Abstract
Background
Alcohol use has been consistently found to have a J-shaped association with coronary heart disease, with moderate drinkers exhibiting a decreased risk compared to both heavy drinkers and non-drinkers. However, studies of the association between alcohol use and subclinical coronary artery disease have conflicted.
Objective
To determine whether alcohol is associated with the presence, amount, or progression of coronary calcium over a 2- to 4-year period.
Design
MESA is a prospective community-based cohort study of subclinical cardiovascular disease in a multi-ethnic cohort. In 2000–2002, 6814 participants free of clinical cardiovascular disease were enrolled at 6 participating centers.
Results
There were 3766 (55.5%) current drinkers, 1635 (24.1%) former drinkers, and 1390 (20.5%) never drinkers included in the analysis. Although light to moderate alcohol consumption was associated with lower coronary heart disease risk, we found no evidence of a protective or J-shaped association of alcohol and coronary artery calcium (CAC). In fact there was evidence that heavy consumption of hard liquor was associated with greater CAC accumulation. Other alcoholic beverages were not associated with CAC prevalence, incidence or progression.
Conclusions
This is the first large study to evaluate the association of alcohol and coronary artery calcium in four racial/ethnic groups, and to evaluate progression of calcification. These results suggest that the cardiovascular benefits that may be derived from light to moderate alcohol consumption are not mediated through reduced CAC accumulation.
Introduction
Alcohol use has been consistently found to have a J-shaped association with coronary heart disease, with moderate drinkers (up to 2 drinks per day) exhibiting a decreased risk compared to both heavy drinkers and non-drinkers. This relationship has not been studied extensively across race/ethnicities, but the limited evidence to date consistently shows a protective effect [1–3]. Alcohol intake greater than approximately 2 drinks per day is associated with higher blood pressure [4]. Alcohol intake is also associated with higher HDL-cholesterol [5, 6], lower inflammation [6], lower fibrinogen [5], and a lower risk of type 2 diabetes [7]. Results of studies of the association between alcohol use and subclinical coronary artery disease as measured by computed tomography have conflicted. One possible mechanism for such an association is via the positive association of alcohol with HDL, which in turn has an inverse association with subclinical CAD. However, results to date have included no association [8–10], a U-shaped association [11], and a dose-response relationship [12]. The explanation for these conflicting results is unclear. The relationship between alcohol and subclinical CAD could provide clues about the mechanisms underlying the relationship between alcohol and CHD. Studies have been limited to White or African-American participants, and have not assessed change in coronary artery calcium in relation to alcohol. In this paper we explore the association of alcohol consumption and the prevalence, incidence, and progression of CAC, as well as with CHD events, in a large multi-ethnic cohort.
Methods
(i) Study Participants
MESA is a prospective cohort study of the prevalence, risk factors and progression of subclinical cardiovascular disease in a multi-ethnic community-based cohort. A detailed description of the study design and methods has been published previously [13]. Briefly, 6814 participants aged 45–84 years who identified themselves as White, African-American, Hispanic, or Chinese were recruited from six United States communities from 2000 to 2002. All participants were free of clinically apparent cardiovascular disease at study entry (self reported history of physician diagnosed heart attack, angina, stroke, transient ischemic attack, or heart failure, current atrial fibrillation, taking nitroglycerin, or having undergone angioplasty, coronary artery bypass graft, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries). The communities were Forsyth County, North Carolina; Northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California. Each site recruited an approximately equal number of men and women, according to pre-specified age and race/ethnicity proportions. All participants gave informed consent, and the institutional review boards of the six field centers have approved the study protocol.
(ii) Alcohol Consumption
As part of a personal history questionnaire participants were asked “Have you ever consumed alcoholic beverages?” If yes, they were then asked “Do you presently drink alcoholic beverages?” The answers to these two questions determined our categorization into never, former, and current drinkers. Both current and former drinkers were asked about number of years of drinking, and usual number of drinks consumed per week (prior to stopping drinking in the case of former drinkers). Current drinkers were also asked about number of drinks consumed during the past 24 hours, and the largest number of drinks consumed in one day in the past month. Binge drinking was defined as consumption of 5 or more drinks on one occasion in the past month.
In addition to the personal history questionnaire, participants completed a 120-item food-frequency questionnaire. Participants were asked to consider their usual eating habits over the past year, and record the usual serving size (small, medium, or large) and average consumption of specific beverages and foods. For beverages, nine options were given that included: “rare or never”, “1–3 per month”, “1 per week”, “2–4 per week”, “5–6 per week”, “1 per day”, “2–3 per day”, “4–5 per day”, and “≥6 per day”. The alcohol percentage in wine, beer and liquor were assumed as 9.3%, 3.6% and 14.2%, respectively. Age, sex and beverage specific portion sizes were used to convert small, medium and large servings to grams. These assumptions were used to estimate grams per day for each of beer, wine and liquor. The food frequency questionnaire used in MESA is based on a questionnaire originally designed for the Insulin Resistance and Atherosclerosis Study, which was previously validated in a sample of whites, blacks, and Hispanics [14].
(iii) Measurement of Coronary Artery Calcium
Coronary artery calcium was measured using either electron-beam computed tomography at 3 field centers, or multi-detector computed tomography at 3 field centers. Each participant was scanned twice consecutively and these scans were read independently at a centralized reading center. Measurements were made on all participants at baseline (2000–2002) and at one of two follow-up examinations in either 2002–2003 or 2003–2005. The methodology for acquisition and interpretation of the scans has been documented previously [15]. The results from the two scans were averaged in order to provide a more accurate point estimate of the amount of calcium present. The amount of calcium was quantified using the Agatston scoring method [16]. Calcium scores were adjusted using a standard calcium phantom that was scanned along with the participant [17].
(iv) Coronary Heart Disease Ascertainment
At intervals of 9–12 months, a telephone interviewer contacted each participant to inquire about interim hospital admissions and cardiovascular outpatient diagnoses. Trained personnel abstracted medical records suggesting possible cardiovascular events, and two physicians independently classified the events. For purposes of this study, we used all incident CHD events as the endpoint, including definite or probable MI, resuscitated cardiac arrest, fatal CHD, and definite angina. Probable angina was included only if accompanied by revascularization (n=2). In most cases, definite or probable MI required either abnormal cardiac biomarkers regardless of pain or ECG findings; evolving Q waves regardless of pain or biomarker findings; or a combination of chest pain, and ST-T evolution or new left bundle branch block, and abnormal biomarker levels. Angina required symptoms of typical chest pain or atypical symptoms. Probable angina required, in addition to symptoms, a physician diagnosis of angina and medical treatment for it. Definite angina required one or more additional criteria, including revascularization; 70% or greater obstruction on coronary angiography; or evidence of ischemia by stress tests or resting ECG. Fatal CHD required a documented MI within the previous 28 days, chest pain within 72 hours, or a history of CHD, and required the absence of a known non-cardiac cause of death.
(v) Measurement of Other Variables
Information on demographics, smoking, medical conditions, and family history, were collected via questionnaire at the initial examination. Current smoking was defined as answering yes to the question “Have you smoked cigarettes during the last 30 days?” For those answering no, former smoking was defined as answering yes to the question “Have you smoked at least 100 cigarettes in your lifetime?” Pack years was calculated as the number of packs smoked per day on average, multiplied by years of smoking. Height and weight were also measured at the baseline exam, and blood was drawn for measurements including lipids, inflammation, fasting glucose, and fibrinogen. Resting blood pressure was measured three times in the seated position, and the average of the last two measurements was used in analysis. Hypertension was defined as diastolic blood pressure >=90mmHg, systolic blood pressure >=140mmHg or use of antihypertensive medications. Diabetes was defined as fasting glucose >125 mg/dL or taking diabetes medication. Income level was categorized into 4 levels based on total gross family income in the past 12 months.
(vi) Statistical Analysis
Relative risk regression was used to model prevalent and incident CAC>0 as a function of alcohol consumption. That is, the probability of incident CAC was modeled as a function of covariates using a generalized linear model with log link, Gaussian error and used robust standard error estimates [18]. Among those with some detectable CAC at Exam 1, we fit robust regression models for the amount of CAC. The robust regression algorithms in Stata [19] were used, which iteratively downweight observations with large residuals [20]. This modeling strategy was necessary to deal with influential outliers in the CAC measurements. Additionally, among those with CAC>0 at baseline we defined progression as the absolute difference between follow-up and Exam 1 CAC, and this was treated as a continuous endpoint. The modeling strategy for this endpoint was analogous to the models for CAC amount at baseline, and we used robust regression.
Generalized additive models [21] were used to explore non-linearity in the alcohol associations with CAC, adjusting for age, gender, race/ethnicity, smoking, income and education. Smoothed terms for either total alcohol, beer, wine or hard liquor were included in the model, with 3 degrees of freedom allowed for each. We found the shape of the smoothed functions to be well approximated by a linear model, with the possible exception of beer consumption. However, for comparison with prior studies, and to allow us to easily include former drinkers in the models we focused mainly on the categorized variables: never drinker, former drinker, <1 drink/day, 1–2 drinks per day, and >2 drinks per day. For the food-frequency questionnaire alcohol variables we assumed a standard drink contains 10 grams of alcohol and categorized accordingly.
We considered gender, race, smoking, income and education as potential confounders, and all comparisons adjust for these factors (Model 1). Additionally, a second model was fit for each endpoint which additionally included potential mediators of the association between alcohol and CAC (Model 2). These included: HDL cholesterol, hypertension, diabetes, body mass index, IL-6 and fibrinogen. Models for incident CAC or CAC change also controlled for the time between scans. Additional adjustment for type of scanner did not impact the findings, and was not included in the final models. P-values were considered statistically significant at the p<0.01 level, to account for the fact that many statistical comparisons were performed. Finally, since women were very rarely heavy drinkers, simply including a term for gender in the models may not have provided adequate adjustment for gender differences. To explore this issue we repeated the analyses stratified by gender.
To evaluate whether alcohol use was associated with incident coronary heart disease (CHD) we used Cox proportional hazards models, adjusting for all the variables in Model 2 described above. A second model also include coronary artery calcium, expressed as log(CAC+1). The distribution of CAC has a very long tail and the logarithmic transformation helped to moderate this. The constant of 1 was added to allow the zero CAC participants to be included.
Results
(i) Alcohol Consumption by Race/Ethnicity
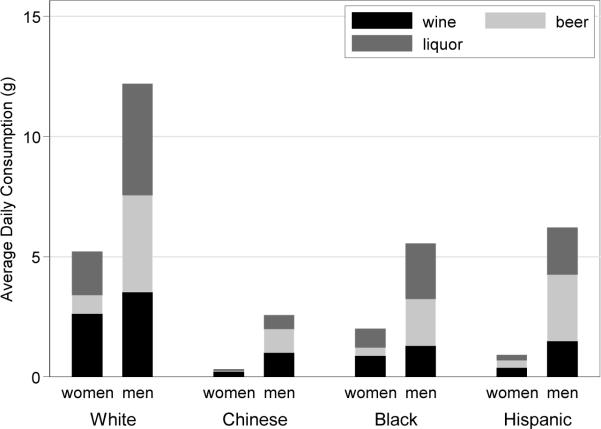
The total sample size for this study was 6791, after exclusion of 23 participants due to missing data on alcohol consumption at baseline. Of these, 3766 (55.5%) were current drinkers, 1635 (24.1%) former drinkers, and 1390 (20.5%) were never drinkers. Light drinking (greater than zero but less than one drink per day) was reported by 2947 (43.4%) participants, moderate drinking (1–2 drinks per day) by 599 (8.8%) participants, and heavy drinking (>2 drinks per day) by 220 (3.2%) participants. Table 1 provides a detailed breakdown of alcohol consumption by race/ethnicity. Generally speaking, White participants tended to consume the most alcohol, Chinese the least, with Blacks and Hispanics intermediate and quite similar to one another. Figure 1 displays average daily consumption for each type of beverage by gender and race/ethnicity.
Table 1.
Alcohol Consumption By Race/Ethnicity
| White (n=2616) | Chinese (n=802) | Black (n=1880) | Hispanic (n=1493) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Personal History Questionnaire Data | ||||
| Usual Consumption: | ||||
| never | 244 (9.3) | 431 (53.7) | 327 (17.4) | 388 (26.0) |
| former | 492 (18.8) | 120 (15.0) | 623 (33.1) | 400 (26.8) |
| <1 drink/day | 1374 (52.5) | 224 (27.9) | 773 (41.1) | 576 (38.6) |
| 1–2 drinks/day | 358 (13.7) | 22 (2.7) | 127 (6.8) | 92 (6.2) |
| >2 drinks/day | 148 (5.7) | 5 (0.6) | 30 (1.6) | 37 (2.5) |
| Binge drinking past month | 243 (9.3) | 11 (1.4) | 111 (5.9) | 174 (11.7) |
| Food Frequency Questionnaire Data | ||||
| Wine Consumption: | ||||
| >0 but <1 drink/day | 1077 (42.8) | 115 (14.5) | 428 (24.4) | 267 (18.7) |
| 1–2 drinks/day | 182 (7.2) | 10 (1.3) | 30 (1.7) | 21 (1.5) |
| >2 drinks/day | 68 (2.7) | 0 | 12 (0.7) | 9 (0.6) |
| Beer Consumption: | ||||
| >0 but <1 drink/day | 755 (30.0) | 96 (12.1) | 304 (17.3) | 300 (21.0) |
| 1–2 drinks/day | 76 (3.0) | 3 (0.4) | 34 (1.9) | 36 (2.5) |
| >2 drinks/day | 79 (3.1) | 3 (0.4) | 12 (0.7) | 19 (1.3) |
| Liquor Consumption: | ||||
| >0 but <1 drink/day | 764 (30.4) | 47 (5.9) | 370 (21.1) | 231 (16.1) |
| 1–2 drinks/day | 101 (4.0) | 0 | 33 (1.9) | 13 (0.9) |
| >2 drinks/day | 74 (2.9) | 2 (0.3) | 19 (1.1) | 9 (0.6) |
| Total Consumption: | ||||
| >0 but <1 drink/day | 1271 (48.6) | 230 (28.7) | 746 (39.7) | 580 (38.9) |
| 1–2 drinks/day | 316 (12.1) | 13 (1.6) | 125 (6.7) | 81 (5.4) |
| >2 drinks/day | 293 (11.2) | 8 (1.0) | 59 (3.1) | 44 (3.0) |
p<0.001 for all comparisons across race/ethnicity; binge drinking was defined as consumption of 5 or more drinks on one occasion during the past 30 days; food frequency questionnaire data has somewhat reduced sample sizes of 2516, 796, 1757, and 1431 for Whites, Chinese, Blacks and Hispanics respectively.
Figure 1.
Distribution of Average Daily Consumption and Beverage Type Among Current Drinkers by Race/Ethnicity and Gender
Total average daily consumption within each gender and race/ethnicity is represented by the full bar, with each section denoting the proportion due to wine, beer and liquor respectively from the bottom up.
(ii) Possible confounders and mediators of the alcohol/CAC association
In Table 2 we examine the distribution of potential confounders and mediators across categories of alcohol consumption. Relative to non-drinkers, alcohol consumers were more often high school graduates, had higher income, had somewhat lower body mass index, and considerably higher HDL. Family history of MI, LDL cholesterol, and use of lipid lowering medications did not differ by alcohol consumption. Among drinkers, increasing alcohol consumption was associated with more hypertension, though never and former drinkers had a greater prevalence of hypertension than those drinking modestly. Rates of diabetes and glucose levels were much lower for alcohol consumers even at more than 2 drinks per day. Generally speaking, inflammation levels were lower among alcohol consumers, although among consumers there was no consistent pattern with increasing amounts of alcohol consumed.
Table 2.
Adjusted Means and Proportions of Potential Confounders and Mediators Across Categories of Alcohol Consumption
| Variable | Alcohol Consumption | |||||
|---|---|---|---|---|---|---|
| never | former | <1 | 1–2 | >2 | P | |
| drink/day | drinks/day | drinks/day | ||||
| Education: <high school (%) | 18.1 | 15.9 | 9.6 | 6.4 | 9.5 | <0.001 |
| Income: <$25,000 (%) | 35.7 | 36.5 | 22.1 | 16.6 | 19.2 | <0.001 |
| Body mass index (kg/m2) | 28.6+/−0.15 | 28.8+/−0.13 | 28.1+/−0.10 | 27.4+/−0.22 | 28.1+/−0.26 | <0.001 |
| Family history of MI (%) | 44.4 | 41.4 | 41.3 | 42.5 | 43.3 | 0.47 |
| Diabetes (%) | 14.6 | 14.4 | 8.8 | 7.3 | 6.7 | <0.001 |
| HDL (mg/dl) | 48.0+/−0.4 | 48.7+/−0.3 | 51.6+/−0.3 | 55.6+/−0.6 | 59.6+/−0.7 | <0.001 |
| LDL (mg/dl) | 118.0+/−1.0 | 116.1+/−0.8 | 117.5+/−0.6 | 118.0+/−1.4 | 116.1+/−1.4 | 0.47 |
| Lipid lowering medications | 15.2 | 14.8 | 15.9 | 13.2 | 15.2 | 0.55 |
| Hypertension (%) | 48.2 | 45.2 | 40.9 | 40.8 | 53.4 | <0.001 |
| Systolic bp (mmHg) | 128.0+/−0.6 | 126.7+/−0.5 | 125.5+/−0.4 | 127.2+/−0.9 | 128.8+/−1.0 | <0.001 |
| Diastolic bp (mmHg) | 72.1+/−0.3 | 71.3+/−0.2 | 71.6+/−0.2 | 72.8+/−0.4 | 73.8+/−0.5 | <0.001 |
| CRP (mg/l) | 3.6+/−0.2 | 4.1+/−0.2 | 3.7+/−0.1 | 3.3+/−0.2 | 3.5+/−0.2 | 0.020 |
| IL-6 (pg/l) | 1.52+/−0.03 | 1.70+/−0.03 | 1.53+/−0.02 | 1.39+/−0.05 | 1.47+/−0.05 | <0.001 |
| Fibrinogen (mg/dl) | 355+/−2.1 | 355+/−1.8 | 344+/−1.4 | 330+/−3.1 | 330+/−3.6 | <0.001 |
Numbers in cells are adjusted means+/− standard error of the mean, or adjusted percentages. Adjustment is for average age, gender, race and smoking status based on the whole MESA cohort. Alcohol consumption category determined based on applying cut points to the food frequency questionnaire data.
We noted some interesting effect modification by race/ethnicity, although the small number of heavy drinkers in each minority precludes a full assessment of these findings. Specifically, in contrast with Whites consumption of more than two drinks per day was associated with much higher average glucose and diabetes prevalence in Hispanics and Chinese. The lower inflammation levels noted among alcohol consumers were observed exclusively among White participants. Inflammation tended to increase with alcohol consumption for Hispanics, and to be stable for Chinese and Blacks. The inverse association between alcohol consumption and fibrinogen was much stronger among Whites than the other race/ethnicities.
(iii) Alcohol consumption and the prevalence and extent of CAC at baseline
Among the overall cohort, alcohol consumption was not associated with the prevalence of any CAC>0 at baseline. This was true regardless of alcohol type, and there was no evidence of a J-shaped association (see Table 3). After adjustment for all mediators and confounders there was a trend for binge drinking to be associated with a slightly increased rate of prevalent CAC (12% higher rate, 95% CI 3% to 22%, p=0.01). A significant interaction between beer consumption and race (p=0.0015) indicated that among Hispanics consumption of >20grams of beer per day increased CAC prevalence by 40% (stratified RR 1.40, 95% CI 1.09 to 1.80, p=0.008). Out of 19 Hispanics reporting consumption of >20grams of beer per day, only 3 were free from CAC.
Table 3.
Alcohol Consumption and Baseline CAC Prevalence and Amount
| CAC Prevalence (CAC>0 at Baseline) | Baseline CAC Amount (among those with CAC>0) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| RR (95% CI) | P | RR (95% CI) | P | coef (95% CI) | P | coef (95% CI) | P | |
| Personal History Questionnaire Data | ||||||||
|
| ||||||||
| Usual Consumption: | 0.43 | 0.70 | 0.63 | 0.57 | ||||
| never | ref | ref | ref | ref | ||||
| former | 0.97 (0.91, 1.04) | 0.98 (0.92, 1.04) | −7.5 (−21.6, 6.7) | −8.2 (−22.5, 6.1) | ||||
| <1 drink/day | 0.95 (0.90, 1.01) | 0.98 (0.92, 1.04) | −3.3 (−16.4, 9.8) | −1.7 (−15.1, 11.6) | ||||
| 1–2 drinks/day | 0.94 (0.86, 1.01) | 1.00 (0.92, 1.08) | −7.3 (−25.7, 11.0) | −4.2 (−23.0, 14.6) | ||||
| >2 drinks/day | 0.99 (0.88, 1.11) | 1.04 (0.93, 1.17) | 7.4 (−17.6, 32.3) | 8.4 (−17.1, 33.9) | ||||
| Binge drinking past month: | 0.01 | 0.001 | 0.70 | 0.52 | ||||
| no (never) | ref | -- | ref | -- | ref | ref | ||
| no (current) | 0.94 (0.89, 1.00) | 0.05 | 0.97 (0.92, 1.03) | 0.34 | −3.2 (−16.3, 9.9) | −1.4 (−14.7, 11.9) | ||
| yes | 1.06 (0.97, 1.16) | 0.19 | 1.12 (1.03, 1.22) | 0.01 | −7.4 (−26.7, 12.0) | −6.7 (−26.4, 13.0) | ||
|
| ||||||||
| Food Frequency Questionnaire Data | ||||||||
|
| ||||||||
| Wine Consumption: | 0.64 | 0.95 | 0.81 | 0.68 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 0.95 (0.89, 1.01) | 0.98 (0.92, 1.05) | −6.1 (−20.7, 8.5) | −4.1 (−18.9, 10.8) | ||||
| 1–2 drinks/day | 0.94 (0.83, 1.05) | 1.00 (0.90, 1.12) | 3.1 (−22.3, 28.4) | 6.9 (−19.0, 32.7) | ||||
| >2 drinks/day | 0.97 (0.82, 1.14) | 1.03 (0.88, 1.21) | 3.9 (−34.7, 42.6) | 8.0 (−31.0, 47.0) | ||||
| Beer Consumption: | 0.39 | 0.41 | 0.20 | 0.16 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 0.94 (0.88, 1.01) | 0.98 (0.92, 1.05) | −6.8 (−22.2, 8.6) | −5.2 (−20.8, 10.4) | ||||
| 1–2 drinks/day | 1.00 (0.87, 1.15) | 1.05 (0.93, 1.19) | 0.2 (−30.4, 30.8) | 1.5 (−29.3, 32.3) | ||||
| >2 drinks/day | 1.02 (0.90, 1.15) | 1.08 (0.96, 1.22) | 29.7 (−1.3, 60.8) | 31.4 (0.01, 62.8) | ||||
| Liquor Consumption: | 0.44 | 0.72 | <0.001 | <0.001 | ||||
| 0 (never) | ref | ref | ref | -- | ref | -- | ||
| <1 drink/day | 0.96 (0.90, 1.03) | 1.00 (0.93, 1.07) | −16.6 (−32.3, −0.9) | 0.04 | −14.2 (−30.0, 1.5) | 0.08 | ||
| 1–2 drinks/day | 0.91 (0.80, 1.03) | 0.92 (0.82, 1.04) | 14.0 (−16.6, 44.6) | 0.37 | 14.6 (−16.1, 45.3) | 0.35 | ||
| >2 drinks/day | 0.90 (0.77, 1.04) | 0.94 (0.81, 1.09) | 56.7 (22.2, 91.2) | 0.001 | 57.0 (22.5, 91.6) | 0.001 | ||
|
|
||||||||
| Total Consumption: | 0.44 | 0.94 | 0.014 | 0.012 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 0.96 (0.90, 1.02) | 0.98 (0.93, 1.04) | −4.8 (−18.2, 8.6) | −2.8 (−16.5, 10.8) | ||||
| 1–2 drinks/day | 0.92 (0.85, 1.01) | 0.97 (0.89, 1.06) | −9.7 (−29.3, 9.9) | −7.8 (−27.8, 12.1) | ||||
| >2 drinks/day | 0.94 (0.86, 1.03) | 0.99 (0.91, 1.09) | 23.1 (2.3, 43.9) | 24.9 (3.5, 46.3) | ||||
Model 1 adjusts for age, gender, race, smoking (never/former/current and pack-years), education and income.
Model 2 adjusts for variables in Model 1, plus body mass index, systolic and diastolic blood pressure, use of anti-hypertensive medications, diabetes, HDL cholesterol, CRP, and fibrinogen. RR=rate ratio. P-values indicate the overall test of the variable in the model. Individual contrast p-values are displayed only if the overall test was significant at the p<0.01 level.
Models for CAC amount at baseline (among those with CAC>0) revealed a significantly higher average CAC amount among participants consuming >2 drinks/day of hard liquor. There were no other significant associations, and there was no evidence of a J-shaped relationship. On average those consuming >2 drinks per day of liquor had 57 units more CAC compared to never drinkers. This was not explained or even attenuated by any of the potential mediators. Stratifying the analysis by gender (data not shown) did not impact the conclusions.
(iv) Alcohol consumption and the incidence and progression of CAC over time
Patterns of associations with incidence and progression of CAC were identical to what was observed for baseline CAC (see Table 4). That is, alcohol consumption in general was not associated with the risk of incident CAC>0. This was true regardless of gender, and for all beverage types. We did observe the same race interaction, whereby Hispanics consuming >20grams of beer per day were at an increased risk of incident CAC, however, there were only 3 participants at risk in this subset (1 developed new CAC).
Table 4.
Alcohol Consumption and CAC Incidence and Progression
| CAC Incidence (CAC<0 at follow-up in those with CAC=0 at baseline) | Change in CAC (among those with CAC<0 at baseline) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| RR (95% CI) | P | RR (95% CI) | P | coef (95% CI) | P | coef (95% CI) | P | |
| Personal History Questionnaire Data | ||||||||
|
| ||||||||
| Usual Consumption: | 0.54 | 0.78 | 0.44 | 0.35 | ||||
| never | ref | ref | ref | ref | ||||
| former | 1.09 (0.81, 1.48) | 1.03 (0.76, 1.40) | −2.4 (−10.2, 5.5) | −2.1 (−9.9,5.7) | ||||
| <1 drink/day | 1.22 (0.93, 1.61) | 1.17 (0.87, 1.57) | 0.7 (−6.5, 7.9) | 2.2 (−5.0, 9.4) | ||||
| 1–2 drinks/day | 1.20 (0.83, 1.73) | 1.19 (0.81, 1.76) | 1.2 (−8.7, 11.0) | 3.5 (−6.5, 13.5) | ||||
| >2 drinks/day | 0.88 (0.44, 1.76) | 1.01 (0.55, 1.85) | 9.7 (−3.9, 23.2) | 9.8 (−3.8, 23.5) | ||||
| Binge drinking past month: | 0.49 | 0.68 | 0.54 | 0.24 | ||||
| no (never) | ref | ref | ref | ref | ||||
| no (current) | 1.22 (0.93, 1.60) | 1.17 (0.87, 1.58) | 1.3 (−5.9, 8.5) | 3.0 (−4.2, 10.3) | ||||
| yes | 1.09 (0.71, 1.69) | 1.14 (0.74, 1.76) | −2.3 (−12.8, 8.2) | −2.1 (−12.8,8.5) | ||||
|
| ||||||||
| Food Frequency Questionnaire Data | ||||||||
|
| ||||||||
| Wine Consumption: | 0.67 | 0.81 | 0.25 | 0.19 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 1.20 (0.88, 1.64) | 1.18 (0.84, 1.66) | −0.6 (−8.5, 7.4) | 1.8 (−6.2, 9.8) | ||||
| 1–2 drinks/day | 0.85 (0.46, 1.58) | 0.92 (0.53, 1.61) | 11.3 (−2.2, 24.7) | 13.4 (−0.2, 27.0) | ||||
| >2 drinks/day | 1.11 (0.47, 2.61) | 1.04 (0.40, 2.69) | 8.7 (−11.4, 28.7) | 9.0 (−11.0, 29.1) | ||||
| Beer Consumption: | 0.05 | 0.10 | 0.47 | 0.34 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 1.42 (1.06, 1.90) | 1.36 (0.99, 1.86) | −0.3 (−8.7, 8.1) | 1.6 (−6.9, 10.1) | ||||
| 1–2 drinks/day | 0.83 (0.33, 2.10) | 0.81 (0.28, 2.33) | −0.2 (−16.8, 16.4) | −1.5 (−18.1, 15.2) | ||||
| >2 drinks/day | 0.70 (0.25, 2.01) | 0.65 (0.22, 1.95) | 11.5 (−5.3, 28.4) | 11.5 (−5.5, 28.5) | ||||
| Liquor Consumption: | 0.66 | 0.13 | 0.003 | 0.006 | ||||
| 0 (never) | ref | ref | ref | -- | ref | -- | ||
| <1 drink/day | 1.27 (0.91, 1.78) | 1.37 (0.96, 1.96) | −0.8 (−9.2, 7.6) | 0.85 | 1.5 (−6.9, 10.0) | 0.73 | ||
| 1–2 drinks/day | 1.11 (0.55, 2.24) | 1.06 (0.49, 2.28) | 5.9 (−10.6, 22.4) | 0.48 | 6.4 (−10.1, 22.9) | 0.45 | ||
| >2 drinks/day | 1.52 (0.85, 2.72) | 1.80 (1.09, 2.97) | 33.2 (15.4, 51.1) | <0.001 | 31.7 (13.8, 49.6) | 0.001 | ||
| Total Consumption: | 0.71 | 0.83 | 0.04 | 0.04 | ||||
| 0 (never) | ref | ref | ref | ref | ||||
| <1 drink/day | 1.21 (0.92, 1.60) | 1.17 (0.86, 1.58) | 0.3 (−7.0, 7.7) | 2.2 (−5.2, 9.5) | ||||
| 1–2 drinks/day | 1.15 (0.78, 1.70) | 1.14 (0.75, 1.74) | 1.9 (−8.8, 12.6) | 2.2 (−8.5, 13.0) | ||||
| >2 drinks/day | 1.04 (0.64, 1.68) | 1.09 (0.66, 1.79) | 13.7 (2.5, 24.9) | 14.2 (2.8, 25.6) | ||||
Model 1 adjusts for time between scans, age, gender, race, smoking (never/former/current and pack-years), education and income. Model 2 adjusts for variables in Model 1, plus body mass index, systolic and diastolic blood pressure, use of anti-hypertensive medications, diabetes, HDL cholesterol, CRP, and fibrinogen. RR=rate ratio. P-values indicate the overall test of the variable in the model. Individual contrast p-values are displayed only if the overall test was significant at the p<0.01 level.
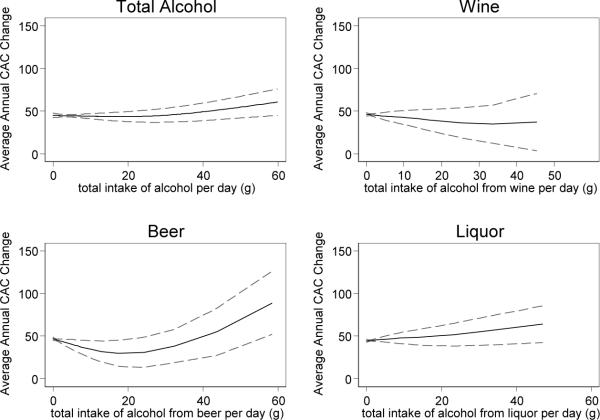
Consumption of >2 drinks/day of hard liquor was associated with faster rates of CAC change (over 30 units more annually) among those with existing CAC at baseline. This was not explained by risk factors or any of the potential mediators we considered. Other types of alcohol did not have a significant association with CAC progression overall, however there was some evidence of racial effect modification. Specifically, within Hispanics heavy wine consumption was associated with more progression and within Chinese both heavy beer and overall consumption were associated with more progression. However, there were only 3 heavy beer consumers (free of baseline CAC) among the Chinese, and 6 heavy wine drinkers among the Hispanics, so these race-specific findings are exploratory only. There was no evidence of any J-shaped association either overall or within racial/ethnic subsets. Stratified analysis indicated that the relationships were the same for men and women. In Figure 2 we show the smoothed association of grams of alcohol per day and average yearly change in CAC, adjusted for age, gender, race/ethnicity, smoking, income and education. For wine the association is nearly flat, and for beer, liquor, and total alcohol consumption there is an increasing trend. Only beer demonstrated significant non-linearity in the association, and was closest to exhibiting a J-shaped association with a slight decrease in CAC progression at lower levels (<2 drinks per day) of consumption, followed by a steep increase at higher consumption. However, the beer consumption associations had the widest confidence intervals, and the slight protective association at low levels is not significant.
Figure 2.
Smoothed Plot of Alcohol Consumption versus Annualized CAC Progression with 95% Confidence Bands
For each alcohol type the estimated grams of alcohol consumed per day is shown based on the food frequency questionnaire data. Average annual CAC change is the change in CAC score between baseline and follow-up, divided by the time between scans in years. The plot includes only those with CAC>0 at baseline, and excludes former drinkers. A generalized additive model was used, adjusting for age, gender, race/ethnicity, smoking, income, and education. The dotted lines indicate the 95% confidence bands.
(v) Alcohol and Incident Coronary Heart Disease
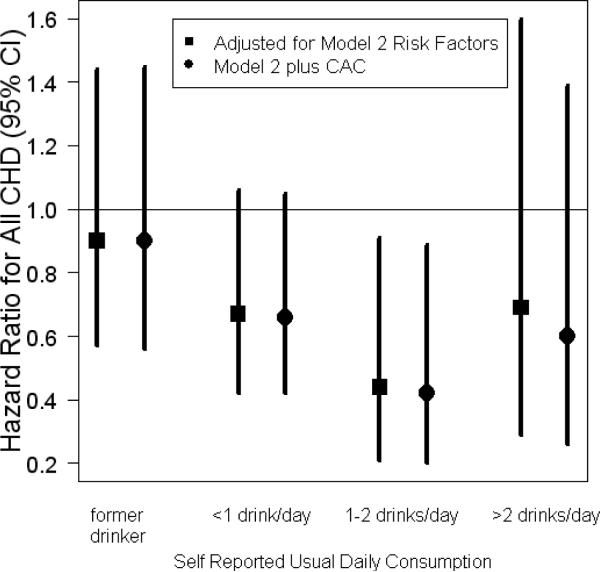
In Figure 3 we display the hazards ratios for incident CHD events for each category of usual alcohol consumption. Never drinkers were used as the reference category. Models are shown with and without adjustment for coronary artery calcium. A total of 189 incident CHD events are included, based on an average of 4 years of follow-up, including 86 myocardial infarctions, 9 resuscitated cardiac arrests, 19 CHD deaths, and 102 participants with angina (numbers add up to slightly more than 189 due to participants experiencing more than one type of event). Alcohol consumption did exhibit the expected J-shaped relationship with incident CHD events in our population, regardless of beverage type. Adjustment for CAC had almost no impact on the association of alcohol and events.
Figure 3.
Usual alcohol consumption and risk of CHD with and without adjustment for CAC Points indicate the hazards ratios relative to never drinkers for each category of self-reported usual daily consumption, and lines indicate the corresponding 95% confidence intervals. Square points adjust for age, gender, race, smoking, education, income, body mass index, systolic and diastolic blood pressure, use of anti-hypertensive medications, diabetes, HDL cholesterol, CRP, and fibrinogen. Round points adjust for those factors and coronary artery calcium.
Discussion
Like previous studies, we found that moderate alcohol consumption has an apparent protective association with coronary heart disease; however, we found no evidence of such a protective association of alcohol consumption and coronary artery calcium. In fact, there was evidence that heavy alcohol consumption, hard liquor in particular, was associated with greater CAC accumulation. This association did not appear to be mediated by hypertension, body size, diabetes, inflammation or cholesterol.
(i) Comparison with Previous Studies
Our findings for are generally consistent with three previous studies of the association of overall alcohol consumption and CAC prevalence. Yang et al [10] found no association between CAC amount and alcohol use among high CHD-risk asymptomatic participants. These participants were primarily White, and among our White participants we found no association between overall alcohol consumption and CAC at baseline. They did not separate out different beverage types, so we cannot assess whether the association of hard liquor consumption and increased CAC amount was present in their population. Tofferi et al [8] found no association among a cohort of 731 U.S. army personnel aged 39–45. These were very healthy participants with a low prevalence of CAC (18% in non-drinkers), and again were primarily White (72%). They did not find any association between hard liquor and CAC amount, although they had only 9 daily consumers of hard liquor with CAC>0. Ellison et al [9] also reported no association of alcohol intake and either CAC or aortic calcium in a study of White and Black participants with an average age of 55. Beverage-specific analyses also showed no relationship, although the highest category of consumption was >=1 drink/day. This would have combined our moderate and heavy drinking categories and diluted the effect we observed for hard liquor. Additionally, their endpoint was CAC>100. These analytic differences could explain the different findings for hard liquor consumption.
In contrast Vliegenthart et al [11] found a J-shaped relationship among 1795 White participants in the Rotterdam Coronary Calcification Study. The Rotterdam population had much higher rates of alcohol consumption than MESA, and many more participants with extensive CAC (26% with CAC>400 versus 13.5% in MESA Whites). It is unclear whether this could explain the different finding. They included former drinkers in the reference category of nondrinkers, which is different from our analysis. However, when we repeated our analysis pooling former and never drinkers we still find that there is no significant association between alcohol and extensive CAC prevalence, even restricting to the White subset of our cohort and examining wine separately (where they found the strongest protective association).
Pletcher et al [12] found a dose response relationship between alcohol consumption and the odds of CAC>0 among White and Black participants aged 33–45 years old. That is, the higher the alcohol consumption, the higher the prevalence of CAC. We did not find such an association in either Whites or Blacks in our study. Additionally they found that binge drinking was strongly associated with CAC prevalence in both races. In our study recent binge drinking was modestly associated with CAC prevalence overall, but not within Whites or Blacks specifically. The association was mainly evident for Chinese participants, although there were only 11 binge drinkers within that subset in our study limiting our ability to investigate this fully. The age distribution of the participants in their study does not overlap with the MESA participants, and hence the different findings may suggest that alcohol has a different association with the onset of coronary calcification very early in life.
Alcohol consumption did exhibit the expected J-shaped relationship with incident CHD events in our population, regardless of beverage type. Adjustment for coronary artery calcium did not modify this association to any noticeable degree, suggesting that slower CAC accumulation is not the mechanism for the cardiovascular benefits of alcohol consumption. This is consistent with Yang et al [10] who found that the protective association of alcohol consumption was independent of coronary artery calcium.
(ii) Study Limitations and Strengths
Although we found no evidence of gender interaction there were relatively few heavy drinkers that were women, limiting our ability to explore alcohol associations among this subset. Alcohol consumption was based on questionnaire, and participants may have underreported heavy consumption. This would most likely have attenuated the associations. For total alcohol consumption we had two measures of consumption: one from asking the participant to categorize their usual consumption, and one derived from the food frequency questionnaire. Results obtained in terms of relationship to CAC were similar, which is reassuring. Also, we did find the expected positive association between alcohol and HDL cholesterol, and the expected J-shaped association with CHD events. Although we have attempted to adjust for many factors, unmeasured confounders may still account for some of the observed associations. Our number of heavy drinkers was small, especially for Chinese participants, limiting our ability to make inferences regarding ethnic differences. The time period between CAC scans was relatively short, however, despite this we did observe considerable CAC incidence and progression over the observation period. Strengths of the MESA study include the large sample size, prospective design, inclusion of four racial/ethnic groups, and the community-based nature.
(iii) Conclusions
We found no evidence of any protective or J-shaped association of alcohol with CAC prevalence, incidence or amount. Consumption of >20g of hard liquor per day was consistently associated with higher CAC accumulation, both in terms of amount at baseline, and progression of CAC over time. Our results suggest that the cardiovascular benefits that may be derived from light to moderate alcohol consumption are not mediated through reduced CAC accumulation.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Author Contributions Robyn L. McClelland: statistical analysis and interpretation; manuscript writing
Diane E. Bild: cardiovascular epidemiology expertise; critical review of manuscript
Gregory L. Burke: cardiovascular expertise; critical review of manuscript
Kenneth J. Mukamal: alcohol and CVD expertise; critical review of manuscript
Joao A. Lima: cardiovascular expertise; critical review of manuscript
Richard A. Kronmal: interpretation of statistical analysis; critical review of manuscript
Conflicts of Interest: The authors have no conflicts to disclose.
References
- [1].Mukamal KJ, Chung H, Jenny NS, Kuller LH, Longstreth WT, Mittleman MA, Burke GL, Cushman M, Psaty BM, Siscovick DS. Alcohol Consumption and Risk of Coronary Heart Disease in Older Adults: The Cardiovascular Health Study. Journal of the American Geriatrics Society. 2006;54(1):30–37. doi: 10.1111/j.1532-5415.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- [2].Kabagambe EK, Baylin A, Ruiz-Narvaez E, Rimm EB, Campos H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. American Journal of Clinical Nutrition. 2005;82:1336–1345. doi: 10.1093/ajcn/82.6.1336. [DOI] [PubMed] [Google Scholar]
- [3].Yuan JM, Ross RK, Gao YT, Henderson BE, Yu MC. Follow up study of moderate alcohol intake and mortality among middle aged men in Shanghai, China. British Medical Journal. 1997;314:318. doi: 10.1136/bmj.314.7073.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary Prevention of Hypertension: Clinical and Public Health Advisory From the National High Blood Pressure Education Program. Journal of the American Medical Association. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- [5].Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. British Medical Journal. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li JM, Mukamal KJ. An update on alcohol and atherosclerosis. Current Opinion in Lipidology. 2004;15:673–680. doi: 10.1097/00041433-200412000-00008. [DOI] [PubMed] [Google Scholar]
- [7].Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Moderate Alcohol Consumption Lowers The Risk of Type 2 Diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- [8].Tofferi JK, Taylor AJ, Feuerstein IM, O'Malley PG. Alcohol intake is not associated with subclinical coronary atherosclerosis. American Heart Journal. 2004;148:803–809. doi: 10.1016/j.ahj.2004.05.023. [DOI] [PubMed] [Google Scholar]
- [9].Ellison RC, Zhang Y, Hopkins PN, Knox S, Djousse L, Carr JJ. Is alcohol consumption associated with calcified atherosclerotic plaque in the coronary arteries and aorta? American Heart Journal. 2005;152:177–182. doi: 10.1016/j.ahj.2005.09.016. [DOI] [PubMed] [Google Scholar]
- [10].Yang T, Doherty TM, Wong ND, Detrano RC. Alcohol Consumption, Coronary Calcium, and Coronary Heart Disease Events. American Journal of Cardiology. 1999;84:802–806. doi: 10.1016/s0002-9149(99)00440-3. [DOI] [PubMed] [Google Scholar]
- [11].Vliegenthart R, Oei HS, van den Elzen APM, van Rooij FJA, Hofman A, Oudkerk M, Witteman JCM. Alcohol Consumption and Coronary Calcification in a General Population. Archives of Internal Medicine. 2004;164:2355–2360. doi: 10.1001/archinte.164.21.2355. [DOI] [PubMed] [Google Scholar]
- [12].Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol Consumption, Binge Drinking, and Early Coronary Calcification: Findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. American Journal of Epidemiology. 2005;161:423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- [13].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, Clark Nelson J, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [14].Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- [15].Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano R. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- [16].Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- [17].Nelson JC, Detrano R, Kronmal RA, Carr JJ, McNitt-Gray MF, Wong N, Loria C, Goldin JG, Williams DO. Measuring Coronary Calcium on CT Images Adjusted for Attenuation Differences. Radiology. 2005;235:403–414. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- [18].Lumley T, Kronmal RA, Ma S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators and Algorithms. University of Washington; 2006. (UW Biostatistics Working Paper Series). Paper 293, http://www.bepress.com/uwbiostat/paper293. [Google Scholar]
- [19].StataCorp . Stata Statistical Software: Release 9. StataCorp LP; College Station, TX: 2005. [Google Scholar]
- [20].Hamilton LC. How robust is robust regression? Stata Technical Bulletin. 1991;2:21–26. [Google Scholar]
- [21].Hastie T, Tibshirani R. Generalized Additive Models. Chapman and Hall; London: 1990. [DOI] [PubMed] [Google Scholar]