Abstract
Objective
To assess the incidence of symptoms (palpitations, syncope) and electrocardiographic signs (increased QT duration and dispersion) of an increased risk of torsades de pointes in youth treated with ziprasidone.
Method
Prospective, observational study of 29 youth (15.3±2.9 years) receiving ziprasidone (112.8±50.6 mg/day, range: 20–240mg/day) for 99.3±108.7 days. All patients had normal electrocardiograms (EKGs) and no serious medical illness at baseline. Patients had a mean of 2.7±1.3 (median=3, range: 1–7, total: 49) follow-up EKGs performed monthly for 3 months and every three months thereafter, with concurrent ziprasidone blood level measurements. Heart rate-corrected QT (QTc) duration and dispersion were measured manually in ≥6 EKG leads. QTc interval > 450msec or ≥60msec increase, and QTc dispersion >100msec were considered abnormal.
Results
No patient reported syncope or symptomatic arrhythmias. Seven patients (24.1%) developed EKG abnormalities; 5 had peak QTc durations >450msec and 2 had peak QTc dispersion >100 msec. The baseline-to-peak QTc duration increased by 22.9±21msec (p<0.0001). The baseline-to-peak QTc dispersion increased by 6.1±31.4msec (p=0.30). The peak QTc duration and dispersion occurred after 47.6±45.2 and 60.4±72.1 treatment days, respectively. Baseline-to-peak QTc duration and dispersion changes were not correlated with ziprasidone dose (p=0.67) or plasma levels (p=0.37).
Conclusions
Ziprasidone was associated with a dose- and level-independent, significant prolongation of QTc duration in one quarter of youth. However, prolongation of QTc dispersion was non-significant, and no patient experienced concomitant abnormal prolongation of both QTc duration and QTc dispersion. The dissociation between prolonged QTc duration and dispersion suggests low arrhythmogenic potential in youth with normal baseline EKGs.
Keywords: Antipsychotic, Ziprasidone, cardiac, QTc, prolongation, dispersion, children, adolescents
Ziprasidone is a second-generation antipsychotic with proven effectiveness for pediatric bipolar disorder Tourette’s syndrome, pervasive developmental disorder and obsessive-compulsive disorder (1–3). In addition to high-affinity antagonist activity at 5HT2A and dopamine D2 receptors, this antipsychotic has partial agonist activity at 5-HT1A receptors and affinity for norepinephrine and serotonin transporters (4). Ziprasidone is associated with substantially less weight gain and adverse metabolic effects than most other widely used atypical antipsychotics, both in adults (5), and in youth (1,6) and is considered a reasonable treatment choice for minimizing these metabolic complications (6). However, ziprasidone has the potential to prolong the rate-corrected QT interval (QTc) in the electrocardiogram. This effect is greater than the QTc prolongation observed during treatment with haloperidol, risperidone, olanzapine and quetiapine (7,8). Significant prolongation of QTc is generally considered a risk factor for torsades de pointes (TdP), ventricular fibrillation and sudden death, particularly when the QTc is greater than 500 msec (9–11).
Antipsychotics produce QTc prolongation by blocking the rapid component of the delayed rectifier current, which promotes potassium efflux from the ventricular myocytes during repolarization (10,11). The mechanism is similar to the QTc prolongation produced by many widely used drugs, such as the antiarrhythmic agents amiodarone and sotalol, quinolone and macrolide antibiotics, and methadone (11). The incidence of TdP in patients treated with these drugs is extremely low, a fact that raises doubts about the value of QTc prolongation as the single electrocardiographic predictor for the risk of TdP. One the other hand, the risk of TdP is clearly increased when the QTc prolongation is associated with a concurrent prolongation of the intramyocardial dispersion of repolarization (10). A larger difference between the action potential duration in adjacent regions of the ventricular myocardium enables circular reentry activity between areas with delayed repolarization and those with excitable myocytes (10,12). The phenomenon is assessed by measuring the QTc dispersion, i.e., the inter-lead variability of QTc on the electrocardiogram. A QTc dispersion >120 msec was shown to be a strong correlate of inducible ventricular tachycardia and its predictive value was not influenced by gender, mean QTc, and left ventricular systolic function (13). QTc dispersion is also a stronger predictor for cardiovascular mortality than QTc duration in the general population (14), patients with myocardial infarction (15), subjects with congenital long QT syndromes (16) and patients who developed TdP after intravenous administration of haloperidol (17).
A four-year post-marketing evaluation (4) and a one-year randomized comparison between ziprasidone and olanzapine in 18,000 adult patients, resulting in similar non-suicide related death rates of 0.9% in both groups (18), have indicated that the ziprasidone-induced QTc prolongation is not linked to the occurrence of TdP in adults, and only one case of TdP related to treatment with ziprasidone has been reported in the literature (19). Although one case of sudden death in a child treated with ziprasidone was reported, the authors made it clear that they believed after extensive investigation that this death was unrelated to the ziprasidone treatment (20).
In contrast to the large adult databases (4,18), the effect of ziprasidone on myocardial repolarization in children and adolescents has been reported only in a total of 102 patients who were included in five published, open-label studies (1–3,21–22). In these studies, the mean QTc change ranged from −3 msec to +28 msec, and none of these pediatric patients was reported to have experienced syncope or other cardiovascular events. The QTc dispersion was not assessed in any of these studies.
In this prospective study, we evaluated the QTc duration and QTc dispersion in 29 children and adolescents monitored carefully during treatment with ziprasidone. Based on the absence of ziprasidone related TdP cases after 8 years of clinical use, we hypothesized that ziprasidone has a low torsadogenic potential because the drug-induced QTc prolongation is not accompanied by a concomitant increase in QTc dispersion.
Methods
Setting and Patient Population
Data required for this study were collected as part of the Second-Generation Antipsychotic Treatment: Indications, Effectiveness and Tolerability in Youth (SATIETY) study, a prospective, observational cohort study of antipsychotics in pediatric patients treated for psychotic, mood or aggressive spectrum disorders. Subjects were recruited from the child and adolescent inpatient and outpatient services of Zucker Hillside Hospital and Schneider’s Children Hospital, North Shore- Long Island Jewish Health System, New York, and from community-based psychiatrists in the catchment’s area of these hospitals.
Subject Eligibility
Patients age 4–19 years were eligible for the study if they had started ziprasidone monotherapy within 7 days of the baseline assessment and had an EKG obtained at baseline and at a minimum of one follow-up visit that included an EKG. Exclusion criteria were baseline QTc >500 ms (N=0), eating disorders, severe medical illness, pregnancy, breastfeeding, and expected move out of the area within one month of starting treatment with ziprasidone. Written informed consent was obtained from patients older than 18 years of age, or from legal guardians if the patient was a minor. According to the Institutional Review Board rules, patients older than 8 years of age gave informed assent. The patients’ mental health care providers selected ziprasidone, and adjusted doses of ziprasidone and other psychotropic drugs based on clinical need. The research protocol was approved by the Institutional Review Board, North Shore – Long Island Jewish Health System, Manhasset, NY.
Assessment of Ziprasidone and Potassium Levels
Ziprasidone and potassium levels were measured in blood samples drawn between 7–11 AM after at least 8 hours fasting at baseline, monthly for the first three months and quarterly thereafter. Ziprasidone levels were assessed at the Thomas Cooper Laboratory, Nathan Kline Research Institute, Orangeburg, New York, using liquid chromatography on sera that had been stored at −40 C. Plasma potassium was measured spectrophotometrically at the North Shore Core Laboratory, Manhasset, New York on the same day of the blood draw.
Measurement of QTc Duration and Dispersion
All patients had a 12-lead electrocardiogram recorded with the same instrument (ELI 1200, Mortara Instruments, Milwaukee) at baseline, monthly for the first three months and quarterly thereafter. All follow-up EKGs and the vast majority of baseline EKGs were obtained at the same time as the AM fasting blood samples. However, a small number of patients had their baseline EKGs obtained at different times upon hospital admission. The electrocardiograph is programmed to calculate the “automated” QTc. Two board-certified internists (VF and PM) with extensive clinical and research experience in the evaluation of EKGs in adults and children and adolescents (23) measured the Q-T intervals in all leads, in which the onset of the QRS complex and the return of T wave to baseline could be clearly identified. These physicians were provided numerically coded EKG’s and had no access to demographic or clinical data. The QT duration was corrected for heart rate according to the Bazett’s formula (9) and averaged for all assessed leads. QTc dispersion was calculated as the difference between the longest and shortest individual lead QTc (13). QTc was considered prolonged if greater than 450 msec or 60 msec longer than at baseline (9). QTc dispersion was considered abnormal if greater than 100 msec (13).
Data Analyses
Analyses of variance and chi-square tests were performed for continuous and categorical variables, respectively. The relationships between QTc duration and QTc dispersion with ziprasidone dose, ziprasidone serum level and plasma potassium level were assessed with Pearson’s product-moment correlation coefficients. All tests were two-sided, with alpha set at <0.05. and calculated with JMP 5.0.1, 1989–2003, SAS Institute Inc, Cary, North Carolina.
Results
Demographic and Clinical Characteristics
Twenty-nine pediatric patients (mean age: 15.3± 2.9 years, 44.8% male, 48.3% White) participated in the study. The three most common diagnostic categories were disruptive behavior disorders (i.e., intermittent explosive disorder (IED), conduct disorder (CD), oppositional defiant disorder (ODD) and impulse control disorder not otherwise specified (55.2%), attention deficit-hyperactivity disorder (37.9%) and psychosis not otherwise specified (27.6%) (Table 1). Patients were treated with ziprasidone at a mean maximum dose of 112.8±50.6 mg/day (median: 80 mg/d; range: 20–240mg/day) and remained in the study for 99.3±108.7 days. The highest mean daily dose of ziprasidone at the time of an EKG was 89.9±52.3 mg (range: 20–180 mg). (The discrepancy between the mean clinical dose of ziprasidone and the dose at the time of the EKG assessment is due to the fact that patients were treated with flexible ziprasidone doses that could be titrated and adjusted during the study and that were sometimes lower at the EKG assessment time point.) In addition to ziprasidone, patients were also treated with mood stabilizers (41.4%), antidepressants (17.2%), anxiolytic/hypnotics (13.8%) and psychostimulants (13.8%) (Table 2).
Table 1.
Baseline Demographic and Clinical Characteristics of 29 Children and Adolescents Treated with Ziprasidone
| Baseline Characteristic | Total (n=29) | Normal QTc Duration and QT Dispersion (n=22) | Abnormal QTc Duration or QT Dispersion (n=7) | P- Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years ± SD) | 15.3 ± 2.9 | 15.5± 2.9 | 14.8 ± 3.0 | 0.60 |
| Adolescents (age 12 or older). (%) | 25 (86.2) | 19 (86.4) | 6 (85.7) | 0.96 |
| Male gender – no. (%) | 13 (44.8) | 10 (45.5) | 3 (42.9) | 0.90 |
| Ethnicity – no. (%) | 0.13 | |||
| White | 14 (48.3) | 9 (40.9) | 5 (71.4) | |
| African-American | 8 (27.6) | 8 (36.4) | 0 (0.0) | |
| Hispanic | 5 (17.2) | 4 (18.2) | 1 (14.3) | |
| Other | 2 (6.9) | 1 (4.5) | 1 (14.3) | |
| Weight and Body Mass Index (BMI) | ||||
| Weight (lbs ± SD) | 163.5 ± 47.9 | 171.4 ± 50.5 | 143.0 ± 36.2 | 0.35 |
| BMI (kg/m2 ± SD) | 27.6 ± 5.9 | 28.3 ± 6.2 | 25.8 ± 4.7 | 0.96 |
| BMI percentile (± SD) | 85.6 ± 21.7 | 85.7 ± 22.7 | 85.3 ± 19.1 | 0.96 |
| Psychiatric diagnoses – no. (%) | ||||
| Disruptive Behavior Disorders (OCD/CD/IED/ICD) | 16 (55.2) | 10 (45.5) | 6 (85.7) | 0.06 |
| Attention Deficit-Hyperactivity Disorder | 11 (37.9) | 8 (36.4) | 3 (42.9) | 0.76 |
| Psychosis Not Otherwise Specified | 8 (27.6) | 7 (31.8) | 1 (14.3) | 0.37 |
| Bipolar Disorder | 7 (24.1) | 5(22.7) | 2 (28.6) | 0.75 |
| Autism Spectrum Disorder | 6 (20.7) | 3 (13.6) | 3 (42.7) | 0.09 |
| Schizophrenia/Schizoaffective Disorder | 5 (17.2) | 4 (18.2) | 1 (14.3) | 0.81 |
| Mood Disorder Not Otherwise Specified | 3 (10.3) | 2 (9.1) | 1 (14.3) | 0.68 |
| Anxiety Disorders | 2 (6.9) | 2 (9.1) | 0 (0.0) | 0.41 |
| Substance Used Disorder | 2 (6.9) | 1 (4.5) | 1 (14.3) | 0.37 |
| Major Depression | 1 (3.5) | 1 (4.5) | 0 (0.0) | 0.57 |
| Clinical Global Impression Severity score (mean ± SD) | 5.1 ± 0.9 | 5.1 ± 0.9 | 5.1 ± 0.9 | 0.83 |
| Global Assessment of Functioning score (mean ± SD) | 39.2 ± 10.6 | 39.6 ± 10.7 | 38.4 ± 10.5 | 0.81 |
IED: intermittent explosive disorder; CD: conduct disorder; ODD: oppositional defiant disorder; ICD: impulse control disorder not otherwise specified
Table 2.
Treatment And Outcome Characteristics of 29 Children and Adolescents Treated with Ziprasidone
| Treatment Characteristic | Total (n=29) | Normal QTc Duration and QT Dispersion (n=22) | Abnormal* QTc Duration or QT Dispersion (n=7) | P-Value |
|---|---|---|---|---|
| Ziprasidone Treatment | ||||
| Duration of Ziprasidone Treatment (days ± SD) | 99.3 ± 108.7 | 96.1 ± 97.8 | 109.4 ± 146.7 | 0.78 |
| Highest Daily Dose of Ziprasidone at Time of EKG (mg ± SD) | 89.0 ± 52.3 | 98.2 ± 49.7 | 60.0 ± 52.9 | 0.093 |
| Mean Ziprasidone Serum level (microg/L ± SD)a | 108.8 ± 75.3 | 109.3 ± 66.2 | 107.5 ± 104.6 | 0.96 |
| Comedications At Longest QTc (No. and %) | ||||
| Total number of comedications (±SD) | 1.48 ± 0.85 | 1.45 ± 0.86 | 1.57 ± 1.13 | 0.77 |
| Patients on > 2 Psychotropic Medications (N, %) | 12 (41.4) | 7 (31.8) | 5 (71.4) | 0.06 |
| Mood stabilizers | 12 (41.4) | 8 (36.4) | 4 (57.4) | 0.09 |
| Antidepressants | 5 (17.2) | 5 (20.0) | 0 (0.0) | 0.16 |
| Anxiolytics/Hypnotics | 4 (13.8) | 4 (16.0) | 0 (0.0) | 0.22 |
| Psychostimulants | 4 (13.8) | 3 (13.6) | 1 (14.3) | 0.19 |
| Anticholinergics | 2 (6.9) | 2 (9.1) | 0 (0.0) | 0.40 |
| Other psychotropics b | 4 (13.8) | 3 (13.6) | 1 (14.3) | 0.97 |
| Longest QTc | ||||
| Duration of Treatment at Longest QTc (days ± SD) | 47.6 ± 46.0 | 48.2 ± 48.9 | 45.7 ± 33.2 | 0.90 |
| Daily Ziprasidone dose at Longest QTc (mg ± SD) | 71.3 ± 48.2 | 76.5 ± 47.0 | 54.3 ± 52.6 | 0.29 |
| Ziprasidone Serum Level at Longest QTc (microg/L ± SD)c | 70.1 ± 51.4 | 67.1 ± 36.1 | 78.6 ± 96.5 | 0.70 |
| Longest QTc Dispersion | ||||
| Duration of Treatment at Longest QTc Dispersion (days ± SD) | 60.4 ± 73.2 | 63.6 ± 81.4 | 51.4 ± 37.1 | 0.69 |
| Daily Ziprasidone dose at Longest QTc Dispersion (level ± SD) | 77.4 ± 49.7 | 84.4 ± 49.7 | 57.5 ± 49.5 | 0.20 |
| Ziprasidone Serum Level at Longest QTc Dispersion (level ± SD)d | 66.1 ± 68.2 | 61.7 ± 41.8 | 82.3 ± 101.3 | 0.55 |
QTc was considered prolonged if greater than 450 msec or 60 msec longer than at baseline (9). QTc dispersion was considered abnormal if greater than 100 msec (13)
Based on 19 patients with information normal QTc: n=14, abnormal QTc: n=5.
Other psychotropic medications: alpha-2 agonists, beta blockers, antihistamines
Based on 19 patients with information normal QTc: n=15, abnormal QTc: n=5
Based on 19 patients with information normal QTc: n=17, abnormal QTc: n=2
Global QTc changes
Patients had a mean of 2.7±1.3 (median=3, range: 1–7, total: 49) follow-up EKGs after baseline. At baseline, the QTc duration and dispersion were 410.5±25.0 msec and 47.0±24.8 msec, respectively (Table 3). During the study, the peak QTc duration averaged 433.4±25.1 msec (mean change: 22.9±21 msec, p<0.0001), while the mean peak QTc dispersion was 53.1±26.6 msec (mean change: 6.1±31.4, msec, p=0.30). The duration of treatment at the time of the longest QTc duration and dispersion were 47.6±46 days and 60.4±73.2 days, respectively (Table 2).
Table 3.
Electrocardiographic Changes Between Baseline and Peak Values In 29 Children and Adolescents Treated with Ziprasidone For Up to 62 Weeks
| EKG Parameter | Baseline | Peak Values | Change | Statistic | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | p | |
| HR (bpm) | 82.7 ± 11.8 | 62 – 108 | 78.6 ± 20.2 | 62 – 113 | −4.1 ± 21.9 | −48 – +37 | 0.32 |
| QRS (ms) | 87.9 ± 9.5 | 70 – 106 | 93.9 ± 10.6 | 72 – 124 | 6.0 ± 8.3 | −10 – +28 | 0.0005 |
| QTc (ms) | 410.5 ± 25.0 | 371 – 460 | 433.4 ± 25.1 | 383 – 507 | 22.9 ± 21.0 | −20 – +68 | <0.0001 |
| QTc dispersion (ms) | 47.0 ± 24.8 | 11 – 118 | 53.1 ± 26.6 | 3 – 103 | 6.1 ± 31.4 | −53 – +59 | 0.30 |
HR = heart rate; bpm = beats per minute; QTc: heart rate corrected QT (Bazett method); ms= milliseconds
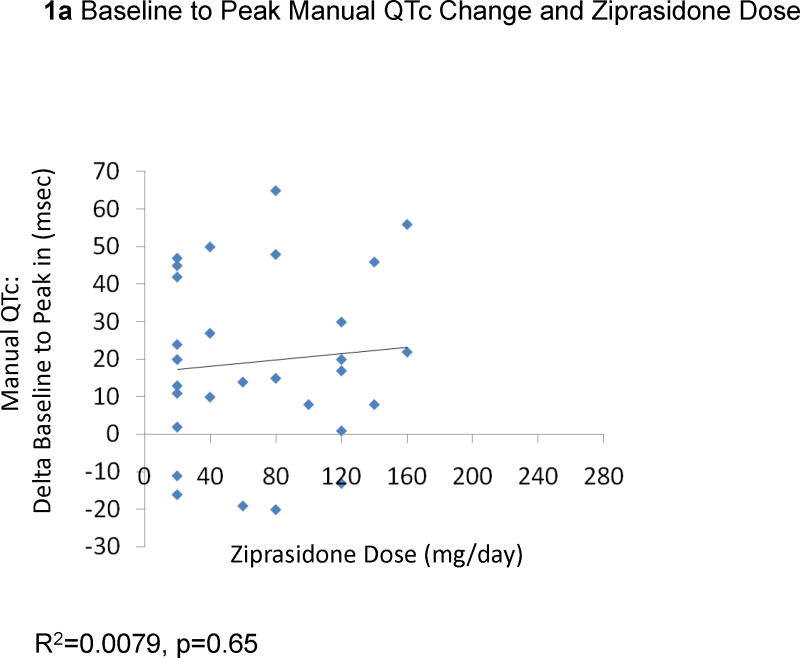
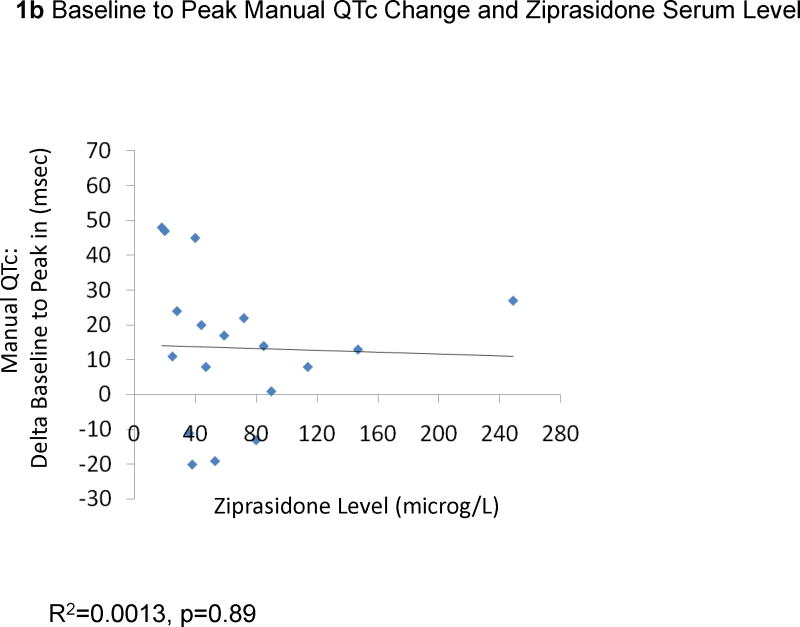
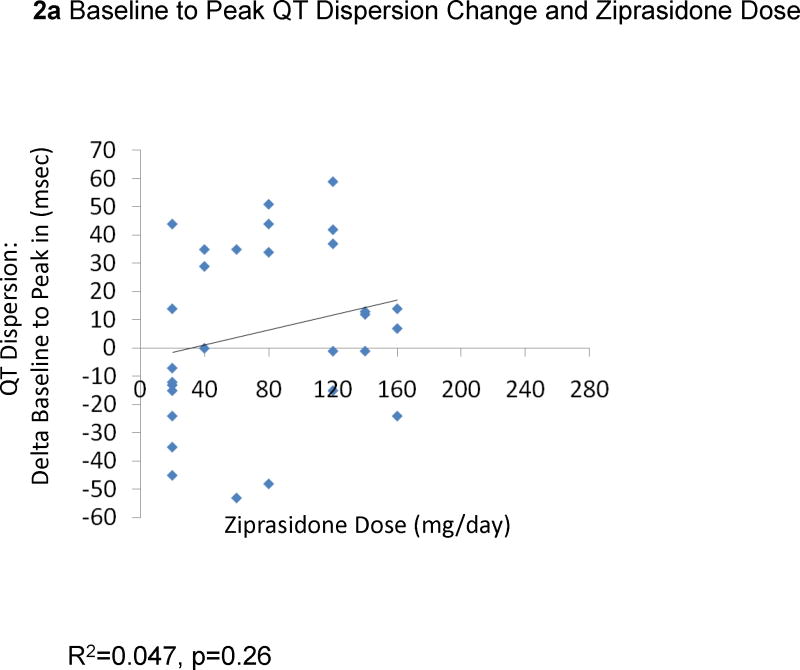
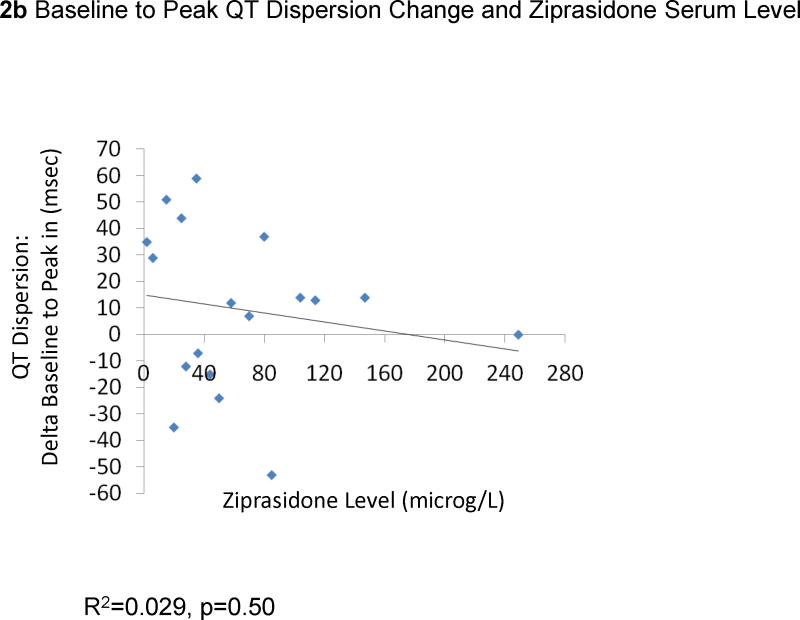
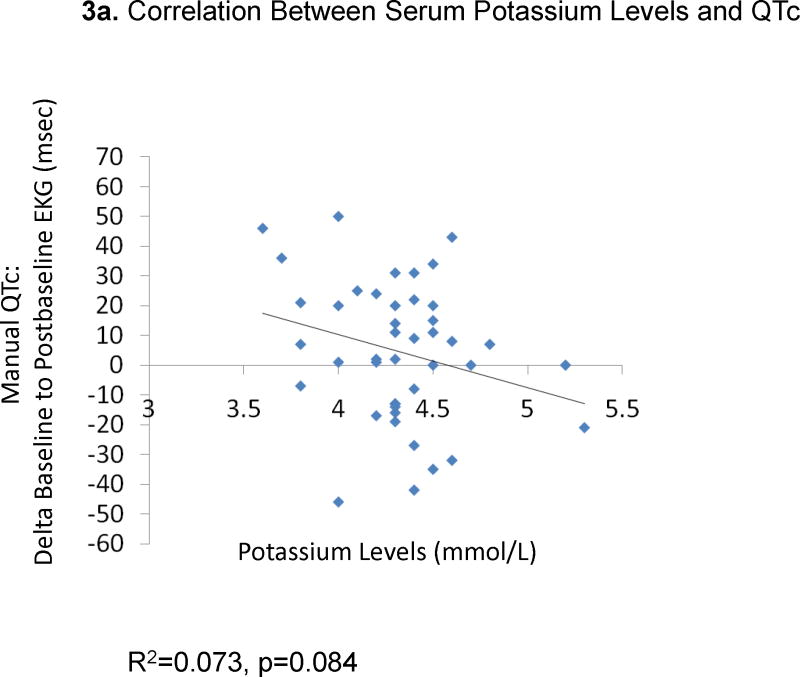
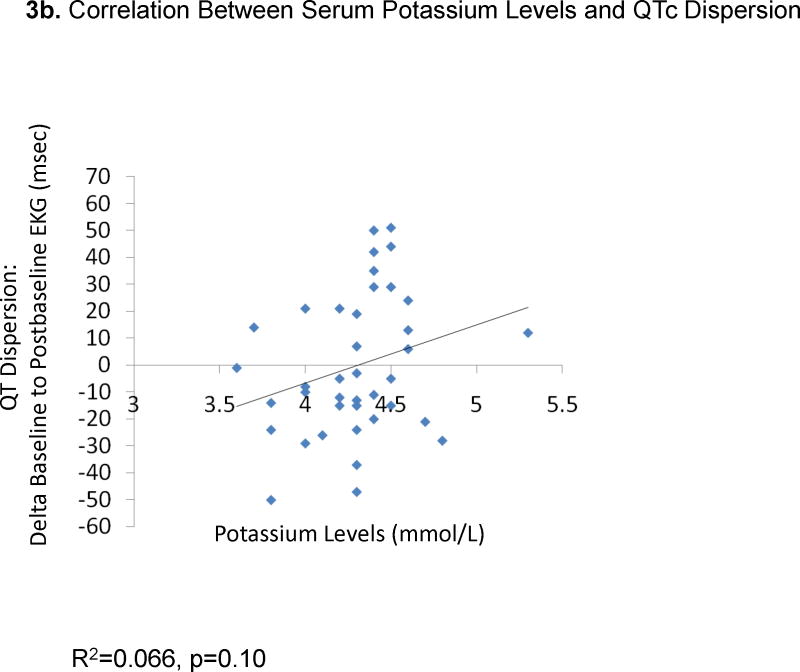
The change from baseline QTc to the peak QTc duration did not correlate with ziprasidone dose (29 EKGs: R2=0.0079, p=0.65, Figure 1a) or ziprasidone serum levels (18 EKGs: R2=0.0013, p=0.89, Figure 1b). Results were similar for the correlations between QTc changes in all available EKGs and ziprasidone dose (78 EKGs: R2=0.0059, p=0.50) and ziprasidone level (51 EKGs: R2=0.000052, p=0.96). Likewise, there were no significant correlations between changes from baseline to peak QTc dispersion and ziprasidone dose (29 EKGs: R2=0.047, p=0.026, Figure 2a) and ziprasidone serum level (18 EKGs: R2=0.029, p=0.50, Figure 2b). Results were similar for the correlations between QTc dispersion changes in all available EKGs and ziprasidone dose (78 EKGs: R2=0.0040, p=0.79), and ziprasidone level (51 EKGs: R2=0.069, p=0.063). Finally, potassium levels were also not correlated with QTc duration (42 EKGs: R2=0.07323, p=0.084, Figure 3a) or QTc dispersion (41 EKGs: R2=0.066, p=0.10, Figure 3b).
Figure 1.
Correlation Between Baseline to Peak QTc Change and Ziprasidone Dose and Serum Level
Figure 2.
Correlation Between from Baseline to Peak QT Dispersion Change and Ziprasidone Dose and Serum Level
Figure 3.
Correlation Between Serum Potassium Levels and QTc and QT Dispersion
Patients With Clinically Significant QTc Changes
Seven patients (24.1% of the sample) had clinically significant changes in QTc duration or QTc dispersion. In five patients (17.2%) the QTc was prolonged to >450 msec; one of these patients had a peak QTc >500 msec and another had a peak QTc prolonged by more than 60 msec compared with baseline. Two patients increased their QTc dispersion to values >100 msec. Patients with (n=7) and without (n=22) clinically significant QTc changes during treatment with ziprasidone were similar with regard to demographic, illness and treatment variables (Table 3).
Discussion
In this prospective study of 29 children and adolescents receiving ziprasidone for a variety of psychiatric disorders, the baseline to peak QTc duration increased significantly and exceeded in five patients 450 msec. In contrast, changes in QT dispersion in the entire sample were modest and statistically insignificant. Two patients acquired QTc dispersion greater than 100 msec. There were no reports of syncope or symptomatic arrhythmias, and the peak QTc prolongation occurred earlier than the peak QTc dispersion. None of the 29 patients had both a prolonged QTc and an abnormal QTc dispersion during ziprasidone treatment at an average daily dose of 112 mg, followed for an average of 99 days. The concomitant use of other psychotropic drugs was not different in patients with or without myocardial repolarization changes during treatment with ziprasidone.
The finding of increased QTc duration with ziprasidone is consistent with studies in adults (7) and in youth treated with ziprasidone (1–3,21–22). While prior studies in youth resulted in a broad range of mean QTc changes (i.e., −3 msec to +28 msec) (1–3,21–22), varying methodologies of using baseline-to-endpoint or baseline-to-peak QTc values may be responsible for these differences. Nevertheless, the 23 msec mean increase found in our study is still within the previously reported range. Furthermore, previously reported QTc prolongation (defined as incidence of QTc > 450 msec or >460 msec) in youth receiving ziprasidone in the aforementioned studies have ranged from 0% (i.e., 0/12 (3) and 0/21) (22) to 15.0% (i.e., 3/20) (21), which was lower than our rate of 24.1%. For additional comparison, 6 months of treatment of 38 children and adolescents (mean age: 15.1 (4–18) years, 68.4% male, 92.1% Caucasian) with risperidone (N=12, mean dose: 2.4 (0.4–5) mg), olanzapine (N=8, mean dose: 10.9 (5–25) mg) or quetiapine (N=18, mean dose 469 (100–1500) mg) was associated with a mean QTc prolongation of 6.3±25.5 msec (range: −38.0 to +70.0 msec) for the combined group (8). However, although based on the reported range, at least, one patient treated with a second-generation antipsychotic other than ziprasidone had a QTc prolongation of more than 60 msec compared to baseline, the authors did not report the percent of patients with newly emerging QTc abnormalities, precluding a comparison with our ziprasidone treated pediatric sample. Moreover, like in all other prior studies of antipsychotic-related QTc changes in pediatric patients, no QTc dispersion values were reported in that study either.
The fact that there were no significant changes in QTc dispersion in this sample of children and adolescents treated with ziprasidone is encouraging. In epidemiological studies, using cardiovascular deaths as a surrogate marker for fatal arrhythmias, prolonged QTc and increased QTc dispersion were significant predictors of all cause mortality and cardiovascular mortality (14). However, after controlling for coronary risk factors, only QTc dispersion and not QTc prolongation remained a strong predictor of cardiovascular mortality (14). Likewise, a controlled study comparing patients with and without TdP after intravenous administration of haloperidol found that QTc dispersion was significantly greater in patients who experienced TdP, particularly when associated with a QTc duration >500 msec (17). Thus, the absence of any clinical or electrocardiographic evidence for arrhythmias in our youth treated with ziprasidone, including one quarter who developed electrocardiographic abnormalities of QTc or QTc dispersion, suggests that the absence of concurrently abnormal QTc dispersion and QTc prolongation might be protective. The same reason might be responsible for the lack of increased cardiac death rates with ziprasidone in adults treated with ziprasidone (18). Although the absence of clinically relevant arrhythmias associated with ziprasidone in our sample could be due to lack of power for its detection, our results and the general conclusions that the cardiac risk potential of ziprasidone that does not seem to be elevated compared to other first-line antipsychotics despite greater numeric increase in QTc seems to be supported by a recently completed, large scale study. In the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC), an open-label, randomized, postmarketing simple trial, 18,154 patients with schizophrenia were randomized to ziprasidone (n=9,077) or olanzapine (n=9,077). At the end of the one-year trial, both medications were associated with an identical and low non-suicide mortality rate of 0.9% (Odds Ratio: 1.0, 95% Confidence Interval: 0.76–1.39) (24). Moreover, even after readjudication, specific cardiac mortality rates did not differ among the two antipsychotics in this large, randomized sample.
Nevertheless, our findings need to be interpreted within the limitations of this prospective study, including the relatively small sample size, EKG assessments that were not conducted at the same time in the morning and in a fasting status in all patients, allowance of clinically indicated co-medications, and absence of a placebo group. Research studies reporting on QTc intervals have been the object of methodological scrutiny in light of the recommendations of the 2005 International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (25), and inclusion of a placebo comparator has been recommended to avoid assessment biases. In our case, we avoided this pitfall by having all QTc intervals measured by experts blinded to baseline or intra-treatment origin of the tracings. In addition, the QT correction for rate according to the Bazett’s formula could produce “longer” QTc at higher heart rates and “shorter” QTc at lower heart rates. However, this limitation does not apply to our study because the mean heart rate did not change from baseline to the time of the peak QTc. Therefore, in this group of pediatric patients with normal baseline EKG, the observed changes are highly likely related to the use of ziprasidone. We also acknowledge that reliance on a single baseline QTc may exaggerate the magnitude of the relative change with respect to the highest of multiple subsequent QTc values, but this would not affect the presence of an absolute abnormal duration and dispersion of QTc intervals.
In conclusion, our findings suggest that ziprasidone-induced changes in QTc duration and dispersion seem to occur independent of each other in a population of children and adolescents without significant medical illnesses. This temporal dissociation may explain why ziprasidone has not been associated with the production of TdP, ventricular arrhythmias or sudden death in children and adolescents, and why there have been no increased sudden cardiac death rates with ziprasidone compared to other first-line antipsychotics in adults. The data question the need for electrocardiographic monitoring of youth treated with ziprasidone who have a normal electrocardiogram prior to taking this antipsychotic drug, are physically healthy and have no family history of premature cardiac death. Moreover, these data suggest that ziprasidone’s advantage of producing relatively limited increase in adiposity and metabolic abnormalities in youth (6) should be given more weight than the effect on QTc prolongation when considering its use in clinical practice.
Acknowledgments
Grant Support: Supported in parts by NIH grant MH01760 (Dr. Malhotra), a NARSAD Independent Investigator Award (Dr. Malhotra), The Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543-01 (Dr. Kane), and by The Feinstein Institute for Medical Research NSLIJHS General Clinical Research Center, Grant #M01 RR018535 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Dr. Lops received support through a Stanley Foundation Fellowship. The article’s content is solely the responsibility of the authors and does not necessarily represent the official view of NCRR, NIH or NIMH. None of these non-commercial funding organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures: Dr. Correll has been a consultant and/or advisor to Actelion, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Janssen/J&J, GSK, Hoffmann-La Roche, Otsuka, Pfizer, Schering-Plough, Supernus, Takeda and Vanda. Dr. Malhotra has been a consultant to or receiving honoraria from Bristol-Myers Squibb, Otsuka, Pfizer and Vanda. Dr. Kane has been a consultant to Janssen, Pfizer, Eli Lilly, and Bristol-Myers Squibb, Otsuka and has received honoraria from Abbott, Bristol-Myers Squibb, and Janssen. Dr. Manu has served on the speaker bureaus and advisory boards of Eli Lilly, Pfizer, Bristol-Myers Squibb and Forest laboratories.
References
- 1.DelBello MP, Versavel M, Ice K, Keller D, Miceli J. Tolerability of oral ziprasidone in children and adolescents with bipolar mania, schizophrenia or schizoaffective disorder. J Child Adoles Psychopharm. 2008;18:491–499. doi: 10.1089/cap.2008.008. [DOI] [PubMed] [Google Scholar]
- 2.Sallee FFR, Kurlan R, Goetz CG, et al. Zpirasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am ACAD Child Adolesc Psychiatry. 2000;39:292–299. doi: 10.1097/00004583-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Malone RP, Delaney MA, Hyman SB, Cater JC. Ziprasidone in adolescents with autism: An open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17:779–790. doi: 10.1089/cap.2006.0126. [DOI] [PubMed] [Google Scholar]
- 4.Nemeroff CB, Lieberman JA, Weiden PJ, et al. From clinical research to clinical practice: a 4-year review of ziprasidone. CNS Spectr. 2005;10:s1–20. doi: 10.1017/s1092852900019842. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 (Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Correll CU. Antipsychotic use in children and adolescents: Minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:9–20. doi: 10.1097/chi.0b013e31815b5cb1. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Package insert, Geodon (ziprasidone) Pfizer, Inc; [Google Scholar]
- 8.De Castro MJ, Fraguas D, Laita P, Moreno D, Parellada M, Pascual D, Alvarez M, Merchán-Naranjo J, Rapado M, Giráldez M, Leiva M, Arango C. QTc changes after 6 months of second-generation antipsychotic treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2008 Aug;18(4):381–3. doi: 10.1089/cap.2007.0115. [DOI] [PubMed] [Google Scholar]
- 9.Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002;62:1649–1671. doi: 10.2165/00003495-200262110-00006. [DOI] [PubMed] [Google Scholar]
- 10.Shah RR. Drug-induced QT dispersion: does it predict the risk of torsade de pointes? J Electrocardiol. 2005;38:10–18. doi: 10.1016/j.jelectrocard.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Internal Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 12.Vieweg WVR. New generation antipsychotic drugs and QTc interval prolongation. Primary Care Companion J Clin Psychiatry. 2003;5:205–215. doi: 10.4088/pcc.v05n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogun F, Kwok Chan K, Harvey M, et al. QT dispersion in nonsustained ventricular tachycardia and coronary artery disease. Am J Cardiol. 1996;77:256–259. doi: 10.1016/s0002-9149(97)89389-7. [DOI] [PubMed] [Google Scholar]
- 14.Cuddy TE, Halli PS, Tate RB. QT dispersion and heart rate predict the risk of sudden unexpected cardiac death in men: the Manitoba Follow-UP Study. Prev Cardiol. 2009;12:27–33. doi: 10.1111/j.1751-7141.2008.00016.x. [DOI] [PubMed] [Google Scholar]
- 15.Higham PD, Furniss SS, Campbell RW. QT dispersion and components of the QT interval in ischaemia and infarction. Br heart J. 1995;73:32–36. doi: 10.1136/hrt.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br heart J. 1990;63:342–344. doi: 10.1136/hrt.63.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisdale JE, Rasty S, Padhi ID, et al. The effect of intravenous haloperidol on QT interval dispersion in critically ill patients: comparison with QT interval prolongation for assessment of risk of Torsades de pointes. J Clin Pharmacol. 2001;41:1310–1318. doi: 10.1177/00912700122012896. [DOI] [PubMed] [Google Scholar]
- 18.Strom BL, Eng SM, Faich G, et al. Comparative mortality associated with ziprasidone vs. olanzapine in real-world use among 18,154 patients with schizophrena: the ziprasidone observational study of cardiac outcomes (ZODIAC). Presented at the Annual Meeting of the American Psychiatric Association; May 2008; [DOI] [PubMed] [Google Scholar]
- 19.Heinrich TW, Biblo LA, Schneider J. Torsades de pointes associated with ziprasidone. Psycvhosomatics. 2006;47:264–2268. doi: 10.1176/appi.psy.47.3.264. [DOI] [PubMed] [Google Scholar]
- 20.Scahill L, Blair J, Leckman JF, Martin A. Sudden death in a patient with Tourett syndrome during a clinical trial with ziprasidone. J Psychopharm. 2005;19:205–206. doi: 10.1177/0269881105049042. [DOI] [PubMed] [Google Scholar]
- 21.Blair J, Scahill L, State M, Martin A. Electrocardiographic changes in children and adolescents treated with ziprasidone: A prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44:73–79. doi: 10.1097/01.chi.0000145372.61239.bb. [DOI] [PubMed] [Google Scholar]
- 22.Biederman J, Mick E, Spencer T, et al. A prospective open-label treatment trial of ziprasidone monotherapy in children and adolescents with bipolar disorder. Bipolar Disorders. 2007;9:888–894. doi: 10.1111/j.1399-5618.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 23.Correll CU, Frederickson AM, Figen V, Ginn-Scott EJ, Pantaleon Moya RA, Kane JM, Manu P. The QTc interval and its dispersion in patients receiving two atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci. 2009 Feb;259(1):23–7. doi: 10.1007/s00406-008-0829-4. [DOI] [PubMed] [Google Scholar]
- 24.Kane JM, Strom BL, Eng SM, Faich G, Reynolds RF, D’Agostino RB, Ruskin J, Geier J, Karayal ON, Kremer C. Primary and Readjudication Mortality Results From ZODIAC, a Large Simple Trial of Ziprasidone vs Olanzapine in Patients With Schizophrenia. Oral Presentation at the 2nd Schizophrenia International Research Society Meeting; Florence, Italy. April 10–14, 2010. [Google Scholar]
- 25.Loebel A, Miceli J, Chappell P, Siu C. Electrocardiographic changes with ziprasidone. J Am Child Adolesc Psychiatry. 2006;45:636–637. doi: 10.1097/01.chi.0000215151.53776.5a. [DOI] [PubMed] [Google Scholar]