Abstract
Genetic alterations and epigenetic dysregulation in cancer cells create a vast array of neoepitopes potentially recognizable by the immune system. Immune checkpoint blockade has the capacity to enhance and sustain endogenous immunity against non-mutated tumor-associated antigens as well as uniquely mutant antigens, establishing durable tumor control. Recent evidence from preclinical models highlights the pivotal role of the Programmed Death-1 (PD-1) T cell co-receptor and its ligands, B7-H1/PD-L1 and B7-DC/PD-L2, in maintaining an immunosuppressive tumor microenvironment. Encouraging early clinical results using blocking agents against components of the PD-1 pathway have validated its importance as a target for cancer immunotherapy.
INTRODUCTION
Ever since it became clear that all cancer cells express tumor-specific and tumor-selective antigens derived from genetic alterations and epigenetic dysregulation, the immunology community has embraced the potential for immune-based therapies to induce targeted anti-tumor responses. However, skepticism about the clinical value of immunotherapies designed to specifically enhance T cell-mediated immunity against preselected commonly expressed tumor antigens, including cancer vaccines and adoptive T cell transfer strategies, has escalated [1]. A game changer for cancer immunotherapy has emerged in the form of monoclonal antibodies (mAbs) that block inhibitory receptors on immune effector cells or their ligands on tumor cells and antigen presenting cells (APCs) – so-called “immune checkpoints”. Immune checkpoint blockade has the potential to enhance and sustain endogenous immunity against non-mutated as well as uniquely mutant antigens, establishing durable tumor control. Recent evidence highlights the pivotal role of the Programmed Death-1 (PD-1) T cell co-receptor and its ligands B7-H1/PD-L1 and B7-DC/PD-L2 in maintaining an immunosuppressive tumor microenvironment. Encouraging early results from clinical blockade of this pathway have validated its potential as a target for cancer immunotherapy.
RECEPTOR/LIGAND BIOLOGY
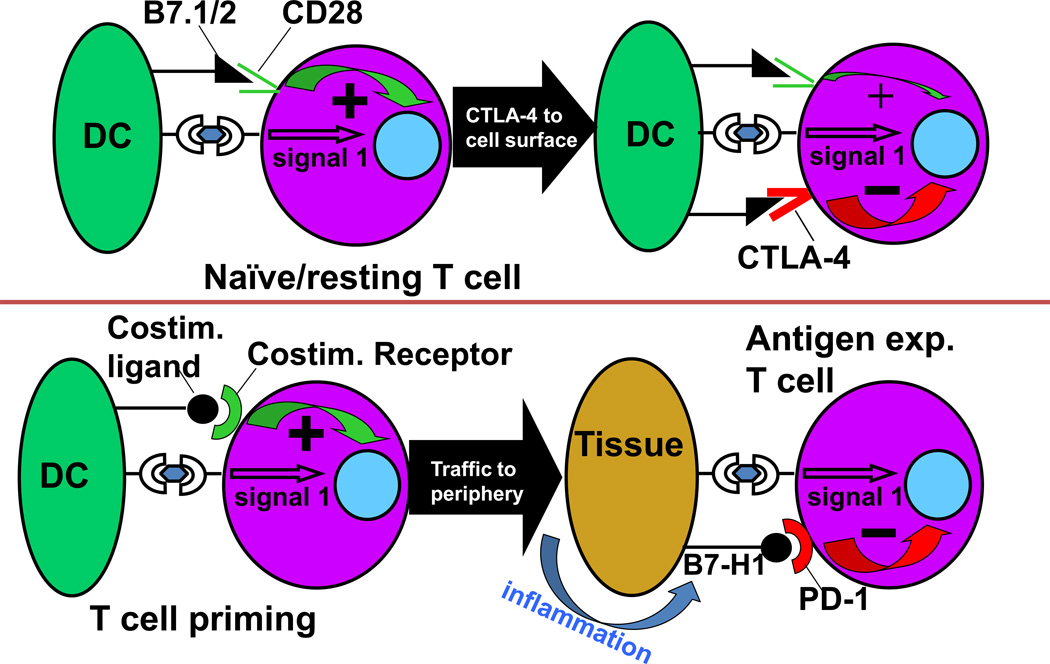
The two checkpoint receptors that have been most actively studied in the context of clinical cancer immunotherapy, CTLA-4 and PD-1 [2–8], play distinct roles in regulating immunity (Figure 1). CTLA-4, which regulates the amplitude of early activation of naïve and memory T cells, is transported to the T cell surface at levels that depend on the strength of the TCR signal. It acts physiologically as a signal dampener. Its importance in modulating T cell responses is demonstrated by the rapidly lethal autoimmune/hyperimmune phenotype of CTLA-4 knockout (KO) mice, which predicted the significant immune toxicity associated with blockade of this receptor.
Figure 1.
PD-1 and CTLA-4 play distinct roles in regulating T cell immunity. CTLA-4 modulates the early phases of activation of naïve or memory T cells in response to TCR stimulation by MHC-peptide complexes displayed by antigen presenting cells (“signal 1”). In contrast, PD-1 is expressed on antigen-experienced T cells in the periphery, and serves to limit the activity of T cells at the time of an inflammatory response, thereby protecting normal tissues from collateral destruction. DC, dendritic cell.
In contrast to CTLA-4, the major role of PD-1 is to limit the activity of T cells in the periphery during an inflammatory response to infection and to limit autoimmunity [5–9]. The basis for this physiology is that the ligands for PD-1, B7-H1/PD-L1 [10,11] and B7-DC/PD-L2 [12,13], are up-regulated in response to inflammation. B7-H1/PD-L1 is up-regulated on many cell types – hematopoietic, endothelial and epithelial - in response to proinflammatory cytokines, notably interferon-gamma, while B7-DC/PD-L2 is upregulated on dendritic cells and macrophages in response to different proinflammatory cytokines such as IL-4 [14,15]. PD-1 is expressed to various degrees on activated T cells; thus, the co-expression of ligand and receptor in inflamed tissues mitigates against collateral tissue destruction by T cells at these sites. In keeping with this biologic role, PD-1 and B7-H1/PD-L1 KO mice do not develop spontaneous autoimmune responses in the first year of age, but do demonstrate exacerbated tissue responses to infection or accelerated disease in autoimmune-prone strains.
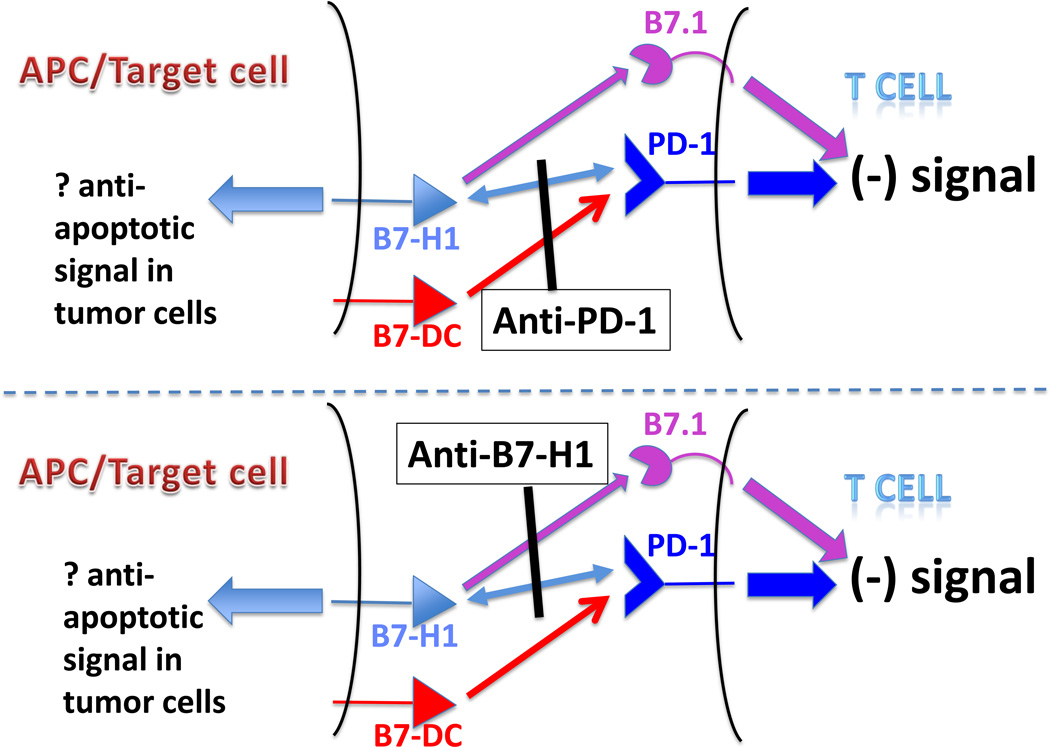
Further complexity to the PD-1/PD-1ligand system was appreciated with the surprising discovery that B7-H1/PD-L1 binds B7.1 in addition to PD-1 [16]. The binding sites appear to be adjacent but not overlapping, allowing for anti-B7-H1/PD-L1 mAbs that block one or both of these interactions. Application of these mAbs in various selective KO models suggests that interactions between B7-H1/PD-L1 and B7.1 on T cells inhibit T cell activity independent of interactions with PD-1 [17,18]. These findings suggest that there are three distinct inhibitory interactions within this pathway and that anti-PD-1 and anti-B7-H1/PD-L1 antibodies might behave differently owing to their ability to block distinct sets of interactions (Figure 2).
Figure 2.
Anti-PD-1 and anti-B7-H1/PD-L1 antibodies (mAbs) might behave differently owing to their ability to block distinct sets of inhibitory interactions. Anti-PD-1 mAbs can block its binding to both B7-H1/PD-L1 and B7-DC/PD-L2, abrogating an inhibitory PD-1-mediated signal in T cells; however, the inhibitory interaction of B7-H1/PD-L1 with B7.1 on T cells is not affected. Conversely, anti-B7-H1/PD-L1 mAbs can block its interactions with both B7.1 and PD-1, but will not block the inhibitory interaction of B7-DC/PD-L2 with PD-1. Both anti-PD-1 and anti-B7-H1/PD-L1 mAbs could potentially block transmission of a retrograde pro-survival signal through B7-H1/PD-L1 into tumor cells [43]. APC, antigen presenting cell.
The finding that B7-H1/PD-L1 is commonly up-regulated on many different tumor types, where it inhibits local anti-tumor T cell responses [19,20], and that PD-1 is expressed on the majority of tumor infiltrating lymphocytes [21,22] creates an important rationale for mAb blockade of this pathway for cancer immunotherapy, as validated by multiple murine tumor studies [23,24].
The clinical development of PD-1 pathway blockers requires an understanding of the signals that induce expression of its ligands within the tumor. In the case of the PD-1 ligands, constitutive oncogene-driven expression of B7-H1/PD-L1 has been suggested. However, recent findings support an alternative model, that B7-H1/PD-L1 up-regulation on tumor cells reflects their adaptation to endogenous immune responses directed at tumor antigens – a process we term adaptive resistance (J. Taube et al., unpublished). In adaptive resistance, the tumor co-opts the natural physiology of the PD-1 pathway for tissue protection in the face of inflammation, to protect itself from an anti-tumor response. Expression of B7-H1/PD-L1 as an adaptive response to anti-tumor immunity likely occurs because this ligand is induced on most epithelial cancers in response to interferon-gamma, similarly to epithelial and stromal cells in normal tissues [25, 26]. In lymphoid malignancies, B7-DC/ PD-L2 is more commonly up-regulated [27], likely in response to different set of proinflammatory cytokine signals. The adaptive resistance mechanism directly implies that any treatment that induces anti-tumor immunity (e.g., vaccination) will provide therapeutic synergy with PD-1 pathway blockade.
PRECLINICAL MODELS
Preclinical evidence for PD-1’s inhibitory role comes from several sources. Knockout mice develop late-onset, strain specific autoimmunity; on a C57/Bl6 background this manifests as sporadic glomerulonephritis [28], on a Balb/c background as an antibody-mediated cardiomyopathy [29]. These findings extend to models of organ-specific autoimmunity; on the non-obese diabetic (NOD) background, PD-1 KO mice develop accelerated insulitis as well as increased T cell production of effector cytokines [30]. Taken together, these data suggest that the PD-1/B7-H1(PD-L1) axis maintains T cell tolerance to persistently expressed antigens. A similar immunological picture emerges in models of chronic infection, where persistent pathogen exposure results in a population of “exhausted” antigen-specific CD8 T cells that express PD-1. In a chronic infection setting, the importance of this axis was elegantly demonstrated by studies in which blockade of PD-1/B7-H1(PD-L1) interactions restored the function of non-functional virus-specific CD8 T cells [31].
While much of this work has focused on CD8+ T cells, recent studies have highlighted a role for PD-1 on other cell types as well. One interesting set of experiments examined the acute B cell depletion that occurs during HIV infection, using a primate model. Here, PD-1 was shown to mediate the depletion of activated memory B cells and PD-1 blockade ameliorated this effect, restoring antibody titers [32]. While expression of PD-1 on B cells has been previously described, these recent data support a potential role for PD-1 in B cell immunity - the relative importance of this mechanism in cancer patients has yet to be explored. Expression of PD-1 on CD4+ T cells may also play a role in their dysfunction; a recent study used MHC class II tetramers to study HCV-specific CD4 T cell function in patients with either chronic or resolved infection. Similar to the case for CD8+ T cells, PD-1 blockade partially restored specific CD4 function in chronically infected patients, but TH1 cytokine production was most effectively restored when PD-1 blockade was combined with TGF-β and/or IL-10 blockade. Finally, PD-1 is highly expressed on induced regulatory T cells (iTregs), and PD-1: PD-L1 interactions appear to promote the induction/conversion and maintenance of Tregs, suggesting an additional mechanism for immunosuppression in a tumor microenvironment rich in PD-1 ligands [33, 34]. These studies reinforce the notion that PD-1 blockade may affect multiple cell types, a critical observation in understanding the mechanism of action of antibody-mediated blockade.
Perhaps even more interesting are accumulating data that non-functional CD8+ cells coordinately express multiple immune checkpoint molecules with PD-1, and that blockade of several checkpoints may augment anti-tumor (as well as anti-viral) immunity. This notion was first demonstrated in the chronic LCMV model, where PD-1 was co-expressed with 2B4 and lymphocyte activating gene-3 (LAG-3) [35]. Recent studies extended this concept to cancer; PD-1/LAG-3 co-expression was documented on tumor antigen-specific CD8+ T cells in women with ovarian cancer, and dual (but not single) blockade was shown to restore CD8+ T cell function in vitro [36]. Indeed, PD-1 and LAG-3 may mediate non-overlapping tolerance-maintaining pathways; an important recent study showed that PD-1/LAG-3 double KO mice developed an accelerated autoimmune phenotype not present in either single KO strain [37]. Another cell surface molecule that may work with PD-1 to maintain tolerance is Tim-3, which is co-expressed with PD-1 in multiple preclinical models. Indeed, in an animal model of acute myelogenous leukemia (AML), blockade of PD-1 or Tim-3 alone was insufficient to rescue mice from cancer progression, while blockade of both pathways resulted in additive disease protection [38]. Similarly, PD-1/Tim-3 co-expression has been reported on NY-ESO-1 specific CD8+ T cells from melanoma patients [39], reinforcing the notion that other checkpoints likely cooperate with PD-1 to control anti-tumor immunity. Determining the relative expression of relevant checkpoint molecules in the tumor microenvironment, or in tumor draining lymph nodes, should provide key insights into combinatorial treatment strategies for clinical exploration.
CLINICAL TRANSLATION
An understanding of PD-1 pathway biology and the preclinical demonstration of its pivotal role in immunosuppression have fueled the clinical development of PD-1 pathway blockade for cancer therapy. There are currently four anti-PD-1 agents in the clinic: MDX-1106/BMS-936558/ONO-4538, CT-011, MK-3475, and AMP-224. The first three are reported to be PD-1 blocking mAbs, while the last is a B7-DC/IgG1 fusion protein (Table 1).
Table 1.
PD-1 and B7-H1/PD-L1 blocking agents currently in clinical testinga
| Target Molecule | ||
|---|---|---|
| Source | PD-1 | B7-H1/PD-L1 |
| Amplimmune Inc./GlaxoSmithKline | AMP-224 (B7-DC/IgG1 fusion protein) | N/A |
| Bristol-Myers Squibb | MDX-1106/BMS-936558/ONO-4538 (fully human IgG4 mAb) | MDX-1105/BMS-936559 (fully human IgG4 mAb) |
| CureTech/Teva | CT-011 (humanized IgG1 mAb) | N/A |
| Merck | MK-3475 (humanized IgG4 mAb) | N/A |
Information about clinical trials can be found at ClinicalTrials.gov, http://www.clinicaltrials.gov.
To date, most clinical experience with PD-1 blockade has been gained with MDX-1106. The first-in-human phase I trial of this fully human IgG4 mAb employed intermittent dosing in patients with treatment-refractory metastatic solid tumors, allowing detailed analysis of pharmacokinetics (PK) and pharmacodynamics over a wide dose range [40]. Clinical activity was observed in patients with melanoma, renal cell carcinoma, colorectal cancer and non-small cell lung cancer (NSCLC). Tumor cell surface expression of B7-H1/PD-L1 in pretreatment biopsies emerged as a potential biomarker of response, consistent with pathway biology. Unexpectedly, the pharmacodynamic effects of PD-1 receptor occupancy by MDX-1106 were prolonged well beyond the measured half-life of 12–20 days, indicating the biological durability of this high-affinity mAb. An ongoing follow-up trial of biweekly MDX-1106 administration has already shown durable complete or partial tumor regressions in approximately one-third of patients with advanced melanoma and kidney cancer, with confirmed activity against NSCLC (M. Sznol et al., abstract 2506 in J Clin Oncol 2010, 28: suppl 15s). The activity of anti-PD-1 against NSCLC, long thought to be a “non-immunogenic” tumor, suggests the potential for PD-1 blockade to reactivate endogenous anti-tumor immunity in a wide spectrum of malignancies. Grade ≥3 adverse clinical events occurred in approximately 12% of patients, and included immune-related phenomena similar to those encountered with anti-CTLA-4 therapy (DF McDermott et al., abstract 331 in J Clin Oncol 2011, 29: suppl 7).
Another PD-1 blocking mAb, MK-3475, recently entered phase I clinical evaluation for cancer therapy. In contrast to the fully human MDX-1106, MK-3475 is a humanized mAb. However, similar to MDX-1106, its engineered IgG4 isotype is designed to obviate antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity (CMC).
Two anti-PD-1 agents with IgG1 isotypes may coordinately activate T cell-mediated and innate anti-tumor immunity. CT-011, a humanized mAb, was first tested in a single-dose regimen in 17 patients with advanced hematologic malignancies [41], and was generally well-tolerated. A complete response in a previously untreated patient with follicular B cell lymphoma and a minor response in a patient with refractory AML were reported. CT-011 is currently undergoing further testing in next-generation clinical trials for patients with advanced hematologic malignancies and a variety of solid tumors. In vitro studies have demonstrated its capacity to activate human NK cells against multiple myeloma, suggesting engagement of the innate immune system [42]. Finally, AMP-224, a recombinant protein fusing the extracellular domain of human B7-DC/PD-L2 to IgG1, also commenced phase I clinical testing in 2011 for patients with treatment-refractory metastatic cancers. Based on its composition, AMP-224 has the potential to block the inhibitory B7-DC:PD-1 interaction while engaging NK cells.
A blocking antibody against the ligand B7-H1/PD-L1 (MDX-1105/BMS-936559) is also currently in phase I clinical testing in patients with advanced solid tumors, and it has shown preliminary evidence of activity against melanoma, kidney cancer and NSCLC. These complementary results further validate the PD-1 pathway as a target for immunotherapy.
CONCLUSIONS
Despite early successes with monotherapies blocking PD-1 pathways, preclinical models indicate that combinatorial therapies will deliver maximum clinical impact. Several clinical trials are already planned or in progress, combining anti-PD-1 mAbs with cancer vaccines (melanoma, prostate cancer, renal cell carcinoma, AML), anti-tumor mAbs (lymphoma), or chemotherapies (pancreatic cancer, NSCLC). These synergistic treatment strategies will provide a foundation for the next generation of clinical investigations.
Highlights.
PD-1/B7-H1 are pivotal in maintaining an immunosuppressive tumor microenvironment.
PD-1 and CTLA-4 play distinct roles in regulating immunity.
B7-H1 up-regulation on tumor cells likely reflects “adaptive resistance”.
PD-1 blockade is active against NSCLC, thought to be a “non-immunogenic” tumor.
Monotherapies blocking PD-1 may be more effective in combinatorial regimens.
ACKNOWLEDGEMENTS
Supported by NIH grants R01 CA142779 (S.L.T., D.M.P.), R01 CA127153 (C.G.D), and 1P50CA58236-15 (C.G.D); a grant from the Melanoma Research Alliance (S.L.T., C.G.D, D.M.P.); the Laverna Hahn Charitable Trust and the Barney Family Foundation (S.L.T.); the Patrick C. Walsh Fund, the OneInSix Foundation, and the Prostate Cancer Foundation (C.G.D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Consulting or advisory role: S.L.T., Bristol-Myers Squibb (uncompensated) and Amplimmune Inc. (spouse); C.G.D., Bristol-Myers Squibb and Amplimmune Inc.; D.M.P., Bristol-Myers Squibb (uncompensated) and Amplimmune Inc.. Stock ownership: C.G.D., Amplimmune Inc.. Research funding: S.L.T., Bristol-Myers Squibb.
REFERENCES
- 1.Topalian SL, Weiner G, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 3. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. This seminal report describes the cloning of PD-1 (named for programmed death-1) from T cells undergoing activation induced cell death. Later studies demonstrated that PD-1 inhibits immunity through mechanisms other than apoptosis induction.
- 4.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 8.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. This paper demonstrated that the PD-1/PD-1 ligand system maintains T cell anergy or exhaustion in the setting of persistent antigen during chronic viral infection.
- 10. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. This paper reports the cloning of B7-H1 (PD-L1) and demonstrates its activity as a co-regulatory ligand.
- 11. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. This paper demonstrates that B7-H1/PD-L1 is a ligand for PD-1 and that its interaction with PD-1 on T cells inhibits their proliferation and cytokine production.
- 12. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. This paper reports the discovery of the second ligand for PD-1.
- 13. Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. This paper reports the discovery of the second ligand for PD-1.
- 14.Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer Immunol Immunother. 2011;60:1529–1541. doi: 10.1007/s00262-011-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–222. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A, Sayegh MH, Blazar BR, Freeman GJ, Sharpe AH. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. This is the first paper demonstrating a role for the major PD-1 ligand in tumor immune evasion. The authors show that forced expression of B7-H1 in tumors that are normally B7-H1 negative inhibits their immune elimination and that antibody blockade restores anti-tumor responses. They also show that many human tumors up-regulate B7-H1 relative to their normal tissue counterparts.
- 20.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 21.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, McDyer JF, Fortuno L, Schleimer RP. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;33:280–289. doi: 10.1165/rcmb.2004-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SK, Seo SH, Kim BS, Kim CD, Lee JH, Kang JS, Maeng PJ, Lim JS. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J Dermatol Sci. 2005;40:95–103. doi: 10.1016/j.jdermsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 32.Titanji K, Velu V, Chennareddi L, Vijay-Kumar M, Gewirtz AT, Freeman GJ, Amara RR. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J Clin Invest. 2010;120:3878–3890. doi: 10.1172/JCI43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. This study demonstrates the importance of PD-L1 in controlling the development and activity of iTegs, and suggests that local expression of PD-L1 in the tumor microenvironment can mediate de novo generation of Tregs thereby enhancing immunosuppression.
- 34.Amarnath S, Mangus CW, Wang JCM, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003130. 111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. The first demonstration that multiple immune checkpoint molecules control tumor-antigen specific CD8+ T cell function in humans.
- 37. Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. A convincing preclinical study showing that knocking out multiple (but not single) checkpoint molecules has profound immunological consequences.
- 38.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, Kirkwood JM, Zarour HM. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brahmer JR, Drake CG, Wollner I, Powderly J, Picus J, Sharfman W, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. This first-in-human study of MDX-1106 demonstrates the clinical activity of PD-1 blockade in patients with treatment-refractory advanced solid tumors, characterizes its pharmacodynamic properties, and provides preliminary evidence for a biomarker predictive of response.
- 41.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 42.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azuma T, Yao S, Zhu G, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]