Abstract
BACKGROUND
Advanced prostate cancer is currently treated with androgen deprivation therapy (ADT). ADT initially results in tumor regression, however, all patients eventually relapse with castration-resistant prostate cancer. New approaches to delay the progression of prostate cancer to castration resistance are in desperate need. This study addresses whether targeting HSP90 regulation of androgen receptor (AR) can inhibit prostate cancer progression to castration resistance.
METHODS
The HSP90 inhibitor 17-AAG was injected intraperitoneally into nude mice bearing LuCaP35 xenograft tumors to determine the effect of HSP90 inhibition on prostate cancer progression to castration resistance and host survival.
RESULTS
Administration of 17-AAG maintained androgen-sensitivity, delayed the progression of LuCaP35 xenograft tumors to castration resistance and prolonged the survival of host. In addition, 17-AAG prevented nuclear localization of endogenous AR in LuCaP35 xenograft tumors in castrated nude mice.
CONCLUSIONS
Targeting Hsp90 or the mechanism by which HSP90 regulates androgen-independent AR nuclear localization and activation may lead to new approaches to prevent and/or treat castration-resistant prostate cancer.
Keywords: Androgen Receptor, HSP90, Castration Resistance, LuCaP35
Introduction
Androgen deprivation therapy (ADT) is the standard treatment for patients with metastatic prostate cancer. Unfortunately, ADT is only palliative and patients will eventually relapse with castration-resistant prostate cancer. The current therapies for castration-resistant prostate cancer, including Docetaxol and Provenge, have limited efficacy and can only extend the life of patients an average of 2–4 months (1,2). New approaches to delay the progression of prostate cancer to castration resistance are desperately needed. Elucidating the molecules involved in the progression of prostate cancer to castration resistance has significant clinical relevance.
Abnormal activation of androgen receptor (AR) is a key mechanism leading to castration-resistance in prostate cancer (3–5). The androgen receptor (AR), a member of the steroid receptor superfamily, is an androgen-dependent transcription factor that controls the expression of androgen-responsive genes (6). Intracellular trafficking represents a key step in the regulation of many transcription factors, including AR. In order to access its target genes, AR must be present in the nucleus. Thus, a key regulatory step in the action of AR is its nuclear localization (7–9). In androgen-sensitive cells, AR resides in the cytoplasm in the absence of ligand. Upon addition of androgens, AR translocates to the nucleus and transactivates target genes. However, in castration-resistant prostate cancer cells, AR remains in the nucleus even in the absence of androgen and transactivates androgen-responsive genes, leading to growth of prostate tumors under androgen-depleted conditions (10,11). Therefore, the development of novel approaches that can block the nuclear localization of AR may be an effective treatment strategy for prostate cancer. A recent study of Zhu et al showed inhibition of androgen-dependent AR nuclear translocation by tubulin-targeting chemotherapeutic agents (12), providing a new mechanism for docetaxel treatment of prostate cancer as well as substantiating the importance of targeting AR nuclear translocation in prostate cancer cells.
Heat shock protein 90 (HSP90) is an abundant chaperone protein involved in the regulation of many key signaling proteins and steroid receptors including AR (13–20). Recent studies showed that HSP90 plays an important role in the regulation of AR nuclear localization and activation in castration-resistant prostate cancer cells (20,21). Another study showed that inhibition of HSP90 by 17-AAG can induce degradation of AR and other important signaling molecules such as HER-2/NEU and inhibition of prostate xenograft tumor growth (16). 17-AAG can inhibit both AR-negative and –positive prostate cancer cells, suggesting that 17-AAG inhibition of tumor growth could bypass AR. Also, inhibition of AR in castration-resistant prostate cancer cells by 17-AAG is not equal to restoration of androgen-responsiveness of the tumor cells. Thus, it will be important to test whether HSP90 inhibition could maintain or prolong the sensitivity of prostate tumors to androgens via preventing androgen-independent nuclear localization and activation of AR under castration conditions.
Here we used a specific HSP90 inhibitor, 17-AAG, to investigate whether HSP90 inhibition could delay the progression of prostate cancer to castration resistance in the LuCaP35 xenograft tumor model. The LuCaP35 xenograft was established from lymph node metastases andexpresses wild-type AR and is sensitive to androgen deprivation (22). LuCaP35 xenograft tumors relapse after initial regression in response to castration in nude mice, providing an excellent model to address the importance of HSP90 in activation of AR under castration conditions and progression of prostate cancer to castration resistance.
MATERIALS AND METHODS
Animal Studies
Four to six week old balb/c nu/nu athymic male mice or SCID mice were purchased from Harlan Labs (Indianapolis, IN). Experiments were carried out under approval through an Institutional Animal Care and Use Committee approved protocol at the University of Pittsburgh. Guidelines for the proper and humane use of animals in research were followed. The mice were maintained one week for recovery from the shipment prior to experimentation. Anesthetized intact mice were implanted subcutaneously with 20–30 mm3 tumor bits from LuCaP35 tumor propagated in vivo as previously described (22). Tumors were measured three times weekly with micro-calipers, and tumor volumes were determined using the formula: (length × width2)/2 (23,24).
Mice were randomized into three groups when tumor volume reached 200mm3: testes intact, castrated with EPL (Egg Phospholipid) vehicle injection, and castrated with 17-AAG injection. EPL and 17-AAG were a generous gift of the NCI (Rockville, MD). 17-AAG or EPL was injected intraperitoneally beginning 10 days post-randomization for 5 days a week for 3 weeks. Blood samples were collected from the saphenous vein the week of castration, one week post-castration, and 5 weeks post-castration to monitor serum PSA levels. Beginning one week post-castration, mice were treated with vehicle alone or 25mg/kg 17-AAG five days per week for three weeks. Tumor volumes were monitored continuously until 150 days post-castration. Mice were sacrificed as indicated or when any one tumor length reached 20mm.
Immunofluorescent Staining
Tumors were harvested and fixed overnight at 4°C in 4% paraformaldehyde. Tissues were washed in PBS and allowed to sit in 30% sucrose overnight at 4°C prior to embedding in TBS Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, N.C.). Embedded tissues were stored at −80°C until sectioning. Tissues were sectioned at 10µm thickness onto charged slides. The slides were allowed to sit at room temperature for 30 minutes and then incubated in ice cold acetone for 5 minutes. Slides were incubated again at room temperature for 10 minutes and stored until imunostaining at −80°C. Before staining, slides were acclimated to room temperature for 10 minutes. They were incubated in 0.5% Triton X-100 in PBS for 10 minutes. Antigen Unmasking Solution (Vector Laboratories, Inc. Burlingame, CA) was used to retrieve antigen by microwaving in solution. Sections were washed 2 times in PBS and endogenous enzyme activity was blocked using 0.3% normal goat serum in PBS for 4 hours. AR antibody N-20 (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted (1:500) in blocking solution at manufacturer’s instructions, applied to sections and incubated overnight at room temperature. The following day sections were washed 2 times in PBS and secondary antibody AlexaFluor conjugated goat anti-rabbit 647(Invitrogen Molecular Probes, Eugene OR) was added at 1:200 in 0.3% normal horse serum. Sections were incubated for 1 hour at room temperature followed by 3 washes with PBS. Vectashield (Vector Laboratories, Inc. Burlingame, CA) containing DAPI nuclear stain was used when viewing tissue sections. Photos were taken at 10 and 40× magnification on a Nikon T2000 microscope with NIS Elements Imaging Software version Br (Nikon, Melville, NY).
Statistics and Data Analysis
Statistics were calculated using Graphpad Software (Graphpad Software, Inc., La Jolla, CA) for Students T test, Fisher’s Exact Test and Kaplan-Meier Test where stated. In this study, we defined statistically significant as p-value less than or equal to 0.05.
RESULTS
LuCaP35 xenograft tumors are androgen-sensitive
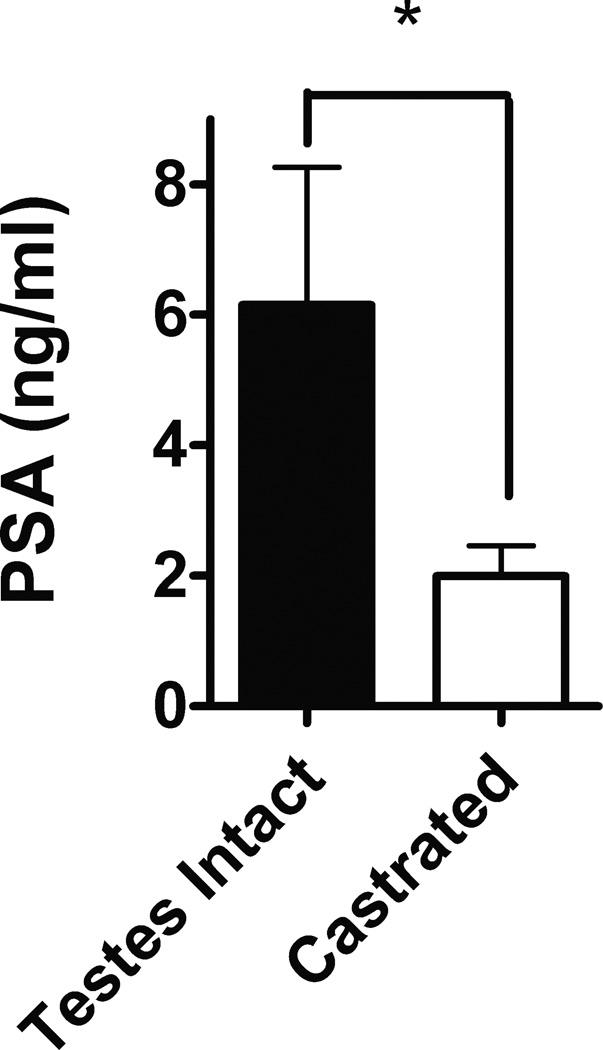
We first evaluated the androgen-responsiveness of LuCaP35 tumors being used in this study. As expected, serum PSA levels were significantly higher in the testes-intact animals compared to the other castrated groups at 2–3 days post-castration or randomization with the castrated animals showing an approximate 3-fold decrease in serum PSA (Fig. 1). These observations show the androgen-responsiveness of LuCaP35, which is in agreement with the previously studies (22).
Fig. 1. Effects of castration on serum PSA of nude mice bearing LuCaP35 xenograft tumors.
LuCaP35 tumor establishment and animal castration were described in Methods section. Mean serum PSA (±SD) were measured 2–3 days post-castration. Numbers of animals for testes intact and castrated were 5 and 32 respectively. *p<0.01
17-AAG inhibits LuCaP35 tumor growth
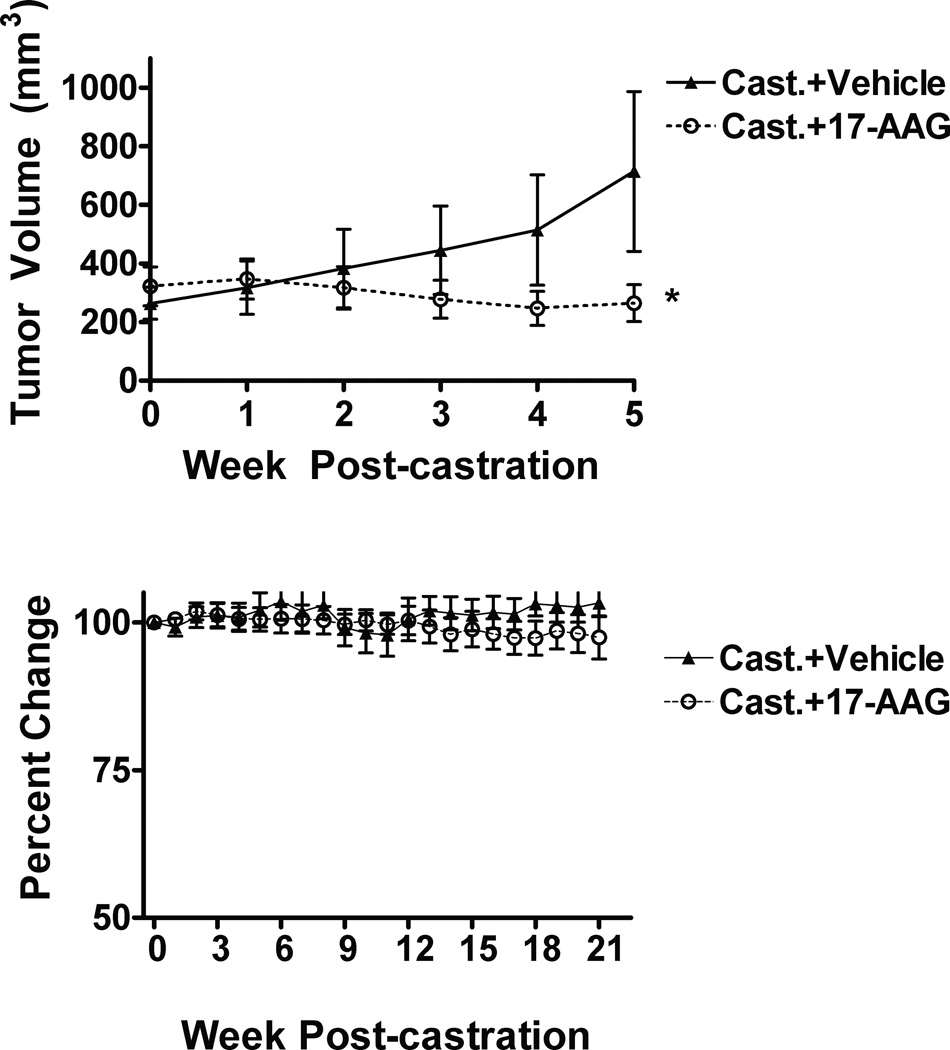
Mice treated with 17-AAG for 5 weeks following castration caused a decrease of less than 30% in mean tumor volume, whereas animals treated with vehicle alone showed a steady increase in volume at 63% increase by week 5 post-castration (Fig. 2a). At the end of the 5-week treatment, the tumor volume in vehicle-treated animals was approximately 3 fold greater than that of the 17-AAG treated mice (p < 0.05). Thus, 17-AAG inhibited the growth of LuCaP35 tumors in castrated mice.
Fig. 2. Effects of 17-AAG on LuCaP35 xenograft tumor growth in castrated mice.
(a) Mean tumor volume in castrated mice treated with vehicle (Cast.+Vehicle) or 17-AAG (Cast.+17-AAG) were measured at indicated time. The tumor volume difference between two groups was statistically significant (*p<0.05). Each group contains 17 animals. (b) Animal body weight was determined at indicated time and was normalized to the initial body weight. The number of animals was 12 for each group. Error bars represent standard deviation.
To determine that differences in tumor volume were not affected by other health issues, we measured body weight throughout the study. Figure 2b shows no significant change in body weight through study end at 21 weeks post-castration.
17-AAG treatment prolonged survival of mice bearing LuCaP35 tumors
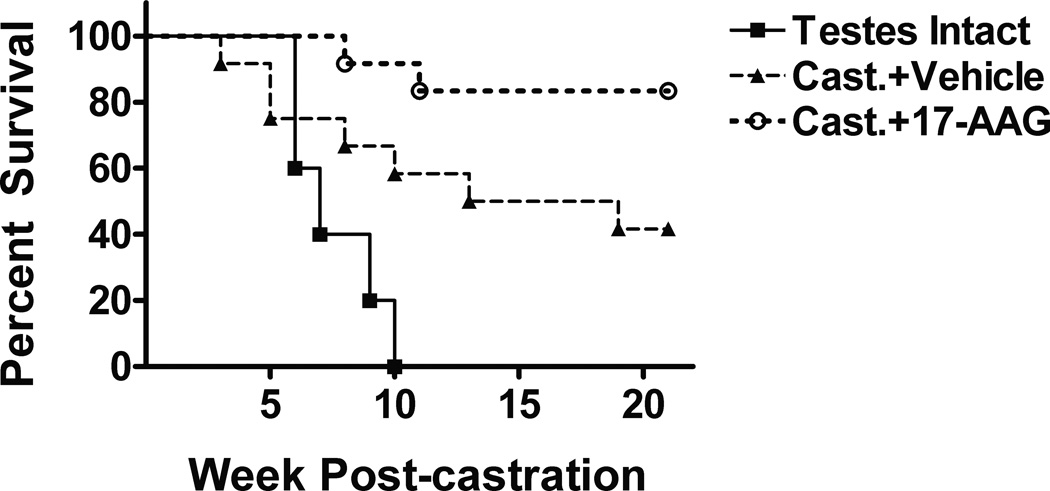
A Kaplan-Meier survival analysis was done on each group out to 21 weeks post-castration. Animals were sacrificed when tumor volume reached 2000mm3 or when any on length on each tumor reached 20mm at which time the mice were euthanized. By week 10 post-castration, 100% (12/12) of the testis-intact animals died or sacrificed due to excessive tumor burden. Of the vehicle-treated castrated group, more than half (7/12) of the mice died or sacrificed within 13 weeks post-castration. As expected, only 2 out of 12 mice in the 17-AAG-treated castrated group reached a tumor burden of 2000 mm3 by 21 weeks post-castration (2/12) (Fig. 3). Thus, 17-AAG treatment prolonged survival of castrated animals bearing LuCaP35 tumors.
Fig. 3. Kaplan-Meier analysis of the effect of 17-AAG on survival of castrated mice bearing LuCaP35 xenograft tumors.
Animals were randomized and castrated when LuCaP35 tumors reach 200 mm3. Numbers of animals in Testis-Intact, Cast.+Vehicle, and Cast.+17-AAG were 5, 12 and 12 respectively. Euthanasia was performed if tumor diameter measured ≥20mm in any direction. The differences in survival were statistically significant according to the Log-rank test (*p<0.05).
17-AAG inhibited progression of LuCaP35 xenograft tumors to castration resistance
To determine if 17-AAG inhibition of LuCaP35 tumor growth in castrated mice is due to the retardation of tumor progression to castration-resistance, we determined the serum PSA levels in mice 1 week after the completion of 17-AAG treatment and in control mice treated with vehicle. By week 5 following castration 35% of animals (6/17) in the vehicle alone treatment groups had progressed to castration-resistance defined by serum PSA measurements greater than 6 ng/ml, however, none of the 17-AAG-treated animals exhibited increased serum PSA (0% or 0/17) (Fig. 4). A Fisher’s Exact log-rank test for these data showed a significant difference between those animals treated with 17-AAG and vehicle alone in progression to a castration-resistant state (*p<0.01). There should be virtually no 17-AAG left in mice by week 5 following castration, because 17-AAG injection was completed by week 4 and the half-life of 17-AAG is only several hours (25). The above observations indicate that transient 17-AAG treatment maintained the sensitivity of AR in LuCaP35 tumors in castrated hosts, delaying their progression to castration-resistance.
Fig. 4. 17-AAG prevention of LuCaP35 tumor progression to castration resistance.
Serum PSA was measure to determine relapse of LuCaP35 tumors 5 weeks after castration, which was 1 week after the completion of 17-AAG or vehicle administration. A PSA value of 6 ng/ml, marked by the dotted line, is considered indicative of tumor relapse. Progression to castration resistance in the vehicle-treated group is statistically different from that in the 17-AAG-treated group according to Fisher’s Exact Test (*p < 0.02). Each group contained 17 animals.
17-AAG prevented nuclear localization of endogenous AR in LuCaP35 xenograft tumors
Previous studies showed 17-AAG inhibition of AR nuclear localization in castration-resistant prostate cancer cells in vitro (21). However, it is not clear whether cytoplasmic localization of AR in androgen-sensitive prostate cancer cells in vivo in castrated mice can be maintained or prolonged after 17-AAG treatment. In order to determine the effects of 17-AAG on AR nuclear localization in LuCaP35 tumors, we performed AR immunostaining of LuCaP35 tumors from testis-intact mice or castrated mice treated with 17-AAG or vehicle. Not surprisingly, AR immunostaining in animals treated with 17-AAG or vehicle alone and previously defined as castration-sensitive and PSA negative was predominantly localized to the cytoplasm of the tumor cells (Fig. 5). The testes-intact animals or those treated with vehicle and previously defined as castration-resistant and PSA positive were found to have a trend toward AR in the nucleus or evenly distributed in the tumor cell (Fig. 5).
Fig. 5. Endogenous AR localization in LuCaP35 xenograft tumors.

Endogenous AR subcellular localization in indicated LuCaP35 xenograft tumors was determined by immunofluorescent staining as described in Methods. Nuclei were stained by Hoechst. Images are the Tumors with serum PSA below 6ng/ml were considered PSA-negative (PSA-), whereas those with serum PSA above 6ng/ml were PSA-positive (PSA+). Tumors from testis-intact control mice (TIC) were included. Isotype control was stained using rabbit IgG in place of primary AR antibody. Magnification of the objectives (10× or 40×) was indicated in the figure.
DISCUSSION
This study provided evidence that 17-AAG can preserve the sensitivity of AR signaling to androgens in LuCaP35 prostate tumors after castration and, as expected, can prolong the survival of animal hosts. Previous studies showed that 17-AAG can inhibit the growth of AR-negative androgen-independent, AR-positive androgen-sensitive, and AR-positive castration-resistant prostate tumors. However, the importance of AR in the inhibition of the AR-positive prostate tumor growth by 17-AAG is not clear and it has not been tested if 17-AAG can delay the progression of androgen-sensitive prostate tumors to castration-resistance. The results presented herein show that 17-AAG can delay the progression of androgen-sensitive LuCaP35 xenograft prostate tumor to castration-resistance and this delay of the progression is accompanied by the lack of androgen-independent AR nuclear localization, a key feature of castration-resistance.
LuCaP35 xenograft tumors provide an excellent model for prostate cancer progression to study the effect of 17-AAG on castration-resistance. LuCaP35 tumors contain wild-type AR and are sensitive to androgen manipulation (22). This is consistent with serum PSA reduction in newly castrated nude mice bearing LuCaP35 tumors (Fig. 1). However, some of the tumors relapsed quickly after castration and about 35% of the tumors became castration-resistant within 5 weeks after castration (Fig. 4). The heterogeneity of LuCaP35 xenograft tumors in their response to castration is consistent with the fact that prostate cancer is highly heterogeneous.
The mechanisms of Hsp90 action in prostate cancer are likely complex since Hsp90 has many client proteins such as Her2/neu, RB, various kinases and AR (16–19,21). 17-AAG can inhibit both AR-negative and –positive cells (16), raising a question on the role of AR in 17-AAG inhibition of AR-positive prostate cancer cells. The results in this study indicate that AR is an important mediator of 17-AAG inhibition prostate cancer progression to castration-resistance, because the sensitivity of AR to androgens was maintained in the presence of 17-AAG after castration (Fig. 4). This finding is consistent with previous finding of AR being a key determinant in castration-resistance (3–5). Hsp90 modulation on AR activity may involve direct and/or indirect interactions between Hsp90 and AR. Elucidating the mechanisms by which Hsp90 modulate castration-resistant AR nuclear localization may lead to additional targets for the treatment and/or prevention of castration-resistant prostate cancer.
A phase II clinical trial of 17-AAG in patients with castration resistant prostate cancer did not show any activity based on PSA response, probably in part due to toxicity that would limit the drug dosage (26). It is possible that new Hsp90 inhibitors with less toxicity and more favorable pharmacokinetics may be developed in the future and they could be used to prevent and/or treat castration-resistant prostate cancer. Previous studies showed that HDAC6 can regulate Hsp90 acetylation (27) and that Hsp90 deacetylation by HDAC6 is required for androgen-independent nuclear localization and activation of AR (28), suggesting that HDAC6 inhibition could also delay the progression of prostate cancer to castration-resistance. De Leon and colleagues recently reported targeting Hsp90 cochaperone FKBP52 in the regulation of AR signaling in prostate cancer cells, which may lead to another approach to inhibit castration-resistance (29).
In summary, the present study argues that Hsp90 is required in the progression of prostate cancer to castration-resistance. Future research aimed at elucidating the mechanism of HSP90 regulation of androgen-independent nuclear localization and activation of AR is warranted. Novel small molecules targeting Hsp90, factors such as HDAC6 that can regulate Hsp90, and/or the mechanisms by which HSP90 regulates androgen-independent nuclear localization and activation of AR may lead to new therapies to treat and/or prevent castration-resistant prostate cancer.
ACKNOWLEDGEMENTS
EPL and 17-AAG were a generous gift of the NCI (Rockville, MD). We would like to thank Laura Pascal for her thorough review of this manuscript. This research was supported in part by NCI R01 CA108675, P50 CA090386 and DOD W81XWH-07-1-0147. This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
REFERENCES
- 1.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PWHC, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New England Journal of Medicine. 2010;(363):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 4.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62(4):1008–1013. [PubMed] [Google Scholar]
- 5.Mellado BCJ, Ribal MJ, Visa L, Gascón P. Molecular biology of androgen-independent prostate cancer: the role of the androgen receptor pathway. Clin Transl Oncol. 2009;11(1):5–10. doi: 10.1007/s12094-009-0304-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Wong C, Sar M, Wilson E. The androgen receptor: an overview. [Review] Recent Progress in Hormone Research. 1994;49:249–274. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 7.Georget V, Lobaccaro JM, Terouanne B, Mangeat P, Nicolas JC, Sultan C. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol Cell Endocrinol. 1997;129(1):17–26. doi: 10.1016/s0303-7207(97)04034-3. [DOI] [PubMed] [Google Scholar]
- 8.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266(1):510–518. [PubMed] [Google Scholar]
- 9.Roy AK, Tyagi RK, Song CS, Lavrovsky Y, Ahn SC, Oh TS, Chatterjee B. Androgen receptor: structural domains and functional dynamics after ligand-receptor interaction. Ann N Y Acad Sci. 2001;949:44–57. doi: 10.1111/j.1749-6632.2001.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 10.Gregory CW, Johnson RT, Jr., Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61(7):2892–2898. [PubMed] [Google Scholar]
- 11.Zhang L, Johnson M, Le KH, Sato M, Ilagan R, Iyer M, Gambhir SS, Wu L, Carey M. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63(15):4552–4560. [PubMed] [Google Scholar]
- 12.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70(20):7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFranco DB, Ramakrishnan C, Tang Y. Molecular chaperones and subcellular trafficking of steroid receptors. J Steroid Biochem Mol Biol. 1998;65(1–6):51–58. doi: 10.1016/s0960-0760(97)00177-5. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 15.Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41(39):11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 16.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8(5):986–993. [PubMed] [Google Scholar]
- 17.Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33(6):341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Münster PNSM, Moasser MM, Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res. 2001;61(7):2945–2952. [PubMed] [Google Scholar]
- 19.Webb CPHC, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, Monks A, Vande Woude GF. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res. 2000;60(2):342–349. [PubMed] [Google Scholar]
- 20.Lamoureux F, Thomas C, Yin MJ, Kuruma H, Fazli L, Gleave ME, Zoubeidi A. A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res. 2011;17(8):2301–2313. doi: 10.1158/1078-0432.CCR-10-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saporita AJ, Ai J, Wang Z. The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. Prostate. 2007;67(5):509–520. doi: 10.1002/pros.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55(4):239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 23.Euhus DMHC, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 24.Tomayko MMRC. Determination of subcutaneous tumor size in athymic (nude) mice, Cancer Chemother. Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 25.Goetz MPTD, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23(6):1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 26.Heath EIHD, Vaishampayan U, Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, Pili R, Carducci MA, Erlichman C, Liu G. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2008;14(23):7940–7946. doi: 10.1158/1078-0432.CCR-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, Wang Z. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009;23(12):1963–1972. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM, Cox MB. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A. 2011;108(29):11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]