immunoreactive glia and Egr-1 early after surgical manipulation. As shown in Figure 5 – Panel C, the Egr-1 positive cells were not also GFAP positive demonstrating that enteric neurons were the Egr-1 positive ganglionic cells. Visually, this early expression of Egr-1 in the smooth muscle and enteric neurons waned substantially 24 hours after surgery. However, what became evident at later time points greater than 6 hours was the expression of Egr-1 in a new round shaped cell within the muscularis externa (Figure 5, Panel D). The cellular identity of the late round shaped Egr-1 expressing cells was identified as extravasating monocytes by double staining the whole-mounts for F4/80 positive monocytes and Egr-1 immunoreactivity 24 hours after surgery (Figure 6 – Panels A, B and C). In the manipulated Egr-1−/− mice, Egr-1 immunoreactivity was not observed in any cell type at early or late time points.
Figure 5.
Immunohistochemistry in control whole-mounts showed no detectable Egr-1 protein (Panel A). Ninety minutes after surgical manipulation Egr-1 was abundantly expressed in smooth muscle nuclei and in nuclei of cells localized to the enteric ganglia (Panel B). Double immunohistochemical stainings of the enteric ganglia for Egr-1 and GFAP localized Egr-1 to a population of enteric neurons 90 minutes after surgical manipulation of the intestine (Panel C). No GFAP positive cells displayed Egr-1 immunoreativity. Twenty-four hours after surgery Egr-1 like immunoreactivity waned in the smooth muscle and enteric neurons, but was visible in a infiltrating cell type (Panel D).
Figure 6.
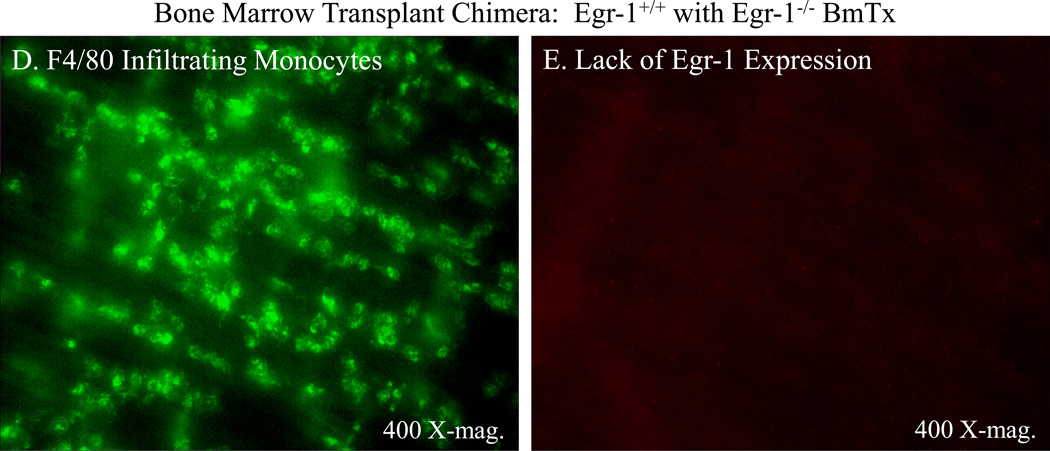
Double immunohistochemical stainings for Egr-1 and extravasated monocytes in a typical jejunal muscularis whole-mount obtained from a surgically manipulated animal at 24 hours. Panel A shows the localization of Egr-1 (red) to a band of invading cells which were not present in control tissues. In the same jejunal whole-mount (Panel B), the same area of tissue was shown to be clustered with F4/80 positive tissue monocytes/macrophages (green). Panel C shows an overlay of these two images demonstrating co-localization of Egr-1 to many, but not all, of the invading monocytes. Panels D and E show the loss of Egr-1 immunoreactivity in infiltrating monocytes. Double immunohistochemical staining for F4/80 positive infiltrating monocytes (Panel D) and Egr-1 immunoreactivity (Panel E) in a jejunal muscularis whole-mount obtained from a surgically manipulated animal at 24 hours after surgery that was transplanted with Egr-1−/− bone marrow after total body irradiation with 10 Gy. Panel D shows the postsurgical recruitment of F4/80 positive monocytes into the muscularis externa in the wild-type mice rescued by Egr-1−/− bone marrow transplantion. The same microscopic field shown in Panel E displays no immunoreactive Egr-1 in the infiltrating monocytes.
Thus, true to form, Egr-1 is expressed in numerous cell types within the postsurgical muscularis externa. The above results would suggest that Egr-1 is playing an important proinflammatory role in postoperative ileus. Therefore, we tested the hypothesis that Egr-1 from bone marrow derived leukocytes was the specific cellular source of Egr-1 that was important in the development of postoperative ileus. Egr-1 wild-type or KO mice were irradiated with 10 Gy to extinguish host bone marrow cells. The animals were then rescued from death by the injection of donor bone marrow from either wild-type animals or isogenic Egr-1−/− mice. This procedure produced chimeras of a normal phenotype (WT-BmTx Egr-1+/+), Egr-1 expressing mice which selectively lacked Egr-1 in their hematopoietic cells (WT-BmTx Egr-1−/−) and KO mice which selectively expressed Egr-1 in their hematopoietic cells (KO-BmTx Egr-1+/+). As shown in Figure 6 – Panels D and E, infiltrating F4/80 positive monocytes 24 hours after surgery in WT-BmTx Egr-1−/− did not display Egr-1 immunoreactivity, where as normal phenotype chimeras, WT-BmTx Egr-1+/+ mice, did express Egr-1 confirming the model.
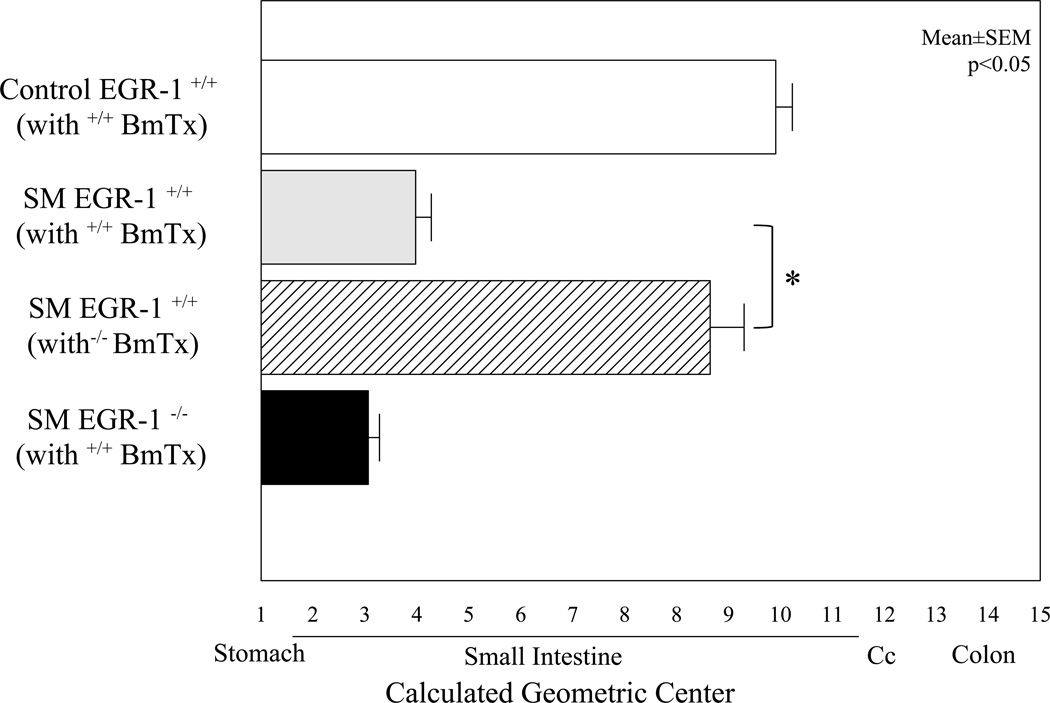
The degree of ileus caused by surgical manipulation of the intestine was studied in the chimera mice to illustrate the specific role of leukocyte derived Egr-1 in the development of postoperative ileus. Previously, we have shown that irradiation and bone marrow transplantation does not alter gastrointestinal motility within this time frame 20. Surgical manipulation of normal WT-BmTx Egr-1+/+ mice displayed the typical delay in gastrointestinal transit, which was similar to untransplanted manipulated wild-type mice reported above. The average geometric center calculated from the individual postsurgical gastrointestinal transit distribution histograms obtained from WT-BmTx Egr-1+/+ mice is shown in Figure 7. In contrast, surgical manipulation of WT-BmTx Egr-1−/− mice had a comparatively improved gastrointestinal transit, as statistically indicated by an improvement in the averaged geometric centers (Figure 7). Furthermore, surgical manipulation of KO-BmTx Egr-1+/+ mice again displayed a significant delay in gastrointestinal transit and averaged geometric centers, which was similar to the non-chimera wild-type mice. These functional changes in gastrointestinal transit measured from the three chimera groups of mice correlated with the observed recruitment of myeloperoxidase positive neutrophils into the postsurgical muscularis externa after 24 hours (WT-BmTx Egr-1+/+ = 115±23.5, KO-BmTx Egr-1+/+ = 90±3.3 and WT-BmTx Egr-1−/− = 56.8±11.2).
Figure 7.
The histogram shows the calculated geometric centers from bone marrow transplanted chimera mice. The first bar demonstrates that the bone marrow transplant procedure itself does not alter gastrointestinal transit, as the fluorescent marker progressed to the distal regions of the small bowel over a period of 90 minutes, similar to naïve controls. The second grey bar shows that surgical manipulation of WT mice transplanted with Egr-1+/+ bone marrow exhibit significant ileus. However, in contrast, manipulated WT mice transplanted with Egr-1−/− bone marrow display significant protection from the development of postsurgical ileus (hatched bar). The fourth solid colored bar representing the averaged geometric center of manipulated KO mice transplanted with Egr-1+/+ bone marrow illustrates that Egr-1 present only in bone marrow derived cells also develop a significant degree of postoperative ileus. (N=4,4,4 and 3, ANOVA with Bonferroni posthoc analysis, *=p<0.05).
Discussion
Previously, much attention has concentrated on discovering the specific mediators that are involved in causing postoperative ileus6. However, in this study we focused on elucidating transcriptional events that transduce these inflammatory mediators. The major finding of the current study is that the transcription factor Egr-1 present in leukocytes plays a major role in the proinflammatory molecular response within the surgically activated postoperative intestinal muscularis externa. The above data show that intestinal manipulation induces Egr-1 mRNA and protein within the inflamed muscularis externa and that Egr-1 can be co-localized early to smooth muscle cells and enteric neurons and later to extravasated monocytes 24 hours after surgery when postoperative ileus is functionally prominent. We also conclude that the functional severity of postoperative ileus is significantly ameliorated in the absence of Egr-1. This conclusion was reached by using homozygous Egr-1−/− mice in which postoperative ileus is prevented, apparently as a consequence of the down-regulation of numerous inflammatory mediators which also resulted in a decrease in the recruitment of leukocytes into the manipulated muscle wall of the intestine. The specific role of leukocyte derived Egr-1 in the causation of postoperative ileus was demonstrated using chimeras of a normal phenotype (WT-BmTx Egr-1+/+) and an Egr-1 expressing mouse which selectively lacked Egr-1 in their hematopoietic cells (WT-BmTx Egr-1−/−).
Although Egr-1 was initially associated with growth and differentiation1, 22, recently its importance as an early inflammatory transcription factor has been revealed5. Here we show for the first time that within the postsurgical intestine muscularis, Egr-1 is rapidly transactivated and induced within minutes after surgical manipulation. This rapid transactivation and up-regulation in Egr-1 has led to the classification of this transcription factor as an immediate early gene responder, which like Fos and Jun are expressed without de novo protein synthesis.
In the current study, however, we observed that Egr-1 levels remained elevated for a considerable period of time. Furthermore, in a lung hypoxic injury model Egr-1 up regulation was observed to be biphasic with a second induction coming 24 hours after injury2. These authors hypothesize that this second surge could be involved in extracellular matrix remodeling in the lung. The prolonged presence of Egr-1 concurs with the potential for sustained Egr-1 levels to be important in chronic inflammatory disorders. Egr-1 protein was localized early by immunohistochemistry to the smooth muscle cells and a population of enteric neurons, whereas, around 24 hours Egr-1 protein expression waned in immunoreactivity in the resident cells and appears more distinctly in recruited monocytes.
Basal constitutively expressed Egr-1 did not appear to regulate the contractions of the normal gastrointestinal musculature as homozygous Egr-1−/− animals had similar gastrointestinal transit distribution histograms and geometric centers as wild-type animals. Additionally, control in vitro jejunal muscle strip contractions in response to bethanechol were also similar in the two homozygous groups of animals. In contrast to the apparent lack of a constitutive role for Egr-1 on gastrointestinal motility, a significant difference was observed following intestinal manipulation in the disparate homozygous animals. As we and others have shown previously23, simple sterile manipulation of the intestinal wall results in a significant and prolonged postoperative delay in gastrointestinal transit and a direct in vitro decrease in jejunal circular smooth muscle contractility 24 hours after surgery. However, as seen above, surgically manipulated Egr-1 gene deficient animals, unlike their wild-type counterparts, failed to develop significant postoperative ileus. And, at least part of this Egr-1 dependent improvement was directly mediate through a local interaction within the muscularis externa, as in vitro jejunal circular smooth muscle contractile function was also significantly improved at higher bethanechol concentrations compared to wild-type animals.
We have previously seen that nitric oxide and prostanoids from their respective inducible synthases, iNOS and PGHS-2, play a significant mediator role in causing postoperative muscle dysfunction11, 20. Here we show that the release of nitric oxide and prostanoids from the inflamed muscularis externa after surgical manipulation is significantly dependent on Egr-1 expression. Although, an apparent directional link between Egr-1 and iNOS induction has not be previously made5, a negative reciprocally relationship has been most frequently reported. Nitric oxide has not only been shown to down-regulate Egr-1 induction through an inhibition in ERK1/2 phosphorylation24, but also to reduce Egr-1 protein expression and transcriptional activity5, 25. Given these observations it would seem most likely that the decrease in Griess reaction determinations for NO from the Egr-1−/− mice in this study is secondarily due to a general suppression in inflammatory mediators, which are known to induce iNOS.
A directional link between Egr-1 and the inducible PGHS-2 pathway with subsequent prostanoid production has been firmly established, as the membrane associated prostaglandin E synthase in highly Egr-1 dependent26. Our ELISA assay for prostanoids showed a marked induction after surgery, which was significantly diminished in the Egr-1 deficient animals. Our previous experiments using selective iNOS and PGHS-2 inhibitors have demonstrated that the end-products of these synthases can directly inhibit gastrointestinal motility11. But, we also hypothesize that these molecules could also play early signaling roles in the inflammatory processes within the manipulated muscularis externa. Although, not studied in this work, a region on the 5-lipoxygenase promoter is known to contain five tandem Egr-1 consensus binding sites27. And hence, possible lipoxygenase end-products may also be down regulated in the postoperative manipulated Egr-1 deficient mice.
Egr-1 appears to be a strategic regulator of an abundant number of inflammatory cytokines/chemokines in the setting of numerous pathological processes, including pulmonary inflammation/fibrosis, pancreatitis, hemorrhagic shock, atherosclerosis, stroke, kidney endotoxemia and liver disease28, 29. In deed, Egr-1 transcriptional activity has been shown to play a critical role in over 300 target genes, including TNF-α, ICAM-1, MCP-1, MIP-1, IL-1β, IL-6, TGF- β, CD44, PAI-1 and tissue factor 3, 4. In this study, we assessed the role of Egr-1 in regulating a spectrum of twenty inflammatory mediators contained on a mouse Luminex 20-plex immune assay kit, as many of these have been shown to be regulated by Egr-1 in other tissues. The release of five of these mediator proteins (GM-CSF, IL-1α, IL-6, MCP-1 and MIP-1α) were significantly down regulated from the inflamed muscularis externa of the surgically manipulated Egr-1 knock-out mice compared to the manipulated wild-types, while none were observed to be up regulated.
In the literature a reciprocal connection has been established between macrophage/monocytes Egr-1 expression and granulocyte colony-stimulating factor (GM-CSF), where GM-CSF has been shown to activate a number of cellular genes, including Egr-130, 31. Consequently, as would be expected, Egr-1 has been implicated in the differentiation of macrophage and megakaryocyte hematopoietic cell lineages32. However, Egr-1 was determined not to be an absolute requirement for macrophage differentiation, as Egr-1−/− mice had comparable monocytes in their peripheral blood and macrophages in their tissues33. The fact that the substantial surgical induction of granulocyte colony-stimulating factor (GM-CSF) within the muscularis externa tissue was decreased by approximately 50% in the Egr-1−/− mice, we believe at this time should be considered to be an indirect effect of the modification of another inflammatory pathway. Recently, concerning another hematopoietic colony stimulating factor, Prince et al. reported a significant decrease in hemorrhagic shock induced liver G-CSF mRNA in Egr-1−/− mice29.
The multiplex protein assay indicated a general down regulation in the postsurgical generation of numerous inflammatory mediators. As part of this quelled response, the lower levels of interleukin secretion (IL-1α and IL-6) could have resulted in improved enteric neural function. Additionally, we speculate that the diminished secretion of GM-CSF, MCP-1 and MIP-1α could be mechanistically behind the observed reduction in the number of neutrophils which were recruited into the manipulated muscularis externa of Egr-1−/− mice. However, Egr-1 has previously been reported to not play a role in leukocyte recruitment into the lung and kidney in response to endotoxin4. But our results showed a significant reduction in neutrophil accumulation into the jejunal muscularis externa in Egr-1 deficient mice, which could be reasoned to be a direct consequence of the diminished induction of the measured chemokines in the Egr-1−/− animals.
As illustrated above, Egr-1 is expressed in smooth muscle cells, enteric neurons and cells of the monocyte cell lineage. Therefore, additional experiments were performed to determine the specific role of leukoyctye-derived Egr-1 by using bone marrow transplant chimeras of a normal phenotype (WT-BmTx Egr-1+/+), a reconstituted Egr-1 expressing mouse which selectively lacked Egr-1 in their hematopoietic cells (WT-BmTx Egr-1−/−) and a KO-BmTx Egr-1+/+. The data from WT-BmTx Egr-1−/− mice clearly showed that the improved gastrointestinal transit reflecting a decreased inflammatory response was due to the selective loss of Egr-1 in the bone marrow derived cells. Additionally, the reverse experiment using KO-BmTx Egr-1+/+ revealed that the Egr-1 deficient mice had greater ileus when reconstituted with Egr-1 expressing normal leukocytes. These selective chimeric changes in motility also correlated to leukocyte recruitment into the muscularis which would be a direct result of Egr-1 modulation of chemokines reported above.
We read with curiosity the recent report that low dose carbon monoxide suppresses ERK-dependent Egr-1 expression in a ischemic lung injury model5. Since, our previous results showed carbon monoxide pretreatment can substantially prevent the development of postoperative ileus13, and because of the marked improvement in postoperative bowel function in the Egr-1 knockout mice and bone marrow Egr-1 deficient mice, it is interesting to speculate that the Egr-1 transcription factor could be a promising check point for the therapeutic targeting of postoperative ileus and other inflammatory bowel disorders.
Supplementary Material
Acknowledgement
Supported by National Institutes of Health grants R01-GM58241, R01-DK068610 and P50-GM53789.
Terms/Abbreviations
- Egr-1
early growth response gene-1
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase
- iNOS
inducible nitric oxide synthase
- SM
surgical manipulation
- KRB
Krebs Ringer buffer
- MPO
myeloperoxidase
- NO
nitric oxide
- PMN
polymorphonuclear neutrophils
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
"No conflicts of interest exist"
Reference List
- 1.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. Journal of Cellular Physiology. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 2.Yan SF, Lu J, Xu L, Zou YS, Tongers J, Kisiel W, Mackman N, Pinsky DJ, Stern DM. Pulmonary expression of early growth response-1: biphasic time course and effect of oxygen concentration. Journal of Applied Physiology. 2000;88:2303–2309. doi: 10.1152/jappl.2000.88.6.2303. [DOI] [PubMed] [Google Scholar]
- 3.Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circulation Research. 2006;98:186–191. doi: 10.1161/01.RES.0000200177.53882.c3. [DOI] [PubMed] [Google Scholar]
- 4.Pawlinski R, Pedersen B, Kehrle B, Aird WC, Frank RD, Guha M, Mackman N. Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood. 2003;101:3940–3947. doi: 10.1182/blood-2002-07-2303. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterology & Motility. 2004;16 Suppl-60 doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakao A, Schmidt J, Harada T, Tsung A, Stoffels B, Cruz RJ, Jr, Kohmoto J, Peng X, Tomiyama K, Murase N, Bauer AJ, Fink MP. A single intraperitoneal dose of carbon monoxide-saturated ringer's lactate solution ameliorates postoperative ileus in mice. Journal of Pharmacology & Experimental Therapeutics. 2006;319:1265–1275. doi: 10.1124/jpet.106.108654. [DOI] [PubMed] [Google Scholar]
- 8.Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR, Bauer AJ, Kalff JC. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436–446. doi: 10.1016/j.surg.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Galeazzi F, Haapala EM, Van Rooijen N, Vallance BA, Collins SM. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am J Physiol Gastrointest Liver Physiol. 2000;278:G259–G265. doi: 10.1152/ajpgi.2000.278.2.G259. [DOI] [PubMed] [Google Scholar]
- 10.Turler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL, Bauer AJ. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Annals of Surgery. 2006;244:220–229. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz NT, Kalff JC, Türler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 12.Moore BA, Otterbein LE, Türler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 13.Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ. Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Critical Care Medicine. 2005;33:1317–1326. doi: 10.1097/01.ccm.0000166349.76514.40. [DOI] [PubMed] [Google Scholar]
- 14.Kilduff TS, Vugrinic C, Lee SL, Milbrandt JD, Mikkelsen JD, O'Hara BF, Heller HC. Characterization of the circadian system of NGFI-A and NGFI-A/NGFI-B deficient mice. Journal of Biological Rhythms. 1998;13:347–357. doi: 10.1177/074873098129000174. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- 16.Lee SL, Wang Y, Milbrandt J. Unimpaired macrophage differentiation and activation in mice lacking the zinc finger transplantation factor NGFI-A (EGR1) Molecular & Cellular Biology. 1996;16:4566–4572. doi: 10.1128/mcb.16.8.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem. 1995;270:9971–9977. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- 18.Eskandari MK, Kalff JC, Billiar TR, Lee KKW, Bauer AJ. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. American Journal of Physiology - Gastrointestinal & Liver Physiology. 1999;277:G478–G486. doi: 10.1152/ajpgi.1999.277.2.G478. [DOI] [PubMed] [Google Scholar]
- 19.Moore BA, Turler A, Pezzone MA, Dyer KF, Grandis JR, Bauer AJ. Tyrphostin AG 126 inhibits development of postoperative ileus induced by surgical manipulation of murine colon. Am J Physiol Gastrointest Liver Physiol. 2004;286:G214–G224. doi: 10.1152/ajpgi.00312.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- 21.Yan SF, Fujita T, Lu J, Okada K, Shan ZY, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress.[erratum appears in Nat Med 2001 Apr;7(4):509] Nature Medicine. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 22.Sukhatme VP. Early transcriptional events in cell growth: the Egr family. Journal of the American Society of Nephrology. 1990;1:859–866. doi: 10.1681/ASN.V16859. [DOI] [PubMed] [Google Scholar]
- 23.The FO, de Jonge WJ, Bennink RJ, van den Wijngaard RM, Boeckxstaens GE. The ICAM-1 antisense oligonucleotide ISIS-3082 prevents the development of postoperative ileus in mice. British Journal of Pharmacology. 2005;146:252–258. doi: 10.1038/sj.bjp.0706303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu JJ, Wung BS, Hsieh HJ, Lo LW, Wang DL. Nitric oxide regulates shear stress-induced early growth response-1. Expression via the extracellular signal-regulated kinase pathway in endothelial cells. Circulation Research. 1999;85:238–246. doi: 10.1161/01.res.85.3.238. [DOI] [PubMed] [Google Scholar]
- 25.Keil A, Blom IE, Goldschmeding R, Rupprecht HD. Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney International. 2002;62:401–411. doi: 10.1046/j.1523-1755.2002.00462.x. [DOI] [PubMed] [Google Scholar]
- 26.Subbaramaiah K, Yoshimatsu K, Scherl E, Das KM, Glazier KD, Golijanin D, Soslow RA, Tanabe T, Naraba H, Dannenberg AJ. Microsomal Prostaglandin E Synthase-1 Is Overexpressed in Inflammatory Bowel Disease: Evidence for Involvement of the Transcription Factor Egr-1. J Biol Chem. 2004;279:12647–12658. doi: 10.1074/jbc.M312972200. [DOI] [PubMed] [Google Scholar]
- 27.Silverman ES, Du J, De Sanctis GT, Radmark O, Samuelsson B, Drazen JM, Collins T. Egr-1 and Sp1 interact functionally with the 5-lipoxygenase promoter and its naturally occurring mutants. American Journal of Respiratory Cell & Molecular Biology. 1998;19:316–323. doi: 10.1165/ajrcmb.19.2.3154. [DOI] [PubMed] [Google Scholar]
- 28.Ji B, Chen XQ, Misek DE, Kuick R, Hanash S, Ernst S, Najarian R, Logsdon CD. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiological Genomics. 2003;14:59–72. doi: 10.1152/physiolgenomics.00174.2002. [DOI] [PubMed] [Google Scholar]
- 29.Prince JM, Ming MJ, Levy RM, Liu S, Pinsky DJ, Vodovotz Y, Billiar TR. Early growth response 1 mediates the systemic and hepatic inflammatory response initiated by hemorrhagic shock. Shock. 2007;27:157–164. doi: 10.1097/01.shk.0000245025.01365.8e. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Kubota H, Sakamoto KM, Arai K. Characterization of cis-acting sequences and trans-acting signals regulating early growth response 1 and c-fos promoters through the granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. Blood. 1997;89:1197–1206. [PubMed] [Google Scholar]
- 31.Li C, Mitchell DH, Coleman DL. Analysis of Egr-1 protein induction in murine peritoneal macrophages treated with granulocyte-macrophage colony-stimulating factor. Yale Journal of Biology & Medicine. 1994;67:269–276. [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnaraju K, Nguyen HQ, Liebermann DA, Hoffman B. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Molecular & Cellular Biology. 1995;15:5499–5507. doi: 10.1128/mcb.15.10.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SL, Wang Y, Milbrandt J. Unimpaired macrophage differentiation and activation in mice lacking the zinc finger transplantation factor NGFI-A (EGR1) Molecular & Cellular Biology. 1996;16:4566–4572. doi: 10.1128/mcb.16.8.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.