Abstract
Beta-amyloid (Aβ), a vasoactive protein, and elevated blood pressure (BP) levels are associated with Alzheimer’s disease (AD) and possibly vascular dementia (VaD). We investigated the joint association of mid-life BP and Aβ peptide levels with the risk for late-life AD and VaD. Subjects were 667 Japanese-American men (including 73 with a brain autopsy), from the prospective Honolulu Heart Program/Honolulu Asia Aging Study (1965 – 2000). Mid-life BP was measured starting in 1971 participants mean age 58 years, Aβ was measured in specimens collected1980/82, and assessment of dementia and autopsy collection started in 1991/93. The outcome measures were prevalent (present in 1991/3) and incident AD (n= 53, including 38 with no contributing cardiovascular disease), and VaD (n=24). Cerebral amyloid angiopathy (CAA), β-amyloid neuritic plaques, and neurofibrillary tangles were evaluated in post-mortem tissue. The risk for AD significantly increased with lower levels of plasma Aβ (Aβ1-40 hazard ratio (HR) 2.1, 95% confidence interval (CI) 1.4 – 3.1; Aβ1-42 HR 1.6, 95% CI 1.1 – 2.3). Evidence of interaction between diastolic BP and plasma Aβ (1-40 pinteraction <0.05; 1-42 pinteraction <0.07) levels, indicated the Aβ-related risk for AD was higher when BP was higher. Low plasma Aβ was associated with the presence of CAA (ptrend<0.05), but not the other neuropathologies. Aβ plasma levels start decreasing at least 15 years before AD is diagnosed, and the association of Aβ to AD is modulated by mid-life diastolic BP. Elevated BP may compromise vascular integrity leading to CAA and impaired Aβ clearance from the brain.
Keywords: Amyloid, blood pressure, brain, aging, dementia
Introduction
Alzheimer’s disease (AD) has been linked to cardiovascular risk factors, such as diabetes, hyperlipidemia, and in particular elevated blood pressure (BP).1, 2 Several reports, including one from the Honolulu Asia Aging Study (HAAS),3, 4 have shown the risk for late life Alzheimer’s disease (AD) and cognitive impairment [5] is more closely associated with elevated BP in midlife compared to late-life, when BP levels tend to decline, possibly as a consequence of the dementing process.4, 6 These findings have importantly contributed to our understanding that dementia processes begin early and may involve vascular changes.
Amyloid beta (Aβ) 1-40 and Aβ1-42 peptides are present in AD neuropathologic lesions 7–9 and it is hypothesized beta-amyloid (Aβ) peptide abnormalities begin early in the neurodegenerative pathological cascade.10,11 However, in addition to neurotoxicity, Aβ peptides also enhance vasoconstriction,12 and contribute to cerebral vascular pathology and dysfunction when deposited in microvessels.13 Either from increased production or decreased clearance of Aβ,14 abnormal processing of amyloid precursor protein leads to higher levels of Aβ in cortical tissue and vessels, and lower levels in the plasma.
Aβ1-40 and Aβ1-42 levels are the two AD biomarkers that can be measured in the plasma, and are thus feasible to determine in large samples. Several large community-based studies 15–19 have reported on the association of plasma Aβ levels to AD. Study results are mixed but do suggest plasma levels may be differentially associated with the risk for AD, reflect the stage of the disease and patterns of production in, and clearance from the brain.
Given the effect of Aβ on the vasculature, and the separate associations of Aβ and BP to dementia, the interaction of the two factors is of interest in the study of the pathophysiology and prevention of AD. Since the associations of both Aβ and BP may differ depending on the stage of disease they are measured, having measures of these exposures years before the clinical onset of disease can contribute to our understanding of the trajectory to AD. Here, we examined the risk for late-life dementia to midlife BP and Aβ measured on average 8 years after BP was measured. We hypothesized high blood pressure would alter the cerebral vasculature, levels of Aβ in the brain, and their association with the risk for dementia. Subjects are Japanese- American men who participated over 30 years in the population-based Honolulu Heart Program/Honolulu-Asia Aging Study.
Methods
Study population
The Honolulu-Asia Aging Study (HAAS) is an extension of the Honolulu Heart Program (HHP), a longitudinal population-based study of heart disease and stroke. The HHP cohort consisted of Japanese-American men born from 1900–1919 and living on Oahu at the time of the enrollment exam in 1965/68 (exam 1, n=8006). Follow-up HHP exams occurred from 1967/70 (exam 2, n = 7498) and from 1971/74 (exam 3, n = 6860), with participation rates among survivors of 85.2% and 83.8%, 20 respectively. A lipoprotein sub-study (L) was also implemented with exams in 1970/72 (L1, n = 2780), 1975/78 (L2, n = 2386), and 1980/82 (L3, n = 1965). The lipoprotein sub-sample included a 30% random sample with the remainder of the sample chosen on the basis of the presence of hyperlipidemia and coronary heart disease.21
From 1991–93, surviving HHP participants were invited to join the HAAS22 80% agreed to participate and had a successfully administered test of global cognitive function (exam 4, n = 3734). Three subsequent follow-up visits (exams 5-7) were conducted through to 2000, the end date of data collected for this analysis. Through the duration of HAAS, the brain of consenting participants was autopsied to evaluate neuropathology. All subjects were invited for inclusion in the autopsy sub-study, with an emphasis on recruiting cases of dementia. Overall 23% consented to autopsy, including, as planned a priori, 35% for those with a dementia diagnosis, and 17% in the non-demented group. The autopsied cases were not different from non-autopsied decedent cases, similarly non-demented autopsied were similar to non-demented decedents.23 The study was approved by the Institutional Review Boards of the Kuakini Medical Center, the Honolulu Department of Veterans Affairs, and the National Institute on Aging. All participants gave written informed consent. Consents for autopsy were provided by a next-of-kin family member or a legally authorized alternative.
Blood pressure measurement
At each exam, blood pressure was measured three times, five minutes apart, on the left arm of a seated subject with a standard sphygmomanometer. Measures were averaged to provide a mean exam value. For this study, mid-life systolic and diastolic BP were calculated by averaging BP measures from HHP exams 1 and 3. We also calculated pulse pressure from the BP variables.
Plasma Aβ determinations
Plasma Aβ was measured with a sandwich ELISA developed by Eli Lilly and optimized by the Laboratory for Clinical Biochemistry Research at the University of Vermont College of Medicine. The antibodies, stock standard proteins, and heat inactivated rat plasma were provided by Eli Lilly. The capture antibody for the Aβ1-40 assay was 2G3 and for the Aβ1-42 assay was 21F12. Plasma samples were denatured using guanidine hydrochloride. Standard stock RS0546 was diluted to range from 250 to3.9 pg/ml for the Aβ1-40 assay. Standard stock RS0548 was diluted to range from 125 to 0.49 pg/ml for the Aβ1-42 assay. Biotinylated 3D6 was the detection antibody for both assays. Streptavidin conjugated HRP provided enzyme activity for detection using TMB as a substrate. Inter-assay coefficients of variation ranged from 3.1% to 7.9% for the Aβ1-40 and 12% to 20% for the Aβ1-42 assay.
Diagnosis of dementia
Diagnosis of prevalent dementia at the first late-life visit (exam 4), or incident dementia at subsequent follow-up visits (exams 5-7), has been previously detailed.22 Briefly, all participants were administered the Cognitive Abilities Screening Instrument (CASI);24 screen positive subjects underwent further neuropsychological25 and neurological evaluation, and a proxy was interviewed. Those with a provisional diagnosis of dementia were further evaluated with blood tests and neuroimaging. A neurologist and at least two additional physicians with geriatrics and dementia expertise evaluated participant data, and came to a consensus diagnosis of dementia based on criteria from the DSM – 3rd edition revised.26 AD was diagnosed using the NINCDS-ADRDA criteria,27 and vascular dementia (VaD) was diagnosed using ADDTC criteria.28 Dementia of other sub-types included that due to Parkinson’s disease, vitamin B12 deficiency, head trauma, progressive supranuclear palsy, or subdural hematoma. Subjects could be diagnosed with a primary and contributing sub-type of dementia. The most common form of mixed sub-types was AD with CVD, which includes subjects with signs and symptoms consistent with diagnostic criteria for AD and VaD.
Neuropathology assessment
Methods for the HAAS autopsy study have been described previously.23 Briefly, initial microscopic examinations were done on paraffin embedded hematoxylin and eosin–stained sections from 32 brain regions. Selected regions were also immunostained for ubiquitin and the 10-D-5 antibody for beta amyloid. Eight micrometer sections of the middle frontal gyrus, superior middle temporal gyri, inferior parietal lobule, and occipital association cortex along the calcarine sulcus were stained by a modified Bielschowsky method. Presence or absence of cerebral amyloid angiopathy (CAA) was assessed in a microscopic examination of parenchymal vessels in four neocortical areas: the middle frontal gyrus, superior-middle temporal gyrus, inferior parietal lobule, and occipital association cortex along the calcarine sulcus. Measures were standardized among three raters according to published guidelines.29 Measures of neuritic plaques and neurofibrillary tangles were recorded as the average number of each across 20 neocortical fields.
Putative confounding factors
Based on previous studies the following putative confounders were considered: age, education, Apolipoprotein E (ApoE) genotype,30 and cardiovascular (CV) risk factors and disease. The CV variables included total cholesterol, triglycerides, insulin, and a history of antihypertensive treatment (self-reported from exams 1 to 3 and obtained from the drug vials presented at exam 4); diabetes (defined as a self reported doctor’s diagnosis of type 2 diabetes, taking oral hypoglycemic medications or insulin, or a fasting and 2-h glucose levels according to published guidelines,31 and coronary and cerebrovascular disease (obtained from the continuous surveillance of hospital discharge and death records on Oahu).
Sub-study sample
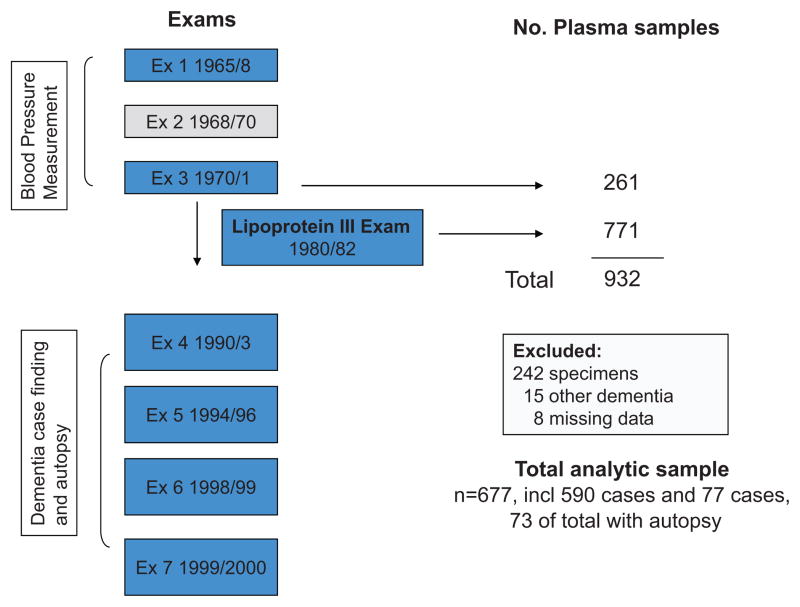
To reduce the variability in storage time, and based on the availability of stored plasma samples in the largest number of subjects at the same exam cycle, we selected subjects from the third HHP exam (n=261) and the third lipoprotein exam (n=771) [Figure 1). This gave us a sample of 932 including 142 dementia cases and 790 controls, as determined by the subject’s status at his last visit prior to 2000. When we assayed the samples, 242 (including all exam 3 and 5 L3 samples) were poor quality and were excluded from the analysis. The 682 with successfully processed plasma Aβ, included 590 controls and 92 cases of dementia. Comparing the included to excluded controls, there were no significant differences in age at exam 3, mid-and late-life blood pressures, or stroke and diabetes history. Similarly, these variables did not differ between the included and excluded cases nor did the distribution of dementia subtype cases differ.
Figure 1.
Design of the HAAS Aβ Sub-Study
For the final analysis we focused on AD and VaD cases; VaD cases were included based on reports of an association between plasma Aβ levels and vascular dementia.17 We excluded the 15 subjects diagnosed dementias other than-AD or –VaD. A further 8 subjects with missing data on any one of the covariates were excluded, giving an analytical sample of 667. This sample included 77 cases of dementia that were sub-typed as follows: 53 ‘All AD,’ (includes participants diagnosed with AD, with or without contributing cardiovascular disease); 38 cases of ‘Pure AD,’ (the sub-set of All AD that were diagnosed with AD and no contributing cardiovascular disease); and 24 cases of VaD (with no contribution of AD pathology). Of the 667, 73 subjects (including 24 cases) also participated in the autopsy sub-study. Compared to the other participants seen at the HHP/HAAS exam 4, this sub-sample was younger, and had significantly higher levels of total cholesterol and triglycerides, and lower levels of HDL (e-Table S1, please see http://hyper.ahajournals.org), as would be expected based on the design of the Lipoprotein sub-study.
Statistical analysis
Subject characteristics were compared across BP strata, Aβ1-40 and Aβ1-42 quartiles and presence of dementia, with linear and logistic regression adjusting for age at exam 3. For analyses of Aβ1-40 and Aβ1-42 as continuous measures the distributions were normalized by log-transforming the values. The Aβ40/42 ratio was calculated as [log(Aβ1-40)]/[log(Aβ1-42)]. Linear regression was used to examine the association of mid-life BP to plasma Aβ.
Cox proportional hazards regression was used to analyze the associations of late-life dementia to midlife BP and Aβ with age at exam 3 as the measure of follow-up time.32. Subjects were left-censored at the age of study entry, and right-censored at the age of event defined as follows: for prevalent dementia cases at exam 4, the event was dated two years before exam 4, for incident dementia cases at exams 5-7, the event was dated halfway between the incident exam and the exam immediately preceding. Participants were censored at the date of death for patients who died with no dementia diagnosis, and at the end of follow-up for participants not diagnosed with dementia. BP and log-transformed Aβ were standardized to evaluate the risk of dementia for every 1 standard deviation change. In the BP and Aβ analyses respectively, non-linear associations were examined by entering quadratic and single terms into the model; the quadratic terms were not significant. To control for confounding and to reduce variation from known factors, our final models were adjusted for: education (number of years of formal education), ApoE genotype (any ε2, any ε4, ε3/ε3, and a dummy variable for missing genotype data (n=34) ); and time between the event and the date of blood draw for the Aβ measures. We included in the models the other putative confounders described above, but the conclusions were not changed. These variables were subsequently removed from the models because the sample size in the higher blood pressure range was relatively small and the models became unstable as more variables were included
For the AD models, we tested the interaction between Aβ and BP by including in the models the cross-product term “Aβ x blood pressure” with main effect terms of each. There was evidence of interaction between BP, Aβ with AD (statistical significance of main effects was indicated at p < 0.05, and of interaction terms at p < 0.10). To better understand and visualize these interactions we examined the association of Aβ and BP to AD within blood pressure strata defined as follows: systolic blood pressure: < 110 mm Hg, 110–139 mm Hg, and ≥ 140 mm Hg; diastolic blood pressure: < 80 mm Hg, 80–89 mm Hg, and ≥ 90 mm Hg. All analyses were adjusted for education, ApoE genotype, and the number of days between the Aβ blood draw and the censor date.
To a large extent, plasma Aβ reflects the balance between the build-up of Aβ in the brain and its clearance through cerebral vessels.33 Therefore there should be evidence in neuropathologic data that is concordant with the findings based on the plasma Aβ data. To this end we examined the association of Aβ tertiles to neuropathology. For plaque and tangle pathology we used regression models based on a Poisson distribution, and for CAA we used models based on a binomial distribution. Due to the relatively small sample size, models were only adjusted for age and interval between blood draw and death.
Analyses were conducted with SAS statistical software, release 9.1 (SAS Institute, Cary, NC).
Results
Participants were a mean age of 58.9 (range 52–73) years at exam 3, mean age of 67.2 (range 61–82) years when the plasma sample for Aβ was obtained, and a mean age of 83.0 (range 72–97) years at the diagnosis/censoring event. The average time between the plasma draw and event was 15.8 years. Plasma Aβ1-40 levels were higher as exam 3 age, blood pressure and lipid levels increased (Table 1). Plasma Aβ1-42 levels increased as exam 3 blood pressure and HDL levels increased. Additional subject characteristics by quartile of plasma Aβ1-40 and -42 levels and by dementia type are given in e-Tables S2 – S4 (please see http://hyper.ahajournals.org).
Table 1.
Subject characteristics and their relationship to plasma Aβ1-40 and Aβ1-42: HAAS Aβ sub-study
| Characteristics* | Study sample | Aβ1-40† | Aβ1-42† |
|---|---|---|---|
| N=667 | Beta (SE)‡ | Beta (SE)‡ | |
| Age at exam 3, years | 58.9 (4.4) | 0.086 (0.04)§ | 0.016 (0.04) |
| Age at event, years | 83.0 (4.4) | −0.018 (0.06) | 0.014 (0.06) |
| Aβ draw to event, years | 15.8 (3.1) | −0.010 (0.04) | −0.002 (0.04) |
| Education, years | 10.6 (3.2) | 0.002 (0.04) | 0.044 (0.04) |
| Stroke, n (%) | 45 (7) | 0.196 (0.15)ll | 0.141 (0.15)ll |
| Diabetes, n (%) | 279 (41) | 0.096 (0.08)ll | 0.132 (0.08)ll |
| ApoE genotype, n (%) | |||
| 33 | 455 (68) | 0.038 (0.05)# | 0.038 (0.05)# |
| 22 or 23 | 53 (8) | −0.075 (0.14)ll | 0.056 (0.14)ll |
| 34 or 44 | 125 (19) | −0.130 (0.10)ll | −0.208 (0.10)ll |
| Missing value | 34 (5) | −0.144 (0.18)ll | −0.076 (0.18)ll |
| Dementia, n (%) | |||
| Total | 77 (12) | −0.121 (0.12)ll | −0.101 (0.13)ll |
| All AD | 53 (8) | −0.236 (0.15)ll | −0.216 (0.15)ll |
| Pure AD | 38 (6) | −0.359 (0.17)§ll | −0.302 (0.17)ll |
| VaD | 24 (4) | 0.137 (0.21)ll | 0.152 (0.21)ll |
| Blood pressure, mmHg | |||
| SBP | 132.7 (16.7) | 0.081 (0.04)§ | 0.111 (0.04)§ |
| DBP | 83.1 (9.5) | 0.058 (0.04) | 0.107 (0,04)§ |
| Pulse pressure | 49.6 (11.0) | 0.074 (0.04) | 0.077 (0.04)§ |
| Lipid at exam 1, mg/dL | |||
| Triglycerides | 295.3 (269.4) | 0.074 (0.04)** | −0.049 (0.04)** |
| Cholesterol | 236.3 (44.5) | 0.066 (0.04) | 0.077 (0.04)§ |
Mean (SD) unless otherwise indicated;
log-transformed and standardized;
adjusted for age at exam 3, beta for 1 standard deviation increase;
p ≤ 0.05;
adjusted difference with reference;
adjusted mean;
triglycerides are log-transformed and standardized for computation of beta.
SBP = systolic blood pressure; DBP = diastolic blood pressure; event includes death, dementia or end of follow-up.
Plasma Aϐ, dementia 15 years later, and neuropathology
One standard deviation decrease in plasma Aβ1-40 and in plasma Aβ1-42 was significantly associated with an increased risk for ‘all AD’ and for ‘pure AD’ (Table 2). The linear trend for risk of AD across Aβ quartiles was also significant (Aβ1-40 plinear trend < 0.001; Aβ1-42 plinear trend = 0.01). A lower Aβ1-40/Aβ1-42 ratio was significantly associated with a decreased risk of AD (plinear trend ≤ 0.05). Plasma Aβ levels were not associated with VaD in the main effects analyses.
Table 2.
Risk of late-life dementia with plasma Aβ levels 15 years prior to dementia diagnosis: HAAS Aβ sub-study
| β Amyloid quartiles (from low to high) | β Amyloid | ||||
|---|---|---|---|---|---|
| Dementia sub-type | Q1 (n=166) | Q2 (n=167) | Q3 (n=167) | Q4 (n=167) | Per -1 S.D. |
| Aβ1-40 | |||||
| All AD | 4.3 (1.9–9.8)* | 3.0 (1.3–7.1) | 1.8 (0.8–4.2) | 1 (ref)† | 2.1 (1.4–3.1) |
| Pure AD | 4.0 (1.5–10.7) | 2.8 (1.0–7.6) | 1.1 (0.4–3.5) | 1 (ref)† | 2.1 (1.3–3.5) |
| Vascular dementia | 1.5 (0.4–5.2) | 1.1 (0.3–3.4) | 1.2 (0.4–3.4) | 1 (ref) | 1.0 (0.6–1.8) |
| Aβ1-42 | |||||
| All AD | 3.1 (1.2–8.0) | 3.5 (1.4–8.7) | 2.5 (1.0–6.3) | 1 (ref)† | 1.6 (1.1–2.3) |
| Pure AD | 4.3 (1.2–15.9) | 4.3 (1.2–15.3) | 3.5 (1.0–12.6) | 1 (ref)† | 1.7 (1.1–2.6) |
| Vascular dementia | 0.8 (0.2–2.9) | 1.4 (0.5–4.3) | 1.2 (0.4–3.6) | 1 (ref) | 0.9 (0.6–1.4) |
| Aβ1-40/Aβ1-42 ratio | |||||
| All AD | 0.5 (0.2–1.2) | 0.7 (0.3–1.6) | 1.0 (0.5–2.1) | 1 (ref) | 0.8 (0.6–1.0) |
| Pure AD | 0.5 (0.2–1.4) | 0.6 (0.2–1.6) | 0.9 (0.4–2.1) | 1 (ref) | 0.7 (0.6–1.0) |
| Vascular dementia | 1.2 (0.4–3.7) | 1.1 (0.3–3.6) | 1.4 (0.4–5.1) | 1 (ref) | 1.1 (0.7–1.7) |
Hazard ratio (95% CI) adjusted for apolipoprotein E genotype, education and time from blood draw to censoring event;
plinear trend < 0.05
The prevalence of CAA increased significantly as the tertile of plasma Aβ1-42 levels decreased, and as the tertiles of Aβ1-40/Aβ1-42 ratio increased (Table 3). No statistical trends were found in the average number of neocortical neuritic plaques or neurofibrillary tangles across tertiles of Aβ1-40, Aβ1-42, or the Aβ1-40/Aβ1-42 ratio (e-Table S5; please see http://hyper.ahajournals.org).
Table 3.
Frequency of cerebral amyloid angiopathy at autopsy by tertile of plasma Aβ: HAAS Aβ sub-study
| Aβ sub-species | Aβ tertile (from low to high)
|
|||
|---|---|---|---|---|
| T1 | T2 | T3 | p trend* | |
| N (%)† | N (%)† | N (%)† | ||
| Aβ1-40 | 18 (24.2) | 27 (42.4) | 28 (33.3) | 0.39 |
| Aβ1-42 | 24 (48.5) | 31 (36.6) | 18 (15.2) | 0.02 |
| Aβ1-40/Aβ1-42 ratio | 20 (12.1) | 25 (33.3) | 28 (54.6) | 0.008 |
Adjusted for age at exam 3, time from blood draw to death;
percent with cerebral amyloid angiopathy.
The interaction between BP, Aϐ and the risk for dementia sub-type
Aβ1-40 and Aβ1-42 levels increased as diastolic and systolic BP increased (Table 1). In the ‘all AD’ models, the interaction between Aβ1-40 and DBP (p < 0.05) and between Aβ1-42 and DBP (p < 0.07) provided significant evidence of interaction. The interaction suggested the risk for ‘all AD’ increased with each standard deviation decrease in plasma Aβ, but the risk logarithmically increased as DBP increased. To better understand the interaction we examined the association of Aβ levels within strata of DBP (Table 4). The risk estimates of Aβ levels to ‘all AD’ were significantly higher in the high DBP group (for Aβ1-40, p =0.05; for Aβ1-42, p=0.03, for the ratio Aβ1-40/Aβ1-42, p=0.08). Trends were similar in the ‘pure AD’ analyses; the interaction between DBP and Aβ1-40 was significant (p=0.1). Models for AD and SBP, or AD and PP did not suggest interaction.
Table 4.
Risk of late-life dementia for every 1 SD decrease in plasma Aβ by mid-life blood pressure group: HAAS Aβ sub-study
| Dementia | Systolic BP* | Diastolic BP* | ||||
|---|---|---|---|---|---|---|
| Low | Normal | High | Low | Normal | High | |
| < 110 mm Hg | 110 – 139 mm Hg | ≥ 140 mm Hg | < 80 mm Hg | 80 – 89 mm Hg | ≥ 90 mm Hg | |
| N=44 | N=432 | N=191 | N=244 | N=271 | N=152 | |
| All AD, n (%) | 6 (14.0) | 37 (8.8) | 10 (5.5) | 27 (11.5) | 19 (7.2) | 7 (4.9) |
| Aβ1-40 | 1.4 (0.2–9.3) | 1.9 (1.2–3.0) | 2.2 (0.9–5.6) | 1.3 (0.7–2.3) | 2.6 (1.3–5.1) | 3.9 (1.5–10.3)† |
| Aβ1-42 | 1.8 (0.4–7.5) | 1.3 (0.9–2.0) | 2.9 (1.1–7.3)† | 1.1 (0.6–1.8) | 1.8 (1.0–3.1) | 4.0 (1.3–11.9)† |
| Aβ1-40/Aβ1-42 | 0.7 (0.2–2.1) | 0.8 (0.6–1.2) | 0.5 (0.2–1.0) | 1.0 (0.6–1.8) | 0.7 (0.4–1.0) | 0.4 (0.2–1.0)† |
| Pure AD, n (%) | 5 (11.9) | 29 (7.1) | 4 (2.3) | 21 (9.2) | 13 (5.0) | 4 (2.9) |
| Aβ1-40 | 0.9 (0.1–8.8) | 2.2 (1.3–3.7) | 3.3 (0.7–15.9) | 1.4 (0.7–2.7) | 2.8 (1.3–6.4) | 5.3 (1.2–22.9)† |
| Aβ1-42 | 2.4 (0.6–9.4) | 1.5 (1.0–2.4) | 1.6 (0.3–7.1) | 1.3 (0.7–2.5) | 1.8 (0.9–3.4) | 3.6 (0.8–15.6) |
| Aβ1-40/Aβ1-42 | 0.5 (0.2–1.5) | 0.8 (0.6–1.1) | 0.9 (0.2–3.5) | 0.9 (0.5–1.6) | 0.6 (0.4–1.0) | 0.5 (0.2–1.8) |
| VaD, n (%) | 1 (2.6) | 13 (3.3) | 10 (5.5) | 9 (4.2) | 6 (2.4) | 9 (6.2) |
| Aβ1-40 | 0.4 (0.0–18.5) | 1.7 (0.7–4.1) | 0.9 (0.5–1.6) | 1.7 (0.6–4.3) | 2.0 (0.5–8.3) | 0.9 (0.4–1.7) |
| Aβ1-42 | 0.5 (0.0–18.7) | 1.5 (0.8–3.0) | 0.6 (0.4–1.1) | 1.6 (0.7–3.7) | 1.0 (0.4–2.4) | 0.8 (0.4–1.6) |
| Aβ1-40/Aβ1-42 | 2.1 (0.0–352.1) | 0.8 (0.5–1.3) | 2.0 (0.7–5.5) | 0.7 (0.4–1.3) | 1.2 (0.4–3.2) | 1.2 (0.5–2.9) |
Hazard ratio (95% CI) for 1 standard deviation decrease in plasma Aβ level, adjusted for apolipoprotein E genotype, education and time from blood draw to censoring event;
p < 0.1 for high BP compared to low BP.
Comment
Decreased plasma Aβ1-40 and Aβ1-42 levels were associated with an increased risk of Alzheimer’s disease ascertained on average 15 years after the plasma sample was obtained. The association was modulated by BP levels measured on average 25 years before AD was diagnosed. We also found evidence of synergism between DBP and Aβ levels such that the risk estimate for AD and decreasing Aβ levels increased as DBP increased. As plasma Aβ1-42 levels decreased, and the Aβ1-40/Aβ1-42 increased, the likelihood of cerebral amyloid angiopathy increased, but not neuritic plaques or neurofibrillary tangles, in brain tissue examined postmortem. This autopsy finding lends support to our finding of lower plasma levels of AB being indicative of higher risk for dementia, and importantly also provides clues about a possible mechanism.
It is increasingly accepted that AD develops over a long period of time.10 Our study provides insight into how the association of plasma Aβ1 to clinical AD and pathologic AD lesions may evolve over a time period that is longer than has previously been reported.15–19. Strengths of this study include: multiple, long-term follow-up visits for diagnoses of dementia in late-life; the availability of Aβ levels measured from plasma drawn 15 years before diagnosis; the measure of mid-life blood pressure, before the age and dementia related drop in blood pressure;6 and the availability of autopsy material to gain insight into possible mechanisms.
There are several factors to take into account when interpreting the results. The number of cases is relatively limited, particularly in the upper strata of BP. Although there are few community-based studies to replicate these findings, these results can be further studied in animal models. Also the sample is composed of Japanese-American men, so differential findings by sex or ethnic/race cannot be evaluated.
Many physiologic issues related to these findings require further research. The cohort included subjects with elevated lipid levels. Lipid transport plays an important role in maintaining neuronal health 34. However, without more understanding about the extent to which peripheral lipid levels reflect cerebral physiology, it is difficult to determine the extent to which having an enriched sample of subjects with high lipid levels affects the results; further investigation is warranted. Another issue in need of investigation is whether there is a physiologic reason why diastolic BP is more significant in this analysis than systolic BP is. Possibly, the strength of the association depends on treatment protocols during the time the BP is measured, or aging of the arteries during the period when the trajectory BP is measured. This cohort was mid-life in the late 1960s when diastolic hypertension was the main reason for treatment. It is not yet clear whether there are other physiologic reasons for differences in results between the two measures, or how measures such as pulse pressure can be associated with late-life cognitive outcomes. Finally, circulating Aβ levels do not reflect non-Aβ amyloid species that might play a role in AD and may reflect infrequent disease states, such as amyloidosis.35 Additionally, plasma Aβ only provides an indirect measure of brain-specific Aβ pathology and we only have one measure. In-vivo analysis of brain-specific Aβ burden (such as with positron emission tomography) could allow more accurate measures of Aβ burden and how it changes over time. Also the sample is composed of Japanese-American men, so differential findings by sex or ethnic/race cannot be evaluated.
Within the limits of what circulating plasma Aβ levels may reflect, there is a strong positive correlation between plasma Aβ and Aβ in cerebrospinal fluid (CSF)33 and studies have shown, for example, significant decreases in CSF and plasma Aβ levels coincident with Aβ deposition in brain tissue of the Tg2576 transgenic mouse model of AD.36 This inverse relationship is consistent with our findings of low plasma Aβ levels from 15 years prior to the clinical presentation of AD, and our findings of more CAA as the level of plasma Aβ decreased.
Results from other studies of AD and Aβ vary from a higher baseline level of plasma Aβ being associated with an increased risk for AD,16, 17 to no association after multivariate adjustment.18 It is important to emphasize that the follow-up time in the present study was longer, and participants were younger at baseline, than in the related studies, and we had autopsy data to assess the consistency of our clinical findings. We also examined plasma Aβ1-40 than Aβ1-42, in the context of blood pressure levels.
Our finding of an interaction between Aβ and diastolic BP suggests BP modulates the association of peripheral Aβ levels and AD. This modulation can reflect different pathways to Aβ deposition. One hypothesis to consider given our autopsy findings is that the vasoactive properties of Aβ in combination with high blood pressure, can greatly weaken vessel walls and reduce amyloid clearance from the brain, lead to capillary occlusion and hypoperfusion, or co-occur with an up regulation of ACE and subsequent adverse changes such as inflammation and apoptosis.37–40 The trajectory of amyloid changes to AD progression is still not well articulated, but our findings suggest vascular integrity is an important component of the trajectory, early on. Importantly, the measures of BP and plasma Aβ levels were made 10 to 20 years prior to the diagnosis of AD, suggesting early intervention for elevated BP is important to reducing the component of AD attributable to high blood pressure.
Perspectives
In summary, we found a joint association of elevated mid-life diastolic BP, lower plasma Aβ levels and the risk for late life AD. The lower levels of Aβ levels may reflect the deposition of the peptides in the brain, as was suggested by the finding of increased CAA and low plasma Aβ levels. These findings bring together research reporting mid-life BP increases the risk for AD, that Aβ plays an important role in the pathophysiology of AD, and separately that Aβ is vasoactive. If problems in amyloid processing begin early in the disease, and mid-life chronic exposure to hypertension damages cerebral micro and small vessels, thus impairing clearance of Aβ from the brain, this argues for an impact on AD prevention of early programs to prevent and treat elevated BP levels
Supplementary Material
Acknowledgments
We thank Margaret Racke for her technical expertise on the Aβ ELISA’s and for the reagent generation.
Funding/Support: This work was supported by the National Institutes of Health, Bethesda, Maryland (National Institute on Aging [NIA] Contract NO1-AG-4-2149, Cooperative Agreements 5U01AG017155-09 and 5U01AG019349-08, and the Intramural Research Program of the NIH and with resources at the VA Pacific Islands Health Care System). The information contained in this article does not necessarily reflect the position or the policy of the United States Government, and no official endorsement should be inferred.
Role of the Sponsor: The sponsor played no role in this work
Footnotes
Author contributions: Dr. Launer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Launer
Acquisition of data: Tilley, Masaki, Ross, Petrovitch, de Mattos
Analysis and interpretation of data: Shah, Vidal, Launer
Drafting of the manuscript: Shah, Vidal, Launer
Critical revision of the manuscript for important intellectual content: Masaki, Tracy, White, Launer, de Mattos, Ross
Statistical analysis: Shah, Vidal
Obtained funding: White, Launer
Administrative, technical, or material support: Tracy, White, Launer
Study supervision: Launer, Tracy, White
Financial disclosures: The authors have no conflicts to disclose
Additional information: e-Tables S1-S5 are available.
Additional Contributions: None
References
- 1.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ. The epidemiologic study of dementia: a life-long quest? Neurobiol Aging. 2005;26:335–340. doi: 10.1016/j.neurobiolaging.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 4.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 5.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 6.Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 8.Roher A, Wolfe D, Palutke M, KuKuruga D. Purification, ultrastructure, and chemical analysis of Alzheimer disease amyloid plaque core protein. Proc Natl Acad Sci U S A. 1986;83:2662–2666. doi: 10.1073/pnas.83.8.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 12.Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–2606. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40(3 Suppl):S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, Dartigues JF, Tzourio C, Alpérovitch A, Buée L, Amouyel P. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73:847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- 16.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 17.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 18.Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, Ravetch J, Mayeux R. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol. 1975;102:477–480. doi: 10.1093/oxfordjournals.aje.a112185. [DOI] [PubMed] [Google Scholar]
- 21.Reed D, Yano K, Kagan A. Lipids and lipoproteins as predictors of coronary heart disease, stroke and cancer in the Honolulu Heart Program. Am J Med. 1986;80:871–878. doi: 10.1016/0002-9343(86)90631-5. [DOI] [PubMed] [Google Scholar]
- 22.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 23.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 24.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 26.Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 29.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- 30.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 31.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 33.Giedraitis V, Sundelof J, Irizarry MC, Garevik N, Hyman BT, Wahlund LO, Ingelsson M, Lannfelt L. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer’s disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 12(5):284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostagno A, Ghiso J. Preamyloid lesions and cerebrovascular deposits in the mechanism of dementia: lessons from non-beta-amyloid cerebral amyloidosis. JNeurodegener Dis. 2008;5:173–175. doi: 10.1159/000113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford F, Suo Z, Fang C, Sawar A, Su G, Arendash G, Mullan M. The vasoactivity of A beta peptides. Ann N Y Acad Sci. 1997;826:35–46. doi: 10.1111/j.1749-6632.1997.tb48459.x. [DOI] [PubMed] [Google Scholar]
- 38.Werner C, Baumhäkel M, Teo KK, Schmieder R, Mann J, Unger T, Yusuf S, Böhm M. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97:418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- 39.Thal DR, Griffin WS, Braak H. Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med. 2008;12:1848–1862. doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.