Abstract
The intracellular protozoan parasite Trypanosoma cruzi is the causative agent of Chagas' disease, a serious disorder that affects millions of people in Latin America. Despite the development of life-long immunity following infections, the immune system fails to completely clear the parasites, which persist for decades within host tissues. Cardiomyopathy is one of the most serious clinical manifestations of the disease, and a major cause of sudden death in endemic areas. Despite decades of study, there is still debate about the apparent preferential tropism of the parasites for cardiac muscle, and its role in the pathology of the disease. In this review we discuss these issues in the light of recent observations, which indicate that T. cruzi invades host cells by subverting a highly conserved cellular pathway for the repair of plasma membrane lesions. Plasma membrane injury and repair is particularly prevalent in muscle cells, suggesting that the mechanism used by the parasites for cell invasion may be a primary determinant of tissue tropism, intracellular persistence, and Chagas' disease pathology.
Keywords: protozoan, parasite, cell invasion, Chagas' disease
Introduction
Trypanosoma cruzi is an obligate intracellular protozoan and the etiological agent of Chagas'disease, a lifelong debilitating illness that still affects millions of people in Latin America. Although the parasite is capable of promoting a strong and long lasting T-cell mediated immunity (Tarleton, 2007), T. cruzi is not completely eliminated and persists indefinitely through the host's lifespan. Hence, unlike other pathogens that are capable of forming dormant cysts and reemerging, T. cruzi persistence appears to be closely linked to its capacity to replicate inside host cells and consequently evade immune system recognition. Interestingly, despite the parasite's remarkable ability to invade any nucleated host cell type that it encounters, the chronicity of the disease is characterized by the infection of specific tissues, such as cardiac and skeletal muscle. The reason for such tropism is still poorly understood, but recent studies of the unique cell entry mechanism used by T. cruzi have revealed features that may explain the preferential infection of these specialized cells, where the parasites persist within the vertebrate host.
Trypanosoma cruzi: history and life cycle
In 1907 the Brazilian physician Carlos Chagas was asked to join a malaria eradication project in the state of Minas Gerais, Brazil. During his interaction with local workers, he became aware of a large heamatophagous insect known as “barbeiro” that infested rural households and drew blood from sleeping people at night. After examining guts of the insects and finding numerous flagellated protozoans, Chagas was able to prove experimentally that these flagellated forms were transmitted to monkeys following contact with the insect. Interestingly, the trypanosome-like organisms found 30 days later in the peripheral blood of the animals presented a completely different morphology than all the currently known species of the genus Trypanosoma. In honor to his mentor, Oswaldo Cruz, Chagas named the new parasite Trypanosoma cruzi. Due to the prevalence of the insect in rural dwellings Chagas suspected that the parasite could infect humans, and began his search for a possible human case of the disease. In 1909 he encountered Berenice, a two-year-old girl who became the first recorded case of acute T. cruzi infection (Chagas, 1909). Chagas' findings represent a unique achievement in the history of parasitology and medicine, since working practically alone he was able to describe most key aspects of a completely new tropical disease: the insect vector, the pathogen and its different developmental stages, the animal reservoirs, the hosts, clinical manifestations and epidemiology.
T. cruzi has a life cycle that alternates between vertebrate hosts (which comprise a wide range of mammals including humans) and invertebrate hosts (insects that belong to the Reduviidae Family, Triatominae sub-Family). Popularly known as kissing bugs, there are three major genus of epidemiological importance: Panstrongylus, Triatoma, Rhodnius. The parasite displays four morphologically and biochemically distinct developmental stages during its life cycle: epimastigotes and amastigotes, the replicative forms of the parasite, and metacyclic and bloodstream trypomastigotes, the infective non-replicative forms. It is important to note that amastigote forms can also infect cells by an actin-dependent mechanism and maintain an intracellular replicative cycle, as discussed below. T. cruzi is transmitted to humans through contaminated urine and feces excreted by the insect vectors during a blood meal. The metacyclic trypomastigotes present in the excreta penetrate through the site of the bite wound after rubbing/scratching by the hosts, or directly through a mucosal membrane, reaching underlying cells. After attaching to host cells, metacyclic trypomastigotes are internalized by the host cell in a membrane bound compartment known as parasitophorous vacuole, from which they subsequently escape and transform into amastigotes. After nine cycles of binary division in the cytosol, amastigotes differentiate into bloodstream trypomastigotes that are released upon rupture of the host cell membrane (Dvorak & Hyde, 1973). The released bloodstream trypomastigotes infect neighboring cells, or are disseminated through the blood, infecting cells at other locations in the body. The reduviid insects are infected by ingesting trypomastigotes circulating in the bloodstream. In the midgut of the insect, the bloodstream trypomastigotes transform into epimastigotes, the replicative developmental stages of the invertebrate host. At the distal part of the intestine, the parasites transform into metacyclic trypomastigotes, the infective forms that are released along with the insect feces during the blood meal, thus completing the T. cruzi life cycle (Figure 1).
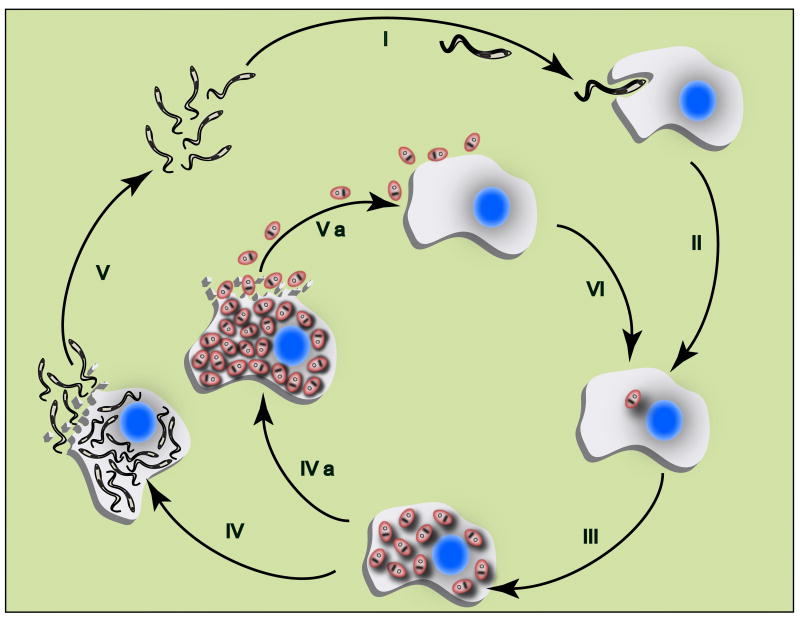
Figure 1. Trypanosoma cruzi replication in the mammalian host.
Flagellated infective forms of the parasite (trypomastigotes) invade mammalian host cells (I) and differentiate into round intracellular parasites (amastigotes) (II), which replicate by binary fission in the cytoplasm (III). Amastigotes can then follow two distinct paths: 1) Parasites differentiate into trypomastigotes, which disrupt the host cell (IV) and invade neighboring cells or enter the bloodstream, disseminating the infection (V); 2) Heavily infected cells may be disrupted prematurely (particularly once immunity is established) releasing amastigotes that can re-invade cells by phagocytosis, thus contributing to parasite persistence (IVa, Va and VI).
Habitat and genetic diversity
Chagas' disease is thought to have originally existed as an exclusively zoonotic disease in the Americas for millions of years. As humans started building homes in remote areas and cutting down forests, wild triatomines gradually adapted to the domestic environment making human infection a part of the parasite life cycle. Nonetheless, T. cruzi DNA has been found in 9,000 year old mummies from northern Chile and Southern Peru (Aufderheide, et al., 2004), indicating that infections with this parasite were already prevalent among humans thousands of years ago. Confined to rural and poor areas of Central and South America, in the 1990's it was estimated that 18-20 million people were infected with T. cruzi. After a series of multinational initiatives organized by the Pan American Health Organization, a significant decrease in the incidence of acute cases of Chagas'disease was effectively achieved, mainly by vector control programs. Thus, in 2006 it was estimated that approximately 10 million people were affected with Chagas' disease (Schofield, et al., 2006) and transmission of T. cruzi by the main domiciliary vector, Triatoma infestans, had been halted in Uruguay, Chile and Brazil, and significantly reduced in Argentina, Paraguay and Bolivia (Dias, et al., 2008). However, Chagas' disease still remains a serious public health problem in several less developed countries of Latin America, where vector control and surveillance programs have not yet been fully implemented.
In nature, over 100 vertebrates species, belonging to different orders, have been reported to be infected with T. cruzi. Two different cycles for parasite transmission can be described based on the vector-vertebrate host niche: the sylvatic cycle, between wild reduviid insects and wild vertebrate hosts, and the domestic cycle, between home-dwelling reduviids and humans and household animals. A connection between the two transmission cycles occurs when infected rats, mice, bats, opossums and reduviids migrate to the human -occupied areas as a result of devastation of forest habitats (Coura & Dias, 2009).
T. cruzi represents a genetically diverse species, comprising of a pool of strains that circulate within its various hosts. Based on analysis of proteins and genetic markers, two major lineages of T. cruzi have been described: T. cruzi I and T. cruzi II (Anonymous, 1999, Zingales, et al., 2009). In addition to the biochemical differences that have been observed between the isolates that comprise these lineages (Miles, et al., 1978, Tibayrenc, 1995, Souto, et al., 1996, Campbell, et al., 2004), important biological differences regarding cell invasion and consequent parasite persistence have been reported, and shall be discussed in greater detail below. T. cruzi I predominates in the sylvatic transmission cycle, and is mainly associated with human disease in all endemic countries north of the Amazon basin (Fernandes, et al., 1998, Zingales, et al., 1998, Yeo, et al., 2005, Rassi, et al., 2010). T cruzi II prevails in the domestic environment in the Southern Cone countries of Latin America (Fernandes, et al., 1998, Zingales, et al., 1998, Rassi, et al., 2010, Risso, et al., 2011), and has been associated with the tissue damage observed during Chagas disease (Di Noia, et al., 2002, Freitas, et al., 2005). Interestingly, the mega syndromes associated with Chagas' disease (marked enlargements of internal organs, particularly the digestive tract) are common in the south of Latin America and rare in the north, implying that T. cruzi II strains may be associated with this disease manisfestation (Yeo, et al., 2005).
Disease pathology and parasite persistence
Chagas' disease presents two clinically distinct phases: the initial or acute phase, which lasts for 4-8 weeks; and the chronic phase that persists for the host's lifespan (Dias, et al., 1956, Rassi, et al., 2010). During the acute phase, a large number of parasites are present in the bloodstream, and all types of nucleated cells in the host are potential targets for infection. Although the parasite is capable of infecting nearly any nucleated cell in vitro, a restricted tissue pool involving cardiac and skeletal muscle (Brener, 1973, Macedo & Pena, 1998), enteric nerves (Tafuri, 1970, da Silveira, et al., 2007, da Silveira, et al., 2008) and adipocytes (Combs, et al., 2005, Ferreira, et al., 2011) seem to be involved in the disease pathology. In particular, an exacerbated infection and inflammatory response is commonly observed in the myocardium (Zhang & Tarleton, 1999). Tissue inflammation is likely to be a consequence of the parasite-induced cytolysis that follows differentiation of amastigotes into bloodstream trypomastigotes and ensures release of parasites from host cells (Bonney & Engman, 2008). However, these acute disease manifestations resolve spontaneously in about 90% of infected individuals, even without trypanocidal drug treatment. After acquired immunity develops, the parasitaemia is reduced to subpatent levels and the number of tissue parasites declines substantially, thereby concluding the acute phase of the infection. About 60-70% of these patients will never develop clinical symptoms, and display what is called the indeterminate form of chronic Chagas disease, characterized by seropositivity for antibodies against T. cruzi, but normal heart, oeasophagus and colon. However, the remaining 30-40% of patients may subsequently develop chronic disease, which is characterized by serious cardiac, digestive or cardio-digestive complications, that usually develop from 10 to 30 years after the initial infection. The serious form of cardiomyopathy associated with the chronic stage of Chagas' disease, leading to heart enlargement, arrhythmias and aneurysms, is still the main cause of death by heart failure in endemic areas of Latin America.
It is not understood why some patients in the indeterminate phase of T. cruzi infection develop chronic disease, while most remain asymptomatic. Although this clearly points to an element of genetic susceptibility of the host, it has been also speculated that the phylogenetic lineage of the infecting parasite strain and/or heterogeneity of the parasite population may play a role in the varying clinical manifestations of Chagas disease (Macedo, et al., 2004). However, specific predictive parameters have not yet been identified, and experimental evidence is still lacking to fully sustain such a correlation. Nonetheless, host resistance, parasitemia and chronicity of infection are determined by the host's innate and acquired immunity, which is known to involve the combined action of a number of immune cell types, including NK cells (Rottenberg, et al., 1988, Gutierrez, et al., 2009), CD8+ (Tarleton, 1995, Bixby & Tarleton, 2008, Gutierrez, et al., 2009) and CD4+ (Nickell, et al., 1987, Gutierrez, et al., 2009) T cells, as well as antibodies produced by B cells (Krettli & Brener, 1976, Gutierrez, et al., 2009). Details of the immune effector mechanisms at play during T. cruzi infections are beyond the scope of this article, and we refer the reader to recent reviews on the subject (Tarleton, 2007, Junqueira, et al., 2010, Kayama & Takeda, 2010).
A notable aspect of the chronic phase of Chagas' disease is the presence of a large number of inflammatory cells in the myocardium, although routine histological techniques often fail to demonstrate the presence of intracellular parasites. Such scarcity of tissue parasites led many investigators to conclude that there was no direct correlation between the presence of T. cruzi and the evolution of Chagas' disease - suggesting, instead, that the pathology could be attributed to the development of autoimmunity after several decades of infection (Kalil & Cunha-Neto, 1996, Kierszenbaum, 1996, Palomino, et al., 2000, Engman & Leon, 2002, Elias, et al., 2003). However, more sensitive detection methods developed in recent years have provided evidence that T. cruzi persists in many tissues including the heart, and that the presence of parasites shows an absolute correlation with areas of tissue inflammation (Bellotti, et al., 1996, Tarleton, et al., 1997, Tarleton & Zhang, 1999, Zhang & Tarleton, 1999). This view is in agreement with the results of clinical trials, showing that treatment of infected patients with trypanocydal drugs can prevent the development of pathological symptoms (Viotti, et al., 1994). Therefore, although a role for parasite-induced autoimmunity cannot be ruled out at present, the prevailing view points towards chagasic cardiomyopathy as being the consequence of a progressive inflammatory process in the heart tissue, caused by parasite persistence.
Cellular invasion: the key to persistence
Despite the development of a robust immune response during the acute phase of infection with T. cruzi, it is well documented that human hosts fail to completely clear the parasites (Tarleton & Zhang, 1999, Zhang & Tarleton, 1999). Intracellularly replicating parasites can be observed in host tissues, particularly in cardiac muscle (Brener, 1973, Macedo & Pena, 1998, Zhang & Tarleton, 1999), and there is a consensus that the in vivo persistence of T. cruzi over many decades is intrinsically related to the parasite's ability to invade a large number of different cell types in their hosts (Burleigh & Andrews, 1995).
Another interesting feature of T. cruzi infections that contributes to parasite persistence is the fact that amastigote forms prematurely released from heavily infected cells are also capable of sustaining the replicative cycle, when taken up by neighboring cells (Figure 1). Interestingly, amastigotes enter host cells by an actin-dependent phagocytic mechanism, which is markedly different from the process by which trypomastigotes invade cells, as discussed below. Amastigotes from the T. cruzi I lineage (G strain) have a remarkable ability of inducing phagocytosis in non-phagocytic cells (Mortara, et al., 2005, Mortara, et al., 2008), while the less infective amastigotes belonging to T. cruzi II (such as the Y strain) are largely phagocytosed by macrophages, and occasionally by other cell types (Ley, et al., 1988, Mortara, et al., 2005). Once inside host cells, amastigotes show the same ability as trypomastigotes to disrupt the parasitophorous membrane, replicate the in the cytosol, and differentiate into infective trypomastigotes. There is also evidence that trypomastigotes can differentiate into amastigotes extracellularly, while circulating in the bloodstream (Andrews, et al., 1987). This adaptation provides an additional mechanism by which T. cruzi can reach an intracellular compartment, evade the immune system, and maintain the infection.
The invasive trypomastigote stages of T. cruzi are remarkably adept at entering a wide range of different cell types. Although considerable advances have been made towards identification of signaling events involved in the early stages of the invasion process (Burleigh & Andrews, 1995, Burleigh & Andrews, 1998, Alves & Colli, 2007, Scharfstein & Lima, 2008, Yoshida & Cortez, 2008) a single candidate for the host cell receptor for T. cruzi has not yet been identified. Furthermore, the infective trypomastigote forms of T. cruzi show marked differences in surface composition, when metacyclic forms (derived from the insect epimastigote stages) are compared to bloodstream forms (Burleigh & Woolsey, 2002, Yoshida, 2006, Alves & Colli, 2007, Yoshida & Cortez, 2008). In addition, trypomastigotes belonging to different lineages have also been reported to express a varying repertoire of molecules that trigger different signaling cascades to achieve cell invasion (Mortara, et al., 2005, Yoshida, 2006, Alves & Mortara, 2009). Intriguingly, despite these differences, both trypomastigote types (metacyclic and bloodstream) induce a very similar process of initial parasitophorous vacuole formation, and early intracellular traffic (Mortara, et al., 2005, Fernandes, et al., 2007, Mortara, et al., 2008). These findings have reinforced the view that host cell invasion by T. cruzi is largely determined by parasite-induced signaling pathways, which enable parasite internalization and vacuole maturation. The striking ability of T. cruzi trypomastigotes to invade essentially any nucleated cell type by a mechanism independent from host actin polymerization, as discussed below, suggests that common cellular features must exist that are capable of responding to stimuli from the parasites, to trigger the internalization process. Below we discuss evidence that has been consistently indicating that lysosomes represent key common organelles of host cells required to promote infection by T. cruzi trypomastigotes.
Trypomastigote invasion and the role of lysosomes
A pioneering video-microscopy study of Dvorak and Hyde (Dvorak & Hyde, 1973) significantly advanced our understanding of the mechanism of host cell invasion by T. cruzi trypomastigotes. The parasites were initially observed invading host cells without any noticeable protrusion of the plasma membrane or formation of spacious phagosomes, leading to the conclusion that T. cruzi exploited a different mechanism of cell invasion than those already described for other intracellular microbes. However, the lack of an obvious intracellular compartment and of plasma membrane disturbance also led to the incorrect assumption that the parasite was capable of directly entering the host cell cytosol, by puncturing the plasma membrane. Subsequent electron microscopy studies showed that this was not the case. In both phagocytic and non-phagocytic cells, a tight association between the trypomastigote and the host cell membrane is maintained during parasite entry, and after invasion the parasite is found inside a tight membrane bound vacuole, prior to escaping into the cytosol (Tanowitz, et al., 1975, Nogueira & Cohn, 1976, de Araujo-Jorge, 1989). Later studies revealed an important aspect of this invasion strategy, that the invasion of non-phagocytic cells by trypomastigotes was independent of host actin polymerization (Schenkman, et al., 1991). This was a surprising finding, given that trypomastigotes are long (10-15 μm) and highly motile parasites that invade cells “backwards” (thorough their posterior end). In the absence of host cell actin polymerization, it was not clear what was the driving force promoting trypomastigote internalization against their direction of movement, and what was the nature of the membrane used for parasitophorous vacuole formation.
In 1992, Tardieux et al. were able to shed light on this issue. Earlier studies had demonstrated fusion of lysosomes with vacuoles containing internalized parasites at later points of infection (Milder & Kloetzel, 1980, Meirelles & De Souza, 1983, de Carvalho & de Souza, 1989). However, this observation was initially interpreted as normal parasitophorous vacuole maturation, following a phagocytic-like entry process. Examining host cells shortly after contact with trypomastigotes, Tardieux observed a striking process of lysosomal recruitment at the parasite invasion site (Tardieux, et al., 1992). Using horseradish peroxidase-loading or antibodies to the lysosomal membrane glycoprotein Lamp1, clusters of lysosomes could be visualized associated with the posterior ends of extracellular parasites, and also with intracellular portions of trypomastigotes in the process of being internalized. Electron microscopy studies confirmed that lysosome recruitment and clustering occurred before internalization was completed, and that newly formed parasitophorous vacuoles already contained lysosomal markers. This finding was fully consistent with the strong inhibition of T. cruzi entry by agents that dissamble microtubules (Tardieux, et al., 1992). Interestingly, Tyler et al. described alterations in host cell microtubule dynamics during T. cruzi invasion, apparently involving de novo microtubule polymerization - a process that may explain the rapid transport of lysosomes to the trypomastigote entry site (Tyler, et al., 2005). Recent studies detected internalized parasites within compartments decorated by LC3, raising the possibility that autophasomes may also act as donor membranes for formation of the T. cruzi parasitophorous vacuole (Romano, et al., 2009).
Subsequent studies found that the parasites triggered Ca2+ signaling in host cells, as NRK fibroblasts loaded with the Ca2+ sensitive dye Fluo3 showed an explosion of Ca2+ oscillations a few seconds after exposure to infective trypomastigotes (Tardieux, et al., 1994). These studies revealed that lysosomal exocytosis was linked to Ca2+ signaling and that Ca2+ transients were a requirement for internalization, since intracellular Ca2+ buffering or depletion significantly inhibited lysosomal recruitment and host cell invasion by T. cruzi. Ca2+- triggered exocytosis of lysosomes at the invasion site provided a plausible answer to the puzzle of the origin of the large amount of membrane required to internalize T. cruzi. However, subsequent studies demonstrated that lysosomal membrane recruitment might not be the only mechanism giving rise to the early T. cruzi-containing parasitophorous vacuole. An alternative entry pathway for T. cruzi mediated by actin-independent plasma membrane invagination was proposed (Woolsey, et al., 2003), based on the use of GFP-labeled reporter molecules that bind to phosphoinositol phosphates at the cytosolic face of the host cell plasma membrane. Markers for the plasma membrane and early endosomes were detected on the parasitophorous vacuole surrounding invading and recently internalized parasites, and the authors also proposed that the two entry pathways, plasma membrane-mediated and lysosome-mediated, could be distinguished by their differential sensitivity to PI-3 kinase inhibitors that block lysosomal fusion. Wortmannin treatment abolished lysosome-mediated entry, whereas a significant fraction of the plasma membrane-mediated pathway remained. These findings suggested that T. cruzi trypomastigotes could also invade non-professional phagocytes by directly invaginating the plasma membrane, in a lysosomal independent fashion (Woolsey, et al., 2003).
However, subsequent studies revealed that when lysosomal fusion was inhibited, internalized parasites failed to be retained inside host cells and escaped to the extracellular environment (Andrade & Andrews, 2004). These findings suggested that cell invasion mediated exclusively by plasma membrane invagination, in the absence of lysosomal fusion with the nascent parasitophorous vacuole, is reversible and does not lead to viable infections (Andrade & Andrews, 2004, Andrade & Andrews, 2005). The very active motility of trypomastigotes seems to play a central role in this process: when lysosomal fusion is blocked, the parasitophorous vacuoles containing parasites fail to associate with microtubules, allowing the parasites to continue to move around the cytosol and eventually reverse the infection process, exiting the cell. Subsequent studies showed that the host cell actin cytoskeleton plays an important role in intracellular retention of the parasites, since cytochalasin D decoupled the process of cell penetration from subsequent fusion with endosomes and lysosomes (Woolsey & Burleigh, 2004).
Recent studies revealed important additional elements of the early steps of T. cruzi invasion, and allowed a functional integration of cell entry pathways previously considered to be independent, such as membrane invagination and lysosome recruitment. As discussed in more detail below, Ca2+-dependent exocytosis of lysosomes results in the extracellular release of acid sphingomyelinase (ASM), a lysosomal enzyme that plays a key role in both plasma membrane repair (Tam, et al., 2010) and in T. cruzi invasion (Fernandes, et al., 2011). Importantly, these studies revealed that the T. cruzi invasion process closely mimics the mechanism by which mammalian cells repair lesions in their plasma membrane (Idone, et al., 2008, Tam, et al., 2010) demonstrating that the parasites subvert a fundamental housekeeping pathway in order to infect their hosts.
Lysosomal exocytosis and plasma membrane repair
The demonstration that T. cruzi promotes Ca2+-dependent lysosomal exocytosis in several cell types (Tardieux, et al., 1992, Tardieux, et al., 1994, Rodriguez, et al., 1995, Burleigh, et al., 1997) suggested that these organelles, once regarded as terminal compartments of the endocytic pathway, could also function as regulated secretory vesicles capable of fusing with the plasma membrane. Further studies confirmed that conventional lysosomes are exocytosed in Ca2+ dependent manner (Rodriguez, et al., 1997) and that synaptotagmin VII (Syt VII), a ubiquitously-expressed member of the synaptotagmin family of Ca2+ sensors, is present in the membrane of lysosomes and is necessary for modulating both lysosomal exocytosis and T.cruzi invasion (Martinez, et al., 2000, Caler, et al., 2001). These findings initially came as a surprise, since regulated secretion was considered to be a property of only certain specialized cell types. However, earlier studies had already detected a large component of Ca2+-regulated exocytosis in a variety of cells previously believed to only be capable of constitutive secretion, such as fibroblasts and epithelial cells (Chavez, et al., 1996, Coorssen, et al., 1996, Ninomiya, et al., 1996), and there is now extensive evidence that lysosomes are Ca2+-responsive organelles in most cell types (Luzio, et al., 2007)
The discovery of a ubiquitous pathway for Ca2+-regulated lysosomal exocytosis raised the question of what could be its physiological role in mammalian cells. Interestingly, several groups had concluded that Ca2+ influx through plasma membrane disruptions triggered vesicular exocytosis at the wound site, an event necessary for plasma membrane resealing (McNeil & Steinhardt, 1997). The precise nature of the intracellular vesicles involved in this process was initially unclear, but conventional lysosomes soon emerged as organelles capable of mediating plasma membrane repair by fusing with the plasma membrane in response to elevations in intracellular Ca2+ (Reddy, et al., 2001, Jaiswal, et al., 2002). While the exact mechanism by which lysosomal exocytosis promoted membrane repair was not yet clear, two models had been proposed: 1) Reduction in plasma membrane tension after Ca2+-triggered exocytosis (Togo, et al., 1999), a process that would preferentially reseal small wounds (McNeil & Steinhardt, 2003); 2) Direct patching of the wound by Ca2+ responsive intracellular vesicles, which would be mostly responsible for the repair of large lesions (McNeil & Steinhardt, 2003). However, these two models failed to explain how stable lesions caused by pore-forming proteins were also promptly removed form the plasma membrane in a Ca2+ dependent manner (Walev, et al., 2001). Neither a patch nor a reduction in membrane tension can explain how transmembrane pores can be effectively removed from the cell surface. An investigation of this issue demonstrated that that Ca2+ influx after cell treatment with the pore forming toxin streptolysin O (SLO) triggers a plasma membrane repair mechanism that is very similar to what is observed after mechanical wounding: Lysosomes are exocytosed and lysosomal enzymes are rapidly released in the extracellular milieu, a process that coincides with cell resealing and prevention of the release of cytosolic proteins (Idone, et al., 2008). As discussed below, host cell exposure to SLO also potentiates T. cruzi invasion (Fernandes, et al., 2011).
Surprisingly, live cell imaging revealed that exocytosis of lysosomes is not the sole membrane trafficking event triggered during plasma membrane repair. A rapid form of endocytosis is observed within seconds of SLO pore formation, but only under conditions that allow plasma membrane repair (in the presence of extracellular Ca2+). Further studies confirmed that this Ca2+ dependent form of endocytosis also occurs in mechanically wounded cells, and suggested that endocytosis promotes resealing by removing lesions from the plasma membrane (Idone, et al., 2008). Subsequent studies demonstrated that Ca2+-dependent lysosomal exocytosis and endocytosis are functionally linked processes that depend on secretion of the lysosomal enzyme ASM (Tam, et al., 2010). ASM cleaves the head group of the abundant plasma membrane lipid sphingomyelin, generating ceramide in the outer leaflet of the plasma membrane (Schissel, et al., 1998). Ceramide-enriched membrane microdomains have the property of coalescing and budding inwards (Holopainen, et al., 2000, Gulbins & Kolesnick, 2003, van Blitterswijk, et al., 2003), suggesting a mechanism for the rapid endocytosis observed during cell wounding and repair (Idone, et al., 2008). In agreement with this view, cells transcriptionally silenced for ASM or cells from Niemann Pick type A patients deficient in ASM still responded to wounding with exocytosis of lysosomes, but injury-dependent endocytosis and plasma membrane repair were severely impaired (Tam, et al., 2010). Moreover, exogenously added recombinant ASM restored endocytosis and plasma membrane resealing in ASM-depleted cells, indicating that ceramide generation by the ASM released from lysosomes can promote lesion internalization (Idone, et al., 2008, Tam, et al., 2010).
The fact that both T. cruzi cell invasion and plasma membrane repair require Syt VII-regulated, Ca2+ dependent exocytosis of lysosomes (Martinez, et al., 2000, Caler, et al., 2001, Reddy, et al., 2001, Chakrabarti, et al., 2003, Chakrabarti, et al., 2005) raised the possibility that the parasites might be taking advantage of this ubiquitous housekeeping mechanism – the resealing of plasma membrane wounds – to gain access into mammalian cells. Extensive evidence in support of this hypothesis was obtained recently (Fernandes et al., 2011), as discussed in detail below. Since skeletal muscle cells and cardiomyocytes are frequently injured in vivo (McNeil & Steinhardt, 2003), it is an intriguing idea that the tissue tropism exhibited by T. cruzi for these cell types may be related to their highly developed capacity for membrane repair.
Subversion of the plasma membrane repair pathway by T. cruzi: a key to tissue tropism?
Following characterization of the molecular mechanism that promotes removal of plasma membrane wounds (Tam, et al., 2010), the following questions began to be considered: Is T. cruzi capable of subverting this highly efficient and widely expressed mechanism of cell defense in order to gain access to essentially any nucleated cell type? Would this provide an explanation for the tissue tropism for cardiac and muscle cells exhibited by the parasites? If so, is the parasite itself capable of wounding the plasma membrane? Recent work allowed us to shed light on these issues (Fernandes, et al., 2011). By performing invasion assays in the presence or absence of Ca2+ (conditions permissive and non-permissive for repair, respectively), we observed that invasion was significantly impaired when Ca2+ was removed from the extracellular medium. In order to investigate whether trypomastigotes were causing plasma membrane damage during the invasion process, we added the membrane impermeable DNA dye propidium iodide (PI) to the invasion assays. While no PI influx was observed during invasion in the presence of Ca2+, PI nuclear staining was readily detected in the absence of Ca2+. These observations suggested that trypomastigotes injure the plasma membrane of host cells, but the wounds are rapidly resealed in a Ca2+ dependent manner. In addition, the efficiency of invasion was significantly enhanced when cells were injured with the pore-forming toxin streptolysin O (SLO) during interaction with trypomastigotes. Altogether, these results provided the first indication that trypomastigotes are capable of injuring mammalian cells during the invasion process. Thus, although earlier studies showed that T. cruzi trypomastigotes trigger signaling events that mobilize Ca2+ from intracellular stores, our recent work shows that Ca2+ influx through membrane lesions also plays a role in activating the cell entry process.
But how does the parasite injure host cells? It was previously shown that T. cruzi secretes a pore forming protein that was proposed to mediate parasite escape from the parasitophorous vacuole (Andrews & Whitlow, 1989, Andrews, et al., 1990). Although this protein has optimum lytic activity in acid pH, it is conceivable that residual activity at the extracellular neutral pH might still be sufficient for pore formation on the plasma membrane of host cells. In addition, live video and scanning electron microscopy analysis of the invasion process revealed that trypomastigotes mechanically deform the plasma membrane during interaction with host cells, suggesting another potential mechanism of injury (Fernandes, et al., 2011). Trypomastigotes attach and invade host cells by their posterior end, where the base of the flagellum is located (Fernandes, et al., 2011). Intriguingly, it is known that trypanosomes move unidirectionaly, with their anterior end oriented forward (Hill, 2003). The nature of the strong attachment that occurs between the posterior end of trypomastigotes and host cell surface, allowing internalization against their direction of movement, is still unclear. Nevertheless, the flagellar motility that propels the parasites away from the cell while still strongly attached may play a role in damaging the host cell plasma membrane.
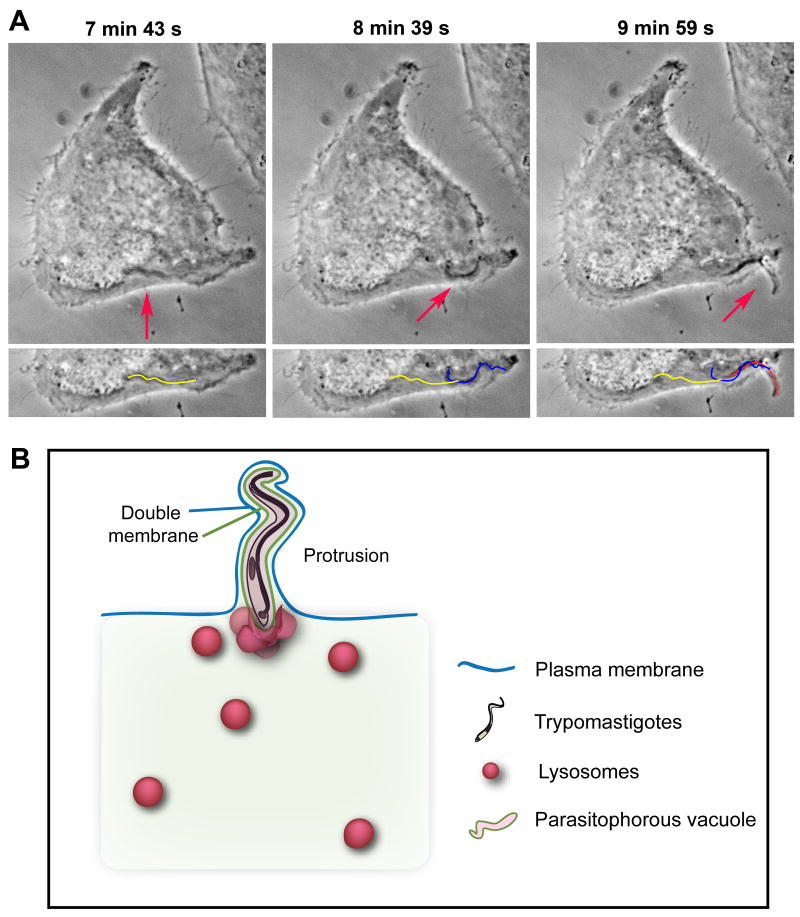
Interestingly, we also observed that recently internalized parasites are highly motile and can protrude from host cells shortly after invasion, stretching the plasma membrane from the inside with its anterior end pointing outwards (Figure 2). Scanning electron micrographs revealed fully internalized trypomastigotes surrounded by a tight membrane layer that was continuous with the plasma membrane, and that stretched outwards for the full length of the parasite (Fernandes, et al., 2011). Given the frequency of such parasite protruding events when recently infected cells are observed by live microscopy, it is tempting to speculate that plasma membrane deformation from the inside, by internalized parasites, may also contribute to further wounding. Rupture of plasma membrane extensions created by this process would allow Ca2+ entry, activating plasma membrane repair and increasing host cell susceptibility to additional invasion events. This phenomenon might explain why T. cruzi infection of mammalian cells does not follow a random Poisson distribution, but rather corresponds more closely to a negative binomial prediction (Hyde & Dvorak, 1973).
Figure 2. Internalized trypomastigotes protrude from host cells.
(A) Time Lapse live imaging (phase-contrast) of a trypomastigote moving inside a HeLa cell, and forming a plasma membrane protrusion (arrows). The yellow line represents the parasite's position at 7 min 43 s, the blue line at 8 min 39 s, and red line at 9 min 59 s (bottom panel). (B) Schematic model for protrusion event. Protruding trypomastigotes are surrounded by two distinct membranes: parasitophorous vacuole (green line), and plasma membrane (blue line). The parasites protrude in the direction of their flagellar movement (anterior end pointing outward) and stretch the overlying plasma membrane, while lysosomes progressively fuse with the vacuole and ultimately anchor trypomastigotes inside the cell.
Considering that the parasite's flagellar movement propels them away, not towards the host cell membrane, the previously described invasion pathway mediated by plasma membrane invagination (Woolsey, et al., 2003) had remained puzzling. The recently established link between T. cruzi invasion and the plasma membrane repair pathway has shed light on this issue. As discussed above, we now know that lysosomal exocytosis, after plasma membrane injury, triggers a rapid form of endocytosis (Idone, et al., 2008) that is dependent on the secretion of lysosomal enzyme ASM (Tam, et al., 2010). Thus, we hypothesized that ASM-dependent, ceramide-enriched microdomains on the plasma membrane played a central role in the early steps of T. cruzi entry into host cells.
Testing this hypothesis, we found that inhibition of ASM activity with desipramine (Hurwitz, et al., 1994, Kolzer, et al., 2004) or RNAi-mediated silencing of ASM expression significantly reduced trypomastigote invasion. Moreover, extracellular addition of purified sphingomyelinase restored invasion in ASM-depleted cells, directly linking sphingomyelin hydrolysis and ceramide generation to the T. cruzi entry process. Interestingly, we found that interaction with T. cruzi trypomastigotes stimulated in host cells the formation of vesicles positive for the endosomal marker EEA1, similar to what is observed in wounded cells (Idone, et al., 2008) or in cells exposed to purified sphingomyelinase (Zha, et al., 1998, Tam, et al., 2010). Furthermore, the increased invasion of cells treated with purified sphingomyelinase correlated with an increase in internalized parasites within vacuoles containing EEA1 (Fernandes, et al., 2011), consistent with an earlier report (Woolsey, et al., 2003). Taken together, these results suggested that by wounding cells and triggering exocytosis of the lysosomal enzyme ASM, trypomastigotes were able to take advantage of the membrane invagination properties of newly-generated ceramide to initiate host cell entry.
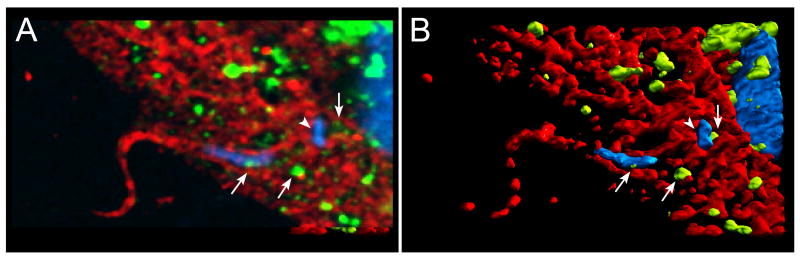
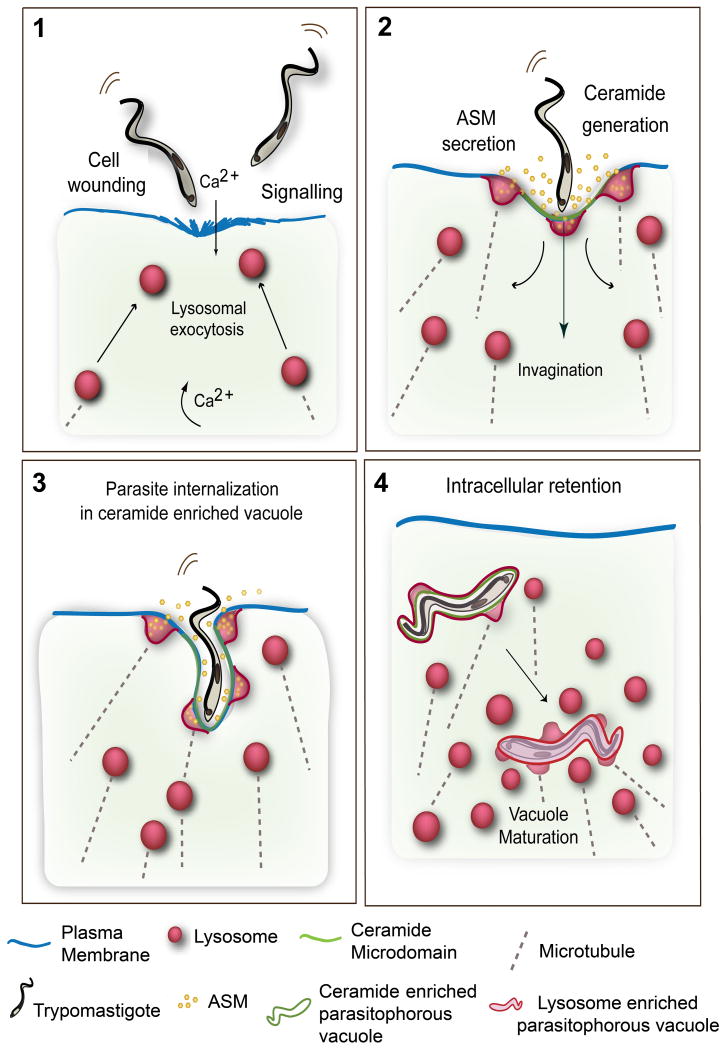
If the above hypothesis was correct, it should be possible to visualize recently Internalized parasites within ceramide-enriched parasitophorous vacuoles. Indeed, over 60% of recently internalized trypomastigotes in HeLa cells were surrounded by host membranes reactive with a monoclonal antibody against ceramide (Figure 3). Lysosomal markers were sparsely detected initially, and become more abundant in the newly formed parasitophorous vacuole at later time points (Fernandes, et al., 2011). These findings agree with the view that ceramide plays an important role in the plasma membrane deformation process required to allow large trypomastigotes to initially enter host cells. The subsequent addition of lysosomal membranes to the parasitophorous vacuole, followed by binding to lysosomes, would then provide the necessary force to retain the parasites intracellularly (Andrade & Andrews, 2004). These results significantly expand the previously reported role of lysosomal exocytosis in T. cruzi invasion of host cells (Reddy, et al., 2001), by showing that a lysosomal enzyme released extracellularly can promote parasite internalization. Our current model (Figure 4) proposes that T. cruzi trypomastigotes induce elevation in intracellular Ca2+ through signaling interactions or transient plasma membrane wounding, which trigger exocytosis of lysosomes and localized ASM release. Sphingomyelin hydrolysis by ASM generates ceramide microdomains and plasma membrane invagination, promoting trypomastigote entry within ceramide-enriched parasitophorous vacuoles. The newly formed parasitophorous vacuoles gradually fuse with lysosomes, which ultimately anchor the parasites inside the host cell.
Figure 3. Parasitophorous Vacuoles of recently internalized trypomastigotes are enriched in ceramide.
(A) Confocal image of an infected HeLa cell (15 min of infection) incubated with anti-ceramide (red) and anti-Lamp1 (green) antibodies. A protruding trypomastigote outlined with ceramide patches is seen on the left, while Lamp-1 enriched lysosomes – the source of acid sphingomyelinase - are observed closely associated with the parasitophorous vacuole (arrows). The DAPI-stained parasite kinetoplast (arrowhead) and the host cell and parasite nuclei are shown in blue. (B) 3D reconstruction of (A) highlighting the ceramide-enriched microdomains and the lysosomes.
Figure 4. Model for T. cruzi cell invasion mediated by wounding and plasma membrane repair.
(1) Trypomastigotes wound mammalian cells, causing influx of extracellular Ca2+ and exocytosis of lysosomes. Signaling events also generate cytosolic Ca2+ transients. (2) Extracellular release of lysosomal ASM generates ceramide on the outer leaflet of the plasma membrane. (3) Ceramide-enriched plasma membrane microdomains facilitate trypomastigote internalization, while lysosomes continue to fuse with the nascent parasitophorous vacuoles, delivering ASM and generating additional ceramide. Lysosomal membrane anchoring to microtubules provides the force for pulling the parasites into the cells. (4) Recently internalized trypomastigotes residing in ceramide/Lamp1-enriched parasitophorous vacuoles fuse with additional lysosomes leading to parasite retention inside the cell.
In addition to representing a major strategy for evading the host immune system, the ability of T. cruzi trypomastigotes to subvert a highly conserved pathway for the repair of injured membranes may explain the observed in vivo tropism of T. cruzi for muscle cells (Brener, 1973, Macedo & Pena, 1998, Zhang & Tarleton, 1999). Considering the frequency by which the sarcolemma of muscle cells is wounded under physiological conditions (McNeil & Steinhardt, 2003), it is tempting to speculate that the tropism of T. cruzi for cardiomyocytes and skeletal miscle is related to the presence of highly developed plasma membrane repair pathways in these cells (Bansal & Campbell, 2004, Han, et al., 2007).
Concluding Remarks
T. cruzi is remarkably well adapted for persistence with the host. A very large number of wild animals carry the parasites, ensuring that eradication from nature is not likely to be ever achieved. However, effective control of human infections is feasible, through measures that limit contact of the insect vectors with humans. As these measures are gradually implemented in the endemic countries of Latin America, public health interventions will become more focused on the treatment of the very large population of patients suffering from the serious pathology associated with the chronic phase of the disease. Although a large fraction of infected individuals remain asymptomatic, live parasites can be recovered from the circulating blood even several decades after the original infection. The intracellular niche occupied by T. cruzi in vertebrates and the remarkable plasticity of its infective developmental forms, alternating between highly motile trypomastigotes and intracellularly-replicating amastigotes, is likely to play a central role in ensuring parasite survival, despite the presence of immunity. Recent findings revealed a very detailed picture of the molecular events involved in host cell invasion by T. cruzi, providing new insights into the remarkable ability of these parasites to infect most nucleated cell types. Hopefully this knowledge will aid in the development of new and less toxic therapeutic agents, which are urgently needed to treat the infected population. However, this goal remains very challenging given the essential role of the plasma membrane repair pathway that is being subverted by the parasites for invasion.
Acknowledgments
We thank Drs. D.C. Miguel (University of Maryland) and R.A. Mortara (UNIFESP, Brazil) for critical reading of the manuscript, and A. Beaven and the Department of Cell Biology and Molecular Genetics Imaging Core for assistance with confocal microscopy. Work on this project was supported by NIH grant R37 AI34867 to N.W.A.
References
- Alves MJ, Colli W. Trypanosoma cruzi: adhesion to the host cell and intracellular survival. IUBMB Life. 2007;59:274–279. doi: 10.1080/15216540701200084. [DOI] [PubMed] [Google Scholar]
- Alves MJ, Mortara RA. A century of research: what have we learned about the interaction of Trypanosoma cruzi with host cells? Mem I Oswaldo Cruz. 2009;104 1:76–88. doi: 10.1590/s0074-02762009000900013. [DOI] [PubMed] [Google Scholar]
- Andrade LO, Andrews NW. Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells. J Exp Med. 2004;200:1135–1143. doi: 10.1084/jem.20041408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- Andrews NW, Whitlow MB. Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol Biochem Parasitol. 1989;33:249–256. doi: 10.1016/0166-6851(89)90086-8. [DOI] [PubMed] [Google Scholar]
- Andrews NW, Hong KS, Robbins ES, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Andrews NW, Abrams CK, Slatin SL, Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990;61:1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- Anonymous. Memórias do Instituto Oswaldo Cruz. Reccomendations from a satellite meeting. Mem I Oswaldo Cruz. 1999;94:429–432. doi: 10.1590/s0074-02761999000700085. [DOI] [PubMed] [Google Scholar]
- Aufderheide AC, Salo W, Madden M, et al. A 9,000-year record of Chagas' disease. P Natl Acad Sci USA. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Bellotti G, Bocchi EA, de Moraes AV, et al. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas' heart disease. Am Heart J. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–2650. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8:510–518. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Burleigh BA, Andrews NW. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu Rev Microbiol. 1995;49:175–200. doi: 10.1146/annurev.mi.49.100195.001135. [DOI] [PubMed] [Google Scholar]
- Burleigh BA, Andrews NW. Signaling and host cell invasion by Trypanosoma cruzi. Curr Opin Microbiol. 1998;1:461–465. doi: 10.1016/s1369-5274(98)80066-0. [DOI] [PubMed] [Google Scholar]
- Burleigh BA, Woolsey AM. Cell signalling and Trypanosoma cruzi invasion. Cell Microbiol. 2002;4:701–711. doi: 10.1046/j.1462-5822.2002.00226.x. [DOI] [PubMed] [Google Scholar]
- Burleigh BA, Caler EV, Webster P, Andrews NW. A cytosolic serine endopeptidase from Trypanosoma cruzi is required for the generation of Ca2+ signaling in mammalian cells. J Cell Biol. 1997;136:609–620. doi: 10.1083/jcb.136.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caler EV, Chakrabarti S, Fowler KT, Rao S, Andrews NW. The Exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J Exp Med. 2001;193:1097–1104. doi: 10.1084/jem.193.9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DA, Westenberger SJ, Sturm NR. The determinants of Chagas disease: connecting parasite and host genetics. Curr Mol Med. 2004;4:549–562. doi: 10.2174/1566524043360249. [DOI] [PubMed] [Google Scholar]
- Chagas C. Nova Tripanosomiaze Humana: Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n., agente etiológico de uma nova entidade mórbida do homem. Mem I Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Chakrabarti S, Andrade LO, Andrews NW. Trypanosoma cruzi invades synaptotagmin VII-deficient cells by a PI-3 kinase independent pathway. Mol Biochem Parasitol. 2005;141:125–128. doi: 10.1016/j.molbiopara.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez RA, Miller SG, Moore HP. A biosynthetic regulated secretory pathway in constitutive secretory cells. J Cell Biol. 1996;133:1177–1191. doi: 10.1083/jcb.133.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Nagajyothi, Mukherjee S, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem I Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- da Silveira AB, Lemos EM, Adad SJ, Correa-Oliveira R, Furness JB, D'Avila Reis D. Megacolon in Chagas disease: a study of inflammatory cells, enteric nerves, and glial cells. Human Pathol. 2007;38:1256–1264. doi: 10.1016/j.humpath.2007.01.020. [DOI] [PubMed] [Google Scholar]
- da Silveira AB, Freitas MA, de Oliveira EC, et al. Neuronal plasticity of the enteric nervous system is correlated with chagasic megacolon development. Parasitology. 2008;135:1337–1342. doi: 10.1017/S0031182008004770. [DOI] [PubMed] [Google Scholar]
- de Araujo-Jorge TC. The biology of Trypanosoma cruzi-macrophage interaction. Mem I Oswaldo Cruz. 1989;84:441–462. doi: 10.1590/s0074-02761989000400001. [DOI] [PubMed] [Google Scholar]
- de Carvalho TM, de Souza W. Early events related with the behaviour of Trypanosoma cruzi within an endocytic vacuole in mouse peritoneal macrophages. Cell Struct Funct. 1989;14:383–392. doi: 10.1247/csf.14.383. [DOI] [PubMed] [Google Scholar]
- Di Noia JM, Buscaglia CA, De Marchi CR, Almeida IC, Frasch AC. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas' disease is due to a single parasite lineage. J Exp Med. 2002;195:401–413. doi: 10.1084/jem.20011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias E, Laranja FS, Miranda A, Nobrega G. Chagas' disease; a clinical, epidemiologic, and pathologic study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- Dias JC, Prata A, Correia D. Problems and perspectives for Chagas disease control: in search of a realistic analysis. Rev Soc Bras Med Trop. 2008;41:193–196. doi: 10.1590/s0037-86822008000200012. [DOI] [PubMed] [Google Scholar]
- Dvorak JA, Hyde TP. Trypanosoma cruzi: interaction with vertebrate cells in vitro. 1. Individual interactions at the cellular and subcellular levels. Exp Parasitol. 1973;34:268–283. doi: 10.1016/0014-4894(73)90087-8. [DOI] [PubMed] [Google Scholar]
- Elias FE, Vigliano CA, Laguens RP, Levin MJ, Berek C. Analysis of the presence of Trypanosoma cruzi in the heart tissue of three patients with chronic Chagas' heart disease. Am J Trop Med Hyg. 2003;68:242–247. [PubMed] [Google Scholar]
- Engman DM, Leon JS. Pathogenesis of Chagas heart disease: role of autoimmunity. Acta Tropica. 2002;81:123–132. doi: 10.1016/s0001-706x(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Fernandes MC, L'Abbate C, Kindro Andreoli W, Mortara RA. Trypanosoma cruzi cell invasion and traffic: influence of Coxiella burnetii and pH in a comparative study between distinct infective forms. Microb Pathogenesis. 2007;43:22–36. doi: 10.1016/j.micpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Fernandes MC, Cortez M, Flannery AR, Tam C, Mortara RA, Andrews NW. Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. J Exp Med. 2011;208:909–921. doi: 10.1084/jem.20102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes O, Souto RP, Castro JA, et al. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. Am J Trop Med Hyg. 1998;58:807–811. doi: 10.4269/ajtmh.1998.58.807. [DOI] [PubMed] [Google Scholar]
- Ferreira AV, Segatto M, Menezes Z, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas JM, Lages-Silva E, Crema E, Pena SD, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35:411–417. doi: 10.1016/j.ijpara.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite immunol. 2009;31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 2007;117:1805–1813. doi: 10.1172/JCI30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL. Biology and mechanism of trypanosome cell motility. Eukaryot Cell. 2003;2:200–208. doi: 10.1128/EC.2.2.200-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JM, Angelova MI, Kinnunen PK. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys J. 2000;78:830–838. doi: 10.1016/S0006-3495(00)76640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol Chem Hoppe-Seyler. 1994;375:447–450. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- Hyde TP, Dvorak JA. Trypanosoma cruzi: interaction with vertebrate cells in vitro. 2. Quantitative analysis of the penetration phase. Exp Parasitol. 1973;34:284–294. doi: 10.1016/0014-4894(73)90088-x. [DOI] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, Rodrigues MM, Gazzinelli RT. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev Mol Med. 2010;12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- Kalil J, Cunha-Neto E. Autoimmunity in chagas disease cardiomyopathy: Fulfilling the criteria at last? Parasitol Today. 1996;12:396–399. doi: 10.1016/0169-4758(96)10058-2. [DOI] [PubMed] [Google Scholar]
- Kayama H, Takeda K. The innate immune response to Trypanosoma cruzi infection. Microbes Infect. 2010;12:511–517. doi: 10.1016/j.micinf.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Chronic chagasic tissue lesions in the absence of Trypanosoma cruzi: a proposed mechanism. Parasitol Today. 1996;12:414–415. doi: 10.1016/0169-4758(96)20050-x. [DOI] [PubMed] [Google Scholar]
- Kolzer M, Ferlinz K, Bartelsen O, Hoops SL, Lang F, Sandhoff K. Functional characterization of the postulated intramolecular sphingolipid activator protein domain of human acid sphingomyelinase. Biol Chem. 2004;385:1193–1195. doi: 10.1515/BC.2004.154. [DOI] [PubMed] [Google Scholar]
- Krettli AU, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–760. [PubMed] [Google Scholar]
- Ley V, Andrews NW, Robbins ES, Nussenzweig V. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J Exp Med. 1988;168:649–659. doi: 10.1084/jem.168.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Macedo AM, Pena SD. Genetic Variability of Trypanosoma cruzi: Implications for the Pathogenesis of Chagas Disease. Parasitol Today. 1998;14:119–124. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem I Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- Meirelles MN, De Souza W. Interaction of lysosomes with endocytic vacuoles in macrophages simultaneously infected with Trypanosoma cruzi and Toxoplasma gondii. J Submicr Cytol. 1983;15:889–896. [PubMed] [Google Scholar]
- Milder R, Kloetzel J. The development of Trypanosoma cruzi in macrophages in vitro. Interaction with lysosomes and host cell fate. Parasitology. 1980;80:139–145. doi: 10.1017/s0031182000000597. [DOI] [PubMed] [Google Scholar]
- Miles MA, Souza A, Povoa M, Shaw JJ, Lainson R, Toye PJ. Isozymic heterogeneity of Trypanosoma cruzi in the first autochthonous patients with Chagas' disease in Amazonian Brazil. Nature. 1978;272:819–821. doi: 10.1038/272819a0. [DOI] [PubMed] [Google Scholar]
- Mortara RA, Andreoli WK, Fernandes MC, da Silva CV, Fernandes AB, L'Abbate C, da Silva S. Host cell actin remodeling in response to Trypanosoma cruzi: trypomastigote versus amastigote entry. Subcell Biochem. 2008;47:101–109. doi: 10.1007/978-0-387-78267-6_8. [DOI] [PubMed] [Google Scholar]
- Mortara RA, Andreoli WK, Taniwaki NN, et al. Mammalian cell invasion and intracellular trafficking by Trypanosoma cruzi infective forms. An Acad Bras Cienc. 2005;77:77–94. doi: 10.1590/s0001-37652005000100006. [DOI] [PubMed] [Google Scholar]
- Nickell SP, Gebremichael A, Hoff R, Boyer MH. Isolation and functional characterization of murine T cell lines and clones specific for the protozoan parasite Trypanosoma cruzi. J Immunol. 1987;138:914–921. [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J Biol Chem. 1996;271:17751–17754. doi: 10.1074/jbc.271.30.17751. [DOI] [PubMed] [Google Scholar]
- Nogueira N, Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976;143:1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino SA, Aiello VD, Higuchi ML. Systematic mapping of hearts from chronic chagasic patients: the association between the occurrence of histopathological lesions and Trypanosoma cruzi antigens. Ann Trop Med Parasit. 2000;94:571–579. doi: 10.1080/00034983.2000.11813580. [DOI] [PubMed] [Google Scholar]
- Rassi A, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Risso MG, Sartor PA, Burgos JM, et al. Immunological identification of Trypanosoma cruzi lineages in human infection along the endemic area. Am J Trop Med Hyg. 2011;84:78–84. doi: 10.4269/ajtmh.2011.10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Rioult MG, Ora A, Andrews NW. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano PS, Arboit MA, Vazquez CL, Colombo MI. The autophagic pathway is a key component in the lysosomal dependent entry of Trypanosoma cruzi into the host cell. Autophagy. 2009;5:6–18. doi: 10.4161/auto.5.1.7160. [DOI] [PubMed] [Google Scholar]
- Rottenberg M, Cardoni RL, Andersson R, Segura EL, Orn A. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand J Immunol. 1988;28:573–582. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Scharfstein J, Lima AP. Roles of naturally occurring protease inhibitors in the modulation of host cell signaling and cellular invasion by Trypanosoma cruzi. Subcell Biochem. 2008;47:140–154. doi: 10.1007/978-0-387-78267-6_11. [DOI] [PubMed] [Google Scholar]
- Schenkman S, Robbins ES, Nussenzweig V. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect Immun. 1991;59:645–654. doi: 10.1128/iai.59.2.645-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schissel SL, Jiang X, Tweedie-Hardman J, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Tafuri WL. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas' disease. Light and electron microscope studies. Am J Trop Med Hyg. 1970;19:405–417. doi: 10.4269/ajtmh.1970.19.405. [DOI] [PubMed] [Google Scholar]
- Tam C, Idone V, Devlin C, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189:1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz H, Wittner M, Kress Y, Bloom B. Studies of in vitro infection by Trypanosoma cruzi. I. Ultrastructural studies on the invasion of macrophages and L-cells. Am J Trop Med Hyg. 1975;24:25–33. doi: 10.4269/ajtmh.1975.24.25. [DOI] [PubMed] [Google Scholar]
- Tardieux I, Nathanson MH, Andrews NW. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J Exp Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Tarleton RL. The role of T cells in Trypanosoma cruzi infections. Parasitol Today. 1995;11:7–9. doi: 10.1016/0169-4758(95)80095-6. [DOI] [PubMed] [Google Scholar]
- Tarleton RL. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Tarleton RL, Zhang L. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol Today. 1999;15:94–99. doi: 10.1016/s0169-4758(99)01398-8. [DOI] [PubMed] [Google Scholar]
- Tarleton RL, Zhang L, Downs MO. “Autoimmune rejection” of neonatal heart transplants in experimental Chagas disease is a parasite-specific response to infected host tissue. P Natl Acad Sci USA. 1997;94:3932–3937. doi: 10.1073/pnas.94.8.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibayrenc M. Population genetics of parasitic protozoa and other microorganisms. Adv Parasit. 1995;36:47–115. doi: 10.1016/s0065-308x(08)60490-x. [DOI] [PubMed] [Google Scholar]
- Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112(Pt 5):719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Luxton GW, Applewhite DA, Murphy SC, Engman DM. Responsive microtubule dynamics promote cell invasion by Trypanosoma cruzi. Cell Microbiol. 2005;7:1579–1591. doi: 10.1111/j.1462-5822.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas' disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. P Natl Acad Sci USA. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey AM, Burleigh BA. Host cell actin polymerization is required for cellular retention of Trypanosoma cruzi and early association with endosomal/lysosomal compartments. Cell Microbiol. 2004;6:829–838. doi: 10.1111/j.1462-5822.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- Woolsey AM, Sunwoo L, Petersen CA, Brachmann SM, Cantley LC, Burleigh BA. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J Cell Sci. 2003;116:3611–3622. doi: 10.1242/jcs.00666. [DOI] [PubMed] [Google Scholar]
- Yeo M, Acosta N, Llewellyn M, et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Yoshida N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. An Acad Bras Cienc. 2006;78:87–111. doi: 10.1590/s0001-37652006000100010. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Cortez M. Trypanosoma cruzi: parasite and host cell signaling during the invasion process. Subcell Biochem. 2008;47:82–91. doi: 10.1007/978-0-387-78267-6_6. [DOI] [PubMed] [Google Scholar]
- Zha X, Pierini LM, Leopold PL, Skiba PJ, Tabas I, Maxfield FR. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J Infect Dis. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- Zingales B, Souto RP, Mangia RH, et al. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int J Parasitol. 1998;28:105–112. doi: 10.1016/s0020-7519(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MR, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem I Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]