Abstract
Mild traumatic brain injuries (TBI) are common in athletes, military personnel, and the elderly, and increasing evidence indicates that these injuries have long-term health effects. However, the difficulty in detecting these mild injuries in vivo is a significant impediment to understanding the underlying pathology and treating mild TBI. In the following experiments, we present the results of diffusion tensor imaging (DTI) and histological analysis of a model of mild repetitive closed-skull brain injury in mouse. Histological markers used included silver staining and amyloid precursor protein (APP) immunohistochemistry to detect axonal injury, and Iba-1 immunohistochemistry to assess microglial activation. At 24 hours post-injury, before silver staining or microglial abnormalities were apparent by histology, no significant changes in any of the DTI parameters were observed within white matter. At 7 days post-injury we observed a reduction in axial and mean diffusivity. Relative anisotropy at 7 days correlated strongly with the degree of silver staining. Interestingly, APP was not observed at any timepoint examined. In addition to the white matter alterations, mean diffusivity was elevated in ipsilateral cortex at 24 hours but returned to sham levels by 7 days. Altogether, this demonstrates that DTI is a sensitive method for detecting axonal injury despite a lack of conventional APP pathology. Further, this reflects a need to better understand the histological basis for DTI signal changes in mild TBI.
Keywords: diffusion tensor imaging, traumatic brain injury, axonal injury
INTRODUCTION
Traumatic axonal injury (TAI) is a hallmark of traumatic brain injury (TBI), and may be a leading cause of cognitive impairment and disability following head injury [14]. Few methods exist for detecting TAI, with a positive diagnosis usually taking place post-mortem following histological analysis for accumulated amyloid precursor protein (APP), an integral membrane protein that is normally transported along the length of the axon [1, 3, 12].
Increasingly, diffusion tensor imaging (DTI) has been used as a non-invasive method for detecting TAI in vivo [9]. DTI measures the directional diffusion of water. In white matter, this diffusion is restricted by the orientation of axon bundles. In regions such as the corpus callosum, water preferentially diffuses along a single axis and has high anisotropy [6]. When white matter is injured, as in traumatic brain injury, the characteristic diffusion of water is altered.
We have previously validated DTI signal changes after TBI with histology for APP-positive injured axons. In these studies, which used a mouse model of moderate-severe TBI, areas with reduced relative anisotropy and axial diffusivity were shown to contain greater numbers of APP-positive swellings [7, 8]. However, from these studies and others, it is not clear whether DTI is sensitive to mild injuries without contusion where the mechanisms of axonal injury may be different.
Recently, our group developed a model of mild repetitive closed-skull TBI (rcTBI) in mouse to study the pathological changes underlying concussion. In this model, young male mice are subject to two concussive impacts within a 24-hour period. Interestingly, only sparse APP-positive axons were visible following injury, though axonal degeneration was evident by both silver staining and electron microscopy and coincided with a prominent microglial response and clear deficits in spatial learning and memory [13].
As DTI is a quantitative method for assessing the integrity of white matter regions, we sought to apply this technique to this mild repetitive closed-skull TBI model to determine if APP-negative axonal injury results in detectable DTI changes 24 hours and 7 days after impact. Results from these experiments have important implications for the use and interpretation of DTI following mild TBI.
MATERIALS AND METHODS
Animals
Six- to eight-week C57BL/6j mice were purchased from Jackson Laboratories and housed in standard cages under a 12-hour light/dark cycle with approval from the Animal Studies Committee at Washington University in Saint Louis. Mice were divided into 3 groups: 7 day sham injury with sacrifice 7 days later, rcTBI with sacrifice 24 hours later, and rcTBI with sacrifice 7 days later Each group initially consisted of six mice. The final n for the rcTBI 24 hour group was 4; a scan of one mouse in this group failed for technical reasons and a second was excluded due to a small hemorrhage within white matter. Repetitive closed-skull injury was performed as described previously [13]. Briefly, mice were anesthetized with inhaled isofluorane and placed in a stereotaxic frame. A midline incision was made to expose the skull and the 9 mm rubber impactor tip was aligned with Bregma. The tip was then moved to the location of the impact (A/P: −1.8 mm, M/L: −3.0 mm) and an electromagnetic impactor (My NeuroLab) was used to drive an impact to a depth of 3.3 mm. Body temperature was closely monitored and maintained throughout the procedure. Following the impact, the incision was sutured closed, antibiotic ointment was applied, and mice were allowed to recover on a heat pad before returning to their cage. Sham-operated mice received the same treatment but an impact was not delivered. This procedure was repeated at 24 hours for a total of two impacts per mouse. Mice in the 24 hour scan group were immediately taken for DTI after the second impact.
Diffusion Tensor Magnetic Resonance Imaging
All scans were performed using a previously published method [8]. Mice were anesthetized with isofluorane maintained at 1% throughout the duration of the scan while circulating warm water preserved a constant body temperature. Images were acquired with a 4.7T scanner (Oxford Instruments 330) and actively shielded gradient coil (180 mT/m, 400 ms rise time) interfaced with a Varian Unity-INOVA console controlled by Sun Microsystems Ultra-60 Sparc workstation. The following acquisition parameters were used: TR= 3s, TE= 40ms, and FOV/Resolution 20 × 20 mm / 192 × 192. Voxel size during acquisition was 104 μm X 104 μm X 0.5 mm (zero padded to 256 × 256). A multislice spin-echo sequence was modified to include a Stejskal-Tanner gradient-sensitizing pair in order to acquire coronal diffusion weighted images. Diffusion gradients were applied in six directions. B-values used were 0 and 764. Each scan required approximately 3 hours. Software written in Matlab (Mathworks, Natick, MA) was used to calculate the six elements of the diffusion tensor and to determine relative anisotropy (RA; overall measure of the asymmetry of diffusion), axial diffusivity (AD, λ1; diffusion along a primary direction), radial diffusivity (RD, λ⊥ diffusion perpendicular to the primary direction), and mean diffusivity (MD).
Diffusion Tensor Imaging Analysis
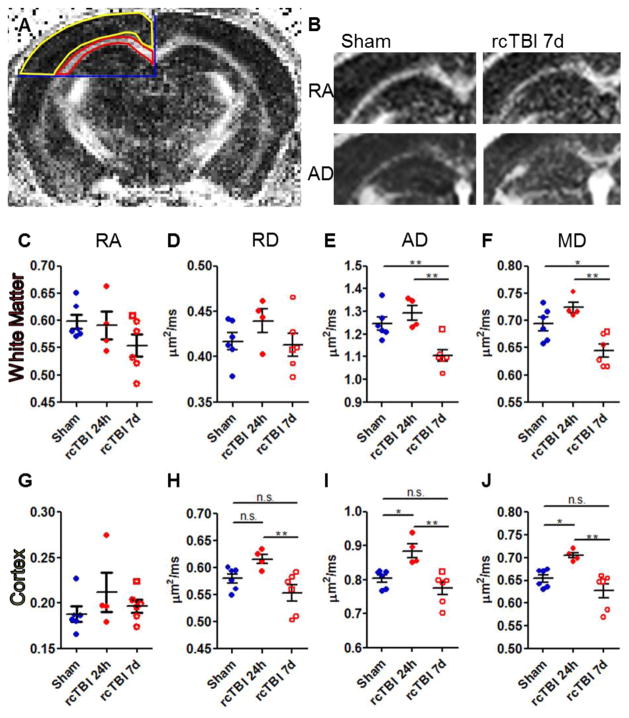
Imaging analysis was performed using Image J. The sync windows plugin was used to trace the regions of interest (ROI) on RA, AD, RD, and MD images simultaneously. The ROIs were carefully defined by anatomical boundaries with the anterior-most boundary being the first section containing hippocampus and the posterior boundary being the last section where the corpus callosum crosses at midline. This resulted in a total of 3 slices per mouse. Corpus callosum and external capsule ipsilateral to midline were included in the white matter ROI with the ventral boundary being drawn at the interface between the hippocampus and thalamus (Fig. 1A red outline). Dorsal cortex ipsilateral to midline was selected as a second ROI using the same boundaries (Fig 1A, yellow outline) Signal intensity within each ROI was measured across slices and weighted by the number of voxels in each sketched region to obtain RA, AD, RD, and MD measures.
Figure 1.
Diffusion tensor imaging in rcTBI mice. (A) The regions of interest (red, yellow) were defined by anatomical boundaries (blue) and were used for quantitative analysis of diffusion tensor images. (B) Representative images of ipsilateral relative anisotropy (RA) and axial diffusivity (AD) in both a sham and an rcTBI mouse at 7 days. White indicates higher signal intensity and greater RA or AD. (C, G) Relative anisotropy (D, H) Axial diffusivity. (E, I) Radial diffusivity. (F, J) Mean diffusivity. (C–F) White matter. (G–J) Cortex (*p<0.05, **p<0.01, ***p<0.001, one-way ANOVA with Bonferroni post-test, error bars represent ±SEM. Square symbol in 7 day group indicates a mouse with histologically mild injury, a possible outlier.)
Histology
Immediately following each scan, mice were sacrificed by isofluorane overdose, perfused with 0.3% heparin phosphate-buffered saline (PBS), and brains were removed and placed in 4% paraformaldehyde (PFA) for 24 hours. After fixation, brains were equilibrated in 30% sucrose PBS and cut into 50 μm-thick sections using a freezing microtome. Sections were then either rinsed in PBS and stored in 4% PFA for 5 days for silver staining or underwent processing for Iba-1 immunohistochemistry. Evaluation was conducted on sections taken at 400 μm intervals along the anterior-posterior axis of the brain. For silver staining, a Neurosilver Kit (FD Neurotech) was used according to the manufacturer’s instructions with the exception of a single two-minute incubation with solution C as previously described [13]. For APP and Iba-1 staining, endogenous peroxidases were quenched with 0.03% H2O2, blocked in 3% normal goat serum in 0.25% Triton-X tris-buffered saline (TBS-X), and incubated overnight in 1:1000 rabbit anti-Iba-1 (Wako) or 1:1000 rabbit anti-APP (Invitrogen) in 3% normal goat serum (NGS) TBS-X. Antibody binding was visualized using 1:1000 goat anti-rabbit (Vector Laboratories) in TBS-X, followed by 1:400 AB complex (Vector Laboratories), and DAB substrate enhanced with nickel chloride (Sigma). All sections were mounted onto slides and digitally scanned using an Olympus Nanozoomer Whole-Slide Imaging System to generate photomicrographs.
Iba-1 stereology and silver stain quantification in corpus callosum was performed as published in Shitaka et al [13]. In short, ROI parameters were the same as used for DTI analysis. Image J was used to measure silver staining by densitometry. The optical fractionator probe in StereoInvestigator (Microbrightfield) was employed to count the number of Iba-1-positive cells. A grid size of 180 × 180 μm and counting frame of 80 × 80 μm ensured that the Gunderson’s coefficient of error was <0.1 for all counts.
Statistical Methods
Prism 5.0 (GraphPad) was used for statistical analysis. All DTI data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. For histology, a one-tailed t test was performed as it was expected that silver staining and Iba-1 positive cells would increase following injury as previously published [13]. Linear regression and Pearson’s product moment correlation was performed to determine the relationship between DTI and histological measures. In all cases, only p-values less than 0.05 were considered significant.
RESULTS
DTI of white matter
Separate cohorts of mice were scanned using DTI 7 days after sham treatment or 24 hours or 7 days after the first closed-skull traumatic brain injury (Fig. 1A–F). Analysis of corpus callosum and external capsule 24 hours after injury revealed no significant changes in DTI measurements compared to shams (Fig.1C–F). At 7 days, relative anisotropy and radial diffusivity remained unchanged (Fig.1C. D), but a statistically significant decrease in axial diffusivity was apparent compared to shams (p<0.01) and injured mice at 24 hours (p<0.01; Fig. 1E). Decreased mean diffusivity was also evident compared to sham (p<0.05) and injured mice at 24 hours (p<0.01; Fig. 1F).
DTI of cortex
Considering injury results in significant histological abnormalities in cortex [13] in addition to the white matter, analysis of DTI signal within cortex ipsilateral to injury was also performed. As expected since cortex is isotropic at the voxel resolution of these scans, little RA was observed within cortex in any injury group (Fig. 1G). No change between sham and injured mice at 7 days was apparent in AD, RD, or MD (Fig. 1H–J). However, AD, RD, and MD were significantly increased in injured mice at 24 hours compared to both shams (p<0.05) and injured mice at 7 days (p<0.01; Fig. 1H–J).
Given the changes in white matter AD and cortical MD, it was possible to separate the three injury groups based on these parameters (Fig. 2). One potential outlier in the 7 day injury group overlapped with sham mice and has been marked with a unique symbol in all figures as this mouse appeared to have minimal injury when analyzed by both DTI and histology.
Figure 2.
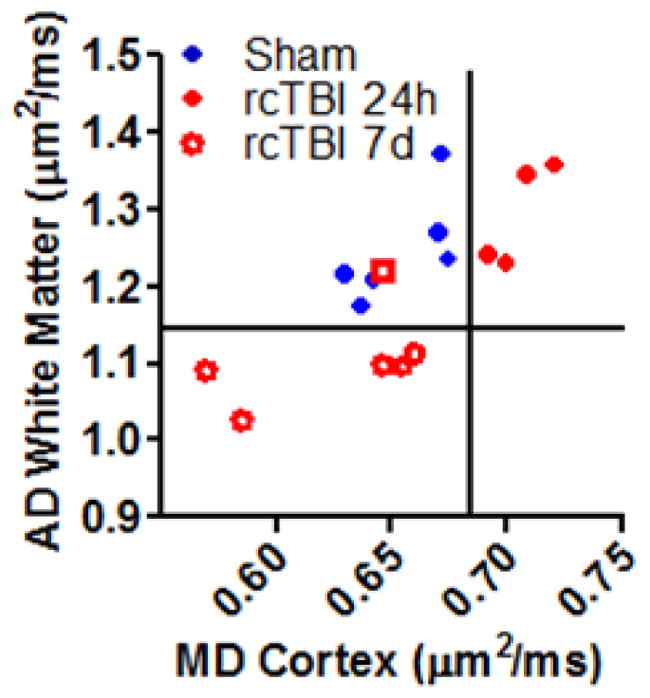
Mean diffusivity in cortex versus axial diffusivity in white matter separates mice into distinct injury groups. (Open Square symbol in 7 day group indicates a mouse with histologically mild injury, a possible outlier.)
Histolological findings
Immediately following DTI scans, mice in the sham and injured cohorts were sacrificed and tissue was processed for APP, Iba-1, and silver staining. As previously reported [13], only occasional APP-positive swellings were detectable in injured mice and were virtually indistinguishable from shams with this marker (Fig. 3A–D). Quantification of Iba-1 and silver staining within corpus callosum and external capsule revealed levels similar to those observed in Shitaka et al [13] in both sham and 7 day injured mice (Fig. 4A–D). This indicates that the additional 3 hours of anesthesia during the MRI scan does not appear to substantially affect these histological markers. Mice sacrificed at the 24 hour timepoint did not have Iba-1 or silver stain abnormalities, as previously reported [13], and were not included in the stereological analysis.
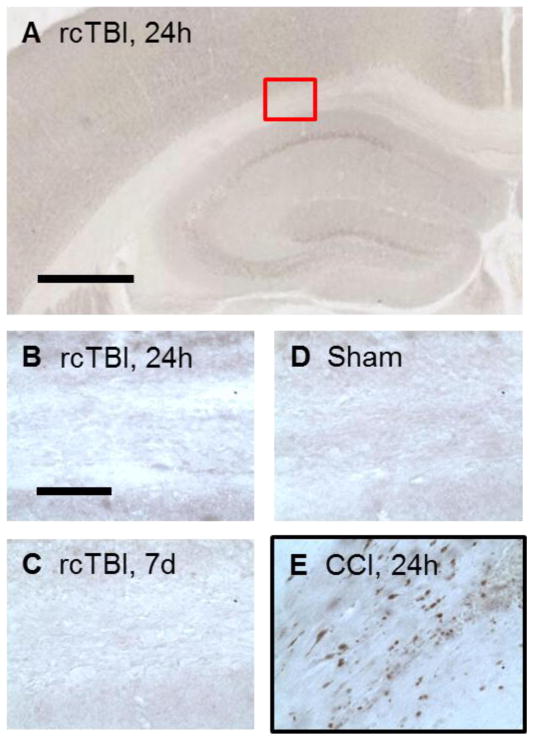
Figure 3.
APP immunohistochemistry. (A, B) Injury did not result in APP positive axons in the corpus callosum of mice analyzed at 24 hours or (C) 7 days following impacts and were similar to (D) sham mice. (E) A mouse subject to moderate controlled cortical impact (CCI) brain injury and sacrificed at 24 hours served as a positive control for APP staining (red box indicates location of B–E, scale bar in A=500 μm, scale bar in B=50 μm).
Figure 4.
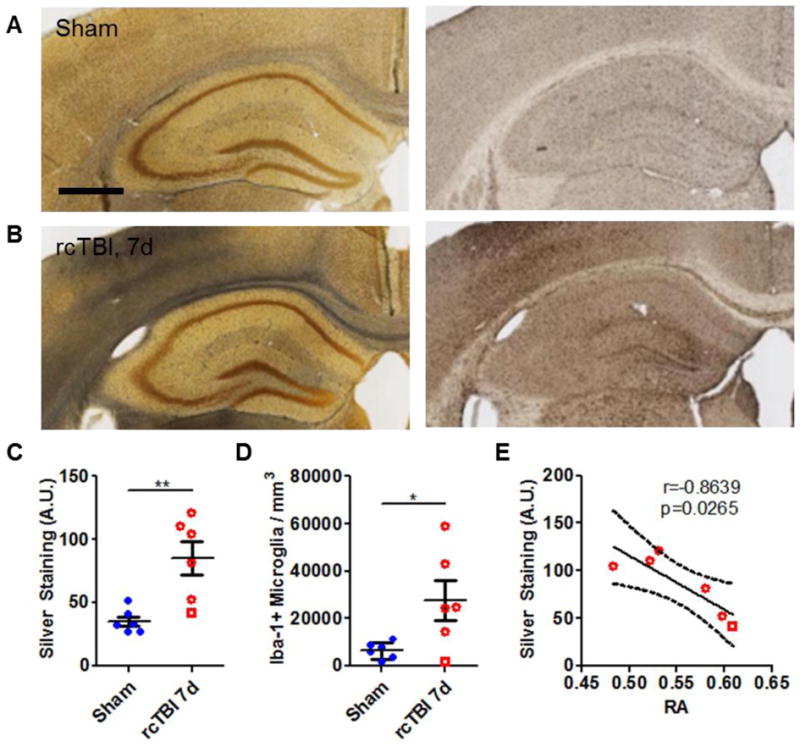
Axonal injury and microglial activation in 7 day rcTBI. (A) Sham operated mice had little silver stain (black precipitate, left panel) and few Iba-1-positive cells (right panel) in the corpus callosum (scale bar=500 μm). (B) Silver staining (left panel) and increased numbers of Iba-1-positive cells (right panel) were evident in the corpus callosum of mice 7 days post-injury. Quantification of silver by densitometry (C, arbitrary units A.U.) and of Iba-1 by stereology (D) confirmed observed changes (*p<0.05, **p<0.01, Student’s one-tailed t test). (E) Silver staining strongly correlated with changes in relative anisotropy at 7 days post-injury (Pearson’s correlation, two-tailed, solid line represents linear regression, dotted lines represent 95% confidence interval. Square symbol in 7 day group indicates a mouse with a histologically mild injury, a possible outlier).
Interestingly, the density of silver staining in the corpus callosum and external capsule correlated strongly with RA (r=−0.8639, p=0.0265; Fig.4E). Silver staining did not correlate with AD (r=−0.6293, p=0.1806), RD (r= 0.5920, p=0.2157), or MD (r=−0.0402, p=0.9397). Iba-1 immunoreactive microglial cell counts were also not significantly correlated with any of the DTI measures: RA (r=−0.7506, p=0.0856), AD (r=−0.3865, p=0.4491), RD (r=0.6238, p=0.1856), MD (r=0.1574, p=0.7658).
DISCUSSION
Here we present findings that mild repetitive closed-skull injuries in mice result in detectable changes by DTI. Importantly, these DTI abnormalities are present despite of a lack of APP-positive histology, which has been considered a “gold standard” for identifying traumatic axonal injury. Previous studies from our group have shown that the numbers of APP-positive axonal varicosities correlate strongly with RA in a model of moderate-severe traumatic brain injury [8]. In this work, we show RA correlates just as strongly with the degree of silver staining in the corpus callosum of mildly injured mice.
Both axial diffusivity and mean diffusivity have been widely used to assess axonal degeneration in the central nervous system [10]. In our model, both of these parameters were reduced within white matter in the injured 7 day group compared to shams. Notably, neither silver stain, a marker of protein aggregates, nor Iba-1-positive microglial cells correlated well with AD and MD changes. While both protein aggregation and microglial pathology may have contributed to some of the reduced diffusivity, weak correlations between these measures indicate other histological markers are needed that better reflect AD and MD changes. In addition, because Iba-1 did not correlate with any DTI parameter, it is important to note that this method may not be suitable to resolving microgliosis. Other imaging modalities may therefore be required to detect this prominent microglial response in vivo [11, 16].
Given the coincident decrease in axial diffusivity and mean diffusivity, it is interesting that no significant changes in relative anisotropy were seen. This is different from TBI with contusion, where AD is reduced, overall diffusivity is unchanged or increased, and subsequently, RA is reduced [8]. In this model, it is possible that a larger sample size may resolve the non-significant reduction in RA. Alternately this could indicate that in mild injuries, analysis of single components of the diffusion tensor may be more sensitive than RA.
Increased radial diffusivity is another common feature of DTI in TBI and in other models with prominent demyelination or edema [5, 7, 15]. We observed no significant change in this parameter in our model, which is in line with previous observations by electron microscopy that revealed myelin degradation is not characteristic of this mild injury [13].
Unlike white matter, imaging of cortex revealed an acute, transient increase in AD, RD, and MD, likely reflecting edema. While these changes were significant at 24 hours, they had resolved by 7 days post-injury. This is potentially useful for determining time since injury. Here, increased mean diffusivity in cortex and unaltered AD in white matter characterized mice imaged 24 hours following initial injury, whereas normalized MD in cortex and decreased AD in white matter characterized mice imaged 7 days post-injury.
These results highlight the evolving nature of traumatic brain injuries, as the DTI changes within white matter were not immediately apparent at 24 hours but were distinct 7 days following injury. Similarly, earlier characterization of this model has shown that Iba-1 and silver stain abnormalities also develop 3–7 days after injury [13]. Thus, it is likely that DTI measurements are reflective of a progressive degenerative process taking place within the corpus callosum and external capsule of these injured mice. Clinically, this may also mean that DTI days or weeks after mild head injury may be more informative for detecting and diagnosing axonal injury. However, given our observed alterations in AD and MD were approximately 10%, it is unclear whether these changes would be significant within a human population where normal white matter diffusion may vary greatly between subjects. This may be one explanation why DTI in human mild TBI patients has resulted in conflicting reports of the observed changes in DTI signal [2, 4, 17].
There are several limitations of this work. First, sample sizes are small and to fully validate this model, larger groups will be required. However, given the small sample and the relatively mild injuries studied, it is encouraging that these DTI changes are evident and correlate with histological abnormalities. Second, this research is limited by the histological markers chosen for analysis. To fully characterize this model, markers for neurofilament, myelin, and activated astrocytes might further inform the nature of these DTI alterations. Quantitative electron microscopy may be the ultimate “gold standard” but is beyond the scope of the current work, Third, mice that received a single injury were not assessed here, and so we did not determine whether single injury alone results in DTI signal change. We have previously demonstrated that mice subject to single injuries do not have significantly more silver staining or microglial activation compared to shams at 7 days post-injury thus it is unlikely that a single impact would be detectable by DTI [13]. Fourth, the interval between injuries was kept fixed at 24 hours; further studies will be required to determine the effect of different intervals between injuries. As a final note, the imaging protocol used was not optimized for measuring MD changes.
Despite these limitations, this study shows that DTI is capable of detecting populations of degenerating axons in the absence of APP pathology. Future studies will be required to determine the distinct DTI signatures of axonal degeneration following repeated mild injuries in order to differentiate injury phenotypes that may or may not include disrupted APP transport. Full validation of detection methods and interpretation of DTI signal changes will be important to providing meaningful evaluation and targeted therapeutics for patients with multiple concussive injuries.
Highlights.
Repetitive closed-skull TBI results in reduced axial and mean diffusivity
DTI alterations were detectable at 7 days but not 24 hours post-injury
Amyloid precursor protein accumulation is absent despite diffusion tensor alterations
Acknowledgments
This work was supported by a NIH R01 NS065069 (Brody), a Burroughs Wellcome Career Award in the Biomedical Sciences (Brody), and an NIH Neuroscience Blueprint Core Grant to Washington University (P30 NS057105).
We thank William Spees, Joel Garbow, and the Biomedical Magnetic Resonance Laboratory at Washington University for advice and assistance.
Abbreviations
- TBI
Traumatic brain injury
- TAI
traumatic axonal injury
- rcTBI
repetitive closed-skull TBI
- APP
amyloid precursor protein
- DTI
diffusion tensor imaging
- RA
relative anisotropy
- RD
radial diffusivity
- AD
axial diffusivity
- MD
mean diffusivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 2.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geddes JF, Whitwell HL, Graham DI. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol. 2000;26:105–116. doi: 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- 4.Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, Theoret H, Ellemberg D, Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- 5.Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bihan D. The 'wet mind': water and functional neuroimaging. Phys Med Biol. 2007;52:R57–90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 7.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- 10.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 11.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 12.Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- 13.Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, Brody DL. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sung P, Tu TW, Trinkhaus K, Klein RS, Cross AH, Song SK. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3587–3598. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]