Abstract
The aquaporins (AQPs) are integral membrane proteins whose main function is to transport water across cell membranes in response to osmotic gradients. At the ocular surface, AQP1 is expressed in corneal endothelium, AQP3 and AQP5 in corneal epithelium, and AQP3 in conjunctival epithelium. AQPs are also expressed in lens fiber cells (AQP0), lens epithelium (AQP1), ciliary epithelium (AQP1, AQP4) and retinal Müller cells (AQP4). Mutations in AQP0 produce congenital cataracts in humans. Analysis of knockout mice lacking individual AQPs suggests their involvement in maintenance of corneal and lens transparency, corneal epithelial repair, intraocular pressure regulation, retinal signal transduction and retinal swelling following injury. The mouse phenotype findings implicate AQPs as potential drug targets for therapy of elevated intraocular pressure and ocular disorders involving the cornea, lens and retina. However, much research remains in defining cell-level mechanisms for the ocular AQP functions, in establishing the relevance to human eye disease of conclusions from knockout mice, and in developing AQP-modulating drugs.
Key terms: aquaporin, AQP, water channel, cornea, retina, water transport, corneal transparency, cell migration, cell proliferation
1. Introduction
The eye is a unique organ containing specialized avascular tissues to maintain its transparency, secretory epithelia for regulation of its internal pressure, and electrically excitable cells to convert incoming light to neural signals. Central to these specialized functions is regulated fluid transport between extravascular spaces and adjacent tissues. As in other mammalian tissues, water movement across membrane barriers in the eye follows osmotic gradients generated by active and secondary active solute transport. One exception is aqueous fluid drainage, in which hydrostatic pressure drives bulk fluid flow. Aquaporin (AQP) water channels are expressed in many cell types in the eye, where their primary function is to facilitate transmembrane flow of solute-free water in response to osmotic gradients. AQP water channels in ocular and extraocular tissues have a variety of interesting roles in cell/organ function and disease pathophysiology.
The AQPs are small integral membrane proteins (~30 kDa/monomer) expressed widely in the animal and plant kingdoms, with 13 members in mammals. AQPs are expressed in epithelia and endothelia involved in fluid transport, as well as in cell types that do not to carry out fluid transport, such as skin and fat cells. In most cell types, the AQPs reside constitutively at the plasma membrane. A notable exception is kidney AQP2, which undergoes vasopressin-regulated trafficking between endosomes and the cell plasma membrane. There is a considerable body of information on AQP protein structure, because, compared to other membrane proteins, the AQPs are relatively easy to isolate and crystallize for structural analysis by x-ray or electron crystallography. High-resolution x-ray crystal structures exist for AQP1 (Sui et al., 2001), the bacterial glycerol-transporter Glpf (Fu et al., 2000), and the major intrinsic protein of lens fiber AQP0 (Harries et al., 2004). AQP1 monomers contain six tilted alpha-helical domains forming a barrel-like structure in which the first and last 3 helices exhibit inverted symmetry (reviewed in Fujiyoshi et al., 2002; Stroud et al., 2003). Two conserved Asn-Pro-Ala (NPA) motifs reside on opposite sides of the AQP monomer. Monomeric AQP units contain independently functioning pores, though they are assembled in membranes as tetramers (Verbavatz et al., 1991). Molecular dynamics simulations based on the AQP1 crystal structure suggest tortuous, single-file passage of water through a narrow pore of less than 0.3-nm diameter, in which steric and electrostatic factors prevent the transport of protons and other small molecules (Tajkhorshid et al., 2002). AQP1, AQP2, AQP4, AQP5 and AQP8 are primarily water-selective, whereas AQP3, AQP7 and AQP9 (‘aquaglyceroporins’) also transport glycerol and possibly other small solutes (reviewed in Rojek et al., 2008). The transport function of some AQPs is inhibited by non-selective mercurial sulfhydral-reactive compounds such as HgCl2. There is considerable interest yet little progress in the identification of non-toxic, AQP-selective inhibitors.
2. Roles of AQPs outside of the eye
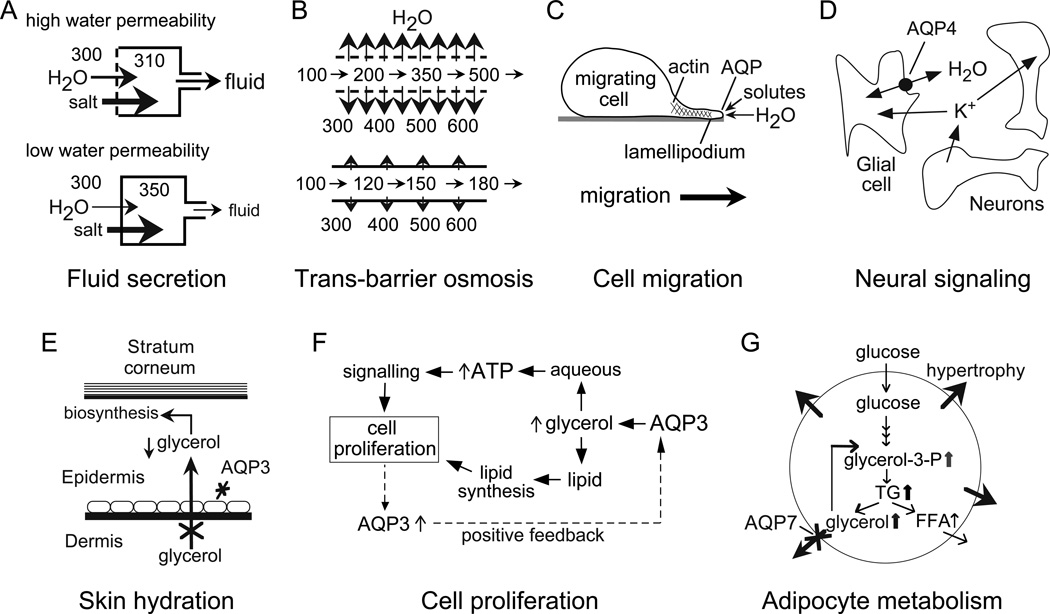
Understanding of the extraocular roles of AQPs provides useful clues about their functions in the eye. Much of our knowledge of AQP physiology has come from phenotype analysis of mice lacking AQPs (reviewed in Verkman, 2005). Some AQPs roles were predictable, such as their function in transepithelial fluid absorption and secretion, while others were unexpected, such as their involvement in cell migration and neural signaling transduction, and aquaglyceroporin involvement in cell proliferation and skin and fat metabolism. Fig. 1 provides an overview of AQP functions in mammalian physiology, which, in most cases, have direct relevance to the eye.
Figure 1. AQP functions in mammalian physiology.
A. Reduced water permeability in an epithelium, such as salivary gland, impairs active, near-isosmolar fluid transport by slowing osmotic water transport into the acinar lumen, resulting in the secretion of a reduced volume of a hypertonic fluid. B. Reduced transepithelial water permeability in kidney collecting duct impairs urinary concentrating ability by preventing osmotic equilibration of luminal fluid. C. Proposed mechanism of AQP-facilitated cell migration, showing water entry into protruding lamellipodia in migrating cells. D. AQP4-dependent neuroexcitation, showing AQP4-facilitated water transport in glial cells, which communicate with neurons through changes in extracellular space volume and K+ concentration. E. Reduced steady-state glycerol content in epidermis and stratum corneum in skin in AQP3 deficiency, accounting for reduced skin hydration. F. Proposed mechanism of AQP3-facilitated cell proliferation involving reduced cellular glycerol and consequent reduced ATP energy and biosynthesis. G. Proposed mechanism for adipocyte hypertrophy in AQP7 deficiency, in which impaired AQP7-dependent glycerol escape from adipocytes results in cellular glycerol accumulation and increased triglyceride content. See text for further explanations.
2.1 AQP roles related to AQP-facilitated water transport
An anticipated phenotype of AQP deficiency is defective urinary concentrating function, which has been found in mice lacking AQPs 1–4 (Ma et al., 1997, 1998, 2000; Yang et al., 2001) and in humans with mutations in AQP1 (King et al., 2001) and AQP2 (Deen et al., 1994). The generation of a concentrated urine involves near-isosmolar fluid absorption by the proximal tubule, generation of a hypertonic extracellular space fluid in the renal medulla by countercurrent multiplication and exchange, and transepithelial osmotic equilibration across a highly water permeable collecting duct. Mice lacking AQP1 show reduced water permeability in proximal tubule with impaired near-isosmolar fluid absorption (Schnermann et al., 1998). AQP1 deletion also reduces water permeability of the thin descending limb of Henle (Chou et al., 1999) and vasa recta microvessels (Pallone et al., 2000), which prevents the generation of a hyperosmolar medullary interstitium. Deletion or mutation of AQPs 2–4 reduces collecting duct water permeability, preventing osmotic equilibration between the renal interstitium and urinary fluid in the collecting duct lumen.
These observations indicate two paradigms regarding AQP functions. AQPs facilitate active, near-isosmolar fluid absorption and secretion in epithelia by increasing water transport in response to small osmotic gradients produced by salt pumping (Fig. 1A). AQP deletion reduces transepithelial water permeability and hence the amount of fluid absorbed or secreted. AQPs also facilitate osmotic equilibration in the presence of a ‘standing’ osmotic gradient (Fig. 1B), as in kidney collecting duct, where reduced transepithelial osmotic water permeability in AQP deficiency impairs the increase in urine fluid osmolality as it traverses the collecting duct.
There is considerable evidence for impairment in active fluid secretion in exocrine glands and other epithelia in AQP knockout mice, supporting the mechanism in Fig. 1A. AQP5 deletion in mice impairs fluid secretion by salivary (Ma et al., 1999) and airway submucosal (Song and Verkman, 2001) glands, resulting in the secretion of a reduced volume of hyperosmolar fluid. Defective fluid secretion has also been found in AQP1 knockout mice in choroid plexus (Oshio et al., 2005), where cerebrospinal fluid is produced, and as discussed in section 4, in ciliary epithelium (Zhang and Verkman, 2002), where ocular aqueous fluid is produced. In each of these systems the rate of transepithelial fluid secretion normalized to epithelial surface area is very high, such that the reduced but non-zero water permeability in AQP deficiency impairs transepithelial osmotic equilibration. In other epithelia, where area-normalized rates of fluid absorption/secretion are much lower, AQPs are not required for transepithelial fluid transport, as found for tear fluid secretion by lacrimal gland (Moore et al., 2000), sweat secretion (Song et al., 2002), alveolar fluid absorption (Bai et al., 1999; Ma et al., 2000), and airway fluid absorption (Song et al., 2001). Involvement of AQP-in the reduced tear fluid secretion in Sjogren’s syndrome has been suggested (Tsubota et al., 2001), though subsequently refuted (Beroukas et al., 2001). AQPs and lacrimal gland function will not be discussed further based on the negative phenotype from our lab and others (Moore et al., 2000; Sasaki et al., 2007).
Another AQP role related to its osmotic water transport function is in the pathophysiology of tissue fluid accumulation, as has been found for AQP4 in brain edema (reviewed in Verkman et al., 2006), and as described later in this review, for AQPs in stress-induced corneal (Thiagarajah and Verkman, 2002) and retinal (Da and Verkman, 2004) edema. AQP4 facilitates water entry into and exit from the brain in response to clinically relevant stimuli, such as altered cellular ionic homeostasis in cytotoxic (cell-swelling) brain edema produced by hyponatremia or ischemic stroke (Manley et al., 2000), and vasogenic (leaky-vessel) brain edema produced by tumor or infection (Papadopoulos et al., 2004).
We discovered an interesting role of AQPs in cell migration, as originally demonstrated in endothelial cells and various transfected cells (Saadoun et al., 2005a), and subsequently in brain astroglial cells (Saadoun et al., 2005b, Auguste et al., 2007), kidney proximal tubule cells (Hara-Chikuma and Verkman, 2006), skin cells (Hara-Chikuma and Verkman, 2008b) and tumor cells (Hu and Verkman, 2006). Fig. 1C shows our proposed mechanism for AQP involvement in cell migration, in which actin cleavage and ion uptake at the tip of a lamellipodium create local osmotic gradients that drive water influx, facilitating lamellipodial extension and cell migration (reviewed in Papadopoulos et al., 2008). Demonstrated consequences of AQP-facilitated cell migration include AQP-facilitated tumor angiogenesis, tumor cell metastasis and spread, and wound healing. As described in section 7.2, AQP3-dependent cell migration is relevant to corneal epithelial wound healing (Levin and Verkman, 2006). As described in section 7.5, AQP1-facilitated migration of corneal stromal keratocytes was found in cell culture and mouse models (Ruiz-Ederra and Verkman, 2008), which may facilitate the repair of corneal wounds. AQP-facilitated cell migration may also promote microvascular proliferation in retinopathies; however, an initial study in mice showed little AQP1 expression during retinal microvascular proliferation in a neonatal model of oxygen deprivation, without impairment of retinal angiogenesis in AQP1 deficiency (Ruiz-Ederra and Verkman, 2007). Whether AQP1 is expressed in other human retinopathies involving neovascularization, such as wet macular degeneration or proliferative diabetic retinopathy, is unknown.
Another unanticipated role of AQP-facilitated water transport is in neural signal transduction (Fig. 1D), which has relevance to light-neural signal transduction in retina. AQP4 is expressed in supportive cells adjacent to electrically excitable cells, as in Müller vs. bipolar cells in retina, glia vs. neurons in brain, and hair vs. supportive cells in the inner ear. Electrophysiological measurements have demonstrated impaired auditory and visual signal tranduction in AQP4 null mice, seen as increased auditory brainstem response thresholds (Li and Verkman, 2001) and reduced electroretinogram potentials (Li et al., 2002). In brain, seizure threshold is reduced and seizure duration prolonged in AQP4 deficiency (Binder et al., 2004a). The mechanisms for altered neuroexcitation in AQP4 deficiency are unclear. Delayed K+ uptake from brain extracellular space (ECS) in AQP4 deficiency has been found (Binder et al., 2006; Padmawar et al., 2005), which may account for the prolonged seizure phenotype (Fig. 1D), though the link between delayed K+ reuptake from the ECS and AQP4 deficiency is not known. It has been proposed that AQP4 associates with the inwardly rectifying K+ channel Kir4.1 (Amiry-Moghaddam et al., 2003), such that reduced K+ channel function in AQP4 deficiency might account for the delay in K+ clearance. However, patch-clamp studies in Müller cells (Ruiz-Ederra et al., 2007) and brain astroglia (Zhang and Verkman, 2008) provide evidence against this mechanism. Another possible mechanism involves ECS expansion in AQP4 deficiency (Binder et al., 2004b; Zador et al., 2008), which may account in part for reduced seizure susceptibility and prolonged seizure duration in AQP4 null mice. An expanded ECS would provide a larger aqueous volume to dilute K+ released into the ECS during neuroexcitation, thereby slowing changes in ECS K+ concentration. Perhaps reduced water permeability in AQP4 deficiency is responsible for defective neuroexcitation by a mechanism involving impaired cell volume responses. Alternative plausible mechanisms include AQP4 interaction with ion channels, perhaps through PDZ-domain interactions, and maladaptive regulation in AQP4 deficiency of other transporters involved in neuroexcitation.
2.2 AQP roles related to AQP-facilitated glycerol transport
The aquaglyceroporins, such as AQP3 in corneal epithelium, have unique biological roles that are related to their glycerol transport function. AQP3-facilitated glycerol transport in skin is an important determinant of epidermal and stratum corneum hydration (Fig. 1E). Mice lacking AQP3, which is normally expressed in the basal layer of keratinocytes in epidermis, manifest reduced stratum corneum hydration and skin elasticity, and impaired stratum corneum biosynthesis and wound healing (Ma et al., 2002). The mechanism responsible for the skin phenotype in AQP3 deficiency involves reduced epidermal cell skin glycerol permeability, resulting in reduced glycerol content in the stratum corneum and epidermis (Hara and Verkman, 2003).
We recently discovered a remarkable skin phenotype–mice lacking AQP3 are resistant to formation of skin tumors (Hara-Chikuma and Verkman, 2008b). AQP3-dependent epidermal cell proliferation appears to involve reduced cellular glycerol metabolism and biosynthesis, resulting in reduced ATP content and impaired MAP kinase signaling (Fig. 1F). AQP3-dependent cell proliferation was also found in cutaneous wound healing (Hara-Chikuma et al., 2008a), colonic epithelial cell regeneration in experimental colitis (Thiagarajah et al., 2007), and, of relevance to the eye, in the repair of the corneal epithelium following mechanical debridement (Levin and Verkman, 2006). Another aquaglyceroporin, AQP7, is expressed in the plasma membrane of adipocytes. Fat mass in AQP7 null mice becomes greater than that in wildtype mice as they age, with remarkable adipocyte hypertrophy and accumulation of glycerol and triglycerides (Hara-Chikuma et al., 2005). We proposed that adipocyte hypertrophy in AQP7 deficiency results from reduced plasma membrane glycerol permeability, with cellular glycerol accumulation and triglyceride biosynthesis (Fig. 1G).
3. Aquaporin expression in the eye
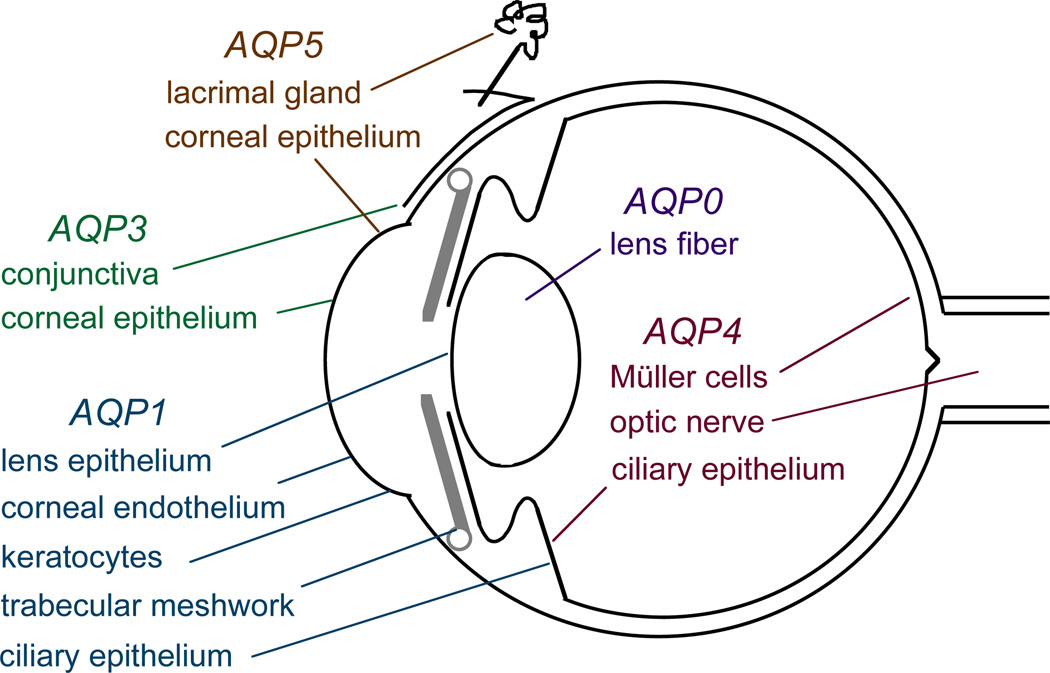
Fig. 2 summarizes the sites of AQP expression in the eye. The expression of MIP (major intrinsic protein, also referred to as AQP0) in lens fiber cells has been known for many years. Our lab first localized AQP1 in ciliary epithelium (Hasegawa et al., 1993), and later AQP3 in conjunctiva and AQP4 in ciliary epithelium and retina (Frigeri et al., 1995). These findings were extended by several laboratories in different mammalian species, including humans, and several groups found AQP5 expression in corneal and lacrimal gland epithelia (Nielsen et al., 1993; Hasegawa et al., 1994; Stamer et al., 1994; Patil et al., 1997; Ishida et al., 1997; Hamann et al., 1998; Funaki et al., 1998; Nagelhus et al., 1998; Kenney et al., 2004; Song et al., 2004; Sasaki et al., 2007). In addition to the AQP expression pattern shown in Fig. 2, which represents a consensus from multiple studies, there are reports of AQP1 expression in retinal pigment epithelial, photoreceptor, glycernic amacrine, and Müller cells (Ruiz and Rok, 1996; Stamer et al., 2003; Kim et al., 1998, 2002; Iandiev et al., 2005). AQP0 protein, which had previously been localized exclusively to lens, was recently detected at low levels in rat and mouse retinal bipolar cells (Iandiev et al., 2007a; Farjo et al., 2008). The ocular expression pattern of AQPs provides indirect evidence for their involvement in intraocular pressure regulation (AQP1 and AQP4), corneal and lens transparency (AQP0, AQP1 and AQP5), visual signal transduction and retinal water balance (AQP4), and corneal and conjunctival barrier function (AQP3, AQP5). As described in the following sections, these and other possible roles of AQPs were tested using knockout mice lacking individual AQPs.
Figure 2. Sites of AQP expression in the eye.
4. Aqueous fluid dynamics and intraocular pressure
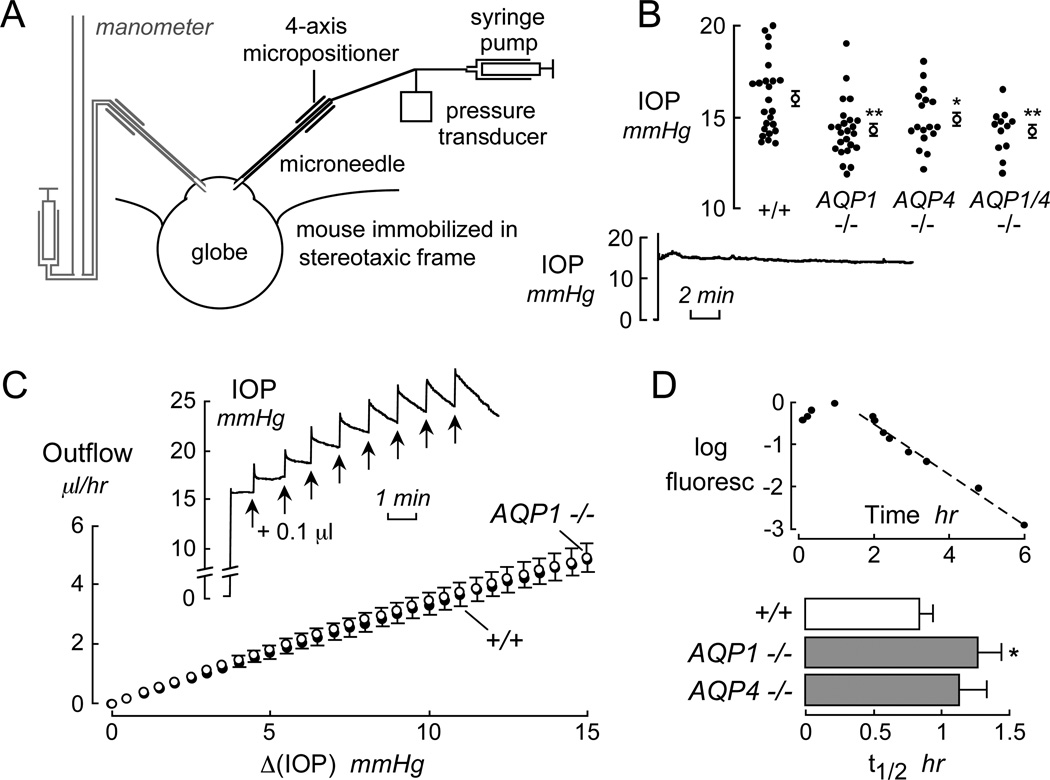
The principal determinants of intraocular pressure (IOP) are the rates of aqueous fluid secretion by the ciliary epithelium and aqueous fluid drainage (outflow) by the canal of Schlemm. As for saliva secretion, aqueous fluid secretion by the ciliary epithelium involves near-isosmolar fluid secretion driven by active salt transport. Aqueous fluid drainage, in contrast, involves pressure-driven bulk flow of fluid through the canal of Schlemm, and fluid seepage through the sclera across ciliary muscles and the supraciliary space. Both AQP1 and AQP4 are expressed in non-pigmented ciliary epithelium, and AQP1 is expressed in trabecular meshwork endothelium. Our lab modified a microneedle method to give stable IOP recordings in mice (Fig. 3A). Fig. 3B shows a small though significant reduction in IOP in mice lacking AQP1 and/or AQP4 compared to wildtype mice (Zhang et al., 2002). We found similar differences in IOP in AQP1 null mice by impact-induction tonometry (unpublished results). It is not known, but of considerable importance with regard to possible AQP-based therapy, whether larger differences in IOP would occur with AQP deficiency (or inhibition) in animal models of glaucoma.
Figure 3. Intraocular pressure and aqueous fluid dynamics.
A. Schematic of measurement method showing the introduction of a fluid-filled microneedle into the anterior chamber through the cornea. In calibration studies, a second micropipette connected to a fluid-filled manometer is inserted (left, shown in gray). B. IOP measurements in wildtype, AQP1 and AQP4 null mice, and AQP1/AQP4 double knockout mice showing data in individual eyes (filled circles) and mean ± SE (open circles). * P < 0.05, ** P < 0.002. (bottom) Stability of IOP measurement in wildtype mouse after microneedle insertion. C. Pulsed infusion method to measure aqueous fluid outflow. (inset) Continuous IOP recording in response to 0.1 µl fluid infusion every minute. The fluid infusions produced a rapid increase in IOP (related to anterior chamber compliance) followed by a pressure-dependent decline due to outflow. D. (top) Aqueous fluid production determined from the kinetics of fluorescein disappearance from anterior chamber fluid after transcorneal iontophoresis. (bottom) Aqueous fluid production, shown as half-times (t1/2) for fluorescein disappearance. *, P < 0.05. Adapted from Zhang and Verkman (2002).
To investigate whether the reduced IOP in AQP1 deficiency is due to reduced aqueous fluid secretion vs. increased fluid drainage, methodology was developed to measure aqueous fluid secretion and drainage in mice. A pulsed-infusion method was used in which IOP was recorded continuously in response to anterior chamber fluid infusions of 0.1 µl each minute (Fig. 3C, inset). From the IOP time course was deduced aqueous fluid outflow as a function of Δ(IOP) (Fig. 3C) and static compliance (0.036 µl/mmHg), neither of which was affected by AQP1 deficiency. The insensitivity of aqueous fluid outflow to AQP1 expression supports a bulk fluid flow mechanism that is predicted to be independent of water-selective AQPs. The reason(s) for AQP1 expression in canal of Schlemm remain unclear. Stamer et al. (2001) suggested that AQP1 may be involved in cell volume regulation, though this possibility is unlikely given the much more rapid kinetics of osmotic cell volume equilibration compared to cell volume regulation.
We used in vivo confocal fluorescence microscopy after transcorneal iontophoretic introduction of fluorescein to measure aqueous fluid secretion (Zhang et al., 2002). The basis of this method is that aqueous fluid inflow and outflow are equal in the steady-state, and that fluorescein disappearance occurs by bulk fluid outflow. Fig. 3D (top) shows a late phase of exponentially decreasing fluorescein disappearance (after 90 min) from the aqueous fluid of wildtype mice, giving an aqueous fluid production rate of 3.6 µl/hr. Aqueous fluid production was significantly slowed in AQP1 null mice (increased t1/2, Fig. 3D, bottom). Thus, the reduced IOP in AQP1 deficiency is a consequence of reduced aqueous fluid production related to impaired near-isosmolar fluid secretion across the ciliary epithelium.
5. Lens
The lens is an avascular tissue composed of concentric layers of epithelial cells at various stages of differentiation (Zampighi et al., 2000). An epithelial cell monolayer extends from the anterior pole of the lens to its equatorial surface with the basement membrane forming a capsule. The interior of the lens contains elongated lens fibers, which are arranged in a stratified manner with the oldest fibers in the lens interior. Upon maturation, lens fibers lose their attachment to the capsule, and cellular organelles are degraded in a synchronized manner (Bassnett, 2002). Nourishment to the lens involves diffusion from the aqueous and vitreous humors. However, it is thought that simple diffusion cannot sustain the metabolic needs of the lens interior (Fischbarg et al., 1999). A circulatory system has been proposed, in which an asymmetric distribution of ion pumps, transporters, channels and cell junctions drive ion-coupled fluid absorption, facilitating the entry of nutrients and metabolites into the inner lens across the polar regions and exit through the lens equator (Fischbarg et al., 1999; Candia, 2004; Mathias et al., 2007).
5.1 AQP0 mutations cause congenital cataracts
The lens contains a uniquely high protein concentration and low water content to maintain an elevated refractive index for transparency. Lens water channels are proposed to facilitate water removal (reviewed in Mathias et al., 2007). Two aquaporins are expressed in the lens: AQP0 (major intrinsic protein-MIP) in the posterior pole and in nuclear fibers, and AQP1 at the anterior pole in epithelial cells. Unlike AQP1, AQP0 has widespread distribution throughout lens fibers, where it constitutes more than 50% of membrane protein (Bok et al., 1982), but is absent in lens epithelial cells. Another difference is that AQP0 (but not AQP1) water permeability is pH and Ca++ regulated, with ~4 fold increase in AQP0 water permeability with reduced pH or [Ca++] (Nemeth-Cahalan and Hall, 2000). AQP0 has at least 40 times lower water permeability than AQP1 (Yang and Verkman, 1997; Chandy et al., 1997).
Because of its low water permeability it has been proposed that AQP0 might be involved in regulating the resistance of the paracellular pathway, rather than in cell membrane water permeability (Nemeth-Cahalan and Hall, 2000; Zampighi et al., 2000). It has also been proposed that AQP0 acts as a scaffold for organizing gamma-crystallins in lens fibers (Fan et al., 2004). Electron crystallography suggested that AQP0 forms not only water pores, but also 11–13 nm ‘thin lens junctions’, providing evidence for AQP0 involvement in fiber-fiber adhesion (Gonen et al, 2004; 2005). The crystal structure also revealed that AQP0 exists in two configurations: a so-called junctional conformation, which forms ‘thin lens junctions’, and a non-junctional conformation, which forms the water pore (Harries et al., 2004; Gonen et al., 2004). Transition from non-junctional to junctional AQP0 occurs as fiber cells mature and become part of the lens core (Gonen et al, 2004).
Mutations in AQP0 are associated with hereditary cataracts in mice and humans (Berry et al., 2000; Shiels et al., 2001). Cataract-producing AQP0 mutations are thought to produce endoplasmic reticulum-retained and non-functional AQP0 (Francis et al., 2000; Geyer et al., 2006); however, the mechanism linking AQP0 loss-of-function and cataracts remains unclear. Proposed mechanisms include loss of AQP0-facilitated fiber-fiber adherence (Shiels et al., 2001), and impaired fiber cell dehydration (Fotiadis et al., 2000).
5.2 Accelerated cataractogenesis in AQP1 deficiency
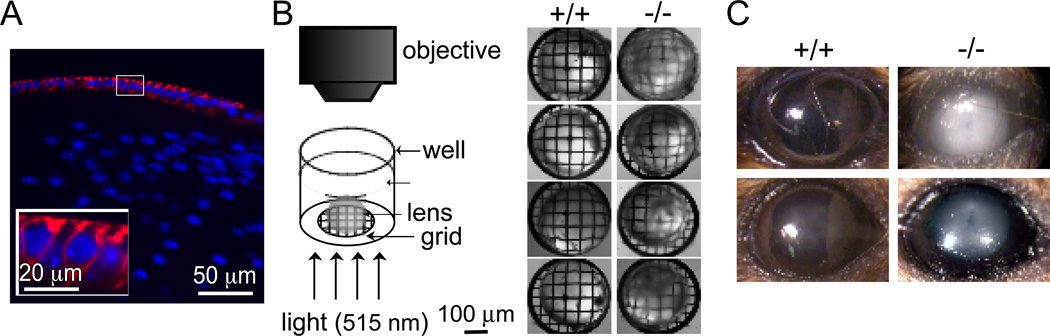
The anterior surface of the lens is covered by an AQP1-expressing epithelium (Fig. 4A), resembling the AQP1-expressing corneal endothelium covering the inner corneal surface. Cataracts were not reported in rare humans with AQP1 deficiency (Preston et al., 1994), nor are spontaneous cataracts seen grossly in AQP1 null mice (Ruiz-Ederra and Verkman, 2006a). Nevertheless, motivated by the marked impairment of AQP1 deletion on corneal transparency and hydration in an experimental model of corneal swelling (Thiagarajah and Verkman, 2002), and by our observations of lens opacification in AQP1 null mice during eye surgery (Ruiz-Ederra and Verkman, 2006b) we tested the possibility that lens AQP1 might be involved in lens transparency (Ruiz-Ederra and Verkman, 2006a). Epithelial cell water permeability was reduced ~3-fold in lenses from AQP1 null mice. Though AQP1 deletion did not alter baseline lens morphology or transparency, basal water content was significantly greater by ~4% in AQP1 null mice as measured by gravimetry using kerosene-bromobenzene gradients and wet/dry weight ratios. Cataract formation was induced in vitro by incubation of lenses in a high-glucose solution. Involvement of AQP1 in cataract development was studied using in vitro and in vivo models. Loss of lens transparency was greatly accelerated in AQP1 null lenses bathed in a 55 mM glucose solution for 18 hours, as measured by optical contrast analysis of transmitted grid images (Fig. 4B). Cataract formation in vivo was greatly accelerated in a mouse model of acetaminophen toxicity, with little opacification in lenses from wildtype mice, and moderate/marked opacification in lenses from AQP1 null mice (Fig. 4C). AQP1 thus facilitates the maintenance of lens transparency and opposes cataract formation, suggesting the possibility of AQP1 induction to retard cataractogenesis.
Figure 4. Accelerated cataractogenesis in AQP1 deficiency.
A. AQP1 protein expression (red) in anterior epithelial cells of lens in wildtype mouse. Nuclei stained blue. White box shows magnified area. B. In vitro cataract induction by incubation with high-glucose solution. (left) Diagram of apparatus used to quantify lens transparency, showing isolated lens in saline on a metal grid, illuminated from below. (right) Grid images transmitted through lenses from 4 wildtype and 4 AQP1 mice after 18 h incubation in high-glucose solutions. C. In vivo cataract induction by acetominophen. Photographs taken 4 h of acetaminophen. Adapted from Ruiz-Ederra and Verkman (2006a).
6. Retina
6.1 Light-neural signal transduction and K+ buffering
As described in section 6.3, light-neural signal transduction is mildly impaired in AQP4 deficiency (Li et al., 2002; Da and Verkman, 2004), suggesting that the AQP4-expressing Müller cells are functionally coupled to the light-transducing bipolar cells. The visual deficit associated with AQP4 deficiency adds to the auditory and olfactory sensory deficits described in AQP4 null mice (Li and Verkman, 2001; Lu et al., 2008), and to the related neural signaling phenotype of altered seizure susceptibility and severity (Binder et al., 2004a; 2006).
How AQP4-expressing Müller cells are functionally coupled to bipolar cells remains a subject of ongoing investigation. AQP4 is expressed most strongly in perivascular and end-foot processes (facing the retinal capillaries and vitreous body) of Müller cells, where it is thought to form a multiprotein complex involving the inwardly rectifying K+ channel, Kir4.1 (Newman, 1987). The polarized distributions of Kir4.1 and AQP4 suggest that they facilitate ‘buffering’ of K+, which is produced during neuronal activity, from inner retina to the vitreous through the Müller cells, by a process known as ‘potassium siphoning’. Immunoelectron microscopy has shown colocalization of AQP4 and Kir4.1 (Nagelhus, 1999), and immunoprecipitation suggested AQP4-Kir4.1 interaction, possibly as a part of a macromolecular complex that includes α-syntrophin and dystrophin (Connors et al., 2004; Connors and Kofuji, 2006). Based on this indirect evidence, a functional interaction between AQP4 and Kir4.1 K+ channel has been inferred and proposed to account for the various neural signaling phenotypes associated with AQP4 deficiency.
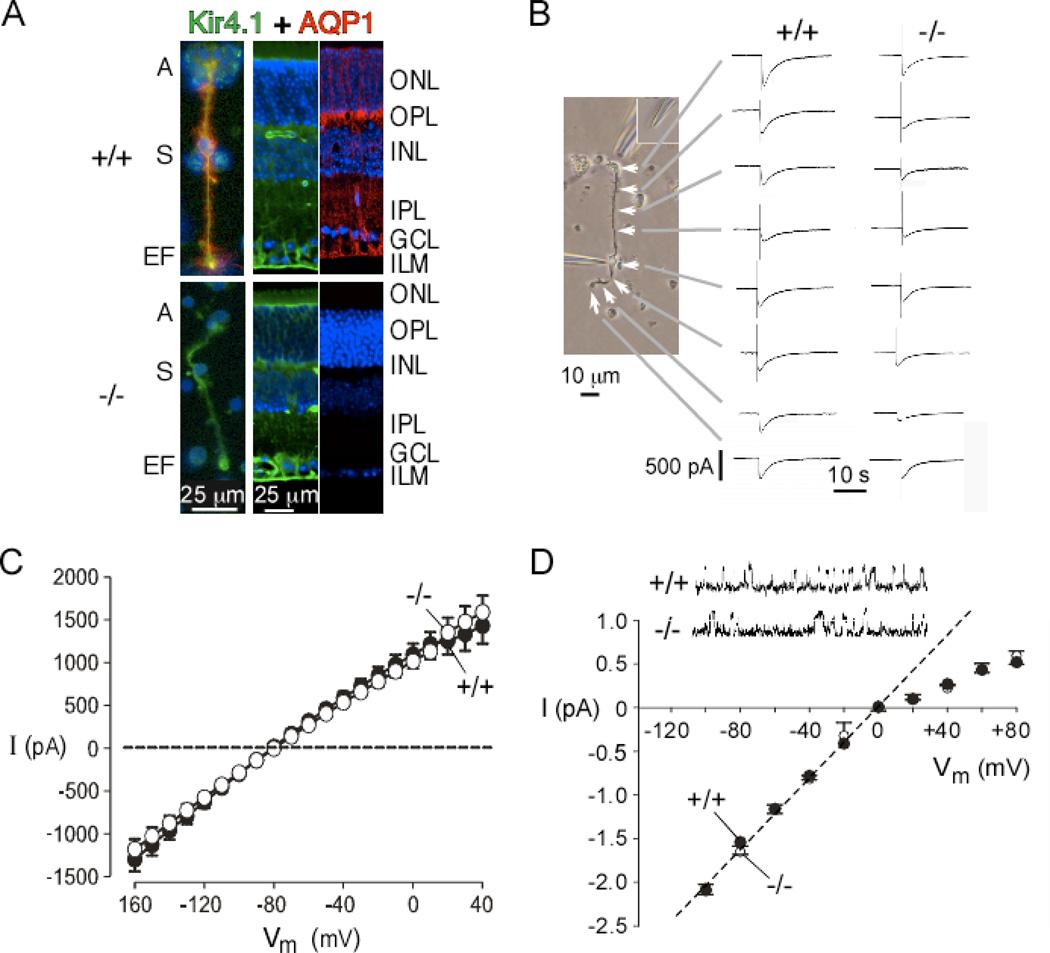
However, recent patch-clamp work from our lab provided evidence against a functional interaction between AQP4 and Kir4.1 (Ruiz-Ederra et al., 2007). Immunofluorescence showed a similar AQP4 and Kir4.1 distribution in Müller cells from wildtype mice, with enriched density of both channels in the end-feet, soma and apical region. The Kir4.1 distribution was not affected by AQP4 deletion (Fig. 5A). Water permeability was greatly reduced in the AQP4 null Müller cells, as measured by calcein quenching method, indicating functional AQP4 expression in Müller cells. Kir4.1 K+ channel function in Müller cells was measured by patch-clamp. Fig. 5B shows representative currents evoked from different regions of Müller cells, with relatively large currents seen at the end-feet, soma and apical region. Fig. 5C summarizes averaged K+ currents from many Müller cells from wildtype and AQP4 null mice, showing no significant differences in current magnitude or current-voltage relationships, which was found as well by single-channel patch-clamp analysis (Fig. 5D). These results are in agreement with a patch-clamp study in brain astroglial cells, showing no effect of AQP4 expression on Kir4.1 function, nor of Kir4.1 expression on AQP4 function (Zhang and Verkman, 2008). The patch-clamp findings challenge the generally accepted view of AQP4-modulated Kir4.1 K+ channel function. Alternative possibilities thus require consideration, such as alterations in ECS volume or expression of other ion transporters in AQP4 deficiency, or direct effects of altered Müller cell water permeability.
Figure 5. Kir4.1 and AQP4 are expressed in retinal Müller cells but are functionally independent.
A. (left panel) Colocalization of Kir4.1 (green) and AQP4 (red) in isolated Müller cells from wildtype (+/+, top) and AQP4 null (−/−, bottom) mice, with nuclei stained with DAPI (blue). A, apical end; S, soma; EF, endfoot. (two right panels) Retinal sections from mice. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; ILM, inner limiting membrane. B. Spatially resolved Kir4.1 K+ conductance in Müller cells. (left) Phase-contrast micrograph showing Müller cell with soma puncture and K+ injection micropipette positioned where indicated by white arrows. Endfoot at the top and apical end at the bottom. (right) Representative whole-cell current recordings (voltage −80 mV) before and following pulsed injections of 50 mM K+ solution for wildtype (left) and AQP4 null (right) Müller cells. C. Mean current-voltage (I–V) curves of whole-cell currents in Müller cells. D. Single-channel patch-clamp of Kir4.1 K+ channels with unitary current-voltage data. Inset shows representative single-channel current traces from cell-attached membrane patches with 145 mM K+ in the pipette at −60 mV. Adapted from Ruiz-Ederra et al. (2007).
The possible involvement of AQPs in the neuroretina also deserves study following reports of AQP1 localization to photoreceptor, glycernic amacrine, and Müller cells (Kim et al., 1998, 2002; Iandiev et al., 2005) and AQP0 in bipolar cells (Iandiev et al., 2007a; Farjo et al., 2008).
6.2 Retinal pigment epithelial fluid transport
The retinal pigment epithelium (RPE) lines the outer blood-retinal barrier, preventing fluid leak between the neural retina and choroidal capillaries. IOP-driven bulk flow and choroidal osmotic pressure promote passive RPE fluid absorption, preventing the build-up of subretinal fluid. Active RPE fluid transport is, however, required to oppose oncotic water accumulation when the blood-retinal barrier is disrupted and protein aberrantly enters the subretinal space, as seen in retinal detachment (reviewed by Marmor, 1999). However, despite the importance of RPE fluid transport, the expression of AQPs in the RPE has not been clearly established. AQP1 mRNA was detected in cultured fetal RPE cells (Ruiz and Rok, 1996), and AQP1 protein detected in human RPE using a highly sensitive membrane biotinylation method (Stamer et al., 2003). Other studies on rat and human tissue have failed to corroborate AQP1 protein expression in RPE (Hamann et al. 1998, and our own unpublished data). A recently reported experimental mouse model of retinal detachment involving transvitreal subretinal saline injection (Nour et al., 2003) might be useful in investigating the potential relevance of AQPs in RPE fluid transport.
6.3 Neuroprotective effect of AQP4 deletion in retinal ischemia
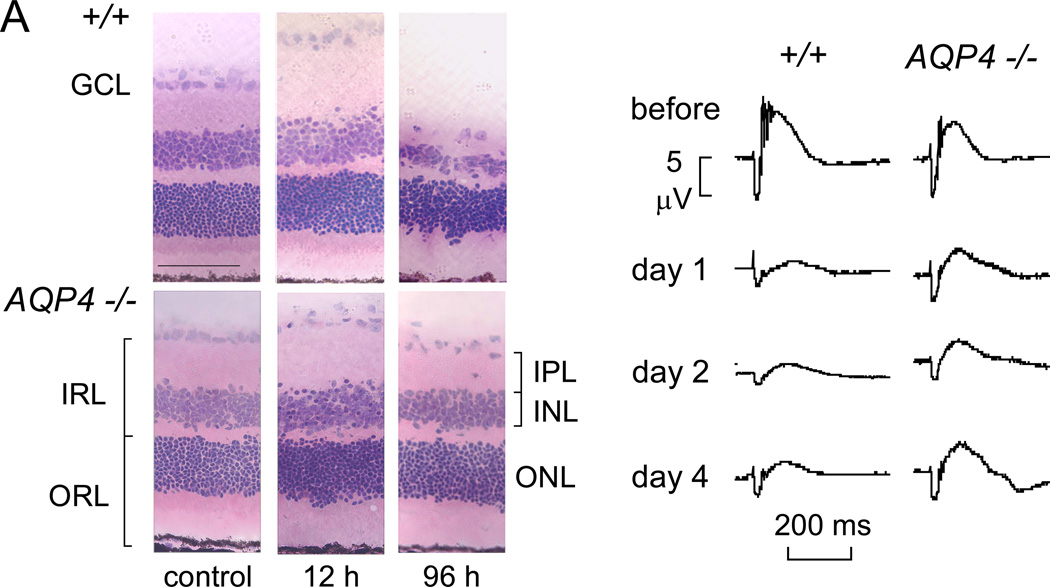
The inner blood-retinal barrier consists of the tight-junction-laden endothelia of retinal blood vessels. The inner retinal extracellular space is regulated primarily by the dehydrating actions of Müller cells. Ischemic disorders, caused by retinal artery occlusion, diabetes, and hypertension, involve fluid accumulation in the inner retina (Bringmann et al., 2005). There is evidence supporting the involvement of AQP4 in retinal swelling. Motivated by the protection against cytotoxic brain edema conferred by AQP4 gene deletion (Manley et al., 2000), the possibility was tested that AQP4 deletion protects the retina in a transient ischemia-reperfusion model produced by 45–60 min elevation in intraocular pressure to 120 mmHg (Da and Verkman, 2004). Retinal structure and cell number were preserved in AQP4 null mice following the ischemic insult, particularly in inner nuclear and plexiform layers of retina where Müller cells are concentrated (Fig. 6A). At 4 days after ischemia, the inner retina was thinned by 43% in wildtype mice vs. 11% in AQP4 null mice. Retinal function and cell survival were also significantly improved in AQP4 deficient mice. By electroretinography, b-wave amplitude prior to ischemia was mildly reduced in AQP4 null mice (Fig. 6B), in agreement with previous data (Li et al., 2002). At 1–4 days after ischemia b-wave amplitude was reduced by 75–83 % in wildtype mice vs. 48–51 % in AQP4 null CD1 mice, and 53–72% vs. <34% in C57/bl6 mice. Possible mechanisms responsible for retinal neuroprotection in AQP4 deficiency that were investigated and ruled out included extracellular space expansion, reduced retinal swelling early after reperfusion, and altered Kir4.1 K+ channel expression (Da and Verkman, 2004). Interestingly, AQP protein expression in glial cells surrounding superficial retinal vessels changes from AQP4- to AQP1-predominant in this mouse model of retinal ischemia, as well as in a rat model of streptozotocin-induced diabetes (Iandiev et al., 2005, 2007b). Notwithstanding our incomplete understanding of the relationship between AQP4 deficiency and retinal neuroprotection in the transient ischemic-reperfusion model, and of the significance of compensatory alterations in AQP protein expression in vivo, the available data suggest the interesting possibility of AQP4 inhibition to limit inner retinal pathology following vascular occlusive and other ischemic diseases associated with cytotoxic edema.
Figure 6. Retinal neuroprotection in AQP4 deficiency in a transient ischemia-reperfusion model.
A. Hematoxylin and eosin-stained retinal sections before and at 12 and 96 hours after 60 min retinal ischemia in wildtype and AQP4 null mice. Note retinal swelling at 12 h and degeneration at 96 h. B. Functional analysis by electroretinography. Representative electroretinograms before and at 1, 2 and 4 days after 45 min retinal ischemia. Adapted from Da and Verkman (2004).
6.4 AQP4 autoantibody as a marker of neuromyelitis optica
AQP4 has recently been implicated recently as a marker of, and perhaps as an autoantigen responsible for the central inflammatory demyelinating disease neuromyelitis optica (NMO), or Devic’s disease. NMO is a unique form of multiple sclerosis (MS) in which inflammatory lesions are restricted to optic nerves and spinal cord, causing acute symptoms of ocular pain with loss of vision, and myelitis with symmetric paraplegia, sensory loss, and bladder dysfunction. NMO is most common in non-white populations, accounting for one-third of MS in Asia. Different disease characteristics for NMO and MS have long suggested different pathogenic mechanisms. The CSF in MS but not NMO generally contains an oligoclonal band reflecting abnormal intrathecal antibody synthesis. The characteristic white-matter lesions of MS seen by magnetic resonance imaging are generally absent in NMO.
A serum immunoglobulin was discovered in NMO subjects, but not in MS or normal subjects, which was subsequently found to target AQP4 (Lennon et al., 2004 and 2005). These findings firmly distinguish NMO and MS as separate disease entities. Seropositivity for NMO-IgG is reasonably sensitive (74%) and specific (>90%) for NMO (Weinshenker, 2007), potentially enabling early diagnostic distinction of NMO from MS, which is of value for disease prognosis and therapeutic decisions.
The characteristic vasculocentric deposition of immunoglobulins and complement activation products in NMO raises the possibility that the AQP4 autoantibody is involved in NMO disease pathogenesis. The high density of AQP4 in perivascular astrocytic foot processes and its homotetrameric structure might enable complement activation by immunoglobulins. The inflammation and demyelination in NMO lesions has also been proposed to involve IgG-mediated dysregulation of tissue water homeostasis by AQP4 water channel disruption. Interestingly, AQP4 immunoreactivity appears to be increased at the periphery of MS lesions, but absent in NMO lesions (Aoki-Yoshino et al., 2005; Misu et al., 2006; Roemer et al., 2007; Sinclair et al., 2007). Moreover, there appears to be a predilection for the development of NMO lesions in regions of enhanced AQP4 expression (Pittock et al., 2006). Other observations challenge the proposed role for AQP4 as the NMO-specific autoantigen, such as the lack of correlation of NMO-IgG antibody titer to disease severity, and the restricted sites of NMO lesions compared to the wide distribution of AQP4 (which includes the cerebellum, lung, stomach, kidney and muscle). Many other questions thus remain about NMO and AQP4 autoantibodies, including how AQP4 autoantibodies cross the blood-brain barrier, whether the autoantibodies directly inhibit AQP4 function, and why autoantibodies are generated against the normally non-antigenic extracellular AQP4 epitopes. It will also be interesting to study whether AQP antibodies are present in other diseases–for example, AQP3 autoantibodies in autoimmune skin diseases.
7. Cornea
7.1 The ocular surface and maintenance of the tear film
The ocular surface is lined by stratified corneal and conjunctival epithelia, which lie in contact with the tear film. Keratoconjunctivitis sicca encompasses a diverse set of conditions that manifest as diminished volume, elevated osmolarity, and resultant ocular surface inflammation (Lemp, 1995). Osmotic flux across ocular surface epithelia, together with the rates of evaporative water loss and tear fluid production and drainage, determine tear film volume and osmolality. A mathematical model of steady-state tear film composition (Levin and Verkman; 2004) demonstrated the sensitivity of tear film osmolarity to excessive tear evaporation and inadequate tear fluid secretion, the two principal causes of keratoconjunctivitis sicca, and indicated the theoretical importance of high ocular surface water permeability, In addition to strong AQP5 expression throughout corneal epithelial cells, AQP3 is expressed in the corneal epithelium at lower levels and primarily in the proliferating basal cell layer. AQP3 expression is strong throughout the neighboring conjunctival epithelium (Patil et al., 1997; Hamann et al., 1998; Levin and Verkman, 2004, 2006).
AQP5-mediated corneal epithelial water transport was first characterized in bovine cells as a mercurial-sensitive flux that was diminished by siRNA-mediated AQP5 knockdown (Kang et al., 1999). Corneal epithelial cell plasma membrane osmotic water permeability was measured in living mice by an ocular surface perfusion - calcein quenching method (Levin and Verkman, 2004). The high osmotic water permeability (0.045 cm/s) measured in wildtype mice corroborated data from earlier studies (Mishima and Hedbys, 1967; Fischbarg and Motoreano, 1982) and suggested an important role for facilitated water transport. However, water permeability was reduced by only ~2-fold in AQP5 null mice, a surprising finding that was later accounted for by a 5-fold up-regulation of AQP3 in AQP5 null versus wildtype mouse corneal epithelium (Levin and Verkman, 2004). Conjunctival epithelial cell membrane water permeability, also assayed by ocular surface perfusion - calcein quenching, was ~4-fold slowed in AQP3 deficiency. These observations implicated corneal AQP5 and conjuctival AQP3 as significant cellular pathways for water movement into the relatively hypertonic tear film. Whether these AQPs play a role in the pathophysiology of dry eye conditions is unknown.
7.2 Corneal re-epithelialization
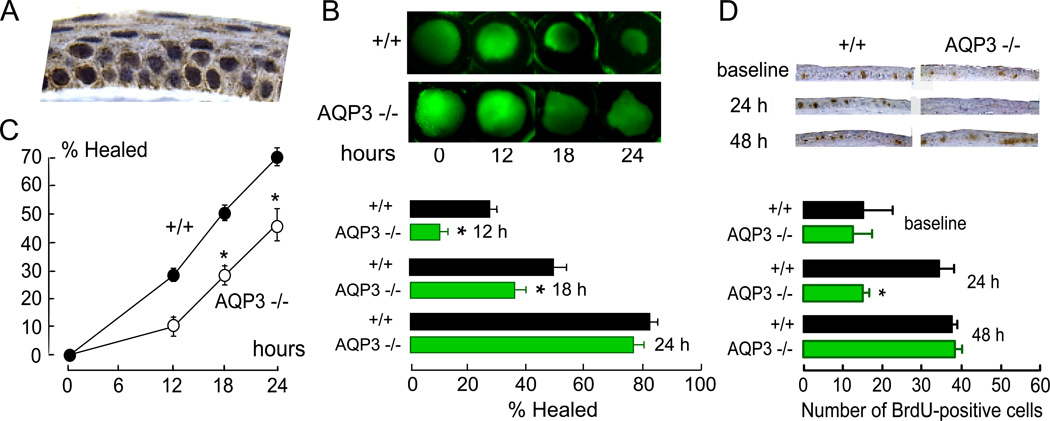
As mentioned above, AQP3 is expressed in corneal and conjunctival epithelia. Fig. 7A shows AQP3 immunostaining in plasma membranes of corneal epithelial cells. As mentioned in section 2.2, involvement of AQP3 in cell proliferation was suggested from skin and intestinal phenotypes in AQP3 null mice. We found significantly reduced osmotic water permeability and 14C-glycerol uptake in corneal epithelial cells from AQP3 null mice (Levin and Verkman, 2006), confirming functional AQP3 expression. Following debridement of a 2.3-mm diameter circular region of corneal epithelium with the limbus intact, re-epithelialization, quantified as defect area by fluorescein pooling, was significantly slowed in AQP3 null mice at 12 and 18 hours (Fig. 7B).
Figure 7. AQP3-dependent corneal epithelial cell proliferation.
A. AQP3 protein expression in mouse cornea. B. AQP3-dependent corneal re-epithelialization following wounding. (top) An epithelial defect (diameter 2.3 mm) was created at the center of corneas, and resurfacing was followed by fluorescein staining. (bottom) Percent of surface defect healed at 12, 18 and 24 h. C. AQP3-dependent corneal epithelial cell migration. Percent of wound resurfaced in organ culture. Eyes were enucleated after corneal scraping and incubated in medium containing serum. D. AQP3-dependent corneal epithelial cell proliferation. (top) Mid-peripheral cornea showing BrdU-immunoreactive cells (dark brown nuclei) before and at 24 or 48 h after corneal scraping. (bottom) Number of BrdU-positive cells in non-injured cornea (baseline) and during healing (SE, * p < 0.01). Adapted from Levin and Verkman (2006).
Distinct defects in corneal epithelial cell migration and proliferation were found to account for the delayed wound repair phenotype in AQP3 null mice (Levin and Verkman, 2006). Delayed closure of corneal wounds was found following corneal epithelial scraping in organ culture of enucleated globes from mice lacking AQP3, in which initial corneal resurfacing results from epithelial cell migration (as shown by BrdU analysis and 5-fluorouracil insensitivity; Fig. 7C). Slowed cell migration was also found in a scratch wound assay in primary cultures of corneal epithelial cells from AQP3 null vs. wildtype mice. A separate defect in corneal epithelial cell proliferation was shown by delayed restoration of full-thickness corneas over days following scraping, reduced density of proliferating BrdU-positive cells in AQP3 deficient mice, and reduced proliferation of AQP3 deficient primary cultures of corneal epithelial cells. Fig. 7D (top) shows representative images of mid-peripheral cornea stained for BrdU. As summarized in Fig. 7D (bottom), while no significant difference in BrdU staining was seen at baseline (non-wounded corneas), greatly diminished cell proliferation was seen in AQP3 deficient corneas at 24 hours after wounding. Based on our recent findings regarding mechanisms of AQP3-dependent epidermal cell proliferation described in section 2.2, we speculate that AQP3-dependent corneal epithelial cell proliferation may involve AQP3-facilitated glycerol transport and consequent metabolic alterations involving MAP kinase signaling and cellular energy balance. However, these mechanisms identified in skin cells will require direct testing in corneal epithelium.
7.3 Corneal endothelial fluid transport
The corneal endothelium is actually a low-resistance epithelium that permits passive hydrostatically driven flow of aqueous humor into the stroma. The endothelium also establishes opposing ion gradients via secondary active anion transport, driving water movement to maintain the relatively dehydrated stromal collagen matrix. Normally, the quiescent corneal endothelial cell population gradually diminishes over the human life span (reviewed by Joyce, 2003). Corneal dystrophies (most notably Fuchs’ dystrophy) and intraocular surgeries (including phacoemulsification) greatly accelerate this process (Yi and Dana, 2002). Decreased endothelial cell density eventually leads corneal edema and opacity, often necessitating penetrating keratoplasty. Stimulation of corneal endothelial pump function could thus reduce corneal swelling.
AQP1 is expressed in mouse, rat, and human corneal endothelial cells (Patil et al., 1997; Hamann et al., 1998; Levin and Verkman, 2004). Compared to primary cultures of corneal endothelia from wildtype mice, cultures from AQP1 knock-out mice had reduced osmotically driven membrane water permeability, but unimpaired near-isosmolar transcellular fluid transport (Kuang et al., 2004). Electro-osmotic coupling of fluid transport to recirculating currents through intercellular junctions has been proposed to explain AQP1-mediated water transport across the corneal endothelium (Fischbarg, 2003; Fischbarg and Diecke, 2005). This model assumes that AQP1 contributes primarily to cell volume regulation rather than to transcellular fluid flow. However, this mechanism cannot be easily reconciled with the thinned corneas of AQP1 null mice (see section 7.4), and the substantially slower rate of cell volume regulation vs. osmotic equilibration.
7.4 Involvement of AQPs in corneal stromal thickness, water permeability, and swelling response
Maintenance of corneal stromal transparency requires precise regulation of extracellular water content (Maurice, 1957; Freegard, 1997). In fixed eyes and in vivo by brightfield scanning confocal microscopy, corneas in AQP5 null mouse were ~20% thicker than corneas in wildtype mice, manifesting stromal, interepithelial, and intraepithelial fluid accumulation (Thiagarajah and Verkman, 2002; Levin and Verkman, 2006). In live mice, corneal swelling, as measured by confocal microscopy, was slowed ~2-fold in AQP5 null mice in response to exposure of the epithelial surface to hypotonic saline. Osmotically induced water transport across the full-thickness intact cornea was slowed ~5-fold in AQP5 null mice, as measured by a steady-state dye-dilution method.
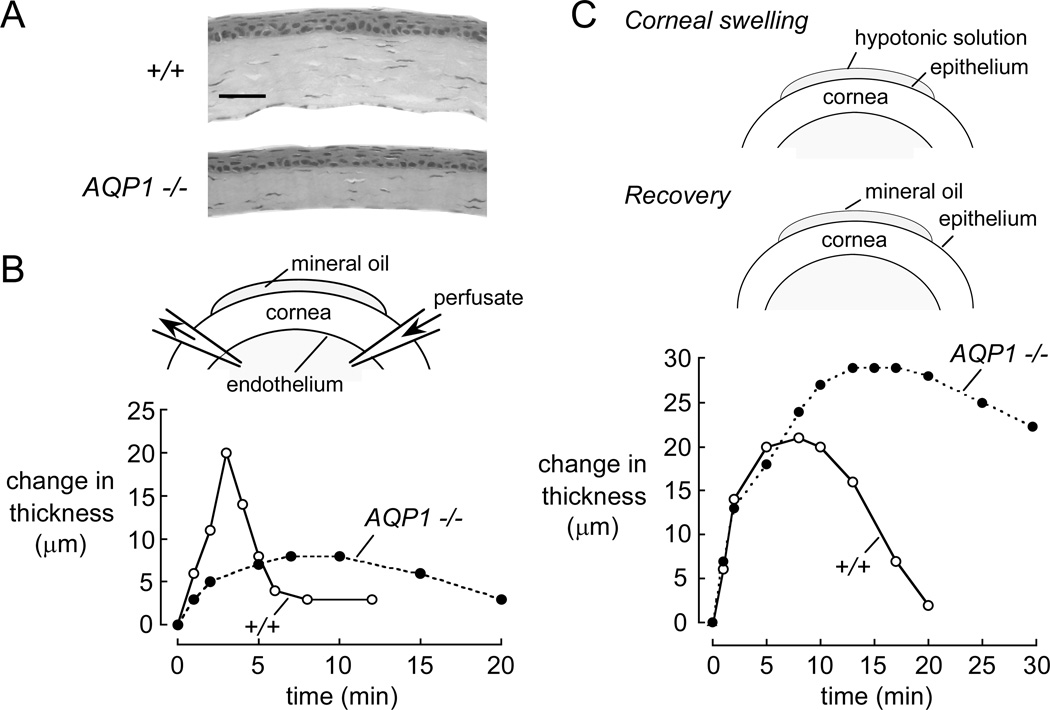
Corneal thickness was ~20% reduced in AQP1 deficiency (Fig. 8A), though baseline corneal transparency was grossly normal (Thiagarajah and Verkman, 2002). Curiously, electron microscopy of corneal stroma of AQP1 deficient mice showed a ~25% reduction in fibril spacing at baseline compared to wildtype stromas, though preserved collagen fibril diameter (unpublished data). This increased stromal collagen density may account for the reduced thickness of AQP1 null corneas and produce subtle impairment in corneal transparency in AQP1 deficiency. AQP1 water transport function was demonstrated in vivo by slowed corneal swelling upon hypotonic challenge at the endothelial surface utilizing an anterior chamber microperfusion method (Fig. 8B). Moreover, in an experimental model of corneal edema produced by 10 min exposure of the corneal surface to hypotonic solution, AQP1 deficiency was associated with remarkably slowed restoration of corneal transparency and thickness (Fig. 8C). The impaired recovery of corneal transparency in AQP1 null mice provides evidence that water extrusion from the corneal stroma traverses the corneal endothelium through AQP1, though the mechanisms responsible for this AQP1-dependent fluid extrusion remain unknown.
Figure 8. Involvement of AQP1 in maintenance of corneal transparency.
A. Reduced thickness in AQP1-deficient adult mouse corneas in paraffin-embedded central corneal sections. Bar, 50 µm. B. Osmotic water transport across the corneal endothelium. (top) Schematic showing micropipette placement for anterior chamber perfusion. (bottom) Time course of corneal thickness following corneal endothelial exposure to hypotonic saline in wildtype (open circles) and AQP1 null (filled circles) mice. C. Restoration of corneal thickness after osmotic swelling. (top) Transient exposure of the corneal surface to hypotonic saline to induce corneal swelling and follow recovery of thickness. (bottom) Time course of corneal thickness, measured in vivo by z-scanning confocal microscopy, following exposure of the corneal surface to hypotonic saline. Adapted from Thiagarajah and Verkman (2002).
7.5 Keratocyte function
AQP1 is also expressed in keratocytes (Hamann et al., 1998; Wen et al., 2001; Levin and Verkman, 2004), the primary resident corneal stromal cell type. Keratocytes synthesize extracellular matrix during development and show enhanced motility and signaling during inflammation and wound healing. Involvement of AQP1 in keratocyte migration in both cell culture and mouse models was found recently (Ruiz-Ederra and Verkman, 2008). In primary keratocyte cell cultures, AQP1 knockout or knockdown reduced keratocyte osmotic water permeability, as measured by calcein quenching, and migration speed, as measured in a scratch wound assay. In mice, following partial-thickness corneal stab injury, AQP1 expression in keratocytes was increased by 24 hours. Wound healing and keratocyte appearance near the wound margin were reduced in AQP1 knockout mice. The impairment in migration of AQP1-deficient keratocytes may be related to their inability to squeeze through the dense stromal matrix, similar to the impaired migration in AQP4 deficient glial cells toward a stab injury in brain (Auguste et al., 2007). The involvement of keratocyte/endothelial AQP1 in stromal dehydration could be further studied by non-invasive measurement of corneal development in wildtype vs. AQP1 knockout mice using confocal microscopy or optical coherence tomography, similar to what has been done in the lumican-deficient mouse (Song et al., 2003). It would also be interesting to study possible differences in stromal ground substance composition in AQP1 deficiency.
8. Directions
Phenotype studies of AQP knockout mice provide compelling evidence for involvement of AQPs in diverse functions outside of the eye, including the urinary concentrating mechanism, glandular fluid secretion, angiogenesis, wound healing, neural signal transduction, cell proliferation, and skin and fat cell glycerol metabolism. In the eye, AQPs appear to be important under some conditions in the maintenance of lens and corneal transparency, regulation of intraocular pressure, retinal signal transduction, and ocular tissue edema following injury. Direct evidence is needed to demonstrate relevance to the human eye of the findings in AQP knockout mice. Such evidence may be difficult to obtain, perhaps ultimately emerging from clinical trials using AQP-selective modulators, when available. Indirect studies, such as altered ocular AQP expression in various eye diseases in humans and animals models (Macnamara et al., 2004; Rabinowitz et al., 2005; Iandiev et al., 2005, 2007b; Kenney et al., 2004), may represent secondary responses and hence do not prove involvement of AQPs in the disease processes. Other important areas of basic research in the biology of ocular AQPs include elucidation of the precise cellular role of endothelial AQP1 in corneal fluid balance, of lens epithelial AQP1 in cataractogenesis, of corneal epithelial AQP3 in cell regeneration, and of retinal AQP4 in light-neural signal transduction and retinal fluid balance.
An intriguing possibility is the clinical development of modulators of AQP function or expression. At the ocular surface, AQP3 and AQP5 up-regulation are predicted to accelerate epithelial resurfacing and reduce corneal edema, respectively. Inducers of AQP1 expression in corneal endothelium might reduce corneal edema and associated opacity following injury. Induction of lens AQPs might slow cataractogenesis. AQP1/AQP4 inhibition represents a possible strategy for reducing intraocular pressure associated with glaucoma. In the retina, AQP4 inhibitors might offer neuroprotection in ischemic and other retinopathies. However, at this time these possibilities remain speculative, and will require experimental verification in large animal models and human clinical trials using small-molecule, genetic, or other types of AQP modulators.
Acknowledgments
Research from our lab was supported by grants EY13574, HL73856, DK72517, DK35124, HL59198, and EB00415 from the National Institutes of Health, and Drug Discovery and Research Development Program grants from the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, FAdams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki-Yoshino K, Uchihara T, Duyckaerts C, Nakamura A, Hauw JJ, Wakayama Y. Enhanced expression of aquaporin 4 in human brain with inflammatory diseases. Acta Neuropathol. (Berlin) 2005;110:281–288. doi: 10.1007/s00401-005-1052-2. [DOI] [PubMed] [Google Scholar]
- Auguste K, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4 deficient astroglial cells in mouse brain toward a site of injury. Faseb. J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Invest. 1999;103:555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp. Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjögren's syndrome. Lancet. 2001;358:1875–1876. doi: 10.1016/S0140-6736(01)06900-8. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant 'polymorphic' and lamellar cataracts linked to 12q. Nat. Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- Binder DK, Oshio K, Ma T, Verkman AS, Manley GT. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport. 2004a;15:259–262. doi: 10.1097/00001756-200402090-00009. [DOI] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 2004b;24:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Bok D, Dockstader J, Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J. Cell. Biol. 1982;92:213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Uckermann O, Pannicke T, Iandiev I, Reichenbach A, Wiedemann P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthal. Scand. 2005;83:528–538. doi: 10.1111/j.1600-0420.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- Candia OA. Electrolyte and fluid transport across corneal, conjunctival, and lens epithelia. Exp. Eye Res. 2004;78:527–535. doi: 10.1016/j.exer.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- Chou CL, Knepper MA, van Hoek AN, Brown D, Ma T, Yang B, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1-null mice. J. Clin. Invest. 1999;103:491–496. doi: 10.1172/JCI5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors NC, Adams ME, Froehner SC, Kofuji P. The potassium channel Kir4.1 associates with the dystrophin-glycoprotein complex via alpha-syntrophin in glia. J. Biol. Chem. 2004;279 doi: 10.1074/jbc.M402604200. 28387-2892. [DOI] [PubMed] [Google Scholar]
- Connors NC, Kofuji P. Potassium channel Kir4.1 macromolecular complex in retinal glial cells. Glia. 2006;53:124–131. doi: 10.1002/glia.20271. [DOI] [PubMed] [Google Scholar]
- Da T, Verkman AS. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest. Ophthalmol. Vis. Sci. 2004;45:4477–4483. doi: 10.1167/iovs.04-0940. [DOI] [PubMed] [Google Scholar]
- Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, Van Os CH, Van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Fan J, Donovan AK, Ledee DR, Zelenka PS, Fariss RN, Chepelinsky AB. Gamma E-crystallin recruitment to the plasma membrane by specific interaction between lens MIP/aquaporin-0 and gammaE-crystallin. Invest. Ophthalmol. Vis. Sci. 2004;45:863–871. doi: 10.1167/iovs.03-0708. [DOI] [PubMed] [Google Scholar]
- Farjo R, Peterson WM, Naash MI. Expression profiling after retinal detachment and reattachment: a possible role for aquaporin-0. Invest. Ophthalmol. Vis. Sci. 2008;49:511–521. doi: 10.1167/iovs.07-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J. Exp. Zoolog. A Comp. Exp. Biol. 2003;300:30–40. doi: 10.1002/jez.a.10306. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP. A mathematical model of electrolyte and fluid transport across corneal endothelium. J. Membr. Biol. 2005;203:41–56. doi: 10.1007/s00232-004-0730-7. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP, Kuang K, Yu B, Kang F, Iserovich P, Li Y, Rosskothen H, Koniarek JP. Transport of fluid by lens epithelium. Am. J. Physiol. 1999;276:C548–C557. doi: 10.1152/ajpcell.1999.276.3.C548. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Motoreano R. Osmotic permeabilities across corneal endothelium and antidiuretic hormone-stimulated toad urinary bladder structure. Biochim. Biophys. Acta. 1982;690:207–214. doi: 10.1016/0005-2736(82)90324-8. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Hasler L, Muller DJ, Stahlberg H, Kistler J, Engel A. Surface tongue-and-groove contours on lens MIP facilitate cell-to-cell adherence. J. Mol. Biol. 2000;300:779–789. doi: 10.1006/jmbi.2000.3920. [DOI] [PubMed] [Google Scholar]
- Francis P, Berry V, Bhattacharya S, Moore A. Congenital progressive polymorphic cataract caused by a mutation in the major intrinsic protein of the lens, MIP (AQP0) Br. J. Ophthalmol. 2000;84:1376–1379. doi: 10.1136/bjo.84.12.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freegard TJ. The physical basis of transparency of the normal cornea. Eye. 1997;11:465–471. doi: 10.1038/eye.1997.127. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Gropper M, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi Y, Mitsuoka K, de Groot BL, Philippsen A, Grubmuller H, Agre P, Engel A. Structure and function of water channels. Curr. Opin. Struct. Biol. 2002;12:509–515. doi: 10.1016/s0959-440x(02)00355-x. [DOI] [PubMed] [Google Scholar]
- Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am. J. Ophthalmol. 2006;141:761–763. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;429:193–197. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- Hamann S, Zeuthen T, La Cour M, Nagelhus EA, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am. J. Physiol. 1998;274:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7 deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 2008a;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 2008b;28:328–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-Angstrom resolution. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Lian SC, Finkbeiner WB, Verkman AS. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am. J. Physiol. 1994;266:C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. Faseb J. 1984;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Iandiev I, Pannicke T, Hartig W, Grosche J, Wiedemann P, Reichenback A, Bringmann A. Localization of aquaporin-0 immunoreactivity in the rat retina. Neurosci. Lett. 2007a;426:81–86. doi: 10.1016/j.neulet.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Iandiev I, Pannicke T, Reichel MB, Wiedemann P, Reichenbach A, Bringmann A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci. Lett. 2005;388:96–99. doi: 10.1016/j.neulet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Iandiev I, Pannicke T, Reichenbach A, Widemann P, Bringmann A. Diabetes alters the localization of glial aquaporins in rat retina. Neurosci. Lett. 2007b;421:132–136. doi: 10.1016/j.neulet.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Kang F, Kuang K, Li J, Fischbarg J. Cultured bovine corneal epithelial cells express a functional aquaporin water channel. Invest. Ophthalmol. Vis. Sci. 1999;40:253–257. [PubMed] [Google Scholar]
- Kenney MC, Atilano SR, Zorapapel N, Holguin B, Gaster RN, Ljubimov AV. Altered expression of aquaporins in bullous keratopathy and Fuchs’ dystrophy corneas. J. Histochem. Cytochem. 2004;52:1341–1340. doi: 10.1177/002215540405201010. [DOI] [PubMed] [Google Scholar]
- Kim IB, Lee EJ, Oh SJ, Park CB, Pow DV, Chun MH. Light and electron microscopic analysis of aquaporin 1-like-immunoreactive amacrine cells in the rat retina. J. Comp. Neurol. 2002;452:178–191. doi: 10.1002/cne.10359. [DOI] [PubMed] [Google Scholar]
- Kim IB, Oh SJ, Nielsen S, Chun MH. Immunocytochemical localization of aquaporin 1 in the rat retina. Neurosci. Lett. 1998;244:52–54. doi: 10.1016/s0304-3940(98)00104-9. [DOI] [PubMed] [Google Scholar]
- King LS, Choi M, Fernandez PC, Cartron JP, Agre P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N. Engl. J. Med. 2001;345:175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- Kuang K, Yiming M, Wen Q, Li Y, Ma L, Iserovich P, Verkman AS, Fischbarg J. Fluid transport across cultured layers of corneal endothelium from aquaporin-1 null mice. Exp. Eye Res. 2004;78:791–798. doi: 10.1016/j.exer.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lemp MA. Report of the National Eye Institute/Industry Workshop on clinical trials for dry eyes. CLAO. J. 1995;21:221–232. [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis, binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest. Ophthalmol. Vis. Sci. 2004;46:1428–1434. doi: 10.1167/iovs.04-0816. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Ophthalmol. Vis. Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- Li J, Patil RV, Verkman AS. Mildly abnormal retinal function in transgenic mice without Müller cell aquaporin-4 water channels. Invest. Ophthalmol. Vis. Sci. 2002;43:573–579. [PubMed] [Google Scholar]
- Li J, Verkman AS. Impaired hearing in mice lacking aquaporin-4 water channels. J. Biol. Chem. 2001;276:31233–31237. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Lu D, Zhang H, Zador Z, Verkman AS. Impaired olfaction in mice lacking aquaporin-4 water channels. Faseb. J. 2008 doi: 10.1096/fj.07-104836. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J. Clin. Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002;277:17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnamara E, Sams GW, Smith K, Ambati J, Singh N, Ambati BK. Aquaporin-1 expression is decreased in human and mouse corneal endothelial dysfunction. Mol. Vis. 2004;10:51–56. [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen A, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema following acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Marmor MF. Mechanisms of fluid accumulation in retinal edema. Doc. Ophthalmol. 1999;97:239–249. doi: 10.1023/a:1002192829817. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. J. Membr. Biol. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. J. Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Hedbys BO. The permeability of the corneal epithelium and endothelium to water. Exp. Eye Res. 1967;6:10–32. doi: 10.1016/s0014-4835(67)80049-6. [DOI] [PubMed] [Google Scholar]
- Misu T, Fujihara K, Nakamura M, Murakami K, Endo M, Konno H, Itoyama Y. Loss of aquaporin 4 in active perivascular lesions in neuromyelitis optica: a case report. Tohoku. J. Exp. Med. 2006;209:269–275. doi: 10.1620/tjem.209.269. [DOI] [PubMed] [Google Scholar]
- Moore M, Ma T, Yang B, Verkman AS. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp. Eye Res. 2000;70:557–562. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Horio A, Inanobe Al, Fujita FM, Haug S, Nielson S, Kurachi Y, Ottersen OP. Immungold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki ML, Torp R, Haug F, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J. Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Newman EA. Distribution of potassium conductance in mammalian Müller (glial) cells: a comparative study. J. Neurosci. 1987;7:2423–2432. [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Smith BI, Christensen EI, Agre PA. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest. Ophthalmol. Vis. Sci. 2003;44:4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. Faseb. J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat. Meth. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Edwards A, Ma T, Silldorff E, Verkman AS. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J. Clin. Invest. 2000;105:215–222. doi: 10.1172/JCI8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Krishna S, Manley GT, Verkman AS. Aquaporin-4 facilitates the reabsorption of excess fluid in vasogenic brain edema. Faseb. J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008 doi: 10.1007/s00424-007-0357-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp. Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 2006;63:964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS, Dong L, Wistow G. Gene expression studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest. Ophthalmol. Vis. Sci. 2005;46:1239–1246. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, WIngerchuk DM, Lucchinetti CF. Pattern specific loss of aquaporin 4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Rojek A, Praetorius J, Frokjaer J, Nielson S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008 doi: 10.1146/annurev.physiol.70.113006.100452. In press. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Rok D. Characterization of the 3’-UTR sequence encoded by the AQP-1 gene in human retinal pigment epithelium. Biochim. Biophys. Acta. 1996;1282:174–178. doi: 10.1016/0005-2736(96)00076-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Accelerated cataract formation and reduced lens epithelial water permeability in aquaporin-1deficient mice. Invest. Opthalmol. Vis. Sci. 2006a;47:3960–3967. doi: 10.1167/iovs.06-0229. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Mouse model of sustained elevation in intraocular pressure produced by episcleral vein occlusion. Exp. Eye Res. 2006b;82:879–884. doi: 10.1016/j.exer.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Aquaporin-1-facilitated keratocyte migration in cell culture and in vivo corneal wound healing models. Invest. Opthal. Vis. Sci. 2008 doi: 10.1016/j.exer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Zhang H, Verkman AS. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Müller cells. J. Biol. Chem. 2007;282:21866–21872. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Aquaporin-1 independent microvessel proliferation in a neonatal mouse model of oxygen induced retinopathy. Invest. Opthalmol. Vis. Sci. 2007;48:4802–4810. doi: 10.1167/iovs.07-0537. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted of aquaporin-1 gene disruption. Nature. 2005a;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 2005b;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr. Eye Res. 2007;32:923–929. doi: 10.1080/02713680701733076. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat. Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, Varadaraj K, Mathias R, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol. Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Kirk J, Herron B, Fitzgerald U, McQuaid S. Absence of aquaporin 4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normalappearing white matter. Acta Neuropathol. 2007;113:187–194. doi: 10.1007/s00401-006-0169-2. [DOI] [PubMed] [Google Scholar]
- Song IK, Joo CK. Morphological and functional changes in the rat cornea with an ethanol-mediated epithelial flap. Invest. Ophthalmol. Vis. Sci. 2004;45:423–428. doi: 10.1167/iovs.03-0947. [DOI] [PubMed] [Google Scholar]
- Song J, Lee YG, Houston J, Petroll WM, Chakravarti S, Cavanagh HD, Jester JV. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest. Ophthalmol. Vis. Sci. 2003;44:548–557. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J. Gen. Physiol. 2001;117:573–582. doi: 10.1085/jgp.117.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Sonawane N, Verkman AS. Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J. Physiol. 2002;541:561–568. doi: 10.1113/jphysiol.2001.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J. Biol. Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Peppel K, O’Donnell ME, Roberts BC, Wu F, Epstein DS. Expression of aquaporin-1 in human trabecular meshwork cells: role in resting cell volume. Invest. Ophthalmol. Vis. Sci. 2001;42:1803–1811. [PubMed] [Google Scholar]
- Stamer WD, Bok D, Hu J, Jaffe GJ, McKay BS. Aquaporin-1 channels in the human retinal pigment epithelium: role in transepithelial water movement. Invest. Ophthalmol. Vis. Sci. 2003;44:2803–2808. doi: 10.1167/iovs.03-0001. [DOI] [PubMed] [Google Scholar]
- Stroud RM, Nollert P, Miercke L. The glycerol facilitator GlpF its aquaporin family of channels, and their selectivity. Adv. Protein Chem. 2003;63:291–316. doi: 10.1016/s0065-3233(03)63011-1. [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O'Connell J, Stroud RM, Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. 2002, [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J. Biol. Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Zhao D, Verkman AS. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut. 2007;56:1529–1535. doi: 10.1136/gut.2006.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjögren's syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- Verbavatz JM, Brown D, Sabolic I, Valenti G, Ausiello DA, Van Hoek AN, Ma T, Verkman AS. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J. Cell. Biol. 1991;23:605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochem. Biophys. Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG. Neuromyelitis optica is distinct from multiple sclerosis. Arch. Neurol. 2007;64:899–901. doi: 10.1001/archneur.64.6.899. [DOI] [PubMed] [Google Scholar]
- Wen Q, Diecke FP, Iserovich P, Kurang K, Sparrow J, Fischbarg J. Immunocytochemical localization of aquaporin-1 in bovine corneal endothelial cells and keratocytes. Exp. Biol. Med. (Maywood) 2001;226:463–467. doi: 10.1177/153537020122600512. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Neonatal mortality in an aquaporin-2 knock-in mouse model of recessive nephrogenic diabetes insipidus. J. Biol. Chem. 2001;276:2775–2779. doi: 10.1074/jbc.M008216200. [DOI] [PubMed] [Google Scholar]
- Yi DH, Dana MR. Corneal edema after cataract surgery: incidence and etiology. Semin. Ophthalmol. 2002;17:110–114. doi: 10.1076/soph.17.3.110.14783. [DOI] [PubMed] [Google Scholar]
- Zador Z, Magzoub M, Jin S, Manley GT, Papadopoulos MC, Verkman AS. Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain. FASEB. J. 2008;22:870–879. doi: 10.1096/fj.07-9468com. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Exp. Eye Res. 2000;71:415–435. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol. Cell. Neurosci. 2008;37:1–10. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Vetrivel L, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J. Gen. Physiol. 2002;119:561–569. doi: 10.1085/jgp.20028597. [DOI] [PMC free article] [PubMed] [Google Scholar]