Abstract
Objective
Hypoglycemia is associated with increased mortality. The reasons for this remain unclear and the effects of low glucose exposure on vascular endothelial function remain largely unknown. We endeavored to determine the effects of low glucose on endothelial cells and intact human arterioles.
Methods and Results
We exposed human umbilical vein endothelial cells to low glucose conditions in a clinically relevant range (40–70 mg/dL) and found rapid and marked reductions in nitric oxide (NO) bioavailability (P<0.001). This was associated with concomitantly increased mitochondrial superoxide production (P<0.001) and NO-dependent mitochondrial hyperpolarization (P<0.001). Reduced NO bioavailability was rapid and attributable to reduced eNOS activity and destruction of NO. Low glucose rapidly activated AMP Kinase but physiological activation failed to restore NO bioavailability. Pharmacological AMP Kinase activation led to phosphorylation of eNOS’s Ser633 activation site, reversing the adverse effects of low glucose, and this protective effect was prevented by L-NAME. Intact human arterioles exposed to low glucose demonstrated marked endothelial dysfunction which was prevented by either metformin or TEMPOL.
Conclusions
Our data suggest that moderate low glucose exposure rapidly impairs NO bioavailability and endothelial function in the human endothelium, and that pharmacological AMP Kinase activation can inhibit this effect in an NO-dependent manner.
Keywords: Low glucose, endothelial function, nitric oxide, mitochondria, AMP Kinase
The disappointing results of recent trials of tight glycemic control in type 2 diabetes highlight the potential risks of hypoglycemia.1–3 Hypoglycemia has been associated with excess mortality in critically ill patients,4 in patients presenting with acute coronary syndromes,5 and in diabetic patients.1, 2 These data strongly suggest hypoglycemia increases the risk of morbidity and mortality in a wide variety of patients.
The mechanisms underlying the risks posed by hypoglycemia are unknown. However, there is considerable biological plausibility implicating hypoglycemia-triggered cardiac ischemia.6, 7 Phenotypic endothelial dysfunction, characterized by inflammation, excessive oxidative stress, and a loss of bioavailable nitric oxide (NO), typically precedes the development of clinical cardiovascular events.8 While there is ample evidence implicating hyperglycemia-induced increases in oxidative stress and reduced NO bioavailability as mechanistically important in the development of endothelial dysfunction in humans,9, 10 little data exist as to the effects of clinically relevant levels of low glucose on oxidative stress, NO bioavailability, and endothelial function. Complete glucose deprivation stimulates the production of mitochondrial reactive oxygen species (ROS) and AMP Kinase (AMPK) activation.11 Activation of AMPK would be expected to boost endothelial production of NO,12 although the response to AMPK activation may be delayed.13 The extent to which these responses occur in endothelial cells under acute, moderate, low glucose conditions (40–70 mg/dL) remains unknown.
In light of these data, we hypothesized low glucose exposure at clinically relevant concentrations would lead to endothelial dysfunction, characterized by reduced NO bioavailability, increased oxidative stress, and impaired endothelium-dependent vasodilation. Further, we endeavored to delineate the interaction between mitochondrial superoxide, NO, and AMPK which occur with acute exposure to low glucose concentrations.
Materials and Methods
Materials
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD) and gifted to our laboratory by Dr. Keith Oldham (Medical College of Wisconsin). Please see the on-line supplement for full details of the materials used in this study.
Human adipose arterioles studied were obtained from volunteers without a history of diabetes or coronary artery disease by either subcutaneous gluteal adipose pad biopsy under sterile conditions using local anesthetic (1% lidocaine) or from subcutaneous or visceral fat discarded at the time of abdominal surgery. All samples were obtained in accordance with the policies and procedures of the Medical College of Wisconsin’s Institutional Research Board (IRB).
Studies were performed primarily under either normal glucose (NG, 5mM) or low glucose (LG, 2.2 mM) conditions unless otherwise specified. Basal and stimulated NO produced by HUVECs under all conditions were measured using a Model 280 NO Analyzer (Sievers Instruments, Boulder, CO, USA), following an established methodology.14 Details on cell culture conditions, mitochondrial and total superoxide measurements performed using MitoSox™ and hydroethidium, mitochondrial membrane potential using membrane potential sensitive fluorophores JC-1 and TMRM, Western Blots for total eNOS, peNOS Ser1177, peNOS Ser633, total AMPK, and pAMPK, measurements of human arteriolar endothelial function by videomicroscopy,15 and our statistical methods are included in our on-line supplement. P-values of <0.05 was considered significant. All data are presented as mean±SE unless otherwise specified.
Results
Endothelial Cell NO Bioavailability, Mitochondrial Superoxide Production, and Cytosolic Superoxide Production Under Low Glucose Conditions
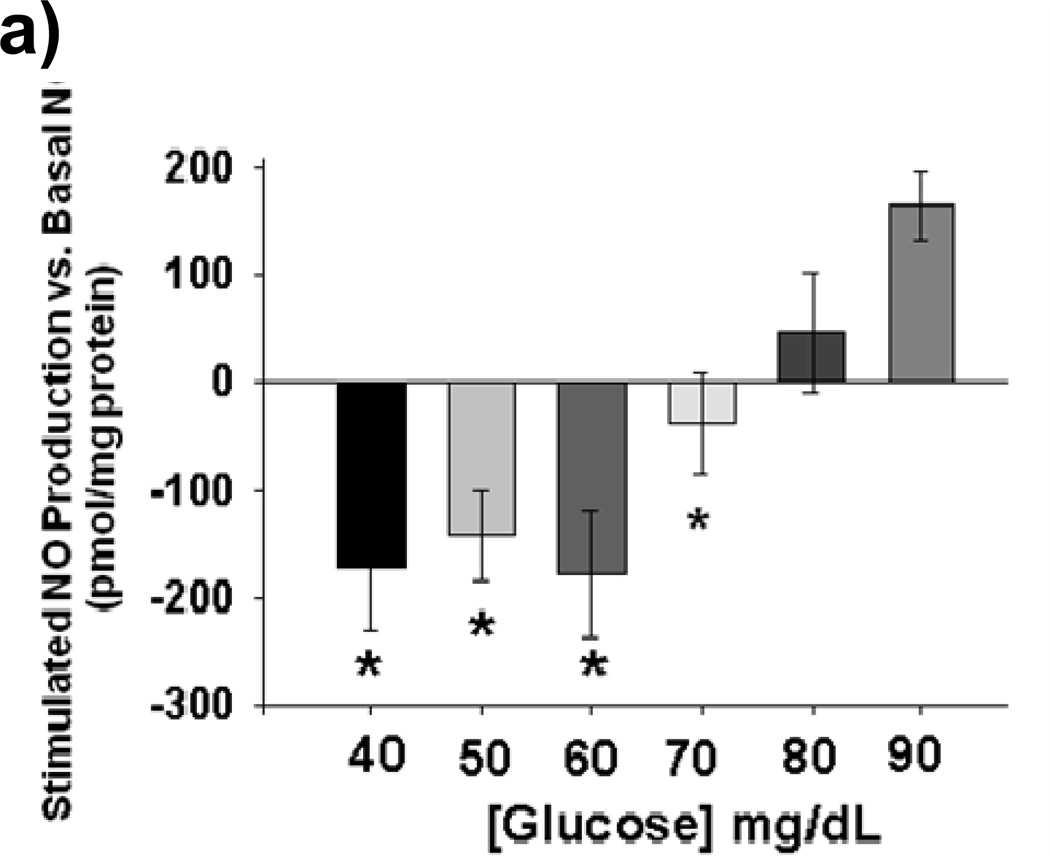
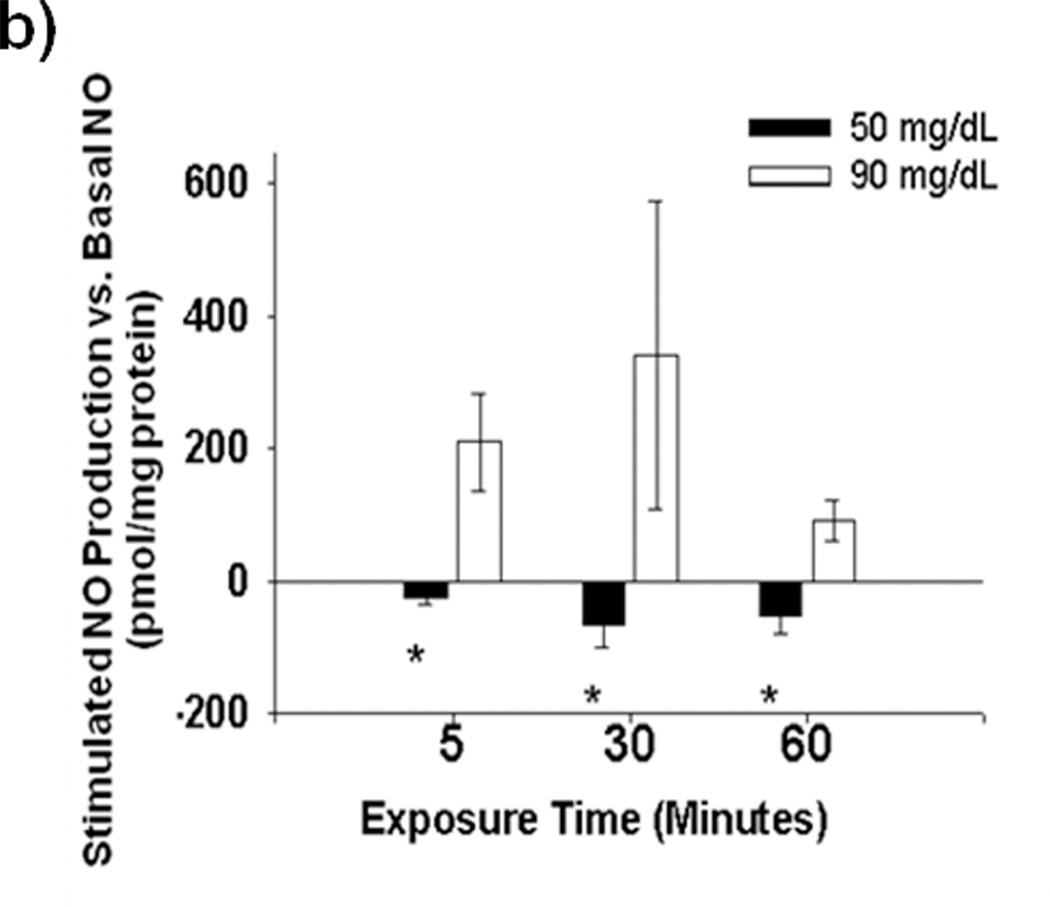
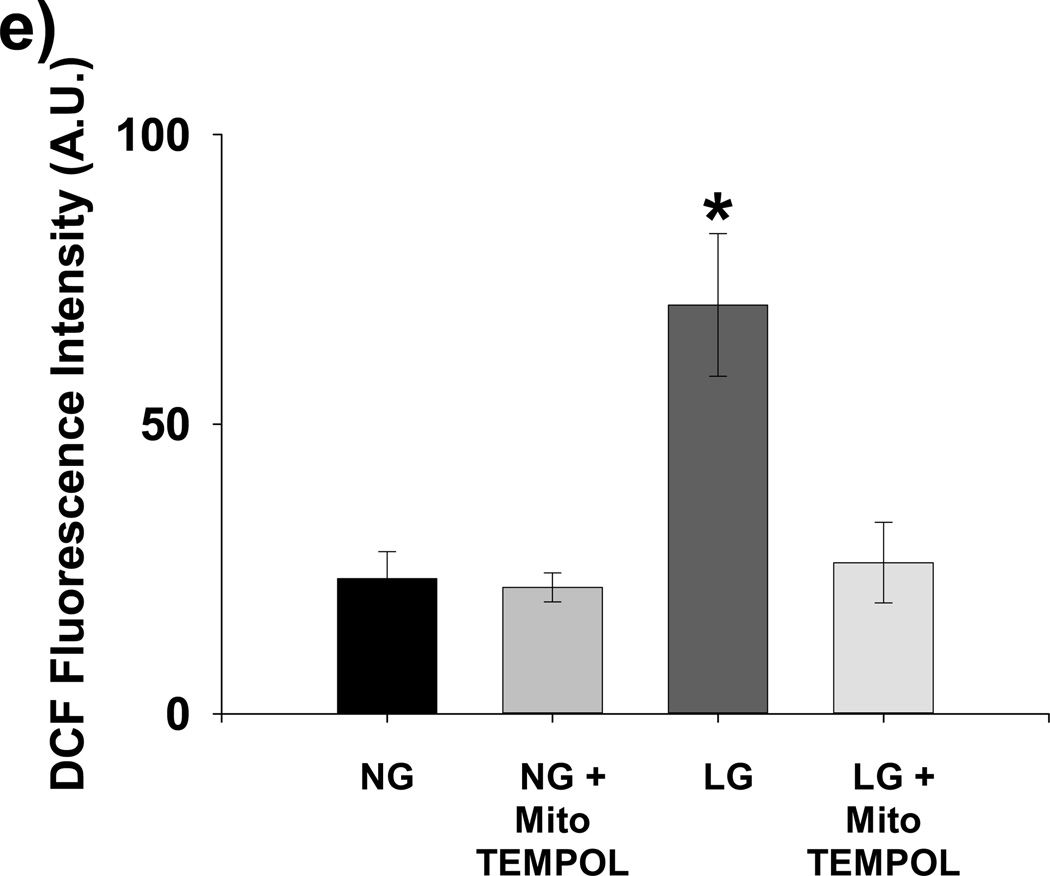
Acute exposure of HUVECs to graded reductions in glucose concentration from 90 mg/dL to 40 mg/dL resulted in rapid impairment stimulated NO bioavailability with 5 minutes of LG exposure (Figures 1a–b). Impaired NO production began to appear at 80 mg/dL and became significant at 70 mg/dL. Basal levels of NO production were not affected by LG (Supplemental Figure I) Concomitantly, mitochondrial superoxide significantly increased relative to baseline beginning at 80 mg/dL. (Figure 1c). PEG-SOD completely prevents LG induced increases in MitoSox™ fluorescence (Figure 1d), suggesting almost all of the MitoSox™ fluorescence signal originates from mitochondrial superoxide production. Total cellular superoxide levels as measured using hydroethidium did not significantly increase with 60 minutes of exposure to LG (50 mg/dL), (Supplemental Figure II). However, cellular hydrogen peroxide concentration more than tripled with low glucose exposure and normalized with concomitant treatment with mito-TEMPOL. (Figure 1e)
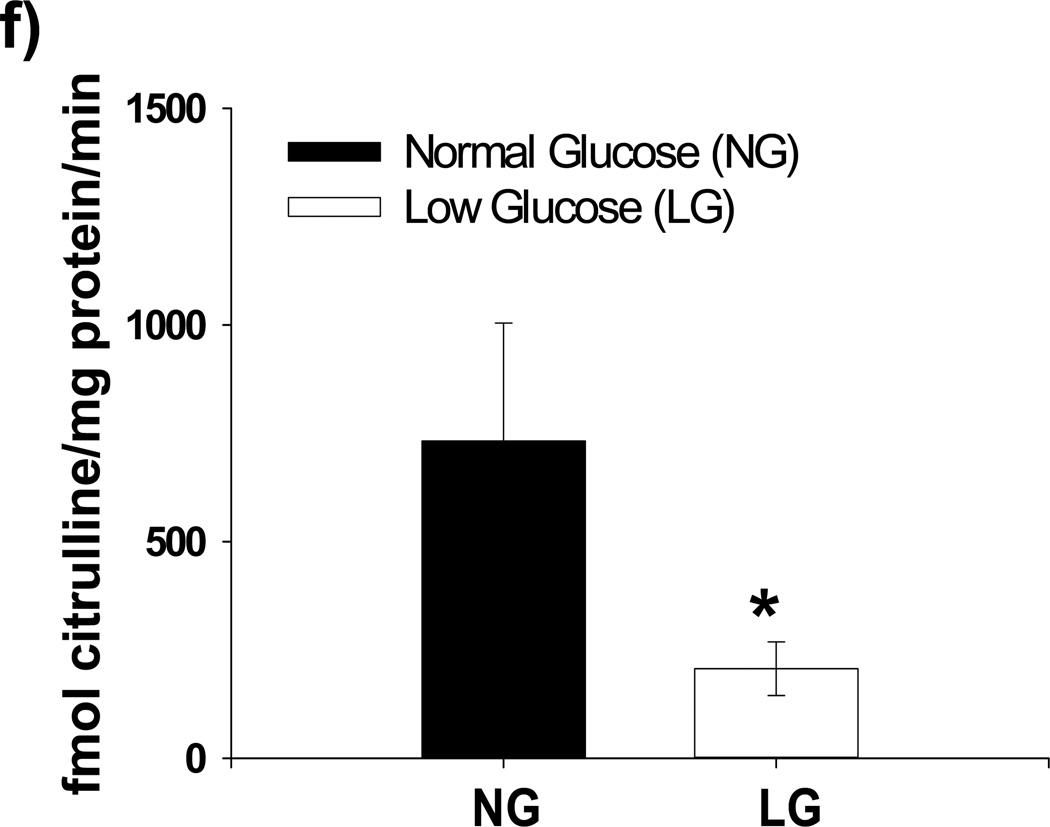
Figure 1. Effect of Low Glucose Exposure on HUVEC NO and mitochondrial superoxide production.
(a) Impairment of VEGF-stimulated NO production relative to basal NO production appears to begin at 80 mg/dL(n = 8, p<0.001 overall), with significant decreases in stimulated NO production at concentrations ≤ 70 mg/dL(*p<0.001 vs. 90 mg/dL). (b) Net NO production following VEGF stimulation is significantly suppressed at 5, 30, and 60 minutes following exposure to LG (n=6, P<0.001 overall, *-P<0.05 at all time points relative NG). (c) Mitochondrial superoxide production as a percentage of control glucose concentration (90 mg/dL) is significantly increased at glucose concentrations of ≤ 80 mg/dL (P<0.001 overall, n = 8, *p<0.05 vs. 90 mg/dL at all time points). (d) MitoSox™ signal under LG conditions is completed eliminated in the presence of either PEG-SOD or PEG-Catalase (n=8, P<0.001 overall, P < 0.05 for LG vs all other conditions). (e) LG induced a significant increase in cellular hydrogen peroxide levels as measured by DCF fluorescence intensity. This increase was completely suppressed by mitochondrial targeted anti-oxidant therapy with mito-TEMPOL (1mM) (*-P=0.01 overall, P< 0.05 for LG versus all other exposure states (f) 30 minutes of low glucose exposure significantly inhibitor NO production as measured by the conversion of L-arginine to L-citruilline (n=12, 731±273 vs. 206±62 fmol citrulline/mg protein/min, p=0.026). (g) The addition of BH4 (100 µM) had no effect on LG-induced inhibition of stimulated NO production. TEMPOL (1 mM) significantly reversed LG-induced inhibition of stimulated NO production with or with the concomitant presence of BH4(n=5–9, P<0.001 overall, P<0.02 for LG vs. all other conditions except LG+BH4)
In order to determine whether free fatty acid supplementation would abrogate LG’s stimulation of mitochondrial superoxide production, we measured MitoSox fluorescence under normal glucose (NG, 90 mg/dL) and LG (50 mg/dL) conditions with and without supplementation with 1% palmitate and L-carnitine, a mitochondrial anti-oxidant that can also improve free fatty acid utilization in endothelial cells to under LG conditions,11 We found that combined treatment of endothelial cells with 1% palmitate and L-carnitine reduced LG-induced mitochondrial superoxide production to normal levels (Supplemental Figure IIIa), However, when examined separately, mitochondrial superoxide production increased under LG conditions with the addition of 1% palmitate while L-carnitine alone returned mitochondrial superoxide levels to those similar to the NG condition (Supplemental Figure IIIb).
To help delineate the potential mechanisms for reduced NO bioavailability with LG, we measured NO production from HUVECs under NG and LG conditions (30 minutes of exposure). 30 minutes of LG exposure significantly inhibited the conversion of L-arginine to L-citrulline by eNOS (Figure 1f). In a subsequent experiments, we supplemented HUVECs with BH4, which failed to improve suppressed NO bioavailability of stimulated HUVECs while treatment with 1 mM TEMPOL, with or without BH4, significantly improved NO bioavailability (Figure 1g).
Effect of Low Glucose on Mitochondrial Membrane Potential and Mitochonrial ROS Production: Regulation by NO
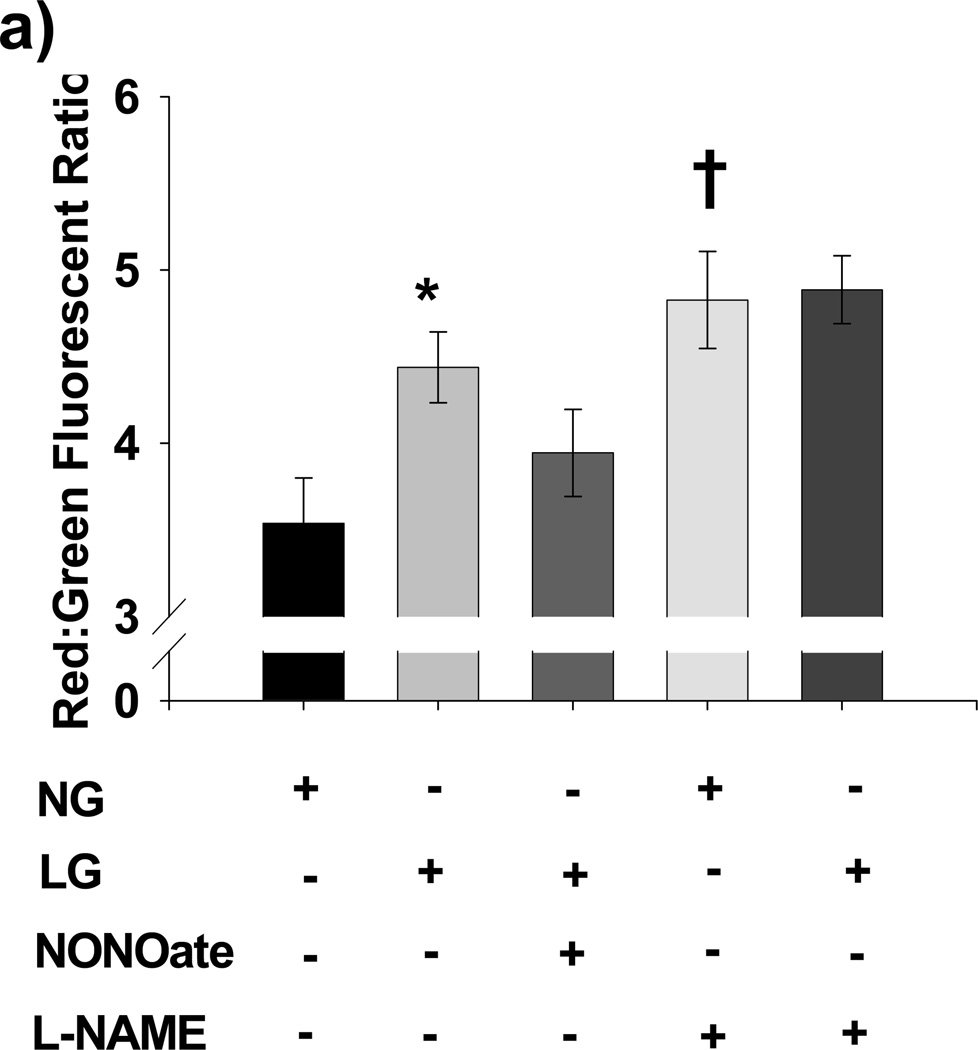
To determine whether LG-induced excess mitochondrial ROS production was associated with alterations in mitochondrial membrane potential, we measured mitochondrial membrane potential under both NG and LG conditions. LG induced mitochondrial hyperpolarization relative to NG based on a significant increase in the red: green ratio of JC-1 fluorescence (P<0.001).
Furthermore, Spermine NONOate, an exogenous source of NO, reversed LG-induced mitochondrial hyperpolarization (P=0.97 vs. NG alone, P=0.007 vs. LG alone), while L-NAME significantly increased mitochondrial membrane potential in the setting of NG (P=0.004 vs. NG alone). No significant differences in mitochondrial membrane potential were seen when comparing LG, LG+L-NAME, or NG+L-NAME (P=NS for all post-hoc comparisons). Our full results are summarized in Figure 2a. These findings were confirmed using a second mitochondrial membrane potential sensitive probe, TMRM (data not shown). 10 µM CCCP significantly reduced mitochondrial membrane potential relative to both NG and LG condition using JC-1, confirming the ability of JC-1 to measure changes in membrane potential (data not shown).
Figure 2. Role of NO in LG-induced Mitochondrial Hyperpolarization and Mitochondrial ROS production.
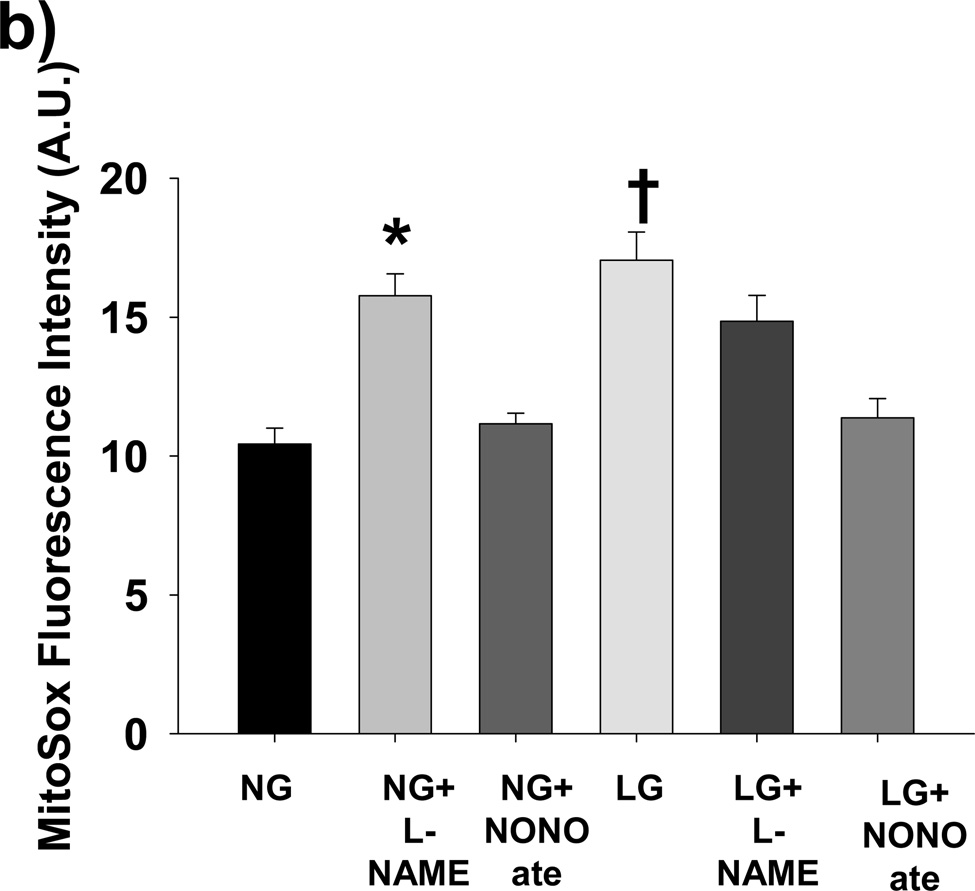
(a) LG induced significant mitochondrial membrane hyperpolarization as reflected by an increased red: green fluorescent intensity ratio from JC-1 relative to NG. Further, exposure to exogenous NO (Spermine NONOate, 10 µM) reversed LG-induced hyperpolarization, while inhibition of eNOS with L-NAME (1 mM) induced hyperpolarization under NG conditions. (N=6–22 for each exposure. *P<0.001 overall and vs. NG, P=0.007 vs. LG+NONOate. †P=0.004 for NG vs. NG+LNAME). (b) L-NAME increases mitochondrial ROS production under NG (90 mg/dL D-glucose) to the level seen under LG conditions (40 mg/dL D-glucose + 50 mg/dL L-glucose). Spermine NONOate exposure prevented LG induced increases in mitochondrial ROS production. No increased in mitochondrial ROS production was seen with the addition of L-NAME under LG conditions (P<0.001 overall, *-P <0.005 for NG+L-NAME vs. NG and LG+NONOate, †- P<0.008 for LG vs. NG, NG+NONOate, and LG+NONOate).
To confirm that losses of NO production lead to increased mitochondrial ROS production, we performed additional experiments under NG and LG conditions with and without L-NAME (1 mM) or Spermine NONOate (10 µM) and measured mitochondrial ROS production (Figure 2B). L-NAME increased mitochondrial ROS production under NG conditions to the level of LG, while NONOate prevented LG induced mitochondrial ROS production.
Effect of Low Glucose on AMP Kinase Activity relative to eNOS phosphorylation at serine 1177 and serine 633 activation sites
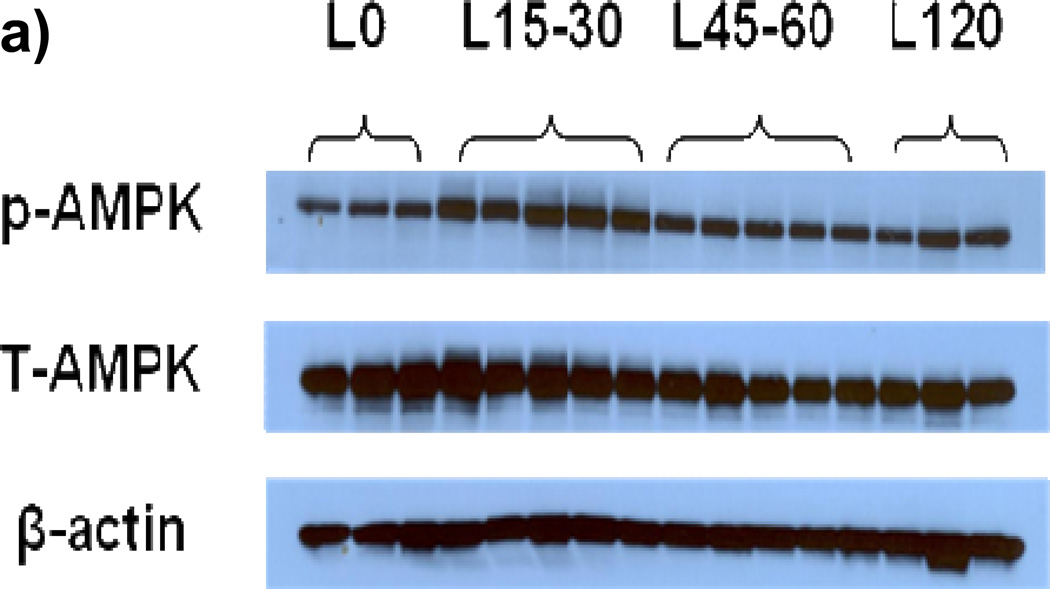
To determine the time course of AMP activation following LG exposure, we measured AMPK phosphorylation (pAMPK) levels between zero and 120 minutes following exposure to LG. As demonstrated in Figures 3a–b, the ratio of pAMPK to total AMPK (tAMPK) increased 75% from time 0 to 15– 30 minutes following LG exposure. While pAMPK/tAMPK remained modestly elevated at 45–60 minutes of LG exposure compared to time zero (32% increase over baseline), the ratio was significantly lower than at 15–30 minutes.
Figure 3. a–h: Effect of Low Glucose Exposure on AMP Kinase Phosphorylation.
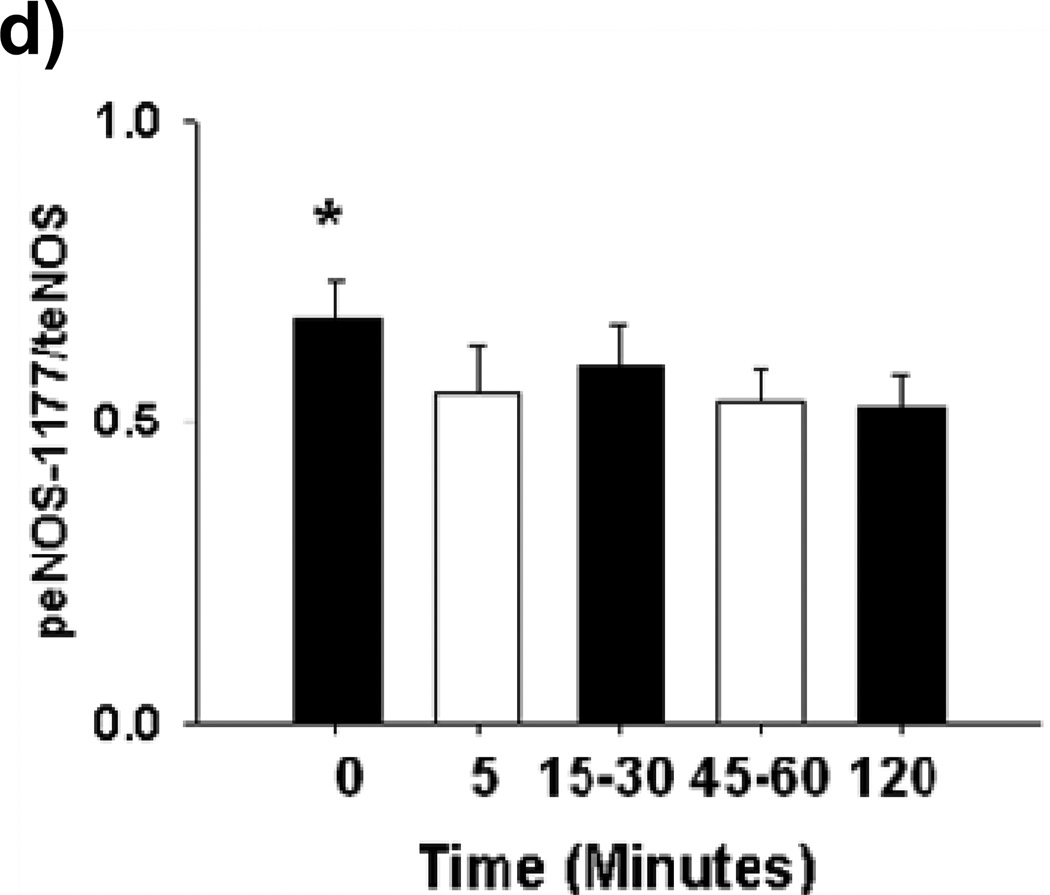
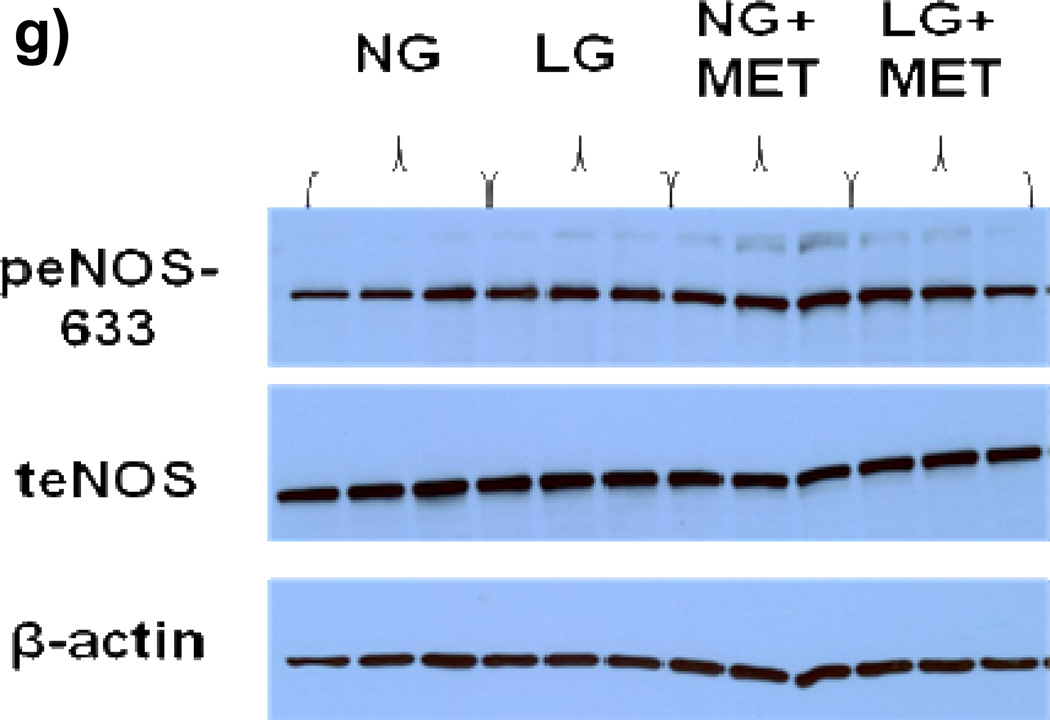
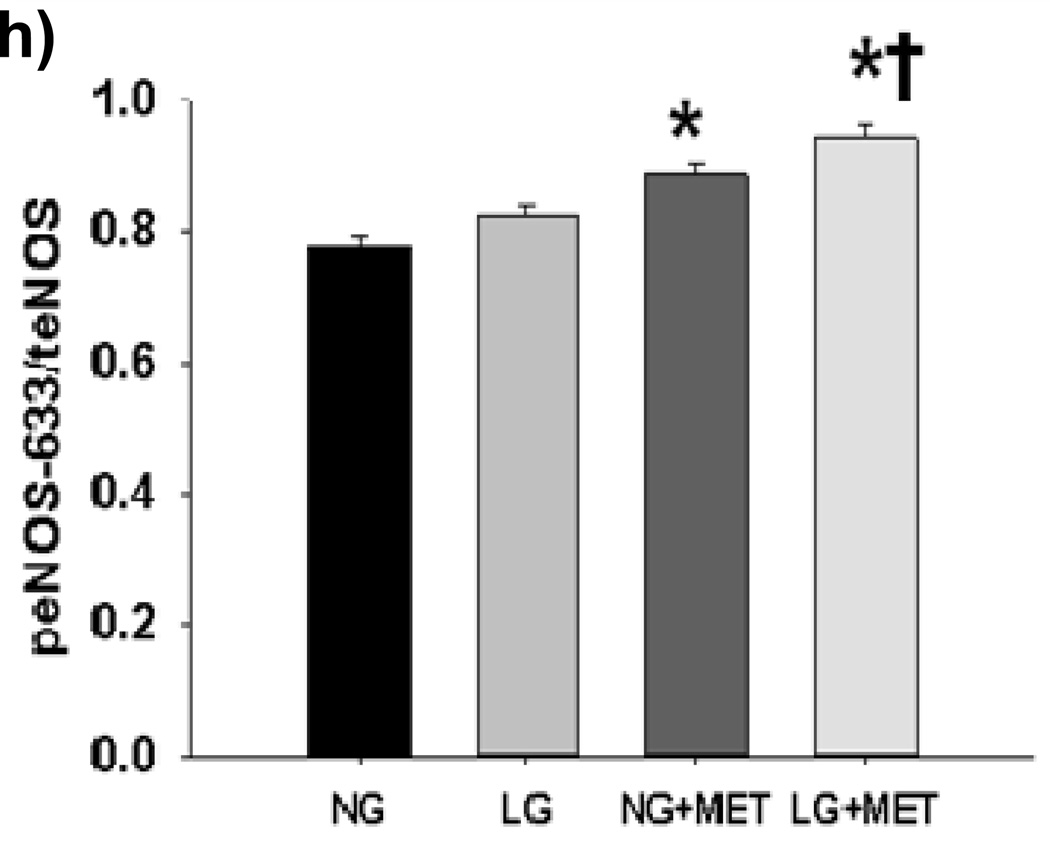
(a) Raw results from Western blot for tAMPK and pAMPK (b) Exposure of HUVECs to LG for 15–30 minutes resulted in an increase in pAMPK/tAMPK levels. While the ratio remained modestly elevated compared to baseline, pAMPK/tAMPK dropped significantly by 45–60 minutes into LG and remained at this level at the 2 hour time point (n=3–5, P<0.001 overall, *-P<0.05 versus time 0, †-P<0.05 versus 45–60 minute and 2 hour time points). (c) Sample raw Western blot result for peNOS1177 and total eNOS (d) peNOS/eNOS ratio was significantly reduced within 5 minutes of LG exposure (N=8, P=0.02overall, *-P=0.03 for 5, 45–60, and 120 minute durations of LG exposure vs.0 minutes). (e) Representative Western Blot result for Figure 3f (f) 1 hour of metformin (10 µM) has no effect on LG induced loss of eNOS phosphorylation at the Ser1177 activation site (n=7, P=0.01 overall, *-P<0.05 vs. NG). (g) Representative Western Blot for Figure 3h. (h) 1 hour of metformin (10 µM) significantly increases eNOS phosphorylation at the Ser633 activation site under both NG and LG conditions. LG alone does not significantly alter eNOS phosphorylation at the Ser633 site (N=10, P<0.001 overall, *-P<0.001 for both LG and NG with metformin vs. NG, †-*P<0.001 LG vs. LG + metformin).
In contrast to AMPK, the eNOS serine 1177 activation site was rapidly de-phosphorylated (within 5 minutes of LG exposure) and this level inhibition was maintained out to 120 minutes of LG exposure (Figures 3c–d).
Effect of Pharmacological AMP Kinase Activation on eNOS Phosphorylation at Activation Sites, Mitochondrial Superoxide Production and NO bioavailability
We exposed HUVECs under NG and LG condition to metformin (10µM) for 1 hour and measured eNOS phosphorylation at the Ser1177 and Ser633 activation sites. Metformin had no significant effect on LG induced dephosphorylation at Ser1177 (Figures 3e–f). However, metformin significantly increased eNOS phosphorylation at Ser633 under both LG and NG conditions while LG alone had no effect on Ser633 phosphorylation (Figure 3g–h)
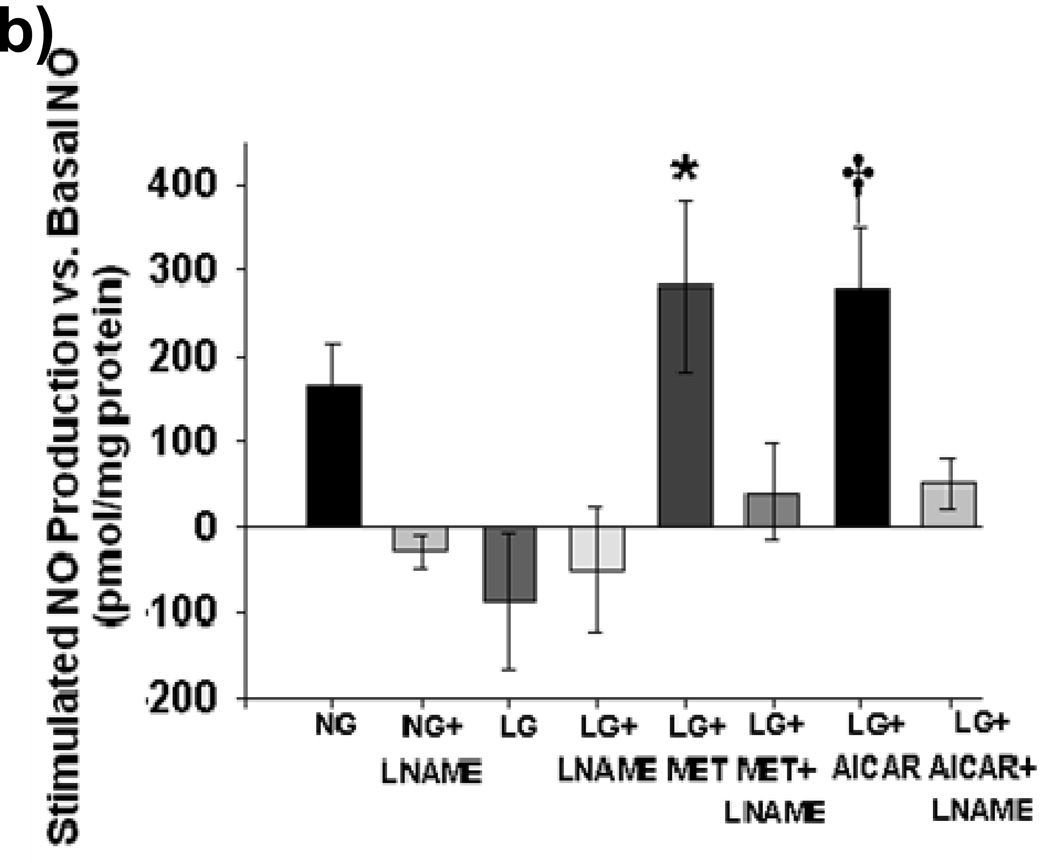
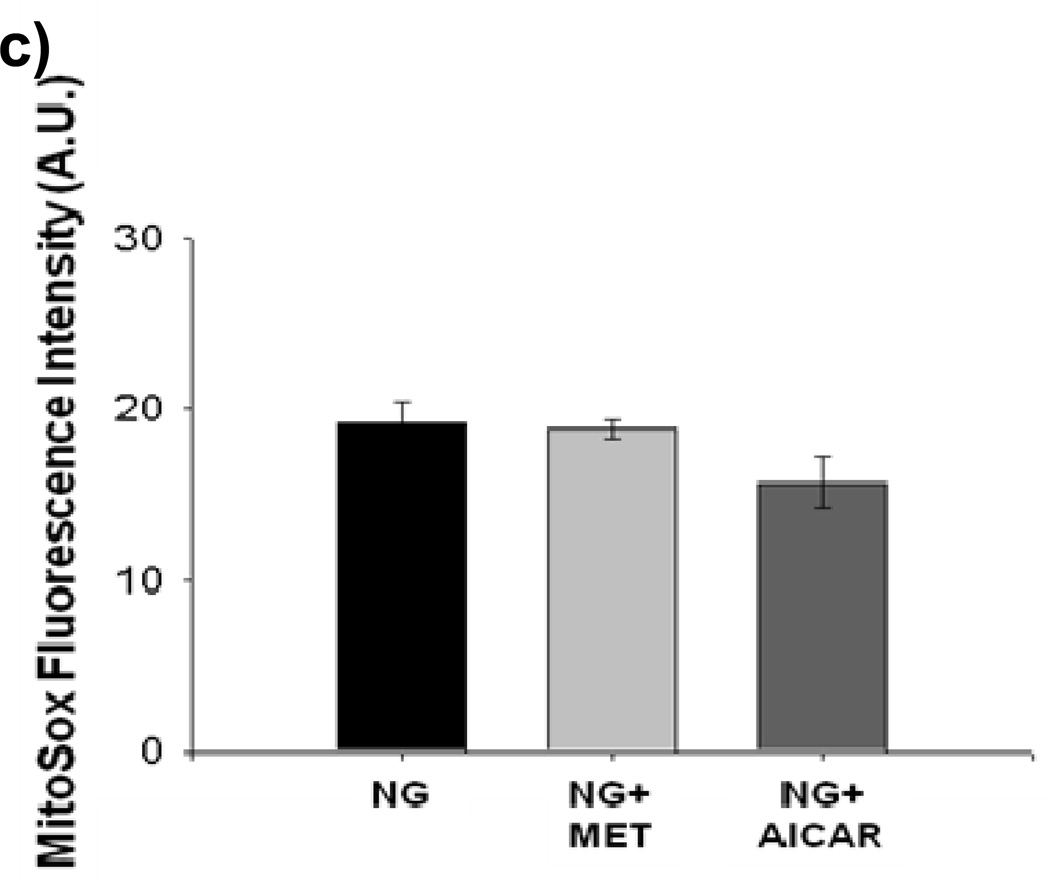
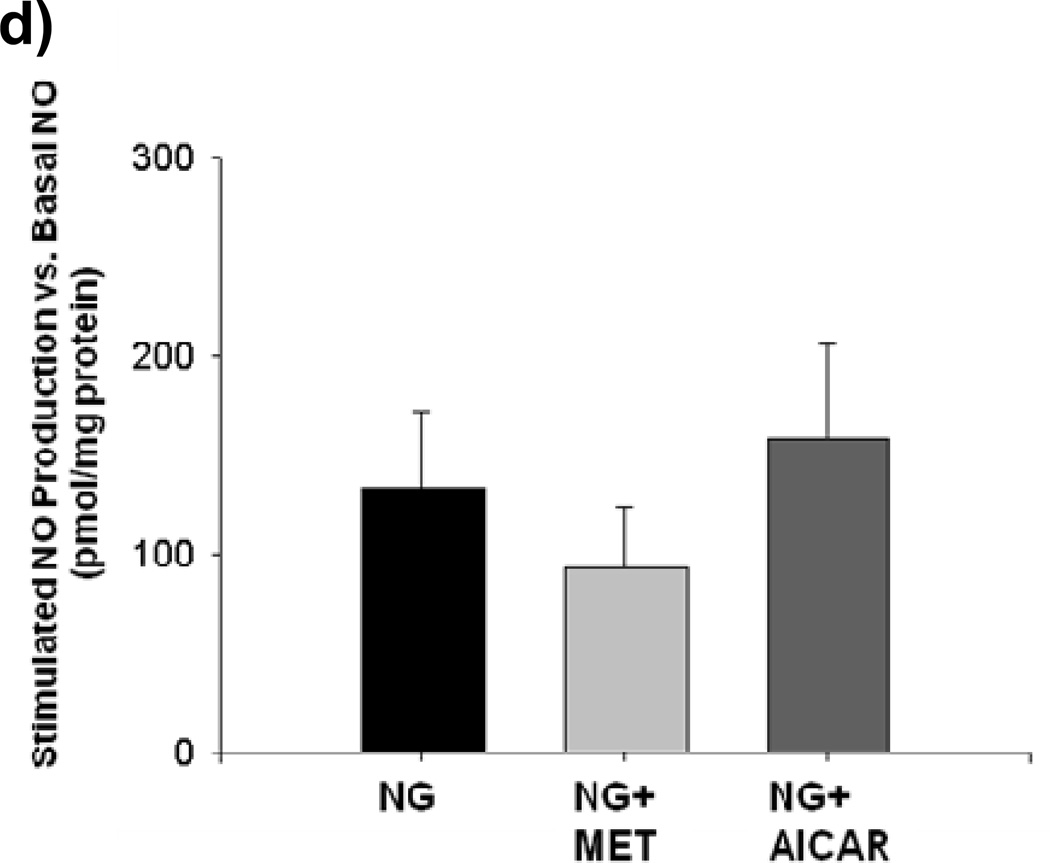
Incubation with either metformin or AICAR for 1 hourblocked mitochondrial superoxide production during LG (Figure 4a). This was accompanied by an increase in NO bioavailability during AICAR and metformin exposure. (Figure 4b) Reductions in mitochondrial superoxide produced by AICAR or metformin treatment were inhibited by concomitant exposure to L-NAME (Supplemental Figure IV).No effect of metformin or AICAR was seen on either mitochondrial superoxide production or NO bioavailability under NG conditions (Figures 4c and 4d).
Figure 4. a–b: Effect of Pharmacological AMPK activation on Mitochondrial ROS Production and NO bioavailability.
(a) Both metformin (10 µM) and AICAR (100 µM) significantly suppressed LG-induced increases in mitochondrial ROS production (n = 3–6, P< 0.001 overall, *-P<0.05 vs. LG,) and (b) reversed LG-inducted inhibition of NO production. (n = 5, P< 0.001 overall *-P<0.05 vs. all other exposure groups except NG). (c) Neither metformin (MET) nor AICAR altered mitochondrial ROS production under NG conditions (n=6, P=0.07). (d) Neither metformin (MET) nor AICAR altered NO production under NG conditions (n=6, P=0.46).
Effect of Acute Low Glucose Exposure on Endothelial Function
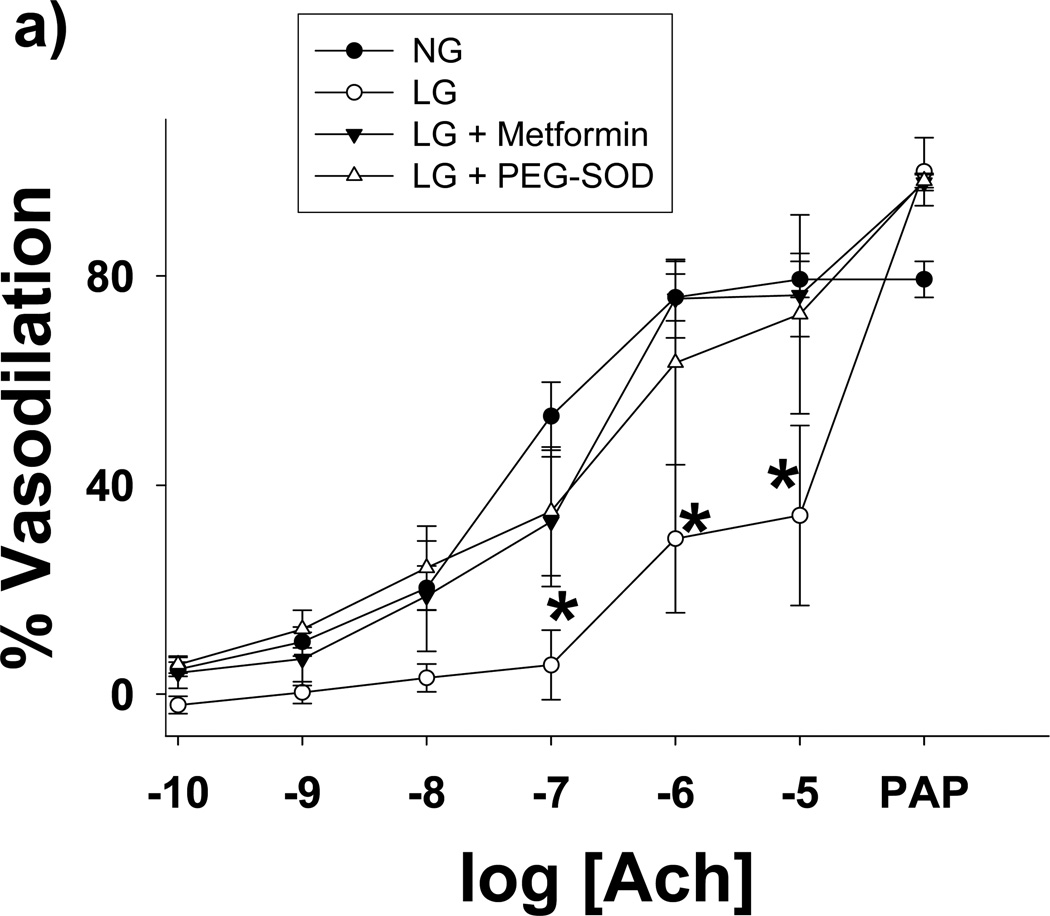
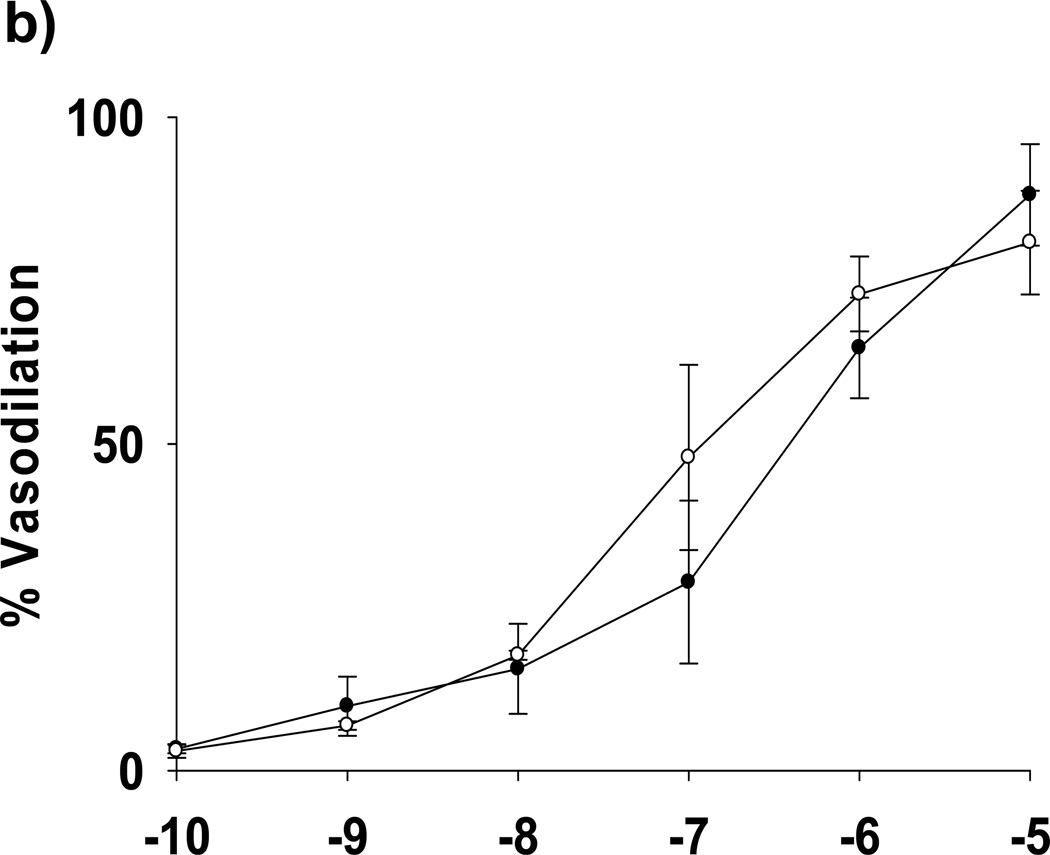
We isolated subcutaneous or mesenteric adipose arterioles from human volunteers without underlying atherosclerotic disease or diabetes for analysis of endothelium-dependent vasoreactivity under NG and LG conditions. Glucose concentration had no effect on the resting diameters of arterioles (100±13 vs. 94±15 nM for NG and LG, respective, P=0.09) As seen in Figure 5A, exposure to LG significantly blunted endothelium-dependent vasodilation to acetylcholine. LG with concomitant metformin or PEG-SOD exposure effectively inhibited the adverse effects of LG on endothelium-dependent vasodilation. No significant differences in endothelial function were found between NG, LG+metformin, and LG+PEG-SOD conditions. There were no significant differences in vasoreactivity to papaverine among exposure conditions (Figure 5A), and there was no difference in vasodilation to NONOate under LG compared to NG (Figure 5B). Using L-glucose (3 mM) to equalize the osmolarity of the buffer had no effect on LG (2 mM) impairment of arteriolar endothelial function (Supplemental Figure V).
Figure 5. Low Glucose Induced Endothelial Dysfunction is Prevented by Treatment with Metformin.
(a) Percent vasodilation from baseline was measured following short-term of exposure to four conditions: NG (n=13) or LG (n=6) with or without concomitant exposure to metformin (10 µM, n=3) or PEG-SOD (150 U/mL, n=3). Overall, low glucose exposure significantly impaired endothelial function relative to all 3 other exposure states (P<0.001 for all comparisons). *-P<0.05 vs. normal glucose, low glucose+metformin, and low glucose + PEG-SOD at the indicated concentration of acetylcholine. (b) LG did not alter endothelium-independent responses to exogenous NO (NONOate, n=3, P= Ach- acetylcholine. P=0.20 for interaction between dose and exposure condition).
Discussion
The present investigation has identified 3 novel findings. First, acute exposure of endothelial cells to low glucose concentrations in a clinically relevant range (40–80 mg/dL) induces a rapid reduction in NO bioavailability at least in part through dephosphorylation of at the serine 1177 site. Second, the loss of NO bioavailability is mechanistically linked to concomitant increases in mitochondria specific superoxide production likely through mitochondrial hyperpolarization. Third, while native AMPK activation is not sufficient to maintain levels of activated eNOS and inhibit excessive mitochondrial superoxide production, pharmacological AMPK activation inhibits excessive mitochondrial oxidative stress through improved NO bioavailability that may involve phosphorylation at eNOS’s Ser633 activation site. Importantly, we demonstrate our findings have relevance to human vascular endothelial function by demonstrating that acute low glucose exposure profoundly impairs endothelial function in intact human adipose arterioles, while treatment with metformin or a superoxide scavenger designed to pass through membranes inhibits the adverse effects of low glucose. Taken together, these data suggest a new mechanism of inducing vascular mitochondrial oxidative stress and endothelial dysfunction; namely, brief hypoglycemia, typical of the clinical situation involving tight glycemic regulation.
The consequences of tight glycemic control include intermittent hypoglycemia in the range that we identify as capable of increasing mitochondrial oxidative stress and endothelial dysfunction. There have been few prior investigations of the effect of low glucose on endothelial function, and prior work in this area has been conflicting. Exposure of porcine aortic endothelial cells to 2-deoxyglucose in a glucose-free environment inhibits bradykinin stimulated production of prostacyclin and possibly nitric oxide as well.16 In a study of rabbit aortas, inhibition of glycolysis with either 2-deoxyglucose or iodoacetate in buffer containing 16.6 mM glucose failed to alter endothelium-dependent vasodilation to acetylcholine.17 A third study assessed the effect of moderate and severe insulin-induced hypoglycemia on cerebral blood flow in goats, finding an NO-dependent increase in cerebral blood flow under hypoglycemic conditions.18. These studies are significantly heterogeneous with respect to their study designs, including significant differences in the species studied, vascular beds tested, and methodologies employed to induce low glucose states. The porcine and rabbit studies employed inhibitors of glycolysis enzymes to mimic the effects of glucose deprivation limiting their generalizability to physiologically relevant low glucose states. Our findings significantly expand our knowledge base on the effects of low glucose on human vascular endothelial function through the use of human tissues and clinically relevant concentrations of glucose.
Our data demonstrate low glucose exposure rapidly inhibits NO bioavailablity at least in part through reducing the level of Ser1177 phosphorylated eNOS. Mechanistically, the loss of NO bioavailability may also relate to our observed increase total cellular oxidative stress observed in this study by an increase in H2O2 under LG conditions. We were unable to demonstrate evidence of excessive total superoxide levels under low glucose conditions. While we cannot exclude an effect on total superoxide concentrations for longer exposure periods, our data suggest that a 60 minute period of LG exposure does not induce excessive total superoxide levels. Our data are also consistent with prior work demonstrating that glucose deprivation inhibits the phosphatydylinositol 3-kinase/Akt signaling pathway intrinsic to the activation of eNOS.19 Our data significantly expand on this finding by 1) extending the prior study’s findings to clearly demonstrate an effect on eNOS and 2) demonstrating the potential clinical relevance of inhibition of this pathway at physiological levels of low glucose. Further work to delineate the mechanisms of eNOS inactivation by low glucose is necessary to determine potential targets for interventions.
NO is a key regulator of the mitochondrial electron transport chain (ETC), the major source of mitochondrial superoxide.20 NO modulates ETC activity through inhibition of complexes I and IV.21, 22 Under normal homeostatic conditions, relatively high levels of NO in endothelial cells significantly inhibit ETC activity.23 Our data suggest that low glucose induced losses of bioavailable NO remove homeostatic restrictions on mitochondrial electron transport chain flux, leading to mitochondrial hyperpolarization and superoxide production. Mitochondrial hyperpolarization, as induced in our study by LG, is well-known to be mechanistically related to increased mitochondrial ROS production. 10, 24, 25, 25, 26 Our novel findings in the setting of LG are consistent with prior work demonstrating that the amount of glucose oxidation via the Krebs cycle, near dormant under normal glucose conditions in endothelial cells, is significantly increased with low glucose exposure.27 Data from the neurological literature demonstrate hypoglycemia shifts mitochondria from state 3 to state 4 respiration in central neurons, an alteration well-known for increasing mitochondrial superoxide production.28 Subsequent work also shows neuronal hypoglycemia induces marked increases in both mitochondrial superoxide and hydrogen peroxide production.29, 30 Further work using inhibitors of the mitochondrial ETC and measurements of specific activity levels of components of the ETC will be necessary to determine the exact ETC sources of mitochondrial ROS under low glucose conditions.
We found that LG exposure enhances endothelial cell H2O2 content with a corresponding increase in mitochondrial superoxide content. Increases in ROS production are linked to increased oxidative damage, reduced NO bioavailability, and endothelial dysfunction.31. In the context of our findings, the biological role of increased mitochondrial superoxide under LG conditions likely includes both the adverse sequelae of increased oxidative damage as well as modulation of important pathological cell signaling cascades. Mitochondrial superoxide can reduce NO bioavailability by rapidly reacting locally with NO to form peroxynitrite. Peroxynitrite formation reduces NO bioavailability, and itself can damage the electron transport chain, antioxidant defenses, and mitochondrial proteins and nucleic acids.32 Our data are also consistent with the emerging concept of that mitochondrial ROS, particularly hydrogen peroxide (H2O2), are central regulators of homeostatic and pathological cell signaling in endothelial cells.33, 34 While superoxide can escape from mitochondria in modest quantities, the concentrations of more stable H2O2 (produced by rapid dismutation of superoxide to H2O2 in both the mitochondrial matrix and intermembrane space) far exceed that of superoxide in mitochondria.35 H2O2 can easily pass through mitochondrial membranes.36
H2O2 has previously been reported to activate AMPK via calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ).37 Prior work also establishes the ability of NO to reduce mitochondrial H2O2 production via suppression superoxide production from the mitochondrial ETC.38 Our data are entirely consistent with this previously established regulatory nexus of H2O2, AMPK, and NO. We have importantly extended these findings by showing exposure to low glucose at clinically relevant concentrations and exposure times triggers this regulatory pathway, beginning with a loss of NO bioavailability, a concomitant increase in mitochondrial H2O2 production, and an increase in AMPK activity.
Interestingly, we found treatment with either metformin or AICAR, known pharmacological activators of AMPK through activation of tumor suppressor product LKB1, reversed the loss of NO bioavailability and inhibited excessive mitochondrial oxidative stress. Further, metformin reversed endothelial dysfunction in intact human arterioles acutely exposed to moderate low glucose concentrations. These data are consistent with prior work demonstrating AMPK activation of eNOS and NO suppression of mitochondrial ETC activity.12, 23 Inhibition of eNOS using L-NAME prevented the effects of metformin and AICAR, further suggesting that the ameliorative effects of pharmacological AMPK activation are mechanistically linked to increased NO bioavailability.
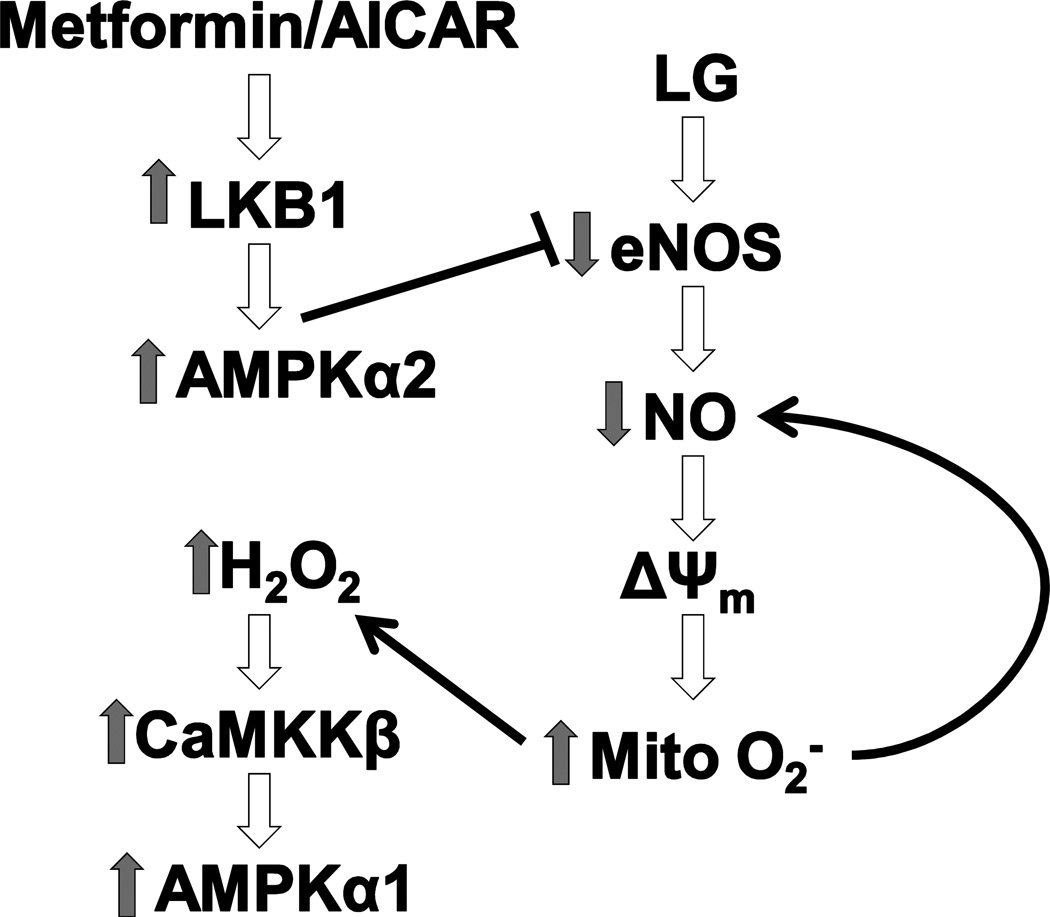
One potential paradox in our findings is that increased pAMPK with LG exposure alone failed to increase NO levels even out to 2 hours of LG exposure. Based on prior work and our data in Figure 3, we suspect this initially puzzling finding relates to differences in the isoform of AMPK activated by metformin and AICAR (AMPKα2 via activation of LKB1)39 versus the isoform activated by H2O2 (AMPKα1 via activation of CaMKKβ)37 and the relative importance of each isoform in regulating eNOS activity. While AMPKα1 is more abundant in endothelial cells, AMPKα2 activity appears to be of significantly greater importance in eNOS regulation. In endothelial cells, pAMPKα2 plays a key role in activating eNOS and increasing NO bioavailability, while there is minimal to no role for AMPKα1 in eNOS regulation.12 Further, these data would suggest that pharmacological AMPKα2 activation may have a protective effect against LG-induced endothelial dysfunction. Future work is clearly indicated to confirm this attractive hypothesis. Figure 6 provides a graphical synthesis of our findings with previously reported work in this area.
Figure 6. LG-Induced Endothelial Dysfunction: Role of Mitochondrial Superoxide Production and Prevention by AMPK Activation.
Acute LG exposure leads to rapid inhibition of eNOS and a reduction in bioavailable NO. This leads to a subsequent increase in mitochondrial ETC activity, mitochondrial membrane hyperpolarization, and increased mitochondrial superoxide production. Increased mitochondrial superoxide production can further reduce NO bioavailability and leads to increased cellular hydrogen peroxide levels that activate AMPK isoform α1 through a CaMKKβ dependent pathway. LG induced losses of NO bioavailability can be prevented/reversed by treatment with AMPKα2 activating agents acting through an LKB1 dependent activation pathway.
Our findings with respect to fatty acid and L-carnitine suggest mechanistically that free fatty acid utilization in vivo would not be sufficient to suppress LG induced NO suppression and excessive mitochondrial superoxide production, particularly in diabetic patients who have significant impairment in free fatty acid utilization and reduced basal plasma and tissue carnitine levels. 40, 41 Interestingly, our data does suggest that pharmacological supplementation with L-carnitine may be able to inhibit the adverse effects of LG on mitochondrial superoxide production and perhaps improve endothelial dysfunction. L-carnitine plays a key role in both transporting and intracellular handling of fatty acids,42 and L-carnitine supplementation has been shown to improve NO bioavailability and improve free-fatty acid induced endothelial dysfunction.43 The mechanism of the vascular benefits of L-carnitine remains unclear, and further work is necessary to delineate the efficacy and mechanism of action of L-carnitine in the setting of low glucose.
Our data have limitations. First, HUVECs may not be representative of other vascular endothelial cells. However, we tested arteriolar endothelial function of intact human vessels and verified that LG induces a state of endothelial dysfunction reversible with metformin and anti-oxidant therapy, suggesting our findings have physiological relevance to human arteriolar endothelial function. Second, the mechanism of eNOS inactivation beyond dephosphorylation at Ser1177 under LG condintions remains unknown and will need to be elucidated in future work. While our data demonstrate eNOS phosphorylation at the 1177 serine site occurs upon exposure to low glucose conditions in a time-dependent manner, experiments with eNOS 1177 mutants would reinforce our findings. Third, we did not specifically measure the contributions of AMPKα1 and AMPKα2. Future work will be necessary to verify the AMPK isoform specific effects we suspect explain the differential effects of physiological H2O2 based AMPK activation and pharmacological AMPK activation. Finally, metformin’s ameloriative effects may also include augmentation of endothelial cell glucose uptake through activated AMPK directed increases in GLUT1 activity and/or blockade of mitochondrial ETC complex I.44, 45 Both mechanisms could also blunt mitochondrial superoxide production. Further investigation will be necessary to determine the contribution of this metformin effect to our findings. Balanced against these limitations is the novelty of our findings, our evidence of physiological relevance in human arterioles, and the potential clinical implications of these data regarding the cardiovascular dangers of hypoglycemia.
Conclusions and Clinical Implications
Acute exposure to clinically relevant levels of low glucose rapidly induces a state of endothelial dysfunction, characterized by reduced NO bioavailability, mitochondrial hyperpolarization, increased mitochondrial ROS production, and vasomotor dysfunction. Physiological AMPK activity modestly increases with LG exposure likely secondary to increased mitochondria-derived H2O2 levels. Pharmacological AMPK activation, which relies on a different AMPK isoform than that activated by physiological H2O2, reverses low glucose-induced endothelial dysfunction at least in part through inhibiting excessive mitochondrial superoxide production in an eNOS-dependent manner.
Hypoglycemic symptoms often do not occur until systemic levels drop below 55 mg/dL.46 Our data support the biological plausibility that acute hypoglycemia may result in the acute development or worsening of endothelial dysfunction, triggering cardiovascular events. Further, our data suggest the potential for pharmacological AMPK activation, and perhaps L-carnitine, to inhibit these effects.8 Further work is needed to determine if in vivo hypoglycemia, similar to hyperglycemia, is also a significant risk factor for endothelial dysfunction. Such confirmation would help explain the observed detrimental effect of tight glycemic control on cardiovascular events and have important ramifications in the treatment strategies for glucose control in diabetes.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the help of Carlyn Leahey and Jennifer Mulkerin for their help with fluorescence measurements and Dr. William Campbell for his help with our eNOS activity assays.
Sources of Funding
Dr Widlansky is supported by K23HL089326, the Elsa Shoeneich Medical Research Fund (Greater Milwaukee Foundation), and a T. Franklin Williams Scholars Award provided by Atlantic Philanthropies, the American Heart Association (Grant-in-Aid 10GRNT3880044), the John A. Hartford Foundation, and the Association of Specialty Physicians. This work was supported by the Clinical Translational Research Institute at the Medical College of Wisconsin and NIH (HL081587). Drs. Kizhakekuttu, Dharmashankar, and Wang received supported from a Ruth L. Kirschstein NIH T32 training grant (HL007792-15). The contents of this manuscript are the responsibility of the authors and do not necessarily represent the official views of any of the above funding sources.
Non-Standard Abbreviations and Acronyms
- AMPK
AMP Kinase
- CaMKKβ
calcium/calmodulin-dependent protein kinase kinase β
- ETC
Electron Transport Chain
- eNOS
Endothelium Derived Nitric Oxide Synthase
- H2O2
Hydrogen Peroxide
- NG
Normal Glucose Concentration, 90 mg/dL
- LG
Low Glucose Concentration, 40–50 mg/dL
- pAMPK
Phosphorylated AMP Kinase
- ROS
Reactive Oxygen Species
- tAMPK
Total AMP Kinase
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
Reference List
- 1.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 4.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 6.Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emanuele NV, Levin SR, Henderson W, Lee HS. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995;18:1113–1123. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 7.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 8.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 9.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 11.Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem. 2008;283:25256–25263. doi: 10.1074/jbc.M802455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 16.Richards JM, Gibson IF, Martin W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br J Pharmacol. 1991;102:203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappelli-Bigazzi M, Battaglia C, Pannain S, Chiariello M, Ambrosio G. Role of oxidative metabolism on endothelium-dependent vascular relaxation of isolated vessels. J Mol Cell Cardiol. 1997;29:871–879. doi: 10.1006/jmcc.1996.0286. [DOI] [PubMed] [Google Scholar]
- 18.Dieguez G, Fernandez N, Garcia JL, Garcia-Villalon AL, Monge L, Gomez B. Role of nitric oxide in the effects of hypoglycemia on the cerebral circulation in awake goats. Eur J Pharmacol. 1997;330:185–193. doi: 10.1016/s0014-2999(97)00181-7. [DOI] [PubMed] [Google Scholar]
- 19.Tzatsos A, Tsichlis PN. Energy depletion inhibits phosphatidylinositol 3- kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem. 2007;282:18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 24.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 25.Fink BD, Reszka KJ, Herlein JA, Mathahs MM, Sivitz WI. Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E71–E79. doi: 10.1152/ajpendo.00332.2004. [DOI] [PubMed] [Google Scholar]
- 26.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 27.Krutzfeldt A, Spahr R, Mertens S, Siegmund B, Piper HM. Metabolism of exogenous substrates by coronary endothelial cells in culture. J Mol Cell Cardiol. 1990;22:1393–1404. doi: 10.1016/0022-2828(90)90984-a. [DOI] [PubMed] [Google Scholar]
- 28.Pelligrino DA, Becker GL, Miletich DJ, Albrecht RF. Cerebral mitochondrial respiration in diabetic and chronically hypoglycemic rats. Brain Res. 1989;479:241–246. doi: 10.1016/0006-8993(89)91624-7. [DOI] [PubMed] [Google Scholar]
- 29.Ballesteros JR, Mishra OP, McGowan JE. Alterations in cerebral mitochondria during acute hypoglycemia. Biol Neonate. 2003;84:159–163. doi: 10.1159/000071951. [DOI] [PubMed] [Google Scholar]
- 30.McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006;399:111–114. doi: 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Keaney JF., Jr . Atherosclerosis, oxidative stress, and endothelial function. In: Keaney JF Jr, editor. Oxidative stress and vascular disease. Boston: Kluwer Academic Publishers; 2000. pp. 155–181. [Google Scholar]
- 32.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol. 2009;296:H539–H549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu S, Hiroi T, Ishii M, Hagiwara T, Wajima T, Miyazaki A, Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through activation of the Jak2 tyrosine kinase pathway in vascular endothelial cells. Int J Biochem Cell Biol. 2008;40:755–765. doi: 10.1016/j.biocel.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 36.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Jin BY, Sartoretto JL, Gladyshev VN, Michel T. Endothelial nitric oxide synthase negatively regulates hydrogen peroxide-stimulated AMP-activated protein kinase in endothelial cells. Proc Natl Acad Sci U S A. 2009;106:17343–17348. doi: 10.1073/pnas.0907409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widlansky ME, Gutterman DD. Regulation of Endothelial Function by Mitochondrial Reactive Oxygen Species. Antioxid Redox Signal. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter SC, Simon M, Zorn EM, Szabo-Aczel S, Vance WH, O'Hara T, Higashi L. Relative carnitine insufficiency in children with type I diabetes mellitus. Am J Dis Child. 1989;143:1337–1339. doi: 10.1001/archpedi.1989.02150230095030. [DOI] [PubMed] [Google Scholar]
- 41.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsay RR, Arduini A. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch Biochem Biophys. 1993;302:307–314. doi: 10.1006/abbi.1993.1216. [DOI] [PubMed] [Google Scholar]
- 43.Cameron NE, Cotter MA. Neurovascular effects of L-carnitine treatment in diabetic rats. Eur J Pharmacol. 1997;319:239–244. doi: 10.1016/s0014-2999(96)00844-8. [DOI] [PubMed] [Google Scholar]
- 44.Sasson S, Gorowits N, Joost HG, King GL, Cerasi E, Kaiser N. Regulation by metformin of the hexose transport system in vascular endothelial and smooth muscle cells. Br J Pharmacol. 1996;117:1318–1324. doi: 10.1111/j.1476-5381.1996.tb16731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, Witters LA, Ismail-Beigi F. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- 46.Bolli GB, Dimitriadis GD, Pehling GB, Baker BA, Haymond MW, Cryer PE, Gerich JE. Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N Engl J Med. 1984;310:1706–1711. doi: 10.1056/NEJM198406283102605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.