Abstract
Elucidation of the role of human papillomavirus (HPV) in the etiology and prognosis of squamous carcinomas of the head and neck (HNSCC) is essential to optimize prevention and treatment strategies for this disease. We analyzed 385 HNSCC tissue blocks identified through a population-based cancer registry in Metropolitan Detroit for HPV DNA using a broad-spectrum PCR technique (SPF10-LiPA25) to correlate with patient and tumor characteristics and overall survival. Overall, HPV DNA (any type) was detected in 29.4% of all HNSCC, but it was significantly more prevalent (50.6%) in oropharyngeal sites (N=81), where 90% of HPV were type 16, than in other sites. HPV prevalence (any type) in oropharyngeal sites was highest in patients with a negative smoking indicator, Caucasians, and in regional tumor stage. Likewise, only in oropharyngeal sites did patients overall positive to HPV show significantly better survival compared with HPV-negative patients, notably among those who had been irradiated. The best and the worst survival from cancer in oropharyngeal sites were found, respectively, among HPV-positive patients with negative smoking indicator and among HPV-negative patients with positive smoking indicator. The results of the present study revealed that the presence of HPV DNA was associated with patients’ specific characteristics and better overall survival exclusively in oropharyngeal sites. To define the fraction of HNSCC preventable by HPV vaccination or amenable to less aggressive treatment, however, tobacco exposure and HPV markers other than DNA presence need to be taken into account.
Keywords: Human papillomavirus (HPV); PCR; Surveillance, Epidemiology and End Results; Head and neck cancer; Survival
Introduction
Despite advances in the management of squamous carcinomas of the head and neck (HNSCC) with multimodal treatment, including surgery, radiotherapy and chemotherapy,1 mortality and morbidity associated with these malignancies continue to be high, impacting also on quality of life and cost of care of these patients.2
Recent trends in cancer incidence indicate an increase in HNSCC located in the oropharyngeal sites in the United States3 and in the Northern Europe,4–5 particularly in young men. This trend has been suggested to be related to an increase in human papillomavirus (HPV)-associated HNSCC.4–5 Several studies have implicated high-risk HPV as the cause of a fraction of HNSCC, not explicable with confounding from other risk factors such tobacco consumption and alcohol abuse.6–7 At a molecular level HPV-associated HNSCC have a distinct profile with an absence of TP53 and RB1 mutations and expression of p16.8–9 In fact, HPV protein E6 blocks the action of TP53 tumor suppressor gene, and E7 blocks RB1 tumor suppressor gene without causing gene mutations.9 Several reports have suggested HPV-positive HNSCCs, notably those that arise from the oropharyngeal sites and integrate HPV type 16, have a better prognosis than HPV-negative HNSCC.6,10–13
With the introduction of HPV quadrivalent (Gardasil, Merck) and bivalent (Cervarix, GalxoSmithKline) for the prevention of cervical carcinoma14 and a recent US Food and Drug Administration approval of Gardasil for prevention of anal cancer, the possible benefit of vaccinating adolescents of both sexes, instead of girls only, for non-anogenital cancers, such as HNSCC, has become a significant public health issue. This is especially important since world-wide incidence of and mortality rates from HNSCC are higher than those for cervical cancer15 and screening and early detection of HNSCC are very challenging. Of notice, the fraction of HPV-positive HNSCC in which HPV16 and 18, i.e., the types included in current vaccine, are detected is higher than in cervical.16,17
Prophylactic HPV vaccine strategies for HNSCC are, however, hampered by current uncertainties on burden of HNSCC attributable to HPV in different populations.10,17–19 The aims of the present study were (i) to determine the prevalence of HPV and its genotypes in formalin fixed paraffin embedded (FFPE) HNSCC tissue, using a sensitive, broad-spectrum PCR-based assay; (ii) to correlate HPV DNA status with characteristics of tumors (anatomic sites, nuclear grades, and stages) and patients (age, gender, race, marital status, comorbidites indicative of smoking); and (iii) to evaluate differences in overall survival by HPV status and smoking indicator.
Methods
Study subjects
The Metropolitan Detroit Cancer Surveillance System (MDCSS) was used for case identification. Eligible cases were patients who were diagnosed with squamous cell carcinoma (SCC) (International Classification of Diseases for Oncology version 3 (ICDO3) histology codes 8050-8084) of the mouth, pharynx, nose, and larynx (ICDO3 topography codes C00.0–C14.8 and C30.0–C32.9) at age 20–79 between July 1, 1999 and December 31, 2007 and had surgical resection of the tumors at one of the three Wayne State University (WSU) affiliated hospitals in Detroit, Michigan (Harper University Hospital, Detroit Receiving Hospital and Karmanos Cancer Center). Cases who had undergone radiation therapy or chemotherapy before surgical resection were excluded. Excluded cases were similar in respect to demographic variables (age, sex and race) and tumor stage to those included, but included a significantly large proportion of laryngeal cancer cases (55%). Demographic and clinical characteristics of the patient, including vital status (as of July 27, 2010), were extracted from MDCSS. Mean follow-up time was 3.98 years (range 0.05–10.71). Since smoking status was not directly recorded in the MDCSS, a surrogate variable was generated based on comorbidity and other primary codes and referred to as smoking indicator. Smoking indicator was considered to be (i) positive, if a patient had one of the following conditions; International Classification of Diseases (ICD) version 9 codes, 305.1 (tobacco use disorder), V15.82 (history of tobacco use), 460.0–519.9 (benign respiratory diseases) and ICDO3 codes C320–C349 (respiratory cancers); (ii) negative, if comorbidity information was available but did not include any of these conditions; and (iii) unknown, if comorbidity information was not available. Respiratory comorbidity codes were already used as surrogates of smoking status in previous studies.20 The study was approved with a waiver for informed consent by WSU Human Investigation Committee.
Haematoxylene and Eosin (H&E) slides of eligible cases were retrieved and microscopically reviewed to confirm HNSCC diagnosis and select representative FFPE tumor tissue blocks. Of the 472 potentially eligible HNSCC cases, an archived FFPE tissue block was available for 385. Five sections were cut. Of these the first and last sections (5-microns thick) were placed on glass slides and stained with H&E to ensure the presence of tumor. The middle 3 sections (10-microns thick) were placed in clean eppendorf tubes and used for HPV assays. Between cases the microtome blade was changed and the laboratory instruments were cleaned by ethanol to minimize the risk of DNA cross-contamination.
DNA extraction
DNA was extracted by digesting the tissue with proteinase K buffer (45 mM Tris-HCl, 0.9 mM EDTA in 0.45% Tween 20, pH 8, 1 mg/ml proteinase K). Proteinase K solution (100 µl) was added to each microtube and incubated for 16–24 h at 56°C. Proteinase K solution was inactivated by incubating the tubes at 95°C for 10 min. Resulting DNA preparations were stored at −20°C.
HPV detection and genotyping
HPV DNA was detected and genotyped by using short-fragment PCR (SPF10) primers to amplify a broad spectrum of HPV genotypes (at least 54 HPV types), followed by DNA enzyme immunoassay (DEIA) and line probe assay (LiPA) as described previously.21–22 Each PCR run included a negative control (distilled water) and a positive control (DNA from a Hela culture cell line containing HPV18). Amplimers, synthesized by biotinylated PCR primers, were detected by hybridization to a mixture of HPV-specific but conserved probes by the DNA enzyme immunoassay (DEIA), as described previously, to determine a broad spectrum of HPV genotypes.22 For a borderline value (75 to 100% of the cutoff value), the SPF10 PCR was repeated and the sample is retested by DEIA.
The same SPF10 amplimers (10 µl of SPF10-DEIA-positive samples) were used to identify the HPV genotype by reverse hybridization on a LiPA containing probes for 25 different HPV genotypes (SPF10 HPV LiPA25 version 1, manufactured by Labo Bio-Medical Products, Rijswijk, The Netherlands). The LiPA detects HPV types 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70 and 74 exactly as described.21–22 DEIA positive samples that failed to hybridize on LiPA were classified as unknown (X) HPV type.
Statistical analysis
The HPV prevalence (all types combined) and the corresponding 95% confidence intervals (CI) were calculated according to the anatomic site of HNSCC. When not otherwise specified, HPV prevalence refers to all types combined. Anatomic sites were analyzed firstly according to the ICDO 3 digit topography code. The ICDO 4 digit topology codes were subsequently used to distinguish the anatomic sites known a priori to be the ones most strongly associated with HPV.6,11,17,19 They are in this report referred to as oropharyngeal sites and include: C019–C020 (base and dorsal surface of tongue), C051 (soft palate),C052 (uvula), C090–C103 and C108–C109 (all tonsil sites and all oropharynx sites except branchial cleft). Other HNSCC anatomical subsites will be referred to as other sites. Most analyses in this report are presented separately for oropharyngeal and other sites or restricted to oropharyngeal sites. Odds ratios and 95% CI for the overall HPV-positivity were calculated by unconditional logistic model according to patients’ and tumor characteristics. Time at risk in survival analysis was defined as the time from diagnosis to the date of death or the date of last vital status confirmation ascertained by MDCSS that provided nearly complete follow-up (i.e., 98%),23 using various record linkage and direct physicians/patients contacts. The Kaplan-Meier method and log rank test were used to analyze overall survival and Cox’s proportional hazard models were applied to estimate univariable and multivariable hazard ratios (HR) for deaths and the corresponding 95% confidence intervals (CI) according to overall HPV-positivity as well as to its combination with smoking-indicator. The proportional hazard assumption was checked by inclusion of a time-dependent covariate, log (follow-up time) multiplied by HPV status. Multivariable models included other prognostic variables that were associated with both overall survival and HPV status, i.e., SEER-tumor stage, race and smoking indicator. Statistical analyses were performed by SAS 9.2. All statistical tests were 2-sided.
Results
Table 1 shows HPV prevalence by anatomic site. Among the most common HNSCC locations base of the tongue (46.4%) and tonsil (60.5%) showed the highest HPV prevalence. In the combination of oropharyngeal sites HPV prevalence was 50.6% (i.e., significantly different prevalence in other sites, (23.7%, p<0.001). Despite the substantial variation in HPV-positivity by anatomic site, a predominance of HPV 16 was found in both groups of anatomic sites. For both anatomic groups combined, HPV 16 was by far the most common in both single infections (90/107, 84%) and in multiple infections (5/12, 42%). Other high-risk types including HPV18 (twice in single and 3 times in multiple infections) were rarely detected. The presence of low-risk types (e.g., 6, 11) was, however, found exclusively in non-oropharyngeal sites (P=0.02) (Table 2).
Table 1.
Overall HPV prevalence (%) and 95% confidence intervals (CI) by anatomic site of the head and neck cancer
| HPV prevalence | ||||
|---|---|---|---|---|
| Anatomic sites (ICD-O 3 digits) | ICDO code | No. of subjects |
% | (95% CI) |
| All head and neck sites | C000–C148,C300–C329 | 385 | 29.4 | (24.8–33.9) |
| Detailed subsites | ||||
| Lip | C000–C009 | 4 | 25.0 | (0.0–67.4) |
| Base of tongue | C019 | 28 | 46.4 | (28.0–64.9) |
| Other and tongue NOS | C020–C029 | 54 | 27.8 | (15.8–39.7) |
| Gum | C030–C039 | 10 | 20.0 | (0.0–44.8) |
| Floor of mouth | C040–C049 | 44 | 20.5 | (8.5–32.4) |
| Palate | C050–C059 | 5 | 20.0 | (0.0–55.1) |
| Cheek mucosa | C060–C061 | 8 | 75.0 | (45.0–100) |
| Retromolar area | C062 | 13 | 46.2 | (19.1–73.3) |
| Overlapping and mouth NOS | C068–C069 | 4 | 25.0 | (0.0 –67.4) |
| Salivary glands | C079–C089 | 10 | 0.0 | - |
| Tonsil | C090–C099 | 38 | 60.5 | (45.0–76.1) |
| Oropharynx | C100–C109 | 4 | 25.0 | (0.0–67.4) |
| Pyriform sinus | C129 | 22 | 9.1 | (0.0–21.1) |
| Hypopharynx | C130–C139 | 10 | 20.0 | (0.0–44.8) |
| Overlapping and pharynx NOS | C148 | 7 | 28.6 | (0.0–62.0) |
| Nasal cavity/mid ear | C300–C309 | 3 | 0.0 | - |
| Accessory sinuses | C310–319 | 10 | 30.0 | (1.6–58.4) |
| Larynx | C320–C329 | 111 | 23.4 | (15.5–31.3) |
| Global anatomic sites | ||||
| Oropharyngeal* | 81 | 50.6 | (33.4–50.4) | |
| Other | 304 | 23.7 | (18.9–28.5) | |
| P=<0.001 | ||||
NOS; not otherwise specified;
Oropharyngeal sites were defined a priori by ICD-O 4 digit topology codes, including C019–C020 (base and dorsal surface of tongue), C051 (soft palate)-C052 (uvula), C090–C103 and C108–C109 (all tonsil sites and all oropharynx sites except branchial cleft) and thus can not be marked in ICD-O 3 column
Table 2.
HPV detection rate (%) and HPV type distribution in cancer of the oropharyngeal and other head and neck sites
| HPV results | Oropharyngeal | Other |
|---|---|---|
| Negative | 40(49.38%) | 232(76.32%) |
| Positive | 41(50.62%) | 72(23.68%) |
| Types* | ||
| 6 | 0(0%) | 2(2.78%) |
| 6 33 | 0(0%) | 1(1.39%) |
| 11 | 0(0%) | 2(2.78%) |
| 16 | 36(87.8%) | 54(75%) |
| 16 18 | 1(2.44%) | 2(2.78%) |
| 16 31 | 0(0%) | 1(1.39%) |
| 16 52 | 0(0%) | 2(2.78%) |
| 18 | 0(0%) | 2(2.78%) |
| 31 | 0(0%) | 1(1.39%) |
| 33 | 2(4.88%) | 0(0%) |
| 35 | 1(2.44%) | 0(0%) |
| 45 | 0(0%) | 1(1.39%) |
| 51 | 0(0%) | 1(1.39%) |
| 56 | 1(2.44%) | 1(1.39%) |
| X | 0(0%) | 2(2.78%) |
% was based on HPV-positive subjects only and a single type listed alone does not include multiple infections
X: DEIA positive but unclassifiable by LiPA
Table 3 shows the odds ratio for overall HPV-positivity associated with selected patient’s and tumor characteristics separately for oropharyngeal and other sites. HPV prevalence did not significantly vary by age, gender or tumor differentiation in either group of anatomic sites. However, some differences were found and were restricted to oropharyngeal sites. HPV prevalence was significantly higher in Whites (70.5%) than other racial groups (African Americans except one Asian patient) (OR=6.44 95% CI: 2.44–17.03), and in patients with a negative smoking indicator (69.2%) than those with a positive smoking indicator (OR = 3.05, 95% CI: 1.04–9.01). An excess of HPV prevalence of borderline statistical significance was found among married patients compared to not-married patients (OR= 2.53 95% CI: 0.96–5.74). HPV prevalence also varied significantly by tumor stage (P=0.022) with the highest level in regional stage (OR=5.25 95% CI 1.00–27.45 in comparison with local stage). The association of HPV-positivity with race, marital status and tumor stage remained or became statistically significant with adjustment for smoking indicator among oropharyngeal site patients
Table 3.
Odds ratios (OR) and 95 % confidence intervals (CI) for overall HPV positivity by patients' and tumor characteristic
| Anatomic sites | |||||||
|---|---|---|---|---|---|---|---|
| Oropharyngeal | Other | ||||||
| Characteristics | No. of subjects |
OR | (95% CI) | No. of subjects |
OR | (95% CI) | |
| Age | |||||||
| 20–44 yrs | 10 | 1.00 | - | 33 | 1.00 | - | |
| 45–54 yrs | 27 | 1.08 | (0.25–4.60) | 95 | 1.19 | (0.46–3.09) | |
| 55–64 yrs | 29 | 0.81 | (0.19–3.43) | 95 | 0.99 | (0.38–2.61) | |
| 65+ yrs | 15 | 1.50 | (0.30–7.53) | 81 | 1.39 | (0.53–3.65) | |
| p=0.816 | p=0.795 | ||||||
| Sex | |||||||
| Female | 19 | 1.00 | - | 95 | 1.00 | - | |
| Male | 62 | 2.08 | (0.72–6.00) | 209 | 1.36 | (0.75–2.46) | |
| Race | |||||||
| Other | 37 | 1.00 | - | 128 | 1.00 | - | |
| White | 44 | 6.44 | (2.44–17.03) | 176 | 0.88 | (0.52–1.50) | |
| Marital Status | |||||||
| Not-married | 42 | 1.00 | - | 183 | 1.00 | - | |
| Married | 39 | 2.53 | (0.96–5.74) | 121 | 1.03 | (0.60–1.76) | |
| Tumor stage | |||||||
| In situ | 1 | 0.00 | - | 8 | 2.68 | (0.62–11.66) | |
| Local | 9 | 1.00 | - | 81 | 1.00 | - | |
| Regional | 60 | 5.25 | (1.00–27.45) | 142 | 0.66 | (0.35–1.25) | |
| Distant | 11 | 1.31 | (0.17–10.26) | 18 | 0.88 | (0.43–1.81) | |
| p=0.022 | p=0.217 | ||||||
| Tumor differentiation | |||||||
| Well-Moderate | 34 | 1.00 | - | 155 | 1.00 | - | |
| Poor- Non | 47 | 1.57 | (0.65–3.81) | 149 | 1.22 | (0.72–2.07) | |
| Smoking or respiratory diseases | |||||||
| Yes | 33 | 1.00 | - | 116 | 1.00 | - | |
| No | 26 | 3.05 | (1.04–9.01) | 90 | 0.98 | (0.52–1.85) | |
| Unknown | 22 | 0.94 | (0.31–2.81) | 98 | 0.69 | (0.36–1.32) | |
P-values were based on the likelihood ratio test
Patients with HPV (any type)-positive cancers at oropharyngeal sites showed significantly better survival than HPV-negative cancer (P<0.001) (Fig. 1). The multivariable HR for HPV (any type)-positive versus HPV-negative patients was 0.45 (95% CI: 0.21–0.96) in oropharyngeal sites and 1.08 (95% CI: 076–1.54) in other sites (data not shown). The influence of HPV status on survival in oropharyngeal sites was significant among patients who underwent radiation treatment (N=58; HR=0.25, 95% CI 0.08–0.78) but not among those who did not received radiation treatment (N=23; HR=1.26, 95% CI 0.30–5.26). The interaction term between radiation and the overall HPV-positivity in oropharyngeal sites was statistically significant (P=0.009). Conversely, among patients with other HNSCC sites the HR did not vary by radiation treatment (1.09; 95% CI 0.69–1.73 and 1.02; 95% CI 0.58–1.82). The association between the presence HPV and overall survival among patients with cancer at oropharyngeal sites was further assessed by Cox’s models after stratification by smoking-indicator, using smoking indicator(+)/HPV(−) as the reference category (Table 4) and presented in Kaplan-Meier plots in Supporting Information Figure 1. Smoking indicator(+)/ HPV(−) patients showed the worst survival but the difference was significantly better only than smoking indicator(−)/ HPV(+) patients (HR 0.27; 95% CI 0.08–0.98).
Fig 1.
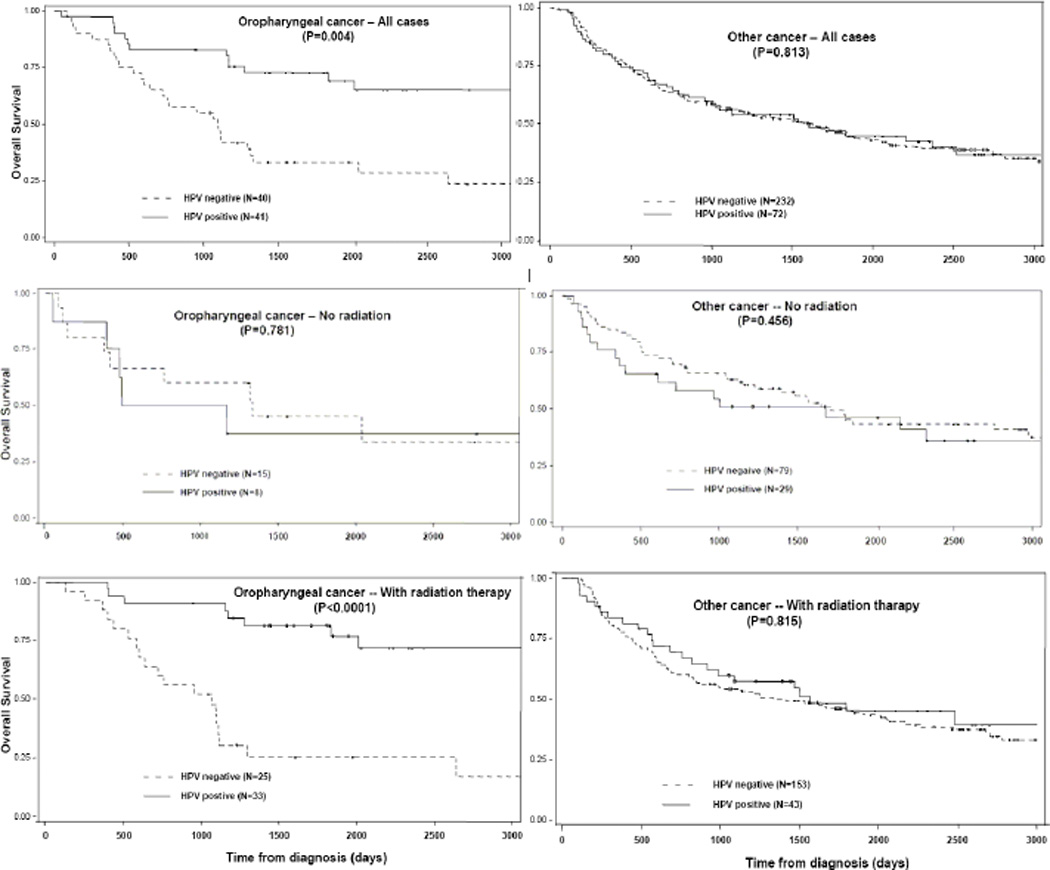
Kaplan-Meier curves for overall survival from cancer of oropharyngeal sites (left) and other sites (right), stratified by global HPV status (solid line: HPV-positive, broken line: HPV-negative). Upper panels indicate all cases combined, middle cases with no radiation, bottom cases with radiation therapy. P-values were based on the long-rank test.
Table 4.
Univariable and multivariable hazard ratios (HR) and 95% confidence intervals (CI) for overall mortality among the patients with cancer in oropharyngeal sites by combinations of HPV (all types combined) and smoking (SM) indicator status
| Combinations | No. | Alive/ Deceased |
Univariable HR (95% CI) |
Multivariable* HR (95% CI) |
|---|---|---|---|---|
| HPV (−) SM(+) | 19 | 6/13 | 1.00 - | 1.00 - |
| HPV(+) SM(+) | 14 | 9/5 | 0.37 (0.13–1.06) | 0.37 (0.12–1.09) |
| HPV (−) SM(−) | 8 | 4/4 | 0.47 (0.15–1.45) | 0.41 (0.13–1.30) |
| HPV(+) SM(−) | 18 | 14/4 | 0.20 (0.06–0.61) | 0.27 (0.08–0.98) |
| HPV(−) SM(?) | 13 | 2/11 | 1.30 (0.57–2.89) | 1.08 (0.43–2.72) |
| HPV(+) SM(?) | 9 | 5/4 | 0.40 (0.13–1.23) | 0.52 (0.14–1.91) |
Adjusted for race and tumor stage (where in situ cases were combined into local stage).
? indicates unknown
Discussion
Our study showed, for the first time in the United States, HPV prevalence in a series of HNSCC patients identified through a population-based cancer registry and obtained using a sensitive and well-validated PCR-based assay. HPV prevalence (any type) in HNSCC tumor blocks was 29% but it reached 51% in oropharyngeal sites. HPV prevalence in our present study was consistent with the findings of previous studies of HNSCC from the United States,6–8 but higher than in previous reports from some other countries.10,17–19 HPV 16 was by far the commonest type (85% of all HPV-positive tumors and 90% in oropharyngeal sites) in agreement with a bulk of previous studies in which HPV positivity in HNSCC was found to be mainly due to the presence of HPV16.8,10,18 Whereas HPV presence is less frequent in HNSCC than in invasive cervical carcinoma, the relative importance of HPV 16, compared to other high-risk types, is greater and similar to what has been showed in anal carcinoma.23 Furthermore, despite the involvement of low risk types, notably HPV 11, in recurrent respiratory papillomatotosis, the presence of HPV 6 and 11 was very rare in HNSCC as it has been reported to be in cervical carcinoma.24 The most important difference between HNSCC and invasive cervical carcinoma is, however, the variability in HPV prevalence across studies.10,17–19 Part of the variability may be an artefact that derived from chance, contamination, or publication bias in the earliest and smallest HNSCC case-series17 and differences in HPV testing protocols. Additional important differences included the type of samples used (i.e., exfoliated cells versus tissue biopsies); the sensitivity of the PCR-based assay;17 and the proportion of cancer of the oropharyngeal sites that were included.
Part of the difference in the fraction of HNSCC across populations may, however, be real and due to variations in the competing role of the other risk factors most notably tobacco use (smoking and chewing included).19 Tobacco and HPV infection can cause HNSCC through at least partly similar mechanisms, i.e., inactivation of host onco-suppressor genes. They therefore somewhat compete in HNSCC causation as shown by a tendency of HPV DNA to be detected more frequently among non-smokers than among smokers in our present study as in previous studies.11,19 It is therefore conceivable that in the United States and in Northern Europe, where smoking has been declining for decades, and a substantial fraction of HNSCC are by now detected among non-smokers or former smokers, a higher proportion of HNSCC may be attributable to HPV infection.
In oropharyngeal sites we also found a significantly lower HPV prevalence among Whites compared to African Americans, in agreement with a previous report from the US.11–12 The difference in HPV prevalence by ethnic group was not eliminated by adjustment for smoking indicator but underadjustment cannot be ruled out. Conversely, we did not find any significant association of HPV-positivity with age and tumor differentiation contrary to some previous reports.6,10,25–26 HPV positivity in cancer of oropharyngeal sites was associated with regional stage disease in several previous studies. 10–12,25 We confirmed an especially high HPV prevalence in cancer of the oropharynx and base of the tongue with regional spread.12,27 The early tendency of even small cancer of the tonsil to metastasize rapidly to regional lymph-node has been attributed to the anatomo-histological characteristics of the tonsil (i.e. thin epithelium and frequent disruption of the basal membrane).28
We also confirmed that HPV positivity, nearly all accounted for by HPV16, was associated with better survival6,10–12,18,25–27 and demonstrated that the survival advantage of HPV-positivity was limited to oropharyngeal sites. The survival advantage associated with HPV detection in the tumor was especially clear among patients who had received radiation therapy pointing to a possible explanation of our finding i.e., better response to radiotherapy in HPV-positive cancers of oropharyngeal sites. Others also have reported better response to radiotherapy and chemotherapy in HPV-positive HNSCC.10,18,26–27 Enhanced radiosensitivity in HPV-associated cancer is biologically plausible. Stable transfection of HPV16 E6 into a human cancer cell line has been found to increase in vitro radiation sensitivity.29 Furthermore, other cellular and molecular characteristics associated with HPV-positive HNSCC, such as P16 and P53 expression, Atk activation and the absence chromosome 11q abnormalities has been linked to increased radiosensitivity.30–32 In addition, HPV-positive carcinomas tend to maintain functional unmutated TP53.9
Smoking history, however, is also an important determinant of survival in HNSCC patients.12,25 We attempted, therefore, to tease out the influence of HPV DNA presence and smoking indicator on survival in patients with cancer of oropharyngeal sites. The best and the worst survival in oropharyngeal sites were found, respectively, among HPV-positive patients with a negative smoking indicator and among HPV-negative patients with positive smoking indicator. HPV-positive patients with positive or unknown smoking indicator as well as those negative for both HPV and smoking showed an intermediate survival probabilities.
Poorer prognosis in smokers has been attributed to smoking-related co-morbidity and a high probability of second primaries12,33 in the head and neck and lung. This can be interpreted in the framework of “field cancerization”, i.e., widespread presence of genetic alterations and precancerous lesions in the head and neck epithelium of smokers.27 Conversely, HPV carcinogenesis in the head and neck shows a special tropism for the crypt epithelium of the tonsil and no field cancerization has been observed.28 In addition, the majority of tobacco-associated carcinomas show TP53 mutations.9
Our present study has strengths and weaknesses. Firstly, it represented one of the first examples of a new efficient strategy of retrieval of archived tumor blocks through a SEER registry and linkage of complete incidence and survival data to important tumor molecular markers. After having shown its feasibility, our current approach can be extended to a full-scale population-based study that includes all medical facilities in registry catchment area. Secondly, the PCR-based assay used for HPV detection combined high sensitivity and robustness in respect to sample quality, notably DNA fragmentation in archival biopsies.8 Contamination was avoided by using a new disposable microtome blade for every tumor tissue block cut, and by cleaning laboratory instruments with ethanol between cases. On the other hand, we also acknowledge the limited statistical power of the present study especially in some sub-group analyse.
Another weakness of our study is the lack of accurate information on smoking and drinking habits in the SEER data. Patient classification by smoking, therefore, had to rely exclusively on comorbidity/other primary codes and could not capture many important aspects of tobacco exposure (duration, intensity, time since smoking cessation). In addition, smoking indicators were missing for the earliest cases (i.e., one third of the present HNSCC cases). We acknowledge the limitation of using comorbidities in predicting individuals’ smoking history. As our study was based on HNSCC patients, a group expected to have had heavy tobacco exposure, smoking indicator is expected to be reasonably specific, but poor sensitivity is of greater concern. The use of an imperfect surrogate for smoking history is likely to have led to an underestimate of real differences between smokers and non-smokers in HPV status and overall survival. So has, however, the use of HPV DNA only as the only marker of HPV-associated cancer as the presence of the virus may be in many instances irrelevant to cancer etiology
As FFPE sections were the sole specimens available in our study and we did not perform a range of addition molecular tests that would have helped predicting if an HPV infection in a HNSCC was trascriptionally active, e.g., p16 immunohistochemistry, in-situ hybridization27, and HPV E2/E6 ratios. 34 Viral E6/E7 transcripts would have been especially informative as the presence of viral RNAs has been shown to influence oropharyngeal cancer survival also among HPV DNA-positive patients.34 Differences in the molecular characteristics of HPV infection (e.g., integrated or extra-chromosomal) probably help explaining the lack of survival advantage among HPV DNA-positive HNSCC other than in oropharyngeal sites. The mucosa lining the tonsil of the oropharynx is notable for its intimate relationship with lymphoid tissue.28 It contains patches of cells, thin and fragile, with disrupted basement membrane, that facilitate contact with HPV and DNA integration. Despite these limitations, our present study was able to contribute to a critical issue: any algorithm to define the fraction of HNSCC preventable by HPV elimination or treatable by less aggressive therapies needs to take the presence of tobacco exposure into account.12,25
In conclusion, quarter of HNSCC and over 50% of those in oropharyngeal sites represent the upper threshold of the fraction of HNSCC theoretically preventable in a country like the United States by vaccination against HPV 16 and 18. However, findings from the United States cannot be readily applied to other populations where the relative importance of HPV infection and smoking may be very different. Even in the United States, the true HPV-attributable fraction would be difficult to be determined as patients with HPV-positive tumors often have a history of smoking. Thus, the most conservative estimate of the fraction of HNSCC potentially preventable by universal HPV vaccination against HPV 16 and 18 or amenable to different treatment protocol may be represented by HPV-positive of cancer at oropharynhgel sites in patients in whom other causes, notably tobacco, can be ruled out.
The novelty and impact of the paper.
Te prevalence of HPV DNA was significantly higher in oropharyngeal sites than the other head and neck sites and associated with patients’ specific characteristics and better overall survival in conjunction with radiation treatment. The results of the present study provide valuable information to define the fraction of HNSCC preventable by HPV vaccination or amenable to less aggressive treatment.
Supplementary Material
Kaplan-Meier curves for overall survival from cancer of oropharyngeal sites stratified by global HPV status and smoking indicator.
Acknowledgment
This work was supported in part by the National Cancer Institute ay the National Institutes of Health (Contract number N01-PC-35145 to IK). We thank Jean-Paul Brunsveld and Jacqueline van Harmelen from DDL for technical assistance in this study.
Abbreviations
- HPV
Human papillomavirus
- SEER
Surveillance, Epidemiology and End Results
- HNSCC
head and neck squamous cell carcinoma
- FFPE
formalin-fixed, paraffin embedded
- DEIA
DNA enzyme immunoassay
- LiPA
line probe assay
Footnotes
Conflict of interest
Thu authors declare no conflicts of interest.
References
- 1.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, et al. Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 2.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:e1–e7. doi: 10.1016/j.ajog.2008.03.064. 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 4.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 5.Attner P, Du J, Näsman A, Hammarstedt L, Ramqvist T, Lindholm J, Marklund L, Dalianis T, Munck-Wikland E. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126:2879–2884. doi: 10.1002/ijc.24994. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 8.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010;17:394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]
- 9.Dai M, Clifford GM, le Calvez F, Castellsagué X, Snijders PJ, Pawlita M, Herrero R, Hainaut P, Franceschi S. Human papillomavirus type 16 and TP53 mutation in oral cancer: matched analysis of the IARC multicenter study. Cancer Res. 2004;64:468–471. doi: 10.1158/0008-5472.can-03-3284. [DOI] [PubMed] [Google Scholar]
- 10.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos TN, Chepeha DB, McHugh JB, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragin CCE, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomvirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Brewer NT, Rinas AC, Schmitt K, Smith JS. Evaluating the impact of human papillomavirus vaccines. Vaccine. 2009;27:4355–4362. doi: 10.1016/j.vaccine.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 16.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 17.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 18.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, Campisi G. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007) Ann Oncol. 2008;19:1681–1690. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 19.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernández L, Idris A, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 20.Cardis E, Gilbert ES, Carpenter L, Howe G, Kato I, Armstrong BK, Beral V, Cowper G, Douglas A, Fix J, Fry SA, Kaldor J, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–132. [PubMed] [Google Scholar]
- 21.Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Yee CL, Dombi GW, Korczak JF, Severson RK. Race and other factors associated with differential follow-up in a population-based cancer registry. J Registr Manag. 2007;34:129–134. [Google Scholar]
- 24.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 25.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 26.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, Urba SG, Eisbruch A, Teknos TN, Chepeha DB, Prince ME, Tsien CI, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Ann Rev Pathol Mech Dis. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HJ, Kim JY, Hampson L, Pyo H, Baek HJ, Roberts SA, Hendry JH, Hampson IN. Human papillomavirus 16 E6 increases the radiosensitivity of p53-mutated cervical cancer cells, associated with up-regulation of aurora A. Int J Radiat Biol. 2010;86:769–779. doi: 10.3109/09553002.2010.484477. [DOI] [PubMed] [Google Scholar]
- 30.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D'Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, Buatti JM, Spitz DR. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928–933. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh RA, White JS, Huang X, Schoppy DW, Baysal BE, Baskaran R, Bakkenist CJ, Saunders WS, Hsu LC, Romkes M, Gollin SM. Loss of distal 11q is associated with DNA repair deficiency and reduced sensitivity to ionizing radiation in head and neck squamous cell carcinoma. Genes Chromosomes Cancer. 2007;46:761–775. doi: 10.1002/gcc.20462. [DOI] [PubMed] [Google Scholar]
- 33.Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, Abecassis J, Clavel C, Wasylyk B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier curves for overall survival from cancer of oropharyngeal sites stratified by global HPV status and smoking indicator.


