Abstract
Inflammatory pain impacts adversely on the quality of life of patients, often resulting in motor disabilities. Therefore, we studied the effect of peripheral inflammation induced by intraplantar administration of complete Freund’s adjuvant (CFA) in mice on a particular form of voluntary locomotion, wheel running, as an index of mobility impairment produced by pain. The distance travelled over 1 h of free access to activity wheels decreased significantly in response to hindpaw inflammation, peaking 24 h after CFA administration. Recovery of voluntary wheel running by day three correlated with the ability to support weight on the inflamed limb. Inflammation-induced mechanical hypersensitivity, measured with von Frey hairs, lasted considerably longer than the impaired voluntary wheel running and is not driving, therefore, the change in voluntary behavior. The CFA-induced decrease in voluntary wheel running was dose-dependently reversed by subcutaneous (s.c.) administration of anti-inflammatory and analgesic drugs, including naproxen (10–80 mg/kg), ibuprofen (2.5–20 mg/kg), diclofenac (1.25–10 mg/kg), celecoxib (2.5–20 mg/kg), prednisolone (0.62–5 mg/kg) and morphine (0.06–0.5 mg/kg), all at much lower doses than reported in most rodent models. Furthermore, the doses that induced recovery in voluntary wheel running did not reduce CFA-induced mechanical allodynia, indicating a greater sensitivity of the former as a surrogate measure of inflammatory pain. We conclude that monitoring changes in voluntary wheel running in mice during peripheral inflammation is a simple, observer-independent objective measure of functional changes produced by inflammation, likely more aligned to the global level of pain than reflexive measures, and much more sensitive to analgesic drug effects.
1. INTRODUCTION
Repeated attempts have been made to generate rodent models that resemble pathological (neuropathic and inflammatory) pain conditions in human patients [2,24,25]. However, the outcome measures used to evaluate whether experimental animals are experiencing pain (or analgesia) rely primarily on the evaluation of withdrawal reflexes evoked by acute (thermal or mechanical) stimulation [25]. Although stimulus-evoked reflexes reflect the hypersensitivity (allodynia and hyperalgesia) that occurs during chronic pain, they do not measure cognitive appraisal, the subjective estimation of the global pain experience, and therefore their assessment in experimental animals only reflects a part of the pain phenotype. Development of behavioral outcomes that better reflect the pain experience of patients should improve translation of preclinical findings to more effective clinical analgesics [24,25,31,45]. Non-reflexive measures of behavior in rodents, that by definition require voluntary decisions and therefore integration of multiple CNS centers, may be a more realistic and sensitive model of clinical pain than stimulus-evoked reflexive measures [24,43].
Inflammatory pain is associated with altered general functioning and activity, particularly reduced mobility [13,41], and can often result in a disability in walking (limp) when affecting the lower limb [17]. Furthermore, analgesic efficacy can be estimated by an ability to decrease interference in motor function [13,17]. Running wheels are of particular interest as a motor test in experimental animals, since the locomotion is not forced (as it is with the rotarod and treadmill), driven by innate exploratory behavior (as in novel environments) or due to a pursuit of food or water. Instead, the running wheels provide a measure of voluntary activity in a known environment, potentially reflecting whether the activity is painful.
The main goal of this study was to develop a pharmacologically relevant and objective measure that reflects inflammatory pain, based on a reduction in voluntary wheel running in mice. To this end, we characterized the degree and time-course of changes in locomotion, using running wheels, in mice with experimentally inflamed hindpaws and compared this with commonly-used measures of inflammatory pain: changes in weight-bearing [15] and punctate mechanical hypersensitivity measured with von Frey hairs, a widely used outcome measure in chronic pain models [24,25]. We then pharmacologically characterized the restoration of the peripheral inflammation-induced decrease in voluntary wheel running, using a representative set of drugs of various chemical and pharmacological classes currently used as therapeutics in humans, including the non-selective non-steroidal anti-inflammatory drugs (NSAIDs) ibuprofen, naproxen and diclofenac [16], the selective cyclooxygenase (COX)-2 inhibitor celecoxib [23,38], the corticosteroid prednisolone [11,17], and the opioid morphine [46]. The sensitivity to drug effects of the voluntary wheel running outcome measure was compared with changes in CFA-induced mechanical hypersensitivity determined with von Frey hairs. Finally, we measured changes in voluntary wheel running of inflamed and non-inflamed mice with the non-analgesic stimulant caffeine [36] and the motor depressant baclofen [37,30] to determine if stimulating or suppressing general motor activity would produce false positives in the model.
2. METHODS
2.1. Experimental animals
Experiments were performed in 9–12 week old C57BL/6J male mice (Jackson Laboratories, ME, USA). Mice were housed in a temperature-controlled room (21±1°C) with an automatic 12-h light/dark cycle (07:00 to 19:00 hours). All experiments were performed during the light phase (10:00–18:00 hours). Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Children’s Hospital Boston, and followed the current guidelines for the investigation of experimental pain with conscious animals [49].
2.2. Drugs and drug administration
To induce peripheral inflammation, mice were injected with intraplantar (i.pl.) complete Freund’s adjuvant (CFA; Sigma-Aldrich, MO, USA), using a 1710 TLL Hamilton microsyringe (Hamilton Company, NV, USA) with a 30½-gauge needle. To assess changes in paw volume, mechanical threshold or hindpaw weight-bearing during inflammation, mice were injected in the left hindpaw with 20 µl of CFA. To characterize the changes in voluntary wheel running by CFA-induced inflammation, the left or both hindpaws were injected with either 10 or 20 µl of CFA. Control animals received i.pl. injections of sterile physiological saline (Sigma-Aldrich, MO, USA).
The analgesic drugs used were: ibuprofen sodium salt, naproxen sodium, diclofenac sodium salt, celecoxib, prednisolone (all from Sigma-Aldrich, MO, USA) and morphine (Baxter Healthcare Corporation, IL, USA). Control drugs tested included baclofen and caffeine (both from Sigma-Aldrich, MO, USA). All drugs tested were dissolved in sterile physiological saline, with the exception of celecoxib and prednisolone which were suspended in 5% gum arabic (Sigma-Aldrich, MO, USA). All drugs or their solvents were administered subcutaneously (s.c., 5 ml/kg) into the interscapular area.
2.3. Plethysmography
Hindpaw volume was determined using a plethysmometer (Bioseb, Boulogne, France). The hindpaw was immersed in a conductive solution (0.02% Triton X-100 in 0.1% NaCl solution) up to the junction of hairy and glabrous skin, and the displaced volume measured with a resolution of 10 µl. Values were recorded before and up to seven days after the i.pl. administration of CFA or saline in the left hindpaw.
2.4. Assessment of mechanical allodynia
Mice were placed individually in transparent test compartments (7.5 cm wide × 7.5 cm long × 15 cm high), separated from each other by an opaque divider, placed on an elevated mesh-bottomed platform with a 0.5-cm2 grid (to provide access to the ventral side of the hindpaws). Two habituations to the evaluation chambers of 1 h duration each were performed on two different days. A baseline measurement was taken on a separate day. Mechanical threshold was defined as the minimum weight of the filament that induced at least 5 responses (fast paw withdrawal, flinching or licking/biting of the stimulated paw) out of 10 stimulations. Mice were then i.pl. injected with 20 µl CFA or saline in the left hindpaw and tested for up to seven days to evaluate the temporal progression of the mechanical allodynia induced by CFA.
To test drug effects on mechanical allodynia, mice were evaluated for thresholds 24 h after CFA injection; they were then distributed into homogeneous groups according to their threshold values and injected with different drugs. Mice were evaluated by an observer blinded to the treatments. For comparison of drug effects in CFA-induced mechanical allodynia and decreases in voluntary wheel running, drugs were evaluated in the von Frey test at the mid-point of the time chosen for the evaluation in the activity wheels. This corresponds to 2.5 h after the administration of naproxen, ibuprofen, diclofenac, celecoxib and prednisolone, and 70 min after the administration of morphine (see Section 2.6).
2.5. Assessment of changes on weight-bearing distribution in freely moving mice
We assessed changes in weight-bearing using a dynamic weight-bearing device (Bioseb, Boulogne, France), developed to measure the weight borne in each limb in freely moving animals. This device has been recently used in rats [39]. Animals were video recorded in a transparent plexiglass evaluation cage (11 cm wide × 11 cm long × 20 cm high) with an instrumented floor, which allowed us to perform this assessment in freely moving mice. A small mirror was placed behind the evaluation cage to allow clear observation of the position of the paws. The video recording and the paw pressure prints obtained by the instrumented floor were synchronized by the provided software (Bioseb). Pressure prints were manually corrected by an observer using the images seen in the video recording as a reference, and the weight borne by each limb during the evaluation period was automatically calculated. Frames in which the reading of the paw pressure was not stable because of excessive movement of the animal, were automatically discarded by the software from analysis. Frames in which it was not possible to match a pressure print to a specific part of the mouse (i.e. when it was not evident if a pressure print was made by a paw or a different part of the mouse, such as the tail or the genitals) were manually discarded by the observer. To ensure the reliability of the determination of the weight borne by each limb, the duration of each experimental session was 10 minutes, to obtain an averaged analyzed time of about 4 minutes per mouse, per day of evaluation.
Mice were habituated to the evaluation chamber for two sessions on two different days. A baseline recording was taken on a separate day, and the mice were then homogeneously distributed in two groups with similar averaged body weights. The mice then received an i.pl. injection of CFA or saline in the left hindpaw and were tested for up to seven days. The primary dependent measure was the difference in weight between the injected and the non-injected hindlimb.
2.6. Assessment of the distance travelled during free access to running wheels
Voluntary wheel running was assessed in polycarbonate cages (20.5 cm wide × 36.5 cm long × 14 cm high) with free access to stainless steel activity wheels (diameter 23 cm; width 5 cm) with a ball-bearing axle (Bioseb, Boulogne, France). The wheel could be turned in either direction. Multiple activity cages were contained within a testing room. The wheels were connected to a computer that automatically recorded the distance travelled by each animal in the wheel during each 1 h evaluation session. No experimenters were present in the room during the evaluation sessions.
Mice were habituated in individual activity cages for three sessions over at least three days. In the first habituation session, each mouse was randomly assigned to a specific cage in which they were always tested for the duration of the experiment. A baseline measurement was recorded one day after the last habituation. The mice that refused to run in the wheels during the baseline measurement (distance travelled less than 275 meters) were discarded from further evaluation (representing 4–6 % of the animals tested). Baseline measurements were considered as a reference for detecting the effect of the treatments tested, and therefore most data will be presented as percentage of the distance travelled during the baseline. After the baseline values were taken, the mice were i.pl. injected with CFA to induce peripheral inflammation, or with saline as a control.
To elucidate the time-course of the changes in voluntary wheel running induced by peripheral inflammation, mice were repeatedly evaluated for up to 7 days after i.pl. administration of CFA/saline. Since it has been shown that exercise can decrease pain behavior in rodents [19], we tested if wheel running during the first days after the induction of peripheral inflammation could alter the subsequent evaluations. Therefore, a separate group of mice was evaluated only 3 and 4 days after CFA injection, and compared with those evaluated daily from days 1 to 4 after CFA injection.
The analgesic effect of drugs was always tested 24 h after the i.pl. injection of 20 µl CFA in both hindlimbs, when a maximum change in voluntary wheel running was detected (see Fig. 2 for details). Drug effects in non-inflamed animals were tested 24 h after the bilateral i.pl. injection of 20 µl saline. All drugs tested were administered 2 hours before the beginning of the test, with the exception of morphine, caffeine and baclofen, which were injected 40 min before the beginning of the test. Since change in body weight (weight loss or retardation of weight gain) is considered to be a sign of ongoing distress induced by pain [2], body weight was monitored daily in animals bilaterally injected with CFA to ensure that this protocol did not induce excessive disturbance to the welfare of the animals.
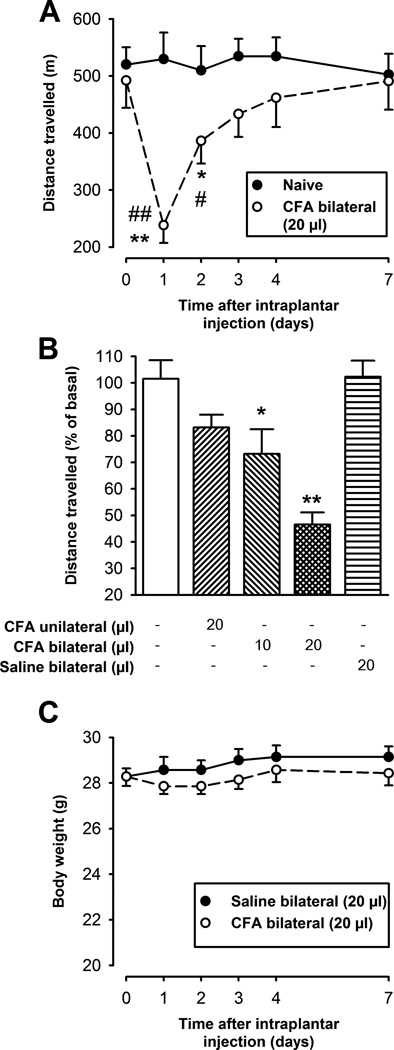
Fig. 2.
Effect of CFA-induced inflammation on voluntary wheel running and body weight. Each point or bar and vertical line represents the mean ± SEM of the values obtained (n=8–14 per group). (A) Time-course of the effect in the distance travelled induced by the intraplantar administration of 20 µl of CFA in both hindpaws (○), in comparison to naïve mice (●), during 1 h of free access to the activity wheel. Statistically significant differences between the baseline (time 0) and the values obtained after the administration of CFA: *p < 0.05; **p < 0.01; and between CFA-treated and naïve mice in the same day of evaluation: #p < 0.05; ##p < 0.01 (two-way repeated measures ANOVA followed by Tukey post-hoc test). (B) Percentage of distance traveled, relative to basal values, in naïve and intraplantar saline or CFA injected mice 24 h after injection. Mice were injected either unilaterally (left hindpaw) or bilaterally (both hindpaws). Statistically significant differences between the naïve group (white bar), and the different experimental groups: *p < 0.05; **p < 0.01 (one-way ANOVA followed by Tukey post-hoc test). (C) Body weight of mice after bilateral intraplantar injection of saline or CFA. There were no statistically significant differences between the values obtained in the inflamed and non-inflamed mice at any time-point tested (two-way repeated measures ANOVA followed by Tukey post-hoc test).
2.7. Assessment of ambulatory locomotion
Ambulatory locomotion was monitored with an infrared detector (InfraMot-Activity System; TSE, MO, USA). Mice were habituated to the evaluation chambers (445 cm2) 90 min before injection of the following drugs: caffeine, baclofen or morphine, or the solvent control saline. After drug injection, mice were immediately returned to the evaluation cages. Animals were then placed in an isolated room for the recording of the locomotor activity. The number of ambulatory events was collected 40 to 100 minutes after the drug injection, since this was the period chosen for assessing the effects of these drugs in voluntary wheel running (see Section 2.6.).
2.8. Data analysis
The differences between values obtained in the time-courses were analyzed with two-way repeated measures analysis of variance (ANOVA), and the effect of drugs was compared to their controls by a one-way ANOVA; a Tukey post-hoc test was performed in both cases. All tests were performed with the SigmaStat 3.5 program (Systat Software Inc., CA, USA). Differences between means were considered statistically significant when p<0.05.
The dose of drug that produced half of the maximal effect against the CFA-induced decrease in voluntary wheel running (ED50), and the maximum recovery induced by each drug (Emax) were calculated from the dose-response curves using non-linear regression analysis of the equation for a sigmoid plot. The degree of recovery was calculated as the percentage of improvement of drug-treated mice over vehicle-treated inflamed mice, using the latter groups as the 0% marker for each drug under study. The value obtained from non-inflamed mice treated with the vehicle of each respective drug was used as 100 % of possible recovery. Parameters obtained from sigmoid plots and their standard errors were calculated as the best-fit values ± standard errors of regression with SigmaPlot (v. 10.0).
3. RESULTS
3.1. Time-course of CFA-induced edema, mechanical allodynia and changes in hindlimb weight-bearing
Administration of CFA into a paw induced a large increase in paw volume that was apparent 24 h after the injection (approximately 50 % increase from the pre-injection values), and then remained virtually unaltered during all time-points tested up to 7 days (Fig. 1A). Mice injected with the same volume of saline did not show any significant change in paw volume at any time-point tested (Fig. 1A). Inflamed mice developed a pronounced and long-lasting decrease in their mechanical threshold measured with von Frey hairs, with the mechanical allodynia still robust and significant 7 days after CFA injection. The mechanical threshold of mice injected with saline into the paws, was unaltered (Fig. 1B).
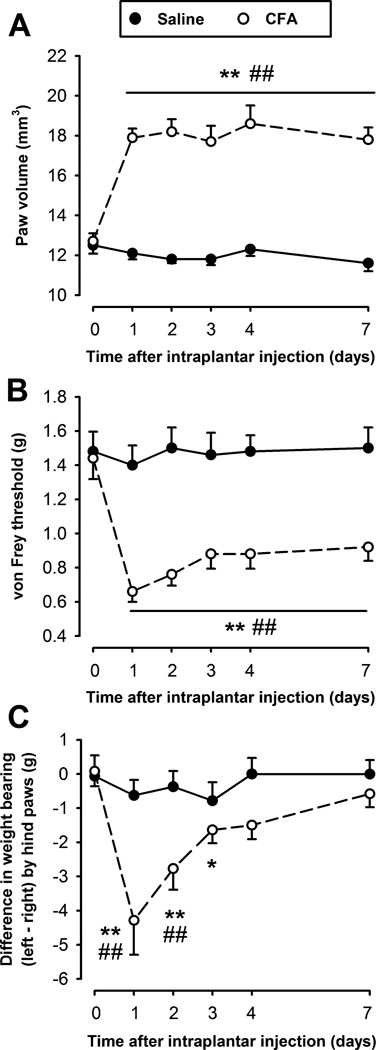
Fig. 1.
Time-course of paw volume (A), mechanical threshold to punctuate stimulation (von Frey) (B), and changes in hindpaw weight-bearing distribution (C), after intraplantar injection of 20 µl of CFA (○) or saline (●) in the left hindpaw. Each point and vertical line represent the mean ± SEM of the values obtained (n=6–10 per group). Statistically significant differences between the baseline (time 0) and the values obtained after the administration of CFA: *p < 0.05; **p < 0.01; and between CFA- and saline-treated mice on the same day after treatment: #p < 0.05; ##p < 0.01 (two-way repeated measures ANOVA with post-hoc Tukey test).
Changes in weight-bearing during the CFA-induced paw inflammation were measured in freely moving mice. Mice symmetrically distributed the weight borne by their hindlimbs during baseline measurements (Fig. 1C; time 0). However, 24 h after induction of peripheral inflammation in the left hindlimb, mice decreased the weight borne by the inflamed paw, and shifted more weight to the non-injured hindlimb to compensate, increasing the difference in weight borne by their hindlimbs up to 4.29 ± 1.00 g (p<0.001). This difference in weight-bearing by the hindlimbs progressively diminished, and 3 days after the induction of the inflammation this difference was only hardly significant compared to the baseline value (p = 0.047), and returned to baseline values by day 4 (Fig. 1C), in contrast to the longer lasting mechanical hypersensitivity detected by stimulation with von Frey hairs which was still significant at 7 days (Fig 1B). Saline-treated mice did not show any significant change in weight-bearing distribution of their hindpaws (Fig. 1C). We also assessed possible changes in the weight loaded by the forepaws, which may compensate for a decrease in the weight borne by the injured paw of CFA-treated mice, but did not find any significant differences (data not shown).
3.2. Characterization of voluntary wheel running in inflamed mice
We first assessed the effect of repeated exposure to voluntary wheel running in mice. After three habituation sessions (see “Methods” section for details), naïve mice travelled approximately 500 m over 1 hour of free access to the wheel (Fig. 2A; time 0). The distance travelled was not significantly different in several independent sets of mice tested at different times in the day (data not shown). Repeated exposure of naïve mice to the wheels did not significantly change the distance travelled during the experimental session (Fig. 2A, closed circles). This effect is not strain-dependent since BALB/cJ mice also needed only three sessions of 1 h to reach stable values (data not shown). Since we found that the distance travelled by the mice was stable over several repeated sessions with this experimental protocol, the effect of a treatment on each mouse was analyzed as percentage of the distance travelled during the baseline.
To find the optimal conditions of peripheral inflammation that produce detectable changes in voluntary wheel running, different protocols of CFA administration were tested, in each case evaluating the mice 24 h after CFA administration. This time-point corresponds to peak paw edema, mechanical allodynia and weight-bearing difference in the hindpaws after an i.pl. injection of 20 µl of CFA into the left hindpaw (Fig. 1A, B and C, respectively). Mice injected unilaterally with CFA showed a modest, non-significant decrease in distance travelled, compared to naïve mice (Fig. 2B). This suggests that the mice can compensate for the inflammation in one hindlimb with the other three non-inflamed limbs, in agreement with previously reported gait analysis experiments showing adaptive changes in coordination after unilateral injury [10] and that the degree of pain did not interfere with the choice to continue running normal distances. We then tested the effect of a low volume (10 µl) of CFA injected in both hindpaws. However, although the mice showed a significant decrease in comparison to naïve mice (Fig. 2B), we judged this difference too modest for pharmacological studies. We next tested the effect of a standard dose of CFA (20 µl) injected bilaterally in the hindpaws, and found that the mice showed a marked decrease in distance travelled (53.4 ± 4.5 % of decrease in comparison to baseline values; p<0.001) (Fig. 2B). This did not produce any significant decrease in body weight (Fig. 2C), and the mice did not show any other behavioral phenotype that might reflect distress (hunched posture, mottled fur) during the time-course of the experiment, indicating that they were not ill, seriously uncomfortable or disturbed. Indeed they still continued to choose to run on the wheels, albeit less. To determine whether the injection procedure alone might produce an effect on voluntary wheel running, bilateral injections of saline were used as a control and were found not to be statistically different from non-injected controls (Fig. 2B).
Analysis of the temporal progression of the decrease in voluntary wheel running after bilateral administration of 20 µl CFA showed that it was maximal at 24 h, returning to baseline levels by day three (Fig. 2A; open circles). This temporal course resembled the time course of recovery in weight-bearing after unilateral injury (Fig. 1C). Both of these non-reflexive outcomes are shorter lasting than the mechanical hypersensitivity detected by von Frey hairs (Fig. 1B).
An independent group of mice was submitted to voluntary wheel running only on days 3 and 4 after the induction of peripheral inflammation. We did not find significant differences in the distance traveled between mice evaluated daily after CFA injection and those evaluated 3 and 4 days after CFA administration indicating that the recovery was not due to repeated exercise (data not shown).
3.3. Effects of NSAIDs, prednisolone and morphine on inflammation-induced decreases in voluntary wheel running
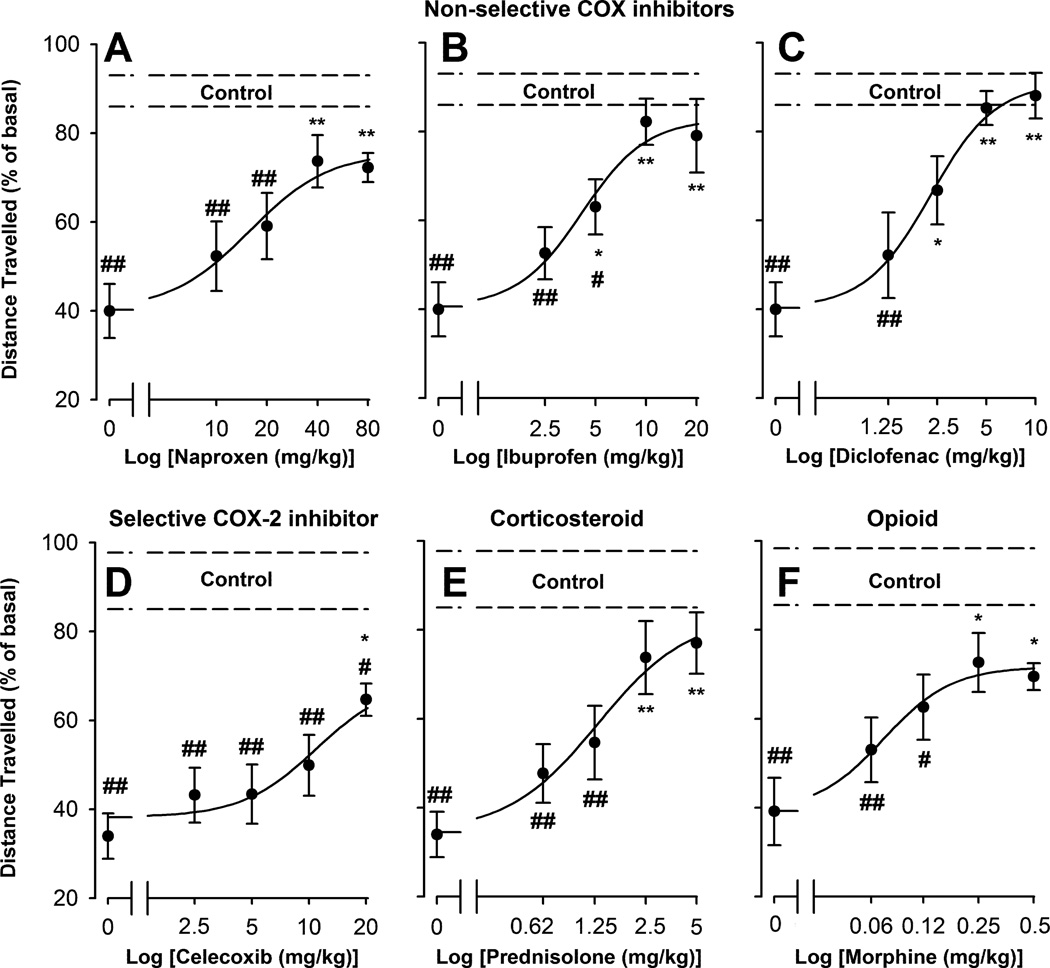
We used a panel of drugs with different pharmacological properties to investigate if common analgesics can prevent the decrease in voluntary wheel running induced 24 h after bilateral hindlimb CFA injection. The non-selective COX inhibitors naproxen (10–80 mg/kg), ibuprofen (2.5–20 mg/kg), and diclofenac (1.25–10 mg/kg) all produced a dose-dependent and significant reduction in the decrease in running-wheel performance compared to inflamed mice treated with vehicle (dose 0) (Fig. 3A, B and C, respectively). The order of potency (ED50) of these compounds on the recovery of wheel running in inflamed mice was: naproxen (17.13 ± 6.70 mg/kg) < ibuprofen (4.40 ± 1.45 mg/kg) < diclofenac (2.29 ± 0.32 mg/kg) (Table 1). The distance travelled by inflamed mice treated with the highest doses tested of these drugs was not significantly different from that found in non-inflamed mice treated with their respective vehicles (Fig. 3A, B and C), with the calculated Emax values close to 100 % recovery (Table 1). This indicates that inflamed mice are able to fully recover their voluntary wheel running performance after suitable pharmacological treatment.
Fig. 3.
Effect of different doses of the non-selective non-steroidal anti-inflammatory drugs naproxen, ibuprofen and diclofenac, the selective COX-2 inhibitor celecoxib, the corticosteroid prednisolone and the opioid morphine, administered subcutaneously (s.c.), on CFA-induced decrease of voluntary wheel running. Data are presented as the percentage of the distance travelled, relative to their basal value, 24 h after the intraplantar (i.pl.) injection of 20 µl of saline or CFA in both hindpaws. Each point and vertical line represent the mean ± SEM of the values obtained (n=8–12 per group). The dashed lines (control) represent the mean ± SEM of non-inflamed mice (intraplantarly injected with saline) treated with the vehicles of the drugs (n=10–13). Statistically significant differences between the values obtained in drug-treated and vehicle-treated groups, either intraplantarly injected with CFA (dose 0): *p<0.05; **p<0.01, or with saline (control): #p<0.05; ##p<0.01 (one-way ANOVA followed by Tukey post-hoc test).
Table 1.
Potency and efficacy of several anti-inflammatory and analgesic drugs against CFA-induced decrease of voluntary wheel running.
| Class | Drug | ED50 (mg/kg) | Emax (%) | |
|---|---|---|---|---|
| COX inhibitor | Non-selective | Naproxen | 17.13 ± 6.70 | 71.45 ± 20.04 |
| Ibuprofen | 4.40 ± 1.45 | 85.25 ± 22.07 | ||
| Diclofenac | 2.29 ± 0.32 | 102.28 ± 11.07 | ||
| Selective COX-2 | Celecoxib | - | 53.45 ± 6.38 | |
| Corticosteroid | Prednisolone | 1.47 ± 0.66 | 85.05 ± 26.82 | |
| Opioid | Morphine | 0.08 ± 0.02 | 61.5 ± 10.40 | |
The effects of subcutaneously (s.c.) administered drugs on voluntary wheel running were tested 24 h after the intraplantar injection of CFA in both hind paws. The ED50 (dose of drug producing half of the maximal effect) and the Emax (maximum recovery induced by each drug) values were obtained from dose–response curves of the drug’s effects. Results are presented as best-fit values ± standard error of regression (see “Methods” section for details), with the exception of the values obtained with celecoxib, as the ED50 could not be calculated and the Emax value was taken from the recovery detected with the highest dose tested (20 mg/kg).
We also tested the effects of two additional anti-inflammatory compounds: the selective COX-2 inhibitor celecoxib (2.5–20 mg/kg) and the steroid prednisolone (0.62–5 mg/kg). Both compounds significantly increased voluntary wheel running of CFA-treated mice in a dose-dependent manner (Fig. 3D and E, respectively). The effect of celecoxib was only significant at the highest dose tested (20 mg/kg) (Fig. 3D). This effect was limited (53.45 ± 6.38 % of recovery; Table 1), and lower than the effect of the other NSAIDs tested (Fig. 3 A, B and C). Higher doses of celecoxib decreased the distance travelled by inflamed mice (data not shown), which is why we could not reliably calculate an ED50 for this compound in this model (Table 1). The corticosteroid prednisolone showed a higher potency than all the other anti-inflammatory drugs tested (ED50= 1.47 ± 0.66 mg/kg). The Emax value of this compound was comparable to those calculated for the non-selective COX inhibitors tested, reaching values close to the full recovery (Fig. 3E and Table 1).
To characterize the response of this outcome behavior specifically to analgesics, we tested the effect of morphine (0.063–0.5 mg/kg), which elicited a dose-dependent reversal of the decrease in locomotor performance of bilaterally inflamed mice in the activity wheels (Fig. 3F). A dose as low as 0.08 ± 0.02 mg/kg was determined to be the ED50 of morphine in this outcome (Table 1), showing an Emax value of 61.5 ± 10.4 % of recovery (Table 1).
3.4. Effects of NSAIDs, prednisolone and morphine on CFA-induced mechanical allodynia
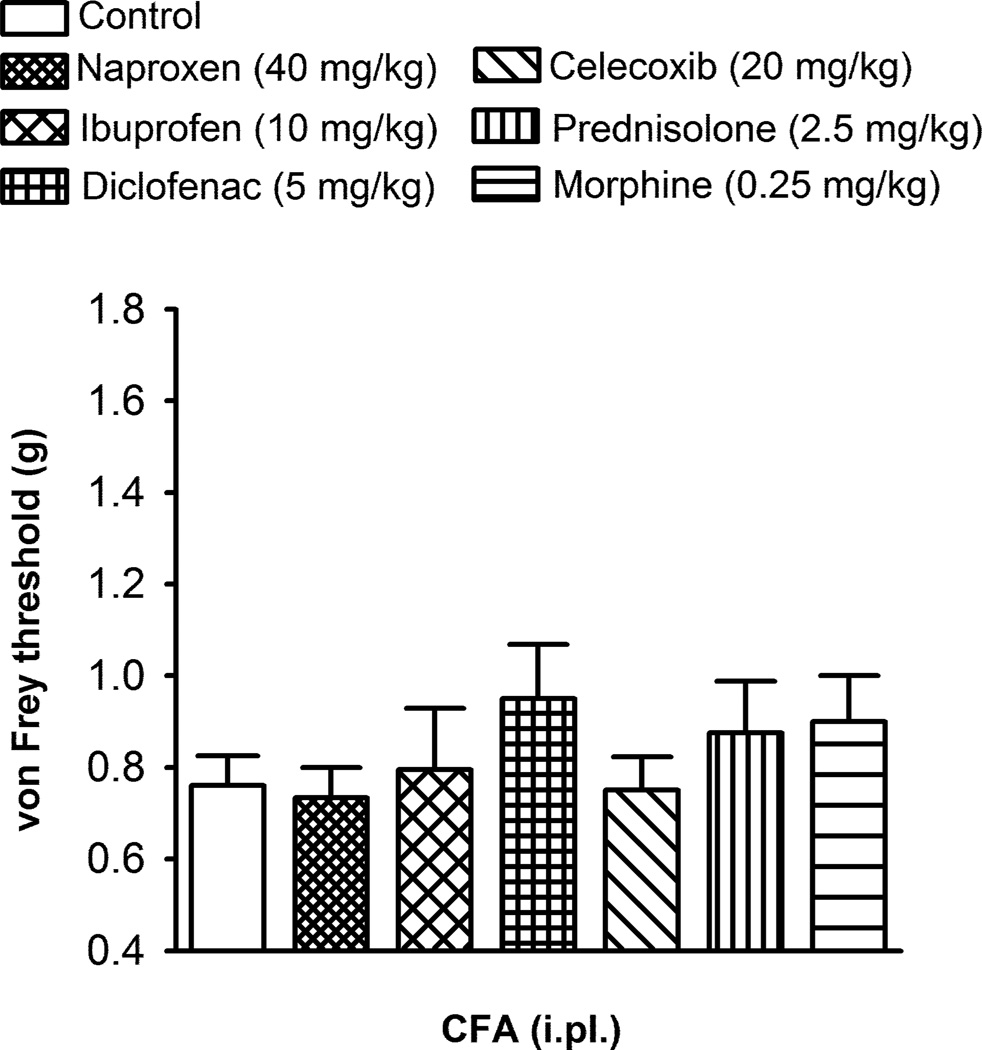
We studied the effects of the same drugs on mechanical allodynia evaluated by von Frey filaments 24 h after CFA-injection using the doses effective on voluntary wheel running performance in inflamed mice. Naproxen (40 mg/kg), ibuprofen (10 mg/kg), diclofenac (5 mg/kg), celecoxib (20 mg/kg), prednisolone (2.5 mg/kg) and morphine (0.25 mg/kg), did not significantly increase the mechanical threshold of CFA-treated mice (i.e. they were devoid of any anti-allodynic effect at the doses tested; Fig. 4).
Fig. 4.
Effect of the subcutaneous administration of the non-steroidal anti-inflammatory drugs naproxen, ibuprofen, diclofenac and celecoxib, the corticosteroid prednisolone and the opioid morphine, on CFA-induced mechanical allodynia. Mechanical threshold was assessed in the left hindpaw 24 h after the intraplantar (i.pl.) injection of 20 µl CFA. Each bar and vertical line represent the mean ± SEM of the values obtained (n=8–12 per group). There were no statistically significant differences between the values obtained in the control non-drug injected group and the drug-treated groups (one-way ANOVA).
3.5. Effects of NSAIDs, prednisolone and morphine on voluntary wheel running in noninflamed mice
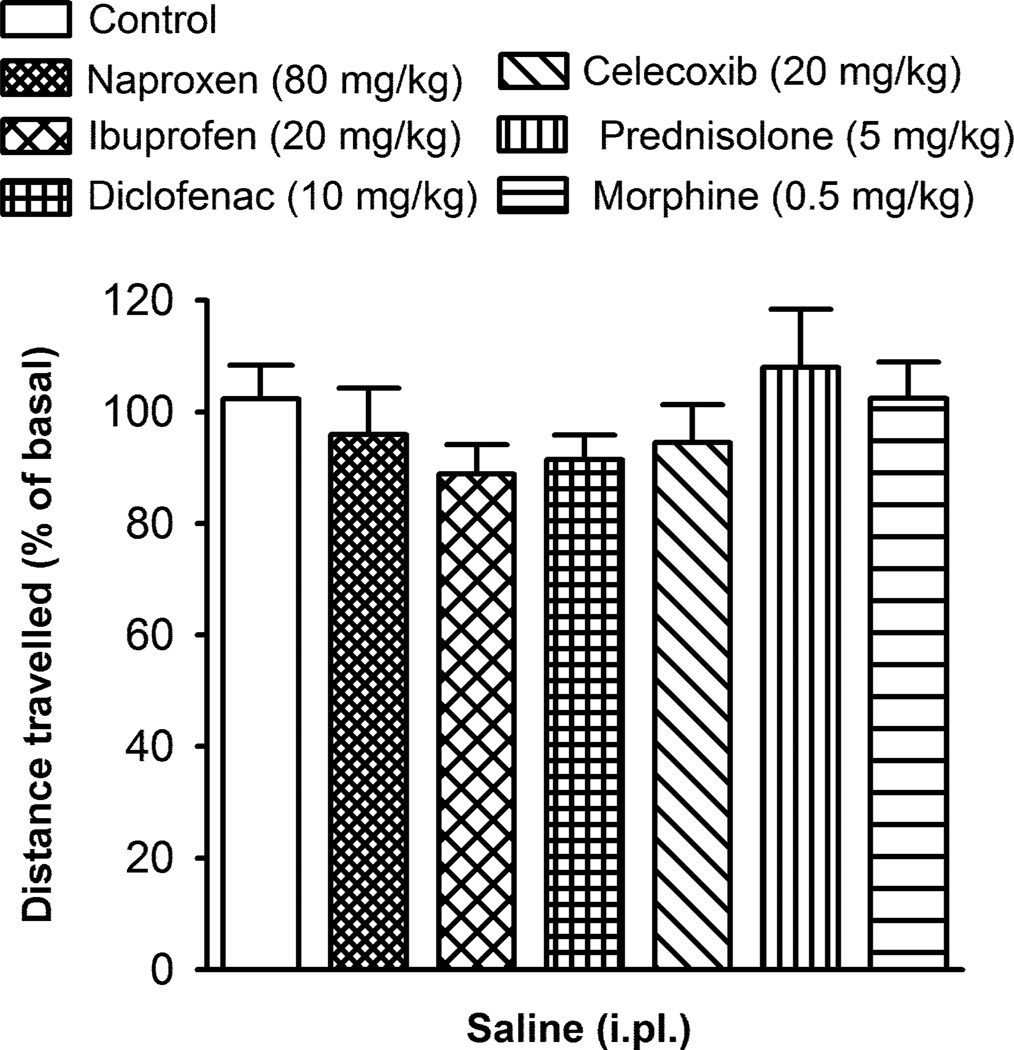
To explore whether any of the drugs tested had effects on activity in the running wheels that could interfere with the interpretation of the results, we administered the highest dose of each drug that had been tested in the inflamed mice (Figs. 3 and 4) in mice bilaterally injected with saline (20 µl, i.pl.). Naproxen (80 mg/kg), ibuprofen (20 mg/kg), diclofenac (10 mg/kg), celecoxib (20 mg/kg), prednisolone (5 mg/kg) and morphine (0.5 mg/kg) failed to produce any significant effect on voluntary wheel running in these non-inflamed mice (Fig. 5). Therefore, the reversal of the decline in the motor activity produced by these drugs in CFA-injected mice cannot be attributed to a nonspecific effect on voluntary wheel running.
Fig. 5.
Effect of the subcutaneous administration of the non-steroidal anti-inflammatory drugs naproxen, ibuprofen, diclofenac and celecoxib, the corticosteroid prednisolone and the opioid morphine, on voluntary wheel running in non-inflamed mice. Data are presented as the percentage of the distance travelled, relative to their basal value, 24 h after the intraplantar (i.pl.) injection of 20 µl saline in both hindpaws. Each bar and vertical line represent the mean ± SEM of the values obtained (n=7–9 per group). There were no statistically significant differences between the values obtained in the control non-drug injected group and drug-treated groups (one-way ANOVA).
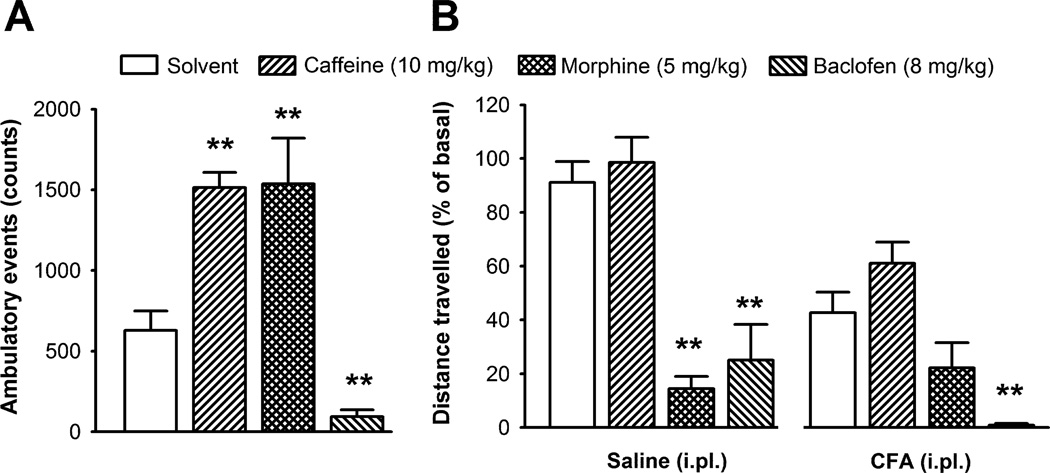
3.6. Effect of caffeine, morphine and baclofen on ambulatory locomotion and voluntary wheel running
To determine if drugs that alter locomotion alter wheel running performance, we compared the effect of caffeine (10 mg/kg) and baclofen (8 mg/kg) on ambulatory locomotion and voluntary wheel running. Caffeine induced a nearly 3-fold increase in ambulatory locomotion, compared to vehicle treated mice (Fig. 6A), in agreement with previous data [8] but did not modify voluntary wheel running in either non-inflamed or inflamed mice (Fig. 6B). A higher dose of caffeine (30 mg/kg) induced a 60 and 76 % decrease in voluntary wheel running of non-inflamed and inflamed mice, respectively. Baclofen, at a dose known to impair locomotion in the rotarod test [28], induced a significant decrease in ambulatory behavior (Fig. 6A) and in running-wheel performance in both inflamed and non-inflamed mice (Fig. 6B).
Fig. 6.
Effect of the subcutaneous administration of caffeine (10 mg/kg), a moderate dose of morphine (5 mg/kg), baclofen (8 mg/kg) or their solvent in locomotor activity measured as ambulatory events (A); and in voluntary wheel running 24 h after the intraplantar (i.pl.) injection of saline or CFA (20 µl) in both hindpaws (B). Each bar and vertical line represent the mean ± SEM of the values obtained (n=7–10 per group). Statistically significant differences between the values obtained in the drug- and solvent-treated groups under the same experimental conditions: **p<0.01 (one-way ANOVA followed by Tukey post-hoc test).
Morphine at 5 mg/kg, considered to be a moderate analgesic dose in mice, increased ambulatory locomotion as previously described [9]. However, voluntary wheel running was significantly reduced in non-inflamed mice (Fig. 6B, left panel), and was not increased in inflamed mice (Fig. 6B right panel). This indicates that the systemic effects of morphine on locomotion and alertness masked the specific analgesic effects seen at lower doses. These results suggest that pharmacological treatments may not affect ambulatory locomotion and wheel running behavior in the same way and that change in voluntary wheel running may be a useful way of detecting potential CNS adverse effects.
4. DISCUSSION
In this study we show that mice provided with an opportunity to voluntarily run in activity wheels choose to travel a reduced distance if they have bilateral hindpaw inflammation. This decrease closely follows their reduced ability to support weight by an inflamed hindlimb, but does not correlate with mechanical hypersensitivity, which lasts much longer. The decrease in voluntary wheel running can be reversed by anti-inflammatory/analgesic drugs at doses that do not alter von Frey thresholds in inflamed mice or locomotion in non-inflamed mice.
While reflexes evoked by acute stimuli in models of chronic pain are a powerful tool to discern mechanisms of pain hypersensitivity, they may not fully reflect the complexities of global changes associated with chronic pain [2,24,25,31,43]. Pain is a perceptual experience which depends upon cerebral processing and is associated with changes in motivational behavior. Consequently, a number of non-reflexive measures have been developed as potential surrogates of chronic pain in rats [1,3,6,12,22,23,42,44]. In spite of the increased use of mice for pain research due to the development of genetically engineered animals, there are virtually no pain studies using non-reflexive or operant measures in mice, for the latter mainly because they are harder to condition than rats in most paradigms. However, mice require less training sessions of voluntary wheel running than rats to reach stable values [34]. Our protocol differed from that used in most studies using running wheels, where the rodents are housed in cages with 24 h free access to the wheel [34]. Here we show that mice when given access to the wheel only for an hour per day reach high and stable values of distance travelled in only three sessions. We found that this behavior is both modulated by peripheral inflammation and highly sensitive to anti-inflammatory and analgesic drugs.
A recent study found that only a select group of rats decreased the distance travelled in activity wheels during unilateral experimental osteoarthritis [37]. This is in agreement with our study since we show that unilateral peripheral inflammation is insufficient to drive a significant decrease in voluntary wheel running (presumably due to compensation with the non-injured limbs) and bilateral injury is needed, as in other non-reflexive measures [23,42,44].
We found that CFA-induced mechanical allodynia is longer-lasting than the impairment in wheel running performance. This difference cannot be attributed to the possible ameliorative effects of the running activity itself on inflammatory pain, since mice not submitted to voluntary wheel running during the first days after CFA injection do not show any alteration in the speed of recovery of wheel running performance. In addition, changes in hindpaw weight-bearing (another non-reflexive outcome) in mice not submitted to any form of exercise were also much shorter-lasting than mechanical allodynia, as previously described in the rat [15,47], and followed a similar time-course to the recovery in voluntary wheel running. These results indicate that the difference in the time-course of these outcomes cannot be explained by effects of physical activity and that the presence of a pronounced mechanical allodynia in the hindlimb does not necessarily induce a significant impairment in voluntary performance. Similarly, it has been recently shown that inflammation-induced mechanical allodynia does not alter home cage locomotor activity and several other putative “quality of life” indexes [40].
There is no agreement on whether changes in hindpaw weight-bearing reflect spontaneous pain [2], increased touch sensitivity of the injured foot [15], or pain-avoidance behavior [26]. Similarly, voluntary wheel running could be affected by a decreased willingness to perform the task due to ongoing pain or by the pain induced by movement/impact (mechanical hypersensitivity) of the inflamed tissue during or after the performance of the target behavior. This reflects human patients, whereby a painful condition may induce a loss of motivation and avoidance of activities that may evoke pain in the injured area or that aggravate the pain that they are already suffering [17,33]. Therefore, regardless of the exact mechanistic nature of the changes in weight-bearing or in voluntary wheel running during inflammation, these outcomes might more realistically reflect the “everyday pain experience” as well as the well-being of the mice than the stimulus-evoked reflexes and we assume that the choice of the animal to run or not must reflect in some way its level of discomfort.
Several anti-inflammatory/analgesic drugs of different pharmacological classes dose-dependently increased the motivation/choice of the mice to run on the wheels in spite of peripheral inflammation, without altering the behavior in non-inflamed mice. Among the NSAIDs tested, diclofenac showed the highest potency, as expected from patients [16]. The selective COX-2 inhibitor celecoxib showed lower efficacy than the non-selective COX inhibitors tested, which agrees with clinical studies showing a higher analgesic efficacy of ibuprofen over celecoxib [7,21]. The ED50 of prednisolone was lower than that of the NSAIDs tested, which is also in agreement with the higher potency of this corticosteroid in reversing inflammation, and thereby inflammatory pain, in comparison to NSAIDs in short-term treatment [11] and shows the sensitivity of the test to anti-inflammatory drugs. Finally, morphine showed the highest potency of all the compounds tested, as expected [18,23,27,47] indicating that the test is suitable for testing analgesics. Its efficacy was lower than that observed in reflexive outcomes [18,27,47]. This could be due to the inability of morphine to induce a maintained analgesia during the full period of evaluation in the activity wheels, because of its short-lasting analgesic effect [47]. Drug doses that induced recovery of voluntary wheel running during peripheral inflammation were devoid of an anti-allodynic effect, which is in agreement with previous reports showing that higher doses of these drugs are needed to induce obvious analgesic effects in mechanical hypersensitivity [3,18,27,32,47] or in chemically (formalin)-induced pain [14,29,38,48]. Ibuprofen plasma concentration in rats after a dose of 100 mg/kg, which is needed to inhibit mechanical [18] or thermal hypersensitivity [1] and formalin-induced pain [48], is 20-fold higher than the therapeutic plasma concentration in humans [1]. Interestingly, this dose is about 20-fold higher than the ED50 found in our model (4.40 ± 1.45 mg/kg). Therefore the higher sensitivity of voluntary wheel running may more accurately predict efficacious drug doses in humans than antiallodynic actions. Other non-reflexive outcomes are also more sensitive than reflexive endpoints in detecting drug-induced analgesia [1,3,20,22,23,44]. Therefore, high sensitivity to drug effects might be an intrinsic quality of non-reflexive outcomes.
Analgesic drug development requires reliable models of both efficacy and toxicity. Here we show that while low doses of morphine (0.06–0.5 mg/kg), showed an ameliorative effect on voluntary wheel running in inflamed mice, a moderate dose of morphine (5 mg/kg) induced ambulatory hyperlocomotion, yet almost completely inhibited voluntary wheel running. Similar inhibition of wheel running performance was found after the administration of a high dose of caffeine (30 mg/kg). These data indicate that locomotion in activity wheels can be used to test acute toxicity and the effects on general well-being of putative analgesics, and that analgesic treatment at a dose also inducing clear adverse effects would not result in an improvement of this outcome. One obvious advantage is use of the same outcome measure in injured and non-injured mice to test drug-induced analgesia and acute toxicity, respectively.
Drugs may inhibit the performance of pain-like reflexes due to effects on motor function rather than on nociception, resulting in false positive results. We found that the muscle relaxant baclofen, which can decrease pain-related reflexes due to motor impairment (i.e. analgesic-like effect) [30,35], did not improve voluntary wheel running performance in inflamed mice. Therefore, and contrary to reflexive models, motor impairment does not lead to a false analgesic-like effect in this behavioral model, as seen in other non-reflexive outcomes [1,23,36].
We tested if caffeine, a stimulant, would result in a false positive result since the return towards baseline of wheel running during inflammation is interpreted as an analgesic-like effect in our model. While caffeine did increase ambulatory behavior, it did not modify voluntary wheel running of either non-inflamed or inflamed mice; therefore, the general arousal induced by a non-analgesic drug did not produce a false positive result.
Standard rodent pain models are prone to experimenter subjectivity [23,24,31], and are susceptible to confounds related to human-animal interactions [4,5]. In our model, data collection is completely automatized and the experimenter is not needed in the room during the evaluation of the mice, fully mitigating against these confounders.
We conclude that a reduction in voluntary wheel running during peripheral inflammation is a simple and objective surrogate measure of inflammatory pain. It provides theoretical and operational differences to current reflexive standard outcomes, and has high sensitivity to detect analgesic and anti-inflammatory effects of compounds. In addition, this non-reflexive outcome can clearly dissociate the effects of drugs on inflammation/analgesia with motor impairment, and is able to detect drug-induced adverse effects. Therefore, non-reflexive behavioral endpoints should improve the reliability of evaluation of new targets contributing to inflammatory pain and candidate analgesics that act on these, by providing a closer measure to what the animal is feeling and how this affects the choices it makes.
ACKNOWLEDGMENTS
We thank the NIH (Clifford J. Woolf, 5 R01 NS039518-08 and 1P01NS072040-01), the MICINN/Fulbright program (Enrique J. Cobos), the Fonds de la recherche en santé du Québec and Banting Fellowships (Nader Ghasemlou), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Dionéia Araldi) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
References
- 1.Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2011.07.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends Pharmacol Sci. 2004;25(6):299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, Decker MW, Honore P. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology. 2010;58(2):537–543. doi: 10.1016/j.neuropharm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26(8):907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 5.Chesler EJ, Wilson SG, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5(11):1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- 6.Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91(1–2):33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- 7.Doyle G, Jayawardena S, Ashraf E, Cooper SA. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol. 2002;42(8):912–919. doi: 10.1177/009127002401102830. [DOI] [PubMed] [Google Scholar]
- 8.El Yacoubi M, Ledent C, Parmentier M, Ménard JF, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148(2):153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- 9.Elhabazi K, Trigo J, Mollereau C, Moulédous L, Zajac JM, Bihel F, Schmitt M, Bourguignon J, Meziane H, Petit-Demouliere B, Bockel F, Maldonado R, Simonin F. Involvement of neuropeptide FF receptors in neuroadaptative responses to acute and chronic opiate treatments. Br J Pharmacol. 2012;165(2):424–443. doi: 10.1111/j.1476-5381.2011.01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabriel AF, Marcus MA, Honig WM, Walenkamp GH, Joosten EA. The CatWalk method: a detailed analysis of behavioral changes after acute inflammatory pain in the rat. J Neurosci Methods. 2007;163(1):9–16. doi: 10.1016/j.jneumeth.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Gøtzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal anti-infl ammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2009;1:CD000189. doi: 10.1002/14651858.CD000189. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez T, Crystal JD, Zvonok AM, Makriyannis A, Hohmann AG. Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain. 2011;152(9):1976–1987. doi: 10.1016/j.pain.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinman RS, Bennell KL, Crossley KM, McConnell J. Immediate effects of adhesive tape on pain and disability in individuals with knee osteoarthritis. Rheumatology (Oxford) 2003;42(7):865–869. doi: 10.1093/rheumatology/keg233. [DOI] [PubMed] [Google Scholar]
- 14.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30(1):103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 15.Huntjens DR, Spalding DJ, Danhof M, Della Pasqua OE. Differences in the sensitivity of behavioural measures of pain to the selectivity of cyclo-oxygenase inhibitors. Eur J Pain. 2009;13(5):448–457. doi: 10.1016/j.ejpain.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Insel PA. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman Gilman A, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill; 1996. pp. 617–657. [Google Scholar]
- 17.Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854–1860. doi: 10.1016/S0140-6736(08)60799-0. [DOI] [PubMed] [Google Scholar]
- 18.Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther. 2005;312(2):726–732. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- 19.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8(12):989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.LaBuda CJ, Fuchs PN. Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain. Neurosci Lett. 2001;304(3):137–140. doi: 10.1016/s0304-3940(01)01787-6. [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom K, Daniels S, Kotey P, Seidenberg BC, Desjardins PJ. Comparison of rofecoxib and celecoxib, two cyclooxygenase-2 inhibitors, in postoperative dental pain: a randomized, placebo- and active-comparator-controlled clinical trial. Clin Ther. 1999;21(10):1653–1663. doi: 10.1016/S0149-2918(99)80045-9. [DOI] [PubMed] [Google Scholar]
- 22.Martin TJ, Zhang Y, Buechler N, Conklin DR, Eisenach JC. Intrathecal morphine and ketorolac analgesia after surgery: comparison of spontaneous and elicited responses in rats. Pain. 2005;113(3):376–385. doi: 10.1016/j.pain.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320(1):194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 24.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151(1):12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306(2):490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- 28.Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137(3):520–531. doi: 10.1016/j.pain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz MI. Blockade of the antinociception induced by diclofenac, but not of indomethacin, by sulfonylureas and biguanides. Pharmacol Biochem Behav. 2011;99(1):1–6. doi: 10.1016/j.pbb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, Fox A. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90(3):217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 31.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stöhr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139(2):243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Sahin NO, Librowski T. Investigations of anti-inflammatory and analgesic activities of prednisolone solid dispersion prepared with skimmed milk. Pol J Pharmacol. 2003;55(2):261–265. [PubMed] [Google Scholar]
- 33.Sanchez K, Papelard A, Nguyen C, Bendeddouche I, Jousse M, Rannou F, Revel M, Poiraudeau S. McMaster-Toronto Arthritis Patient Preference Disability Questionnaire Sensitivity to Change in Low Back Pain: Influence of Shifts in Priorities. PLoS One. 2011;6(5):e20274. doi: 10.1371/journal.pone.0020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56(1):11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 35.Smith GD, Harrison SM, Birch PJ, Elliott PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/−)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33(9):1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85(7–8):309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98(1):35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tegeder I, Niederberger E, Vetter G, Bräutigam L, Geisslinger G. Effects of selective COX-1 and -2 inhibition on formalin-evoked nociceptive behaviour and prostaglandin E2 release in the spinal cord. J Neurochem. 2001;79(4):777–786. doi: 10.1046/j.1471-4159.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 39.Tétreault P, Dansereau MA, Doré-Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav. 2011;104(3):495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152(5):990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhoeven AC, Boers M, van der Liden S. Validity of the MACTAR questionnaire as a functional index in a rheumatoid arthritis clinical trial. The McMaster Toronto Arthritis. J Rheumatol. 2000;27(12):2801–2809. [PubMed] [Google Scholar]
- 42.Vierck CJ, Acosta-Rua AJ, Johnson RD. Bilateral chronic constriction of the sciatic nerve: a model of long-term cold hyperalgesia. J Pain. 2005;6(8):507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Vierck CJ, Jr, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119(1):223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva L. Is there a gap between preclinical and clinical studies of analgesia? Trends Pharmacol Sci. 2000;21(12):461–462. doi: 10.1016/s0165-6147(00)01577-7. author reply 465. [DOI] [PubMed] [Google Scholar]
- 46.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 47.Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004;141(1):85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Eur J Pain. 1998;2(1):63–68. doi: 10.1016/s1090-3801(98)90047-7. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]