Abstract
We evaluated the prevalence of colonization with all gram-negative bacilli (GNB) and with ciprofloxacin-resistant GNB among nursing home residents with and without indwelling devices. We found that device presence increases the risk of colonization with all GNB and with ciprofloxacin-resistant GNB. Colonization with ciprofloxacin-resistant GNB increases with decreasing functional status.
Multidrug-resistant organisms are endemic in nursing homes in the United States.1 Infection prevention initiatives in these facilities have focused predominantly on methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci. However, there is emerging concern that multidrug-resistant gram-negative bacilli (GNB) may be more prevalent and result in greater health care costs than MRSA.2,3
The main objective of the present study was to evaluate the epidemiology of GNB colonization at various anatomic sites, as well as ciprofloxacin-resistant GNB colonization patterns, in a discrete, well-defined, high-risk group of nursing home residents. We have shown previously that nursing home residents with indwelling devices are more likely to be colonized with both methicillin-sensitive S. aureus and MRSA at multiple body sites.4 Our goal was to define the frequency of GNB colonization in this group, as well as ascertain the risk of carriage at various anatomic sites. We also investigated the relationship between ciprofloxacin-resistant GNB and functional status in this population.
METHODS
We conducted a cross-sectional surveillance study involving 14 nursing homes in southeastern Michigan from March 2003 through November 2004.4 Our study cohort has been described elsewhere.4 Briefly, after obtaining written informed consent, nursing home residents with an indwelling device (urinary catheter, percutaneous enteral gastrostomy feeding tube, or peripherally inserted central catheter) and randomly selected control subjects were enrolled. One hundred twenty-five residents with indwelling devices and a similar number of control subjects qualified for our study. Thirty-seven residents refused to provide consent. Therefore, 105 residents formed the device group, and 108 formed the control group. The Charlson Comorbidity Index was used to assess comorbidity, and the Lawton and Brody Physical Self-Maintenance Scale (PSMS) was used to assess functional status.5,6 Residents were categorized from fully independent to fully dependent as follows: category 1, PSMS score 6–11; category 2, score 12–17; category 3, score 18–23; and category 4, score 24–30.
To assess colonization with GNB, culture samples were obtained using Culturette rayon-tipped swabs (Becton Dickinson) from the nares, oropharynx, groin, perianal area, feeding tube, and wound. Swab samples were plated on Mac-Conkey agar, and phenotypically different colonies were identified to the species level by means of API-20E test strips (Analytab Products). Isolates were screened for ciprofloxacin resistance by disc diffusion, using 5 µg/mL disks. Isolates from 11 residents in the device group and 13 in the control group were inadvertently lost and could not be tested for ciprofloxacin resistance.
Colonization density was defined as the number of unique GNB organisms per resident. Categorical and continuous variables were compared by the χ2 test and the Student t test, using Stata software, version 9.0 (StataCorp). Multivariate logistic regression models were adjusted for age, comorbidity, and functional status. The χ2 test for trend was used to assess the relationship between colonization with ciprofloxacin-resistant GNB and functional status.
RESULTS
Indwelling device use and colonization with GNB
A total of 733 GNB isolates were cultured and identified from 213 nursing home residents. In the device group, 101 (96%) of 105 residents were colonized with GNB at any site, compared with 89 (82%) of 108 in the control group (odds ratio [OR], 3.0; P = .02). The device group had a significantly higher average colonization density than did the control group (4.3 vs 3.3 GNB isolates per resident; P = .03) (Table 1). When comparing GNB colonization rates by anatomic site, the device group was also more likely than the control group to be colonized in the oropharynx (OR, 2.6; P = .004), groin (OR, 2.6; P = .001), and perianal area (OR, 2.5; P = .01). In the multivariate model, the odds of oropharyngeal colonization with GNB in the device group remained significantly higher than that the control group.
TABLE 1.
Epidemiology of Gram-Negative Bacilli (GNB) Isolated from Nursing Home Residents in Each Study Cohort
| Category, parameter | Device group (n = 105) |
Control group (n = 108) |
OR (95% CI) |
P for OR |
aOR (95% CI) |
P for aOR |
|---|---|---|---|---|---|---|
| All GNB | ||||||
| Total no. of GNB isolates recovered | 440 | 293 | ||||
| Proportion (%) of patients colonized at any site | 101/105 (96) | 89/108 (82) | 3.0 (1.2–7.5) | .02 | 2.1 (0.6–5.8) | .2 |
| GNB colonization densitya | 4.3 | 3.3 | .03 | |||
| Proportion (%) of GNB that were cipro resistant | 124/331 (38) | 56/221 (25) | ||||
| Proportion (%) of patients with cipro-resistant GNBb | 51/94 (54) | 35/95 (37) | 2.0 (1.1–3.6) | .01 | 1.0 (0.5–2.1) | .97 |
| Cipro-resistant GNB colonization densitya | 2.5 | 1.6 | <.001 | |||
| No. of patients colonized, by anatomic site | ||||||
| Oropharynx | 33 | 16 | 2.6 (1.3–5.2) | .004 | 3.1 (1.3–7.3) | .009 |
| Groin | 81 | 62 | 2.6 (1.5–4.5) | .001 | 1.3 (0.6–2.8) | .5 |
| Perianal area | 92 | 80 | 2.5 (1.2–5.2) | .01 | 1.9 (0.7–4.9) | .2 |
| Nares | 10 | 13 | 0.8 (0.3–1.8) | .4 | 0.8 (0.3–2.4) | .7 |
| Wounds | 10 | 2 | 2.5 (0.2–27.2) | .5 | 0.5 (0.2–10.2) | .6 |
| Proportion (%) of cipro-resistant GNB, by organism | ||||||
| Escherichia coli | 59/119 (50) | 25/95 (26) | ||||
| Proteus species | 21/66 (32) | 21/62 (34) | ||||
| Pseudomonas aeruginosa | 17/50 (34) | 5/13 (39) | ||||
| Klebsiella species | 6/59 (10) | 1/30 (3.3) | ||||
| Morganella morganii | 13/18 (72) | 1/10 (10) | ||||
| Acinetobacter baumanii | 8/9 (89) | 2/3 (67) | ||||
| Citrobacter species | 0/10 (0) | 1/8 (13) |
NOTE. aOR, adjusted odds ratio; CI, confidence interval; cipro, ciproflozacin; OR, odds ratio.
No. of unique organisms per resident.
Isolates from 94 residents in the device group and 95 residents in the control group were subsequently screened for ciprofloxacin resistance.
Indwelling device use and colonization with ciprofloxacin-resistant GNB
Ninety-four residents in the device group and 95 in the control group were further screened for ciprofloxacin-resistant GNB. Fifty-one (54%) of 94 residents in the device group were colonized with ciprofloxacin-resistant GNB, compared with 35 (37%) of 95 in the control group (OR, 2.0; P = .01). Ciprofloxacin-resistant GNB colonization density was significantly higher in the device group than in the control group (2.5 vs 1.6 ciprofloxacin-resistant GNB isolates per resident; P < .001) (Table 1). Among residents with urinary catheters, 29 (63%) of 46 in the device group were colonized, compared with 35 (37%) of 95 in the control group (OR, 3.4 [95% confidence interval, 1.5–7.6]; P = .002). Among residents with feeding tubes, 25 (50%) of 50 in the device group were colonized, compared with 35 (37%) of 95 in the control group (OR, 1.8 [95% confidence interval, 0.9–3.8]; P = .12). There was no significant difference in antimicrobial use between residents in the device group who were colonized with ciprofloxacin-resistant GNB (33 [65%] of 51) and residents in the device group who were not (28 [65%] of 43).
The prevalence rates for ciprofloxacin-resistant GNB were determined among the most common species isolated (Table 1). Escherichia coli and Proteus species were the most common organisms isolated in both the device and control groups. In the device group, ciprofloxacin-resistant GNB was highest among Acinetobacter baumanii (89% of isolates), Morganella morganii (72%), and E. coli (50%).
Ciprofloxacin-resistant GNB and functional status
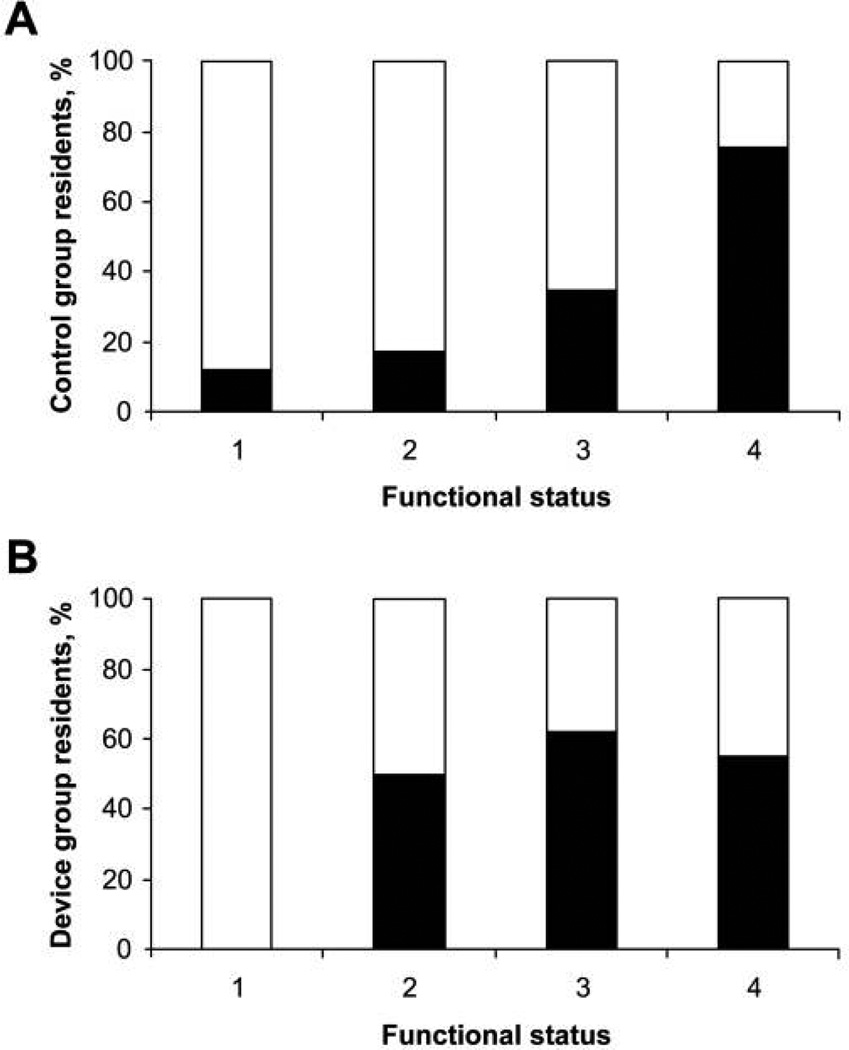
To understand the role played by functional status in ciprofloxacin-resistant GNB carriage among these patients, we evaluated the association between ciprofloxacin-resistant GNB carriage in the control group residents (n = 87) and the level of functional dependency. Residents with poor functional status were more likely to be colonized with ciprofloxacin-resistant GNB, and the prevalence increased with worsening status (P < .001, χ2 test for trend). Of functionally independent control group residents (represented by category 1), 13% (1 of 8) were colonized. This increased in a stepwise fashion (18% [6 of 34] and 35% [7 of 20] for categories 2 and 3, respectively), with ultimately 76% (19 of 25) of functionally dependent residents (category 4) being colonized. Similarly, 37 (55%) of 67 of those in the device group who were functionally dependent were colonized with ciprofloxacin-resistant GNB, although the results were not statistically significant because of the interaction between indwelling device use and functional status (Figure 1).
FIGURE 1.
Ciprofloxacin-resistant gram-negative bacilli (GNB) colonization and functional status. Shown are the percentages of nursing home residents colonized with ciprofloxacin-resistant GNB by increasing functional status category in control group residents (A) and device group residents (B). Functional status categories are defined in the Methods section. White bars, percentage of patients colonized with ciprofloxacin-sensitive GNB; black bars, percentage of patients colonized with ciprofloxacin-resistant GNB. For the control group, P < .001 (χ2 test for trend).
DISCUSSION
Our results show that while all nursing home residents carry GNB, those with indwelling devices had a greater colonization density and were more likely to be colonized with ciprofloxacin-resistant GNB, identifying this group as being at high risk.4 The prevalence of ciprofloxacin-resistant GNB was substantially higher than ceftazidime-resistant GNB and quite impressive. Fluoroquinolone use has substantially increased in the United States over the last decade.7 In 1998, the prevalence of ciprofloxacin-resistant GNB among rectal surveillance cultures was 2.6% in California nursing homes.8 In the present study, more than 54% of residents in the device group and 35% of those in the control group were colonized with ciprofloxacin-resistant GNB. Although residents with devices were colonized more often with ciprofloxacin-resistant organisms, both groups had high rates of colonization with resistant A. baumanii, P. mirabilis, and P. aeruginosa organisms.
Additionally, we found that colonization with ciprofloxacin-resistant GNB increases with decreasing functional status. To our knowledge, this is the first study to show this association. Previous reports have shown that patients with decreased functional status are more likely to be colonized with opportunistic organisms, but the reason for this association is not fully understood.9 Future studies should examine functional dependence and the risk of colonization with all resistant pathogens in greater depth, as well as design interventions for residents with decreased functional status.
Although only the oropharynx remained an independent risk factor for GNB colonization in the multivariate analysis, we believe colonization at other anatomic sites to be important for further study. Considering the high numbers of organisms that colonize these sites in all residents, it is possible that our study was not powered to detect a statistically significant difference. It is also possible that impaired functional status may play a larger role in groin and perianal GNB colonization and thereby confound the association between device use and GNB colonization.9 Clearly, additional studies that investigate this interaction and define clinically relevant differences in colonization in different anatomical sites will be useful in designing appropriate infection prevention interventions.
Antibiotic use is high in any healthcare setting, and resistance will continue to rise. Considering the magnitude of risk that indwelling devices impose on colonization, patients with these devices could be targeted for intensive surveillance and infection control practices.10 By targeting a specific high-risk group, infection prevention initiatives can have a greater effect on controlling the emergence of multidrug-resistant GNB in resource-limited nursing homes.
ACKNOWLEDGMENTS
Financial support. L.M. was supported by the National Institute on Aging (grant K23 AG028943) and an ASP/AGS T. Franklin Williams Research Scholarship. E.L.F. was supported by a Rackham graduate school spring/summer research grant.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Lautenbach E, Marsicano R, Tolomeo P, Heard M, Serrano S, Stieritz DD. Epidemiology of antimicrobial resistance among gram-negative organisms recovered from patients in a multistate network of long-term care facilities. Infect Control Hosp Epidemiol. 2009;30(8):790–793. doi: 10.1086/599070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32(8):1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002;162(2):185–190. doi: 10.1001/archinte.162.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55(12):1921–1926. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units—iplications for fluoroquinolone use. JAMA. 2003;289(7):885–888. doi: 10.1001/jama.289.7.885. [DOI] [PubMed] [Google Scholar]
- 8.Lee YL, Cesario T, McCauley V, Flionis L, Pax A, Thrupp L. Low-level colonization and infection with ciprofloxacin-resistant gram-negative bacilli in a skilled nursing facility. Am J Infect Control. 1998;26(6):552–557. doi: 10.1053/ic.1998.v26.a88774. [DOI] [PubMed] [Google Scholar]
- 9.Tada A, Watanabe T, Yokoe H, Hanada N, Tanzawa H. Oral bacteria influenced by the functional status of the elderly people and the type and quality of facilities for the bedridden. J Appl Microbiol. 2002;93(3):487–491. doi: 10.1046/j.1365-2672.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 10.Harris AD, McGregor JC, Furuno JP. What infection control interventions should be undertaken to control multidrug-resistant gram-negative bacteria? Clin Infect Dis. 2006;43:S57–S61. doi: 10.1086/504479. [DOI] [PubMed] [Google Scholar]