Abstract
Background
Little is known regarding the health-related quality of life (HRQL) trajectory of children with sickle cell disease or thalassemia (“hemoglobinopathies”) following hematopoietic stem cell transplantation (HSCT).
Procedure
We serially evaluated the HRQL of 13 children with hemoglobinopathies who received HSCT during two prospective multi-center studies using the Child Health Ratings Inventories (CHRIs). The HRQL scores among children with hemoglobinopathies, as reported separately by the children and their parents were compared using repeated measures models to scores of a comparison group of children receiving HSCT for malignancies or severe aplastic anemia.
Results
The sample included 13 children with hemoglobinopathies (median age: 8 years, range 5–18) and 268 children in the comparison group (median age: 11 years, range 5–18). There were similar rates of early infection, chronic GVHD and all-cause mortality between the two groups. There was no significant difference in recovery to baseline scores for physical, emotional, and role functioning by three months for either group. Children with hemoglobinopathies had higher HRQL scores for physical (beta=12, se=5.5, p=0.01) and baseline emotional functioning (beta=11.6, se=5.5, p=0.03) than the comparison group. For all domains for both groups, parent reports demonstrated a nadir at 45 days with recovery to baseline by three months following transplant. Children’s ratings were higher than those of their parents in both diagnostic groups.
Conclusions
Children with hemoglobinopathies had higher physical and emotional functioning scores prior to HSCT and experienced a similar pattern of recovery to their baseline functioning by three months post-HSCT when compared to children receiving HSCT for acquired conditions.
Keywords for indexing: sickle cell disease, thalassemia, hematopoietic stem cell transplant, health-related quality of life
INTRODUCTION
Sickle cell disease (SCD) and thalassemia major (“hemoglobinopathies”) are common diseases worldwide responsible for a large burden of disease[1, 2]. Research demonstrates the negative effects of SCD on the HRQL of both children and adults[3–7]. Factors associated with poorer HRQL in children with SCD included physical pain, older age, female gender, and neurobehavioral comorbidities[4, 8, 9]. The HRQL of adults with SCD is severely compromised. One study reported that the HRQL pattern seen in adults with SCD was similar to patients undergoing hemodialysis[6]. Similarly, the life-long need for transfusion and iron chelation impairs the HRQL for children and adults with thalassemia and puts families with lower socio-economic status at greatest risk [10–12].
HSCT offers a cure for children with hemoglobinopathies [13, 14]. The complex, shared decision-making between families and physicians to undergo HSCT for hemoglobinopathies involves weighing acute and chronic complications of HSCT against the shortened lifespan and long-term burden of these chronic illnesses[15–17]. A cross-sectional study of 24 children and adults who underwent HSCT for thalassemia found that HSCT survivors experienced higher HRQL than 74 similar patients treated with transfusion and chelation[18]. More understanding of HRQL post-HSCT may help families and physicians decide when or if to proceed to HSCT for these conditions.
We described the 12-month HRQL trajectory of 13 children with SCD or thalassemia, treated with HSCT and enrolled in the “Journeys to Recovery Study” (JTR) or HSCT-Comprehensive Health Enhancement Support Study (CHESS™)[19, 20]. The principal outcome in both studies was HRQL, as rated from the Child Heath Rating Inventories (CHRIs)-General Health module[21, 22] which was augmented with demographic and clinical information. The purpose of this study was to describe the HRQL trajectory of children with hemoglobinopathies and to compare their results to a contemporaneous comparison group of 268 children with acquired disorders, (malignancies and aplastic anemia), who were also included in the two studies. We hypothesized that children with hemoglobinopathies may have a better HRQL at the time of HSCT than children undergoing HSCT for acquired disorders due to a lack of prior treatment with chemotherapy, radiation, and/or immunosuppressive therapy. Beyond baseline, because of treatment similarities during and following HSCT, we did not expect to see persistent differences in the HRQL trajectories.
METHODS
Participants
Subjects ages 5–18 years were drawn from the JTR study and the HSCT-CHESS™ study, two prospective studies of pediatric HSCT on parent-child dyads conducted at eight HSCT centers in the United States from 2003 to 2011. The objective of the JTR study (N=165 parent-child dyads) was to describe the 12-month HRQL trajectory of pediatric patients following HSCT, as reported by the child and their parents in the 12 months following HSCT, using the CHRIs-General[19]. The HSCT-CHESS™ study was a randomized controlled trial of a web-based eHealth intervention of integrated health information and support for parents of pediatric HSCT recipients designed to improve the health-related knowledge, skills, and quality of life of parents of children undergoing HSCT [20]. The HSCT-CHESS™ trial included 198 parent-child dyads; 147 were between the ages of 5–18 years. For both studies, individual transplant centers determined the criteria for HSCT.
Eligibility for both studies included working knowledge of English and having a parent/legal guardian who could consent on behalf of the child. After consulting with clinical providers and deciding that the child would proceed to transplant, eligible dyads were approached for recruitment. Parental informed consent and age-appropriate assent were obtained. The studies were approved by the institutional review board (IRB) at Tufts Medical Center and at each clinical site; IRB approval was also obtained to combine data from the two studies.
Our analytic sample was restricted to 281 parent-child dyads from both studies based on child age (5 to 18 only) and diagnosis. The diagnostic category of hemoglobinopathies was comprised of patients with SCD or thalassemia. The comparison group of patients with acquired disorders was comprised of patients with malignancies or severe aplastic anemia. Thirteen children with SCD or thalassemia were identified with 85% complete data by 12 months. The remaining 268 children, serving as a comparison group, had a completion rate of 64% by 12 months.
Measures
The CHRIs-General contains 20 items forming three general health status domains: physical, role, and emotional functioning. The measure is designed to assess the child’s status in the week prior to the assessment using separate parent and child versions. The measure also contains a summary item of the child’s general health (parent report, only). For all items, the voice of the child is standard, re-worded for parental ratings from ‘you’ to ‘your child.’ Responses to all health status questions are a five-point Likert scale. For children 5–12 years of age, the responses are pictorially represented; for adolescents and parents, the responses are text-based. Using the half-scale rule, which requires that at least half the items in a scale be completed, the items were averaged together to create a summary score and transformed to a 100-point scale. Higher scores for the CHRIs-General domains indicate better functioning; higher scores for the general health summary item indicate better health. The ten-item CHRIs-HSCT module was also used at each of the follow up assessments. This module contains 10 transplant items forming three dimensions: hassles, body image, and distress/preoccupation. In contrast to the CHRIs-General, higher scores on the CHRIs-HSCT connote more transplant impact (i.e., greater distress or more hassles). The CHRIs-General measure and its HSCT module were used in both studies based on demonstrated psychometric performance in prior applications for children and their parents within the HSCT population [21, 22]. The baseline CHRIs-General assessment was collected before the hematopoietic stem cell infusion. Following transplant, CHRIs-General and CHRIs-HSCT assessments were conducted at 45 days, and three, six, and 12 months.
Medical and Demographic Information
At baseline parents completed the CHRIs demographic questionnaire. Trained research staff recorded information on causal diagnosis, hematopoietic stem cell source, donor type, and intensity of the conditioning regimen.
At the end of the transplant hospitalization and at all follow-up assessments, trained study staff conducted chart reviews to collect clinical information. Major outcomes including engraftment, infections, acute and chronic graft versus host disease (aGVHD, cGVHD, respectively), end-organ toxicity, and death, were collected and scored using standard toxicity scales[23–27].
Data Analysis
Data were stratified by those who received HSCT for hemoglobinopathies versus the comparison group. Demographics and clinical characteristics were described for each patient in the hemoglobinopathies group and summarized using medians, ranges, frequencies, and percentages. Baseline demographic and clinical characteristics for the comparison group were summarized. Psychometric properties of the CHRIs-General were described separately for each of the diagnostic groups (hemoglobinopathies vs. comparison group) and by rater (parent vs. child). Mean, standard deviation (SD), percent missing, percent ceiling, percent floor, and internal consistency reliability (ICR) of all CHRIs-General domains were calculated. ICR was based on Cronbach’s alpha coefficient, where α>0.70 indicates acceptable consistency [28, 29].
Recovery by three months was a binary variable for physical, emotional, and role functioning and general health and was defined as surpassing the baseline score at 45 days or three months. For those at the ceiling at baseline, scoring at the ceiling at 45 days or three months was also considered recovery. Recovery was defined separately for parent and child ratings. Due to small cell size, Fisher’s exact test was used to compare the proportion recovering among the hemoglobinopathies group and the comparison group.
Mean summary scores for parent and child assessment were computed for each CHRIs-General and CHRIs-HSCT domain. Parent assessment only was used to compute the general health scores. Domain scores over time by rater and general health over time were plotted, comparing children in the hemoglobinopathies group to the comparison group.
We looked principally at change from baseline to three months only because of small sample size and because the plots indicated that most changes in HRQL occurred within 100 days post-HSCT. To assess whether CHRIs-General domain scores varied from baseline to three months, and whether there were differences in scores among the two groups, six separate models were constructed representing each CHRIs-General and rater combination: (1–2) child and parent rating of the child’s physical functioning, (3–4) child and parent rating of the child’s emotional functioning, and (5–6) child and parent rating of the child’s role functioning. An additional model was constructed for the parent rating of the child’s general health. An indicator for diagnostic group, time, and an interaction between group and time were included in the models. Time was treated categorically (with baseline as the reference) because of its non-linear relationship with the domain scores. Separate models were constructed for each domain score to assess whether there were differences in parent and child ratings from baseline through three months. An indicator for rater (parent or child), time, and an interaction between rater and time was included. Due to small sample size, we could not explore interactions in rater, time, and diagnostic group in the same model, so we then ran the same three models restricted the to the hemoglobinopathies group. Finally, three models were constructed to look at changes over time by diagnostic group for the three CHRIs-HSCT domains (hassle, body image, distress/preoccupation). In contrast to previous models, only the child rater was used and all times post-HSCT were included. We used maximum likelihood estimation with repeated measures in SAS Proc Mixed to account for the correlations over time, with an unstructured covariance matrix.
RESULTS
Summary of parent and child characteristics
The hemoglobinopathies group included 13 parent-child dyads (Table I). Median ages of parent and child were 36 years (range, 26–54) and 8 years (range, 5–18), respectively. Parents were predominantly female (77%), college-educated (69%), and married (85%). Just over half had private insurance (54%). Most children were male (62%) and Black (55%). Seven transplants were performed for SCD (54%) and six for thalassemia (46%). Most graft sources were bone marrow (85%) while the rest were umbilical cord. Most donors were related (69%) and most of the conditioning regimens were myeloablative (62%). The comparison group consisted of 268 parent-child dyads. Median ages of the parent and child were 40 years (range, 21–59) and 11 years (range, 5–18), respectively. Parents in the comparison group were mostly female (84%), college-educated (68%), and married (78%); most had private insurance (66%). Half of the children in the comparison group were male and only 5% were Black. Children in the comparison group received HSCT for hematologic malignancies (71%), solid tumors (17%), or aplastic anemia (12%). Most (74%) underwent allogeneic HSCT and 69 (26%) underwent autologous HSCT. Within the subgroup receiving allogeneic transplants, 66 (33%) grafts were from matched family donors and 133 (67%) were from unrelated donors.
TABLE I.
Summary of characteristics of HSCT patients receiving transplant for sickle cell disease or thalassemiaa
| Disease | Age (yrs) |
Gender | Blackb | Hispanic | Allogeneic HSCT Type |
Myeloablative Prep |
Max aGVHD grade through 3m |
Max cGVHD grade through 12m |
Max organ toxicity through 3m |
|---|---|---|---|---|---|---|---|---|---|
| SCD | 6 | F | Yes | No | Related | No | 0 | 0 | Good |
| SCD | 7 | M | Yes | No | Related | Yes | 0 | 1 | Good |
| SCD | 7 | M | Yes | No | Unrelated | Yes | 0 | 0 | Good |
| SCD | 10 | M | No | No | Related | Yes | 0 | 0 | Good |
| SCD | 13 | M | Yes | No | Related | Yes | 0 | 0 | Good |
| SCD | 17 | F | Yes | No | Related | No | 0 | 0 | Good |
| SCD | 18 | M | Yes | No | Unrelated | No | 2 | 2 | Good |
| Thal | 5 | M | NSd | No | Related | Yes | 0 | 0 | Good |
| Thal | 7 | F | No | No | Related | Yes | 1 | 0 | Good |
| Thal | 7 | M | No | No | Related | No | 2 | 0 | Good |
| Thal | 8 | M | No | No | Related | Yes | 0 | 0 | Good |
| Thal c | 8 | F | NSd | Yes | Unrelated | Yes | 4 | 2; progressive onset |
Intermediate to poor |
| Thal | 13 | F | No | No | Unrelated | No | 1 | 0 | Good |
SCD=sickle cell disease; Thal=thalassemia; M=male; F=female; aGVHD=acute graft versus host disease; cGVHD=chronic graft versus host disease;
Child race per parent report;
Patient died approx. 3 months after transplant;
Not specified
All patients in the hemoglobinopathies group successfully engrafted, whereas eight (3%) children in the comparison group did not, resulting in a subsequent transplant. The rates of infection and GVHD were similar between the hemoglobinopathies group and the comparison group; 23% in both groups developed systemic infection within the first three months post-HSCT. Late-onset systemic infection was documented in 8% of the hemoglobinopathies and 14% of the comparison group. Three patients with hemoglobinopathies (23%) developed aGVHD of grade 2 or greater in the first three months post-HSCT, similar to 30% (of the 187 evaluable allogeneic HSCT recipients) in the comparison group. Three patients (23%) with hemoglobinopathies developed cGVHD; two cases (15%) were extensive. Both received unrelated donor transplants. One of these children also had substantial end-organ toxicity by three months and subsequently died. Within the comparison group, 77 of the 187 (41%) allogeneic recipients evaluable for cGVHD developed it, of which 33 (18%) were extensive. The one-year rate of all-cause mortality was 8% for the hemoglobinopathies group and 18% for the comparison group.
Summary of CHRIs-General psychometrics
In the hemoglobinopathies group, acceptable ICR was demonstrated for most CHRIs-General domains, as assessed by the parent (physical, α=0.96; emotional, α=0.73; role, α=0.91) and child (physical, α=0.78; emotional, α=0.54; role, α=0.83) (Table II). Similar ICR was demonstrated in the comparison group for measures completed by the parent (physical, α=0.91; emotional, α=0.88; role, α=0.87) and child (physical, α=0.78; emotional, α=0.80; role, α=0.80). Relatively high ceiling effects (>20%) were observed for baseline physical and role functioning as assessed by the parent and child among those with hemoglobinopathies. In the comparison group, child-rated ceiling effects were seen in 10% of the physical functioning scores and in 21% of the role functioning scores; parent-rated ceiling effects in the comparison group were similar. In contrast, floor effects were infrequent at baseline (<10%) as reported by parents and children in both groups. There was almost no missing data (<1%) (Table II).
TABLE II.
Summary of psychometrics for baseline CHRIs-General domains and general health by causal disease as assessed by the child and parenta
| Hemoglobinopathies (N=13) |
Comparison Group (N=268) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (sd) |
α | Missing | Floor | Ceiling | N | Mean (sd) |
α | Missing | Floor | Ceiling | |
| Child Report | ||||||||||||
| Physical Functioning | 13 | 74.7 (25.8) | 0.78 | 0.0% | 0.0% | 30.8% | 267 | 64.2 (24.9) | 0.78 | 0.4% | 2.3% | 9.7% |
| Emotional Functioning | 13 | 80.8 (11.3) | 0.54 | 0.0% | 0.0% | 7.7% | 266 | 69.1 (19.5) | 0.80 | 0.8% | 0.0% | 3.0% |
| Role Functioning | 13 | 79.3 (22.9) | 0.83 | 0.0% | 0.0% | 30.8% | 267 | 72.8 (26.1) | 0.80 | 0.4% | 1.9% | 21.4% |
| Parent Report | ||||||||||||
| Physical Functioning | 13 | 67.3 (28.4) | 0.96 | 0.0% | 7.7% | 23.1% | 267 | 52.4 (32.6) | 0.91 | 0.4% | 7.1% | 9.7% |
| Emotional Functioning | 13 | 67.0 (16.8) | 0.73 | 0.0% | 0.0% | 0.0% | 268 | 59.6 (21.7) | 0.88 | 0% | 0.8% | 2.2% |
| Role Functioning | 13 | 65.4 (32.1) | 0.91 | 0.0% | 0.0% | 38.5% | 267 | 68.8 (27.9) | 0.87 | 0.4% | 1.1% | 25.1% |
| General Health | 13 | 48.1 (27.9) | - - - | 0.0% | 7.7% | 15.4% | 267 | 52.7 (28.3) | - - - | 0.4% | 8.2% | 12.4% |
SCD=sickle cell disease; sd=standard deviation; α=Cronbach’s alpha
Recovery on CHRIs-General domains by three months
There were no statistically significant differences between the proportion recovering to their baseline scores in the hemoglobinopathies group and the comparison group (Table III). For the child report, the majority of the hemoglobinopathies group had recovered their physical (62%) and role (85%) functioning, but not emotional functioning (38%). Nearly two-thirds of the comparison group had recovered by three months for all domains as reported by the child. The majority of parents reported their child recovering by three months for physical, emotional, and role functioning across the hemoglobinopathies and comparison groups. More parents of children in the hemoglobinopathies group reported recovering their general health (75%) than the comparison group (44%) (p=0.08).
TABLE III.
Recovery of CHRIs-General domain and general health scores by three months post-HSCT by causal disease as assessed by the child and parent
| Hemoglobinopathies | Comparison Group | ||||
|---|---|---|---|---|---|
| Recovered | Not recovered | Recovered | Not recovered | p-value | |
| Child Report | |||||
| Physical Functioning | 8/13 (62%) | 5/13 (39%) | 156/242 (64%) | 86/242 (36%) | 1.00 |
| Emotional Functioning | 5/13 (38%) | 8/13 (62%) | 149/242 (62%) | 93/242 (38%) | 0.14 |
| Role Functioning | 11/13 (85%) | 2/13 (15%) | 156/242 (64%) | 86/242 (36%) | 0.23 |
| Parent Report | |||||
| Physical Functioning | 8/12 (67%) | 4/12 (33%) | 139/240 (58%) | 101/240 (41%) | 0.77 |
| Emotional Functioning | 8/12 (67%) | 4/12 (33%) | 132/241 (55%) | 109/241 (45%) | 0.56 |
| Role Functioning | 9/12 (75%) | 3/12 (25%) | 146/241 (61%) | 95/241 (39%) | 0.38 |
| General Health | 9/12 (75%) | 3/12 (25%) | 107/232 (44%) | 134/232 (56%) | 0.08 |
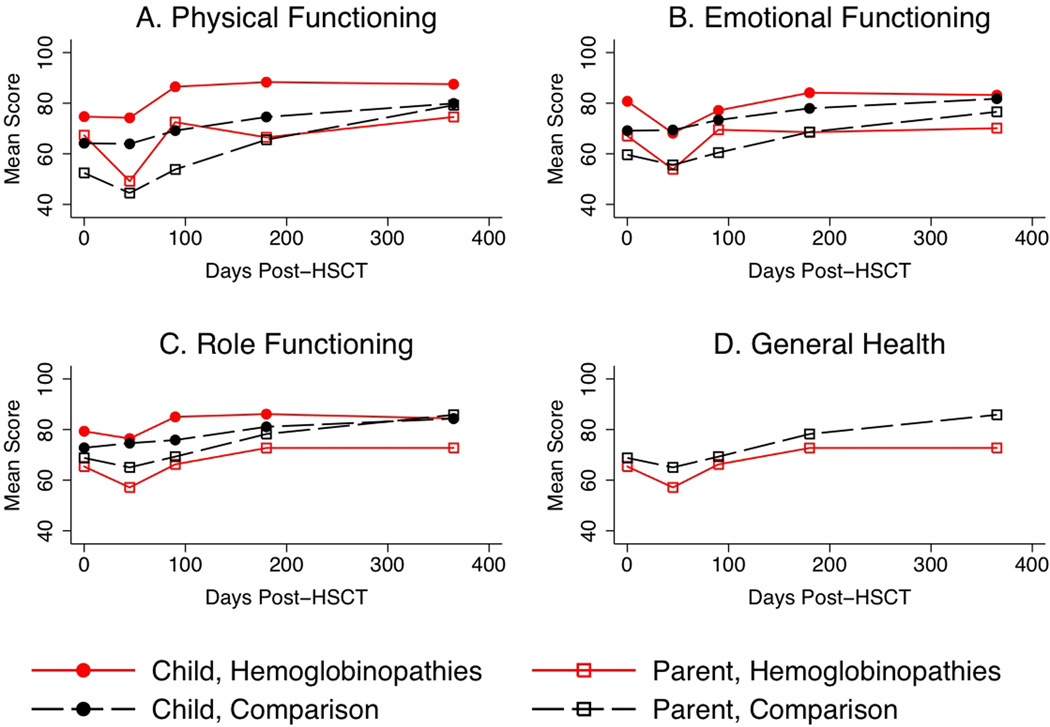
CHRIs-General and CHRIs-HSCT patterns of scores by domain
Figure 1 plots mean CHRIs-General and general health over time, by rater and diagnostic group. Child ratings of their physical functioning were higher among the hemoglobinopathies group than the comparison group (beta=12.0, se=5.5, p=0.03). The three-month physical functioning score was higher than baseline for all children (beta=4.7, se=1.8, p=0.01). Child ratings of baseline emotional functioning were higher for the hemoglobinopathies group (beta=11.6, se=5.5, p=0.03) than the comparison group. The hemoglobinopathies group experienced a larger drop in emotional functioning at 45 days than the comparison group (beta=−12.4, se=6.1, p=0.04), but their three-month scores were not statistically different than their baseline scores (p=0.13). The comparison group exceeded their baseline emotional functioning scores by nearly four points at three months (beta=3.7, se=1.4, p=0.01). There were no time or group effects for the child rating of role functioning.
Figure 1.
CHRIs-General domain and general health scores over time by diagnostic group assessed by the child and parent.
For all parent of child ratings of physical, emotional, and role functioning, there was no effect of group. Each parent rating had a nadir at 45 days (p<0.05 for physical and emotional functioning; p=0.06 for role functioning) and returned to the baseline score at three months (no significant difference from baseline score, p>0.1 for all domains). The interaction between diagnostic group and time in the parent of child physical functioning model showed a sharper decrease in scores from baseline to 45 days compared to the comparison group (effect size = −0.3, p=0.35) and a sharper increase in scores from 45 days to three months (effect size= 0.3, p=0.37). For the general health model, parents of children in both groups rated their child’s general health as similar at baseline. However, parents of children with hemoglobinopathies rated their child’s general health as 18 points higher than the comparison group at three months (se=9.0, p=0.04).
The model that compared rater differences over time regardless of diagnostic group found children rate their functioning higher than their parents. A significant interaction between time and rater indicated that parents reported a drop in scores at 45 days, but children did not. By three months, parents and children had returned to their baseline scores across all domains. The model restricted to those with hemoglobinopathies found that child ratings were higher than parent ratings for physical (beta=17.1, se=8.8, p=0.07), emotional (beta=13.2, se=5.5, p=0.03), and role (beta=18.3, se=7.7, p=0.04) functioning. The interaction between rater and time was not significant.
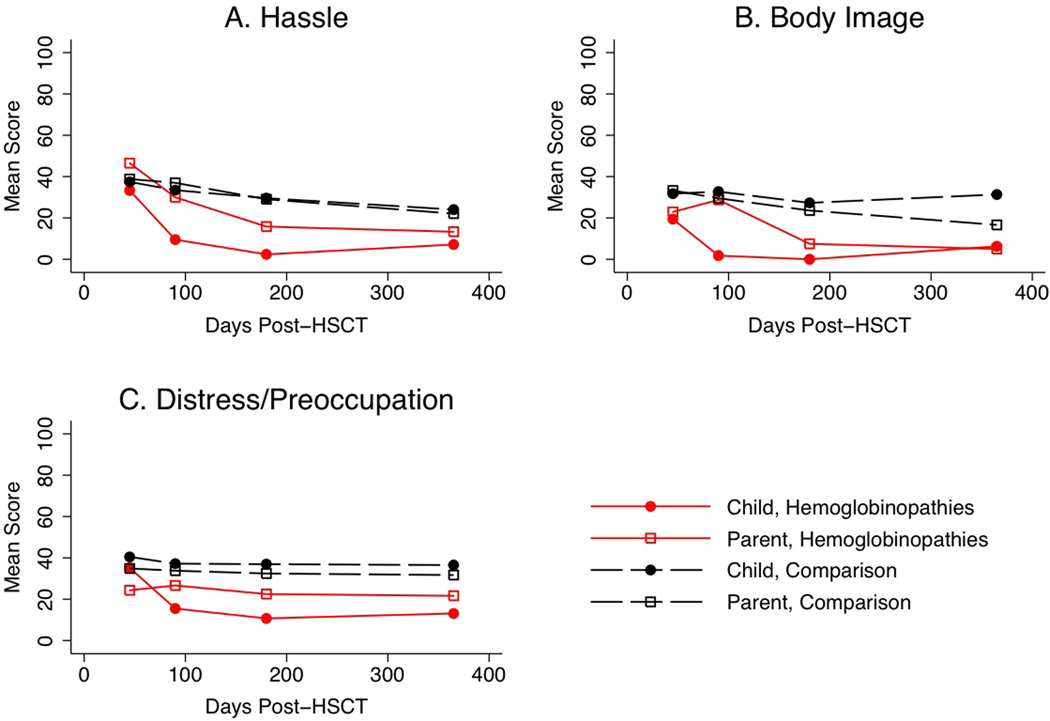
Figure 2 plots mean CHRIs-HSCT over time, by rater and diagnostic group. The models examining child ratings of CHRIs-HSCT domains (hassle, body image, distress/preoccupation) found no effect of diagnostic group. However, effect sizes indicate some evidence that the hemoglobinopathies group had lower scores than the comparison group (hassle: effect size= −0.6, p=0.09; body image: effect size= −0.5, p=0.12; distress/preoccupation: effect size= −0.4, p=0.35).. The scores decreased at 45 days for both diagnostic groups (p<0.05 for one or more time points after 45 days for each domain).
Figure 2.
CHRIs-HSCT domain scores over time by diagnostic group as assessed by the child and parent.
DISCUSSION
This is the first longitudinal evaluation of HRQL of children with SCD or thalassemia post-HSCT. Because many physicians are more familiar with HSCT among children with malignancies or aplastic anemia, we created a comparison group to guide physicians about the similarities and differences between children with hemoglobinopathies who undergo HSCT and others.
There were several similarities in the clinical outcomes and the HRQL among the two groups. Interestingly, the two groups had comparable rates of early infection, aGVHD, and cGVHD. Also the HRQL trajectory followed a similar pattern with a substantial proportion of children in both groups surpassing their baseline physical, emotional, and role functioning by three months post-HSCT. Parent ratings also followed this recovery pattern. For all CHRIs-General domains for both groups, parents reported a nadir at 45 days with recovery to baseline by three months. This pattern highlights the importance of measuring HRQL in the initial weeks following HSCT. The 45-day assessment is necessary to capture HRQL changes related to the intensity of the early transplant period. The lower parental ratings observed across all three CHRIS-General domains at all time points as compared to child reports mirror previous studies of HRQL in children with SCD, and other clinical populations[4, 30–32]. For both diagnostic groups, the child ratings of the CHRIs-HSCT domains improved over time.
There were several differences in HRQL trajectories between the two groups. For the CHRIS-General domains, physical functioning and baseline emotional functioning were higher in the hemoglobinopathies group. As we hypothesized, children with hemoglobinopathies may have higher baseline functioning because they have not recently undergone prior chemotherapy and/or radiation as many children with malignancies and aplastic anemia do prior to HSCT. These higher baseline scores differ from cross-sectional studies, which have shown lower HRQL among children with more severe disease features [3, 4, 8, 33]. Of note, while published criteria for determining HSCT eligibility, principally related to disease status and end-organ functioning, have been available for more than 15 years[13, 14], we did not routinely collect specific information on the high-risk features prompting the decision to proceed with HSCT for children within our case series.
Further, we were struck by the significant drop in emotional functioning from baseline to 45 days among child raters in the hemoglobinopathies group, such that their 45-day emotional functioning was similar to the comparison group. The effect size of −1.1 is within the range of “clinically important difference” [34]. The parallel recovery by three months within the context of comparable rates of clinical complications in both groups highlights the similarities in functional outcomes within the acute post-HSCT period.
We also note that child-rated HRQL trajectories for the hemoglobinopathies group were generally higher throughout the 12-month period (Figure 2) which has important implications for considering HSCT in affected children. Similarly, effect sizes ranging from 0.4 to 0.6 indicated that children in the hemoglobinopathies group reported more favorable scores (e.g., less hassle, distress) on the CHRIs-HSCT domains. Future investigations of HRQL in children with hemoglobinopathies undergoing HSCT should include a larger cohort with descriptive information of disease characteristics, baseline comorbidities, and indications for HSCT.
We acknowledge limitations, principally the small size of the cohort with hemoglobinopathies which likely resulted in insufficient power to detect other differences in HRQL between the groups. Grouping together SCD and thalassemia was also a limitation. While these diseases have similarities and result in chronic treatment and sequelae, there may be important differences in the clinical course and HRQL trajectories post-HSCT, which we are underpowered to investigate. We were also underpowered to explore the impact of race on HRQL. A recent study demonstrated that African-American children reported higher HRQL in the year following HSCT than other racial/ethnic groups.[35] Since SCD is more common in African-Americans, the higher scores in our sample among those with hemoglobinopathies could be due to race. Future research is needed to understand the impact of both race and diagnostic group on HRQL.
Despite these limitations, the investigation of HRQL in children who undergo HSCT for SCD or thalassemia is important. SCD and thalassemia are heterogeneous conditions with a wide spectrum of clinical outcomes. The decision to undergo HSCT for SCD or thalassemia is complex. The risk of morbidity and mortality with HSCT must be considered alongside the shortened life expectancy and chronic complications in adulthood without transplant[15, 16, 36, 37]. In a decision analysis that compared treatment with no intervention, hydroxyurea, chronic transfusions, or HSCT for children with severe SCD, treatment with hydroxyurea or HSCT was preferred [38]. However, the analysis was sensitive to HRQL estimates of children treated with either hydroxyurea or HSCT, indicating that the preferred treatment may change based on the HRQL estimates. Measurements of HRQL post-HSCT are needed to make informed decisions regarding the optimal treatment for these poor-risk patients.
Our results reveal that, based on this case series of 13 children, the HRQL trajectories for children with hemoglobinopathies are similar to a comparison group, treated at the same centers for acquired diseases. Future and more robust studies are needed to further characterize HRQL outcomes for children by disease and severity so that physicians and families can make informed decisions about the role of HSCT within the treatment armamentarium for each child.
Supplementary Material
ACKNOWLEDGEMENT
This project was funded in part from grants from the American Cancer Society (RSGPB-02-186-01-PBP, Parsons, PI) and the National Cancer Institute (R01 CA119196, Parsons, PI). Dr. Kelly is the recipient of a KM1 award from the National Cancer Institute (KM1 CA 156726).
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dampier C, Lieff S, LeBeau P, et al. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55:485–494. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panepinto JA, O'Mahar KM, DeBaun MR, et al. Health-related quality of life in children with sickle cell disease: child and parent perception. Br J Haematol. 2005;130:437–444. doi: 10.1111/j.1365-2141.2005.05622.x. [DOI] [PubMed] [Google Scholar]
- 5.Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer. 2008;51:5–9. doi: 10.1002/pbc.21557. [DOI] [PubMed] [Google Scholar]
- 6.McClish DK, Penberthy LT, Bovbjerg VE, et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual Life Outcomes. 2005;3:50. doi: 10.1186/1477-7525-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dampier C, LeBeau P, Rhee S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86:203–205. doi: 10.1002/ajh.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palermo TM, Schwartz L, Drotar D, McGowan K. Parental report of health-related quality of life in children with sickle cell disease. J Behav Med. 2002;25:269–283. doi: 10.1023/a:1015332828213. [DOI] [PubMed] [Google Scholar]
- 9.Barakat LP, Patterson CA, Daniel LC, Dampier C. Quality of life among adolescents with sickle cell disease: mediation of pain by internalizing symptoms and parenting stress. Health Qual Life Outcomes. 2008;6:60. doi: 10.1186/1477-7525-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke SA, Skinner R, Guest J, et al. Health-related quality of life and financial impact of caring for a child with Thalassaemia Major in the UK. Child Care Health Dev. 2010;36:118–122. doi: 10.1111/j.1365-2214.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 11.Ismail A, Campbell MJ, Ibrahim HM, Jones GL. Health Related Quality of Life in Malaysian children with thalassaemia. Health Qual Life Outcomes. 2006;4:39. doi: 10.1186/1477-7525-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobota A, Yamashita R, Xu Y, et al. Quality of life in thalassemia: a comparison of SF-36 results from the thalassemia longitudinal cohort to reported literature and the US norms. Am J Hematol. 2011;86:92–95. doi: 10.1002/ajh.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucarelli G, Galimberti M, Polchi P, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322:417–421. doi: 10.1056/NEJM199002153220701. [DOI] [PubMed] [Google Scholar]
- 14.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 15.Kodish E, Lantos J, Siegler M, et al. Bone marrow transplantation in sickle cell disease: the trade-off between early mortality and quality of life. Clin Res. 1990;38:694–700. [PubMed] [Google Scholar]
- 16.Davies SC. Bone marrow transplant for sickle cell disease--the dilemma. Blood Rev. 1993;7:4–9. doi: 10.1016/0268-960x(93)90018-y. [DOI] [PubMed] [Google Scholar]
- 17.van BK, Koshy M, Anderson-Shaw L, et al. Allogeneic stem cell transplantation for sickle cell disease. A study of patients' decisions. Bone Marrow Transplant. 2001;28:545–549. doi: 10.1038/sj.bmt.1703208. [DOI] [PubMed] [Google Scholar]
- 18.Cheuk DKL, Mok ASP, Lee ACW, et al. Quality of life in patients with transfusion-dependent thalassemia after hematopoietic SCT. Bone Marrow Transplant. 2008;42:319–327. doi: 10.1038/bmt.2008.165. [DOI] [PubMed] [Google Scholar]
- 19.Parsons S, Terrin N, Ratichek S, et al. Trajectories of HRQL following Pediatric Hematopoietic Stem Cell Transplantation (HSCT) The QLR Journal. 2009;A-44 Abstract#1420. [Google Scholar]
- 20.Parsons SK, Ratichek SJ, Rodday AM, et al. Caring for the caregiver: EHealth interventions for parents of pediatric hematopoietic stem cell transplant recipients. Abstract from the SIOP-psychosocial oncology (SIOP-PPO) meeting, October 21, 2011 Boston, Massachusetts, USA. Pediatr Blood Cancer. 2011;56:1159. [Google Scholar]
- 21.Parsons SK, Shih MC, Mayer DK, et al. Preliminary psychometric evaluation of the Child Health Ratings Inventories (CHRIs) and Disease-Specific Impairment Inventory-HSCT (DSII-HSCT) in parents and children. Qual Life Res. 2005;14:1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- 22.Parsons SK, Shih MC, DuHamel KN, et al. Maternal perspectives on children's health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol. 2006;31:1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Shulman H, Sullivan K, Weiden P, et al. Chronic graft-versus-host disease syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Amer J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 26.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 27.Common Terminology Criteria for Adverse Events v3.0 (CTCAE) National Cancer Institute; [Accessed on July 25, 2011]. In: http://ctep.cancer.gov. [Google Scholar]
- 28.Cronbach L. Coefficient alpha and the internal structure of test. Psychometrika. 1951;16:297–334. [Google Scholar]
- 29.Nunnally J, Bernstein I. Psychometric Theory. New York: McGraw-Hill, Inc.; 1994. [Google Scholar]
- 30.Parsons SK, Fairclough DL, Wang J, Hinds PS. Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: the value of both raters' perspectives. Qual Life Res. 2011 doi: 10.1007/s11136-011-9986-4. -epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Panepinto JA, Hoffmann RG, Pajewski NM. The effect of parental mental health on proxy reports of health-related quality of life in children with sickle cell disease. Pediatr Blood Cancer. 2010;55:714–721. doi: 10.1002/pbc.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClellan CB, Schatz J, Sanchez C, Roberts CW. Validity of the Pediatric Quality Of Life Inventory for youth with sickle cell disease. J Pediatr Psychol. 2008;33:1153–1162. doi: 10.1093/jpepsy/jsn036. [DOI] [PubMed] [Google Scholar]
- 33.Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol. 2011;33:251–254. doi: 10.1097/MPH.0b013e3182114c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 35.Brice L, Weiss R, Wei Y, et al. Health-related quality of life (HRQoL): The impact of medical and demographic variables upon pediatric recipients of hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2011;57:1179–1185. doi: 10.1002/pbc.23133. [DOI] [PubMed] [Google Scholar]
- 36.Angelucci E, Baronciani D. Allogeneic stem cell transplantation for thalassemia major. Haematologica. 2008;93:1780–1784. doi: 10.3324/haematol.2008.001909. [DOI] [PubMed] [Google Scholar]
- 37.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien SH, Hankins JS. Decision analysis of treatment strategies in children with severe sickle cell disease. J Pediatr Hematol Oncol. 2009;31:873–878. doi: 10.1097/MPH.0b013e3181b83cab. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.