Abstract
Objective
Menopause is often accompanied by vaginal discomfort including burning, itching, dryness and spontaneous or provoked pain. While direct effects of estrogen withdrawal on vaginal cells are implicated, surgical menopause in rodents causes autonomic and sensory nerves to proliferate, suggesting that indirect effects mediated by changes in vaginal innervation may contribute. We assessed whether post-menopausal women display hormone-dependent changes in vaginal innervation.
Methods
Vaginal biopsies from 20 postmenopausal women undergoing surgery for stress urinary incontinence and pelvic organ prolapse were fixed and immunostained for the pan-neuronal marker, PGP9.5, the sympathetic marker tyrosine hydroxylase, the parasympathetic marker vasoactive intestinal polypeptide, and the sensory nociceptor marker calcitonin gene-related peptide. Innervation density was measured as apparent percentage of section area occupied by immunofluorescent axons. Specimens were grouped according to whether participants received systemic hormone therapy (HT), topical (vaginal) HT, or no HT.
Results
Women not receiving HT showed relatively high levels of total innervation, with most axons expressing tyrosine hydroxylase or vasoactive intestinal polypeptide immunoreactivity. In patients receiving systemic HT, overall innervation was reduced, as were presumptive parasympathetic, sympathetic and sensory axon populations. Topical HT elicited more dramatic reductions in innervation than systemic HT.
Conclusions
Hormone therapy reduces autonomic and sensory vaginal innervation density, which may in part contribute to relief from vaginal discomfort. Moreover, topical therapy is more effective than systemic therapy, which may help explain the greater improvement reported with topical as compared to systemic HT.
Keywords: Estrogen, menopause, vagina, autonomic, sensory, dyspareunia
INTRODUCTION
The consequences of menopause, with its declining production of reproductive hormones, are well known. In the human female reproductive tract, vaginal and uterine tissues are well imbued with estrogen (E2) and progesterone receptors (ERs and PRs)1–4 and receptor activation results in wide-ranging effects such as endometrial cell proliferation, increased secretions, vascularization and smooth muscle hypertrophy5. Reproductive hormones are therefore essential for the normal maintenance of the human reproductive tract.
Menopause is characterized by reductions in normal circulating levels of reproductive hormones, leading to the loss of fecundity with accompanying changes in reproductive tract structure and function. Changes include atrophy of the vaginal epithelium and in some cases the vulva, decreased secretions, and changes in pH5–7. In some women, however, alterations are more profound and disconcerting. Many perimenopausal women experience itching, burning, dryness and discomfort, and in some cases pain that may be exacerbated with intercourse (dyspareunia). These symptoms are often severe enough to be considered for inclusion in the pelvic pain disorder, spontaneous generalized (dysesthetic) vulvodynia8. Both menopausal discomfort and symptoms of spontaneous vulvodynia are often relieved by systemic or topical hormone therapy (HT)9, 10.
The classical explanation as to why E2 is effective in reversing vaginal menopausal symptoms is that it acts directly on vaginal tissue ERs to restore normal target function. However, recent data suggest that the explanation may be more complex. Autonomic and sensory neurons express ERs11–13 and are responsive to E2 in culture14, 15, 16, supporting the idea that E2 can act directly on peripheral neurons. In addition, E2 induces uterine myometrium to produce neuromodulatory factors17–19, and may do so in other parts of the reproductive tract. Indeed, in a rat model of menopause, we showed that overall vaginal innervation density increases by 50% 2 weeks after ovariectomy as compared to intact cycling rats, and that exogenous E2 reduces innervation to pre-ovariectomy levels20. In the present study, we assessed whether E2 affects vaginal innervation density in post-menopausal women who received no HT, systemic HT, or topical HT.
METHODS
Patient and Sample Information
Post-menopausal women scheduled at the Kansas University Hospital to undergo surgery for treatment of stress urinary incontinence and/or pelvic organ prolapse by a single surgeon were invited to participate. Exclusion criteria included any history of genitourinary malignancy, pelvic radiation therapy, breast cancer, current severe atrophic vaginitis, or dysfunctional vaginal bleeding. No participants complained of vaginal pain or dyspareunia. Two women were also excluded because removing the tissue sample would have resulted in unacceptable tension at the vaginal incision closure site. All participants completed informed consent. The study was reviewed and approved by the KU Medical Center Human Subjects Committee and Institutional Review Board.
Full-thickness vaginal biopsy specimens (~1×2 cm) obtained from the anterior wall at the mid-vaginal level corresponding to the bladder neck from 20 post-menopausal women were included in this study. Specimens were obtained from the anterior vaginal wall in a sagittal orientation and included the edge of the midline vaginal incision. Biopsies were removed using sharp dissection, and hemostasis was achieved using electrocautery prior to closure of the incision.
One group of 9 patients (50–73 years, 59.7±7.3) was not and had no history of receiving HT. A second group of 6 patients (47–75 years, 61.2±4.0) was receiving systemic estrogen treatment prior to and at the time of surgery. Of these, four women were taking oral conjugated estrogen preparations (dose range 0.3–1.25 mg/day), one used a transdermal estrogen patch (0.05 mg applied twice weekly), and one received regular intra-muscular estrogen injections (exact dosing not available). Five patients (47–91 years, 63.6±7.3 years) applied topical vaginal estrogen cream. In these women, therapy was initiated at least two weeks prior to the date of surgery. Participants were administered 1 gram of estradiol vaginal cream 0.01% (0.1 grams active estradiol) three times weekly at bedtime. The decision to begin topical estrogen therapy was made by each patient as part of the routine standard of care and not as part of the study protocol.
Tissue Processing and Immunostaining
Immediately after surgical excision, vaginal biopsy specimens were fixed in Zamboni's solution for 24 h at 4°C and washed daily for 7 days with 0.01 M PBS (pH 7.4). Tissue was transferred to 20% sucrose in PBS and cryoprotected at 4°C overnight, snap-frozen in isopentane pre-cooled with liquid nitrogen, and stored at −80°C until cryosectioning at 10 μm. Stepped series of adjacent sections were thaw-mounted onto Fisherbrand Superfrost/Plus precleaned slides (Fisher Scientific, Pittsburgh, PA) and air-dried.
Sections were rinsed in PBS containing 0.3% Triton X-100 (PBST) and blocked for 60 min at room temperature in PBST containing 5% normal goat serum and 1% bovine serum albumin. Sections were incubated overnight in a humidified chamber at room temperature with antiserum against protein gene product 9.5 (PGP 9.5, 1:1200; rabbit polyclonal IgG; AbD Serotec), tyrosine hydroxylase (TH, 1:200; rabbit polyclonal IgG; Chemicon), vasoactive intestinal peptide (VIP, 1:600; rabbit polyclonal IgG; Chemicon). Slides were then washed three times in PBST, followed by incubation with secondary antiserum for 1h at room temperature (goat anti-rabbit IgG conjugated to Cy3, 1:200, Jackson ImmunoResearch); antibodies used in this study have been characterized previously21, 22. Slides were coverslipped with Fluoromount G.
Quantitative analyses
Digital microscopic images (Nikon Eclipse TE300 with an Optronics MagnaFire camera) were captured from 3–4 randomly selected sections from each vaginal specimen. In each section, 4 randomly selected fields from each of superficial lamina propria (right below dermo-epidermal junction), deep lamina propria, and muscular layer were captured with Cy3 epifluorescence using a 40× objective.
A stereology grid with line intersects at 10-μm intervals was superimposed randomly over the images. All grid intersects overlying nerve profiles were counted and divided by total points over vaginal tissue to yield the innervation density, as reported previously20, 21.
Statistical analysis
All values are presented as the mean ± SEM. Data were normally distributed and statistical comparisons were made using one way anova with post-hoc testing using Student-Newman-Keuls method for marker-positive axon densities across treatment groups. A relation between age and innervation density was explored using linear regression analysis. Differences were considered significant at p ≤ 0.05.
RESULTS
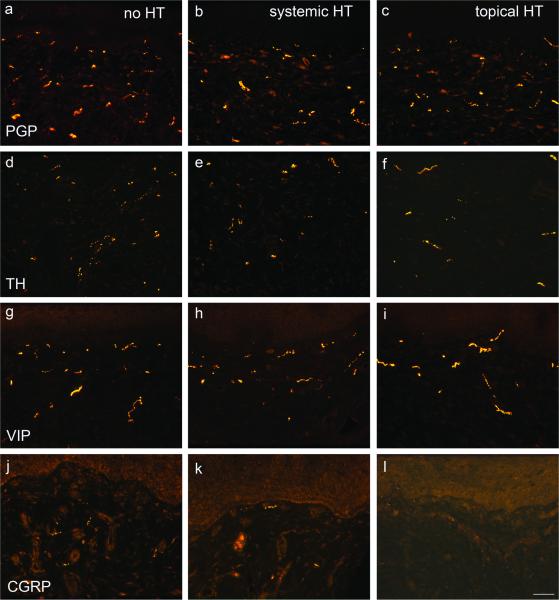
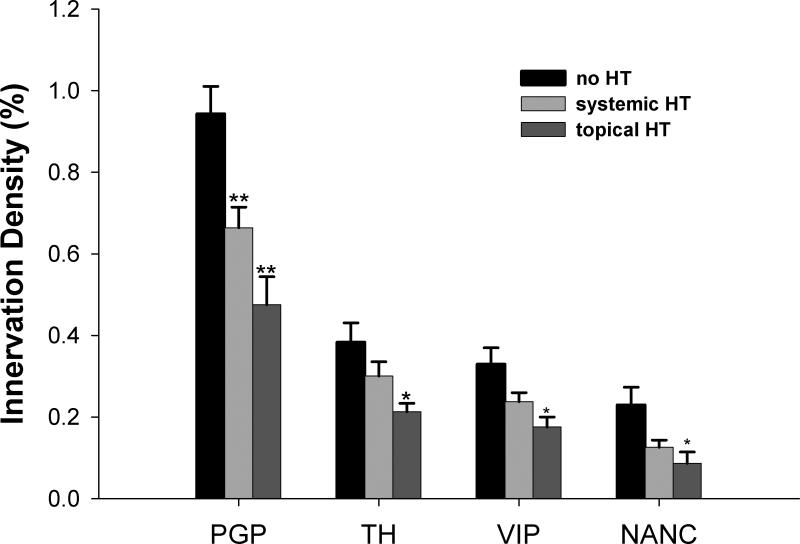
Axons immunostained for the pan-neuronal marker, PGP 9.5, were observed frequently throughout the vaginal lamina propria and muscularis (Fig. 1a–c), while few were found within the vaginal epithelium. PGP 9.5-ir axon density in postmenopausal patients without HT was 0.94 ± 0.07% (Figs. 1a, 2). In postmenopausal patients receiving systemic HT, PGP 9.5-immunoreactive (-ir) fiber density was 0.66± 0.05% (Figs. 1b, 2), and in patients with topical HT it was 0.48± 0.07% (Figs. 1c, 2). Densities in postmenopausal patients with systemic HT and topical HT were both reduced compared to patients without HT (P<0.001). There was no association between innervation density and age in these groups.
Fig. 1.
Immunofluorescence photomicrographs of sections taken from specimens from the posterior vaginal wall of postmenopausal patients. Sections were obtained from patients receiving no hormone replacement therapy (no HT, a, d, g, j), systemic HT (b, e, h, k) and topical HT(c, f, I, l). Sections were immunostained for the pan-neuronal marker, PGP9.5 (a–c), for tyrosine hydroxylase (TH, d–f), vasoactive intestinal polypeptide (VIP, g–i), and calcitonin gene-related peptides (CGRP, j–l). Scale bar in l =50 μm for all panels.
Fig. 2.
Quantitative analysis of nerve density in the vagina from postmenopausal patients. n=9 for no HT, n=6 for systemic HT, n=5 for topical HT. * P<0.05 vs. no HT, ** P<0.01 vs. no HT by one way anova with post-hoc testing using Student-Newman-Keuls method.
TH-ir labels noradrenergic sympathetic fibers, and these were associated predominantly with smooth muscle cells comprising the vasculature and vaginal muscularis layer (Fig. 1d–f); they were rarely seen in the superficial submucosa. TH-ir fiber densities were 0.38± 0.05%, 0.30± 0.04%, and 0.21± 0.02% in postmenopausal patients without HT, with systemic HT, and with topical HT, respectively (Fig. 2). Differences were significant between patients with topical HT and without HT (P<0.05), but not between patients with systemic HT and without HT.
Fibers immunoreactive for VIP, a neuropeptide expressed by cholinergic pelvic parasympathetic innervation23, were distributed beneath the epithelium and associated predominantly with vascular and nonvascular smooth muscle (Fig. 1g–i). VIP-ir nerve density was 0.33% ± 0.04%, 0.24% ± 0.02%, and 0.18% ± 0.02% (Fig. 2) in postmenopausal patients without HT, with systemic HT and with topical HT respectively. VIP density in patients with topical HT was significantly lower than patients without HT.
While TH-ir and VIP-ir fibers comprised the vast majority of vaginal innervation, approximately a fifth of axons in all groups were negative for both markers (non-adrenergic, non-cholinergic, NANC). These NANC fibers are likely to represent predominantly unmyelinated sensory innervation. Indeed, immunostaining for calcitonin gene-related peptide (CGRP), a marker of presumptive sensory nociceptor axons, revealed small numbers of subepithelial fibers, which likely represent the bulk of the NANC population. The numbers of CGRP-ir fibers encountered appeared to be diminished in participants receiving oral or topical HT (Fig. 1j, k and l), but the low density of this population precluded reliable quantitative analysis.
To estimate the effect of HT on the NANC axon population, for each section we summed contributions by TH- and VIP-ir axons, and subtracted this from PGP9.5-ir total innervation (Fig. 2). This derivative estimate showed that putative nociceptor innervation density also decreased in the presence of HT (p=0.035).
DISCUSSION
Our observations revealed a rich plexus of innervation within the submucosal regions of the vaginal tissue, with relatively few intraepithelial axons. The plexus is composed predominantly of TH- and VIP-ir fibers in roughly equal proportions. This suggests that sympathetic and parasympathetic axons comprise the bulk of human vaginal innervation with smaller contributions by sensory fibers, consistent with earlier reports in humans24–26 and rats20, 27, 28. Qualitative analysis showed that the NANC fiber population expresses CGRP, consistent with the presence of putative nociceptor axons.
Our quantitative findings show that innervation density changes as a function of HT status in postmenopausal females. This is concordant with an abundance of information regarding the effects of hormones on reproductive tract innervation. It has long been known that massive degenerative loss of uterine innervation occurs in rats and guinea pigs at term pregnancy29–32, and that sympathetic axons are selectively pruned with rising estrogen levels in the proestrus/estrus phase of the estrous cycle in rats and mice22, 33, 34. This loss of innervation occurs through E2 acting directly on uterine smooth muscle target, leading to the production of pro-degenerative and/or neuro-repulsive proteins17, 18. These local prodegenerative conditions appear to dominate over direct effects of E2, which tend to promote axon outgrowth and regeneration on sensory and sympathetic neurons15, 35,36. We have shown recently that vaginal innervation in rats is also responsive to endogenous and exogenous E2 resulting in reductions in sympathetic and parasympathetic axons and in peptidergic sensory fibers20. Because rising E2 levels at term pregnancy promote this denervation, we've proposed that these hormonal changes may underlie an important form of physiologically adaptive plasticity that may reduce pain sensitivity and facilitate delivery at parturition37.
The findings presented here support the notion that the human vaginal innervation is also responsive to E2. However, there are some limitations relative to studies in animal models. One caveat is that the protocol did not permit us determine overall changes in vaginal volume associated with HT; therefore we can't exclude the possibility that some changes in nerve density occurred passively as a result of increased target size. However, it is generally believed that the primary hypertrophic effect of HT is restricted mainly to the vaginal epithelial cells38, 39, whereas our measurements were from the submucosal region which is not thought to be substantively affected by HT. Further, smooth muscle atrophy in the surgically menopausal rat is only partially reversed by E2. It therefore seems unlikely that the axonal remodeling seen here could be accounted for entirely by changes in target volume.
Reductions in vaginal autonomic nerves seen in women receiving HT are likely to have functional implications. Sympathetic axons from paravertebral ganglion neurons innervate blood vessels and non-vascular smooth muscle of the vaginal wall, and norepinephrine released by these axons elicits α-adrenergic receptor-mediated contraction and vasoconstriction28, 40, 41. Conversely, parasympathetic neurons from the paracervical ganglion42 project to vascular and non-vascular vaginal smooth muscle and release VIP, nitric oxide and acetylcholine, all of which are reported to relax smooth muscle and promote vasodilation28, 40, 43. Accordingly, post-menopausal increases in these fibers could result in changes in vaginal smooth muscle tone and blood flow.
Vaginal smooth muscle tone is reportedly diminished in menopause. At least part of this is due to the reduced smooth muscle content that occurs in the absence of reproductive hormones44. However, parasympathetic axons releasing nitric oxide can strongly inhibit vaginal smooth muscle contraction28, and their proliferation after menopause could also contribute to reduced tone. If so, HT could have beneficial effects by both increasing smooth muscle mass and by reducing numbers of parasympathetic axons. It should be noted, however, that excitatory sympathetic innervation is also reduced by HT, giving rise to a more complex picture, and it will be of interest to assess physiologically which autonomic effects of HT may predominate.
Changes in hormone status also affect vaginal blood flow. The postmenopausal vagina is characterized by reduced vaginal blood flow, leading to decreased secretions and dryness of the vaginal mucosa10. This could be due in part to an increase in sympathetic vasoconstrictor nerves. Hence, reductions in sympathetic vasoconstrictor axons seen in women receiving HT could well help to increase vaginal blood flow. However, decreases in parasympathetic vasodilator fibers could partially offset the increased vasodilatory tone; because the relative distributions of sympathetic and parasympathetic axons to the vaginal vasculature are not well described, the physiological consequences of altered vascular innervation density are yet to be defined. Nonetheless, it is known that E2 strongly suppresses release of pelvic parasympathetic nitric oxide45, which may suggest that improved blood flow with HT could be due preferentially to reductions in vasoconstrictor nerves.
While our sampling methods were not amenable to direct quantitation of sensory innervation, we did confirm the presence of putative nociceptor axons and showed that this non-adrenergic, non-cholinergic population was also responsive to E2. Both quantitation of NANC axons and assessment of CGRP-ir innervation confirmed that putative sensory nociceptor innervation was relatively abundant in postmenopausal women. This is of interest because CGRP is a potent vasodilator, and postmenopausal vulvodynia is frequently associated with neurogenic genital inflammation, which could be caused by increased numbers of vasodilatory fibers. Accordingly, the reduction in NANC and CGRP-ir fibers induced by HT could well explain improvement seen after therapy is initiated. Further, given that sympathetic innervation has been implicated in generalized vulvodynia46, the reduction in both putative nociceptor and sympathetic innervation may act synergistically to improve symptomatology.
With the recognition that systemic HT can present specific health risks to some patients44, there is increased emphasis in pursuing alternative approaches, and topical HT has gained additional favor. Topical estrogen therapy is often used to treat dysfunctional vaginal symptoms in post-menopausal women including burning, pain, and dyspareunia.45 Even in patients already taking systemic therapy, clinicians will frequently prescribe topical HT in order to maximize the concentrations of estrogen in vaginal and periurethral tissues. Our findings show that, at least with regard to HT modulation of vaginal innervation density, topical HT is in fact more effective in reducing density of vaginal autonomic and sensory nerves. Therefore, not only is topical therapy associated with potentially lower health risk, but it represents a more efficacious treatment modality, consistent with its more profound effects on vaginal innervation.
CONCLUSION
We conclude that topical HT, and to a lesser extent systemic HT, represent effective means of reducing populations of sensory nociceptor and autonomic motor nerves in the postmenopausal vagina. Reductions in sensory innervation density are likely to play a role in the relief from burning, itching, and dyspareunia experienced by many postmenopausal women receiving HT. The effects on the autonomic nervous system appear more complex but are likely to impact the reductions in smooth muscle tone and vaginal dryness that occur in many instances following menopause.
Acknowledgments
Funding for this work was provided by NIH NICHD RO1HD049615 with core support from NICHD P30HD002528.
Footnotes
Disclaimer: The authors have no financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000;86(1):32–32. doi: 10.1046/j.1464-410x.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodgins MB, Spike RC, Mackie RM, MacLean AB. An immunohistochemical study of androgen, oestrogen and progesterone receptors in the vulva and vagina. Br J Obstet Gynaecol. 1998;105(2):216–216. doi: 10.1111/j.1471-0528.1998.tb10056.x. [DOI] [PubMed] [Google Scholar]
- 3.MacLean AB, Nicol LA, Hodgins MB. Immunohistochemical localization of estrogen receptors in the vulva and vagina. J Reprod Med. 1990;35(11):1015. [PubMed] [Google Scholar]
- 4.Perez-Lopez FR, Campo LC, Alos L, Juste G, Ibanez F, Martinez-Hernandez H. Oestrogen and progesterone receptors in the human vagina during the menstrual cycle, pregnancy and postmenopause. Maturitas. 1993;16(2):139. doi: 10.1016/0378-5122(93)90058-p. [DOI] [PubMed] [Google Scholar]
- 5.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273(4):195–195. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 6.Lara LAdS, Useche B, Ferriani RA, et al. The Effects of Hypoestrogenism on the Vaginal Wall: Interference with the Normal Sexual Response. Journal of Sexual Medicine. 2009;6(1):30–30. doi: 10.1111/j.1743-6109.2008.01052.x. [DOI] [PubMed] [Google Scholar]
- 7.Semmens JP, Tsai CC, Semmens EC, Loadholt CB. Effects of estrogen therapy on vaginal physiology during menopause. 1985;66(1):15. [PubMed] [Google Scholar]
- 8.Bachmann G, Rosen RC. Vulvodynia and menopause. Menopause Management. 2006:14–21. [Google Scholar]
- 9.McKay M. Vulvodynia. A multifactorial clinical problem. Archives of Dermatological Research. 1989;125(2):256. doi: 10.1001/archderm.125.2.256. [DOI] [PubMed] [Google Scholar]
- 10.Notelovitz M, Mattox JH. Suppression of vasomotor and vulvovaginal symptoms with continuous oral 17beta-estradiol. 2000;7(5):310. doi: 10.1097/00042192-200007050-00005. [DOI] [PubMed] [Google Scholar]
- 11.Zoubina EV, Smith PG. Distributions of estrogen receptors alpha and beta in sympathetic neurons of female rats: enriched expression by uterine innervation. J Neurobiol. 2002;52(1):14–14. doi: 10.1002/neu.10064. [DOI] [PubMed] [Google Scholar]
- 12.Zoubina EV, Smith PG. Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J Urol. 2003;169(1):382–382. doi: 10.1016/S0022-5347(05)64132-8. [DOI] [PubMed] [Google Scholar]
- 13.Papka RE, Srinivasan B, Miller KE, Hayashi S. Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience. 1997;79(4):1153. doi: 10.1016/s0306-4522(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 14.Blacklock AD, Cauveren JA, Smith PG. Estrogen selectively increases sensory nociceptor innervation of arterioles in the female rat. Brain Res. 2004;1018(1):55–55. doi: 10.1016/j.brainres.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarty A, Blacklock A, Svojanovsky S, Smith PG. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinology. 2008;149(7):3452–3452. doi: 10.1210/en.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blacklock AD, Johnson MS, Krizsan-Agbas D, Smith PG. Estrogen increases sensory nociceptor neuritogenesis in vitro by a direct, nerve growth factor-independent mechanism. Eur J Neurosci. 2005;21(9):2320–2320. doi: 10.1111/j.1460-9568.2005.04075.x. [DOI] [PubMed] [Google Scholar]
- 17.Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. European Journal of Neuroscience. 2003;18(10):2760. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 18.Krizsan-Agbas D, Pedchenko T, Smith PG. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res. 2008;86(14):3086–3086. doi: 10.1002/jnr.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorling DE, Beckman M, Clayton MK, Wang ZY. Modulation of nerve growth factor in peripheral organs by estrogen and progesterone. Neuroscience. 2002;110(1):155. doi: 10.1016/s0306-4522(01)00568-1. [DOI] [PubMed] [Google Scholar]
- 20.Ting AY, Blacklock AD, Smith PG. Estrogen Regulates Vaginal Sensory and Autonomic Nerve Density in the Rat. Biology of Reproduction. 2004;71:1397–404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- 21.Clarke GL, Bhattacherjee A, Tague SE, Hasan W, Smith PG. beta-Adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth. J Neurosci. 2010;30(37):12446–12446. doi: 10.1523/JNEUROSCI.1667-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoubina EV, Fan Q, Smith PG. Variations in uterine innervation during the estrous cycle in rat. Journal of Comparative Neurology. 1998;397:561–71. [PubMed] [Google Scholar]
- 23.Gu J, Polak JM, Su HC, Blank MA, Morrison JF, Bloom SR. Demonstration of paracervical ganglion origin for the vasoactive intestinal peptide-containing nerves of the rat uterus using retrograde tracing techniques combined with immunocytochemistry and denervation procedures. Neurosci Lett. 1984;51(3):377–377. doi: 10.1016/0304-3940(84)90406-3. [DOI] [PubMed] [Google Scholar]
- 24.Hilliges M, Falconer C, Ekman-Ordeberg G, Johansson O. Innervation of the human vaginal mucosa as revealed by PGP 9.5 immunohistochemistry. Acta Anat (Basel) 1995;153(2):119. doi: 10.1159/000147722. [DOI] [PubMed] [Google Scholar]
- 25.Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996;188(Pt 3):633–44. [PMC free article] [PubMed] [Google Scholar]
- 26.Pauls R, Mutema G, Segal J, et al. A prospective study examining the anatomic distribution of nerve density in the human vagina. J Sex Med. 2006;3(6):979–979. doi: 10.1111/j.1743-6109.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 27.Adham N, Schenk EA. Autonomic innervation of the rat vagina, cervix, and uterus and its cyclic variation. American Journal of Obstetrics and Gynecology. 1969;15:508. doi: 10.1016/s0002-9378(16)34239-9. [DOI] [PubMed] [Google Scholar]
- 28.Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14(4):271. doi: 10.1038/sj.ijir.3900886. [DOI] [PubMed] [Google Scholar]
- 29.Haase EB, Buchman J, Tietz AE, Schramm LP. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Research. 1997;288(2):293. doi: 10.1007/s004410050815. [DOI] [PubMed] [Google Scholar]
- 30.Sporrong B, Alm P, Owman C, Sjoberg NO, Thorbert G. Ultrastructural evidence for adrenergic nerve degeneration in the guinea pig uterus during pregnancy. Cell Tissue Research. 1978;195(1):189. doi: 10.1007/BF00233686. [DOI] [PubMed] [Google Scholar]
- 31.Owman C, Alm P, Björklund A, et al. Histochemistry and cell biology of autonomic neurons, SIF cells, and paraneurons. New York; Raven Press: 1980. Extensive sympathetic denervation of the uterus during pregnancy as evidenced by tyrosine hydroxylase determinations in the guinea pig; pp. 313–20. [PubMed] [Google Scholar]
- 32.Brauer MM, Shockley KP, Chavez R, Richeri A, Cowen T, Crutcher KA. The role of NGF in pregnancy-induced degeneration and regeneration of sympathetic nerves in the guinea pig uterus. 2000;79(1):19. doi: 10.1016/s0165-1838(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 33.Zoubina EV, Smith PG. Axonal degeneration and regeneration in rat uterus during the estrous cycle. Autonom Neurosci. 2000;84(3):176–176. doi: 10.1016/S1566-0702(00)00209-5. [DOI] [PubMed] [Google Scholar]
- 34.Zoubina EV, Smith PG. Uterine sympathetic hyperinnervation in the estrogen receptor α knock-out mouse. Neuroscience. 2001;103(1):237–44. doi: 10.1016/s0306-4522(00)00549-2. [DOI] [PubMed] [Google Scholar]
- 35.Blacklock AD, Smith PG. Estrogen increases calcitonin gene-related peptide-immunoreactive sensory innervation of rat mammary gland. J Neurobiol. 2004;59(2):192–192. doi: 10.1002/neu.10310. [DOI] [PubMed] [Google Scholar]
- 36.Smith PG, George M, Bradshaw S. Estrogen promotes sympathetic nerve regeneration in rat proximal urethra. Urology. 2009;73(6):1392–1392. doi: 10.1016/j.urology.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Z, Smith PG. Reprod Sci. 2011. Adaptive Plasticity of Vaginal Innervation in Term Pregnant Rats. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsberg JG. A morphologist's approach to the vagina--age-related changes and estrogen sensitivity. Maturitas. 1995;22 Suppl:S7. doi: 10.1016/0378-5122(95)00957-4. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139(10):4345–4345. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- 40.Munarriz R, Kim SW, Kim NN, Traish A, Goldstein I. A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. 2003;170(2 Pt 2):S40–4. doi: 10.1097/01.ju.0000075352.03144.15. [DOI] [PubMed] [Google Scholar]
- 41.Gunn JA, Franklin KJ. The sympathetic innervation of the vagina. Proceediings of the Royal Society of London Series B. 1922;94:197–203. [Google Scholar]
- 42.Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. 1999;296(2):293. doi: 10.1007/s004410051290. [DOI] [PubMed] [Google Scholar]
- 43.Giuliano F, Rampin O, Allard J. Neurophysiology and pharmacology of female genital sexual response. J Sex Marital Ther. 2002;28(Suppl 1):101. doi: 10.1080/00926230252851230. [DOI] [PubMed] [Google Scholar]
- 44.Semmelink HJ, de Wilde PC, van Houwelingen JC, Vooijs GP. Histomorphometric study of the lower urogenital tract in pre- and post-menopausal women. Cytometry. 1990;11(6):700. doi: 10.1002/cyto.990110607. [DOI] [PubMed] [Google Scholar]
- 45.Berman JR, McCarthy MM, Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998;51(4):650. doi: 10.1016/s0090-4295(97)00683-3. [DOI] [PubMed] [Google Scholar]
- 46.Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. 2003;42(6):491. doi: 10.1046/j.1365-4362.2003.01831.x. [DOI] [PubMed] [Google Scholar]