Abstract
Background
Interactions between the gut, immune system, and the liver, as well as the type of fat in the diet, are critical components of alcoholic liver disease (ALD). The goal of the present study was to determine the effects of saturated and unsaturated fat on ethanol-induced gut-liver interactions in a mouse model of ALD.
Methods
C57BL/6N mice were fed Lieber-DeCarli liquid diets containing EtOH and enriched in unsaturated (USF, corn oil) or saturated fat (SF, MCT:beef tallow). Control mice were pair-fed on an isocaloric basis. Liver injury and steatosis, blood endotoxin levels, intestinal permeability and tight junction (TJ) integrity, as well as hepatic Toll-like receptor (TLR) gene expression were evaluated.
Results
After 8 weeks of EtOH feeding liver injury and steatosis were observed in USF+EtOH group compared to control and SF+EtOH. Significantly increased intestinal permeability in conjunction with elevated blood endotoxin levels were observed in the ileal segments of the mice fed USF+EtOH. USF diet alone resulted in down-regulation of intestinal TJ protein mRNA expression compared to SF. Importantly, alcohol further suppressed TJ proteins in USF+EtOH but did not affect intestinal TJ in SF+EtOH group. The type of fat in the diet alone did not affect hepatic TLR expression. Compared to control animals, hepatic TLR (TLR 1, 2, 3, 4, 7, 8, 9) mRNA expression was significantly (p<0.05) increased in USF+EtOH, but not in SF+EtOH group. Notably, TLR5 was the only up-regulated TLR in both SF+EtOH and USF+EtOH groups.
Conclusions
Dietary fat is an important cofactor in alcohol-associated liver injury. We demonstrate that unsaturated fat (corn oil/linoleic acid) by itself results in dysregulation of intestinal TJ integrity leading to increased gut permeability, and alcohol further exacerbates these alterations. We postulate that elevated blood endotoxin levels in response to unsaturated fat and alcohol in conjunction with up-regulation of hepatic TLRs combine to cause hepatic injury in ALD.
Keywords: Alcoholic Liver Disease, Saturated and Unsaturated Fat, Gut Permeability and Intestinal TJ, Liver TLRs
INTRODUCTION
Alcoholic liver disease (ALD) is one of the leading causes of liver diseases and a major cause of morbidity and mortality in the United States and worldwide. Interaction between the gut, immune system, and the liver are critical components of ALD. It is generally accepted that gut-derived endotoxin (lipopolysaccharide - LPS) plays a crucial role in the pathogenesis of ALD (Purohit et al., 2008, Rao et al., 2004). Significantly increased levels of LPS were found in patients with different stages of ALD – fatty liver, hepatitis and cirrhosis (Fukui et al., 1991, Parlesak et al., 2000) and in experimental animal models of ALD (Zhong et al., 2010, Nanji et al., 2001). Several mechanisms have been proposed for alcohol-induced endotoxemia: excessive production of endotoxin through ethanol-induced bacterial overgrowth (Bode et al., 1993); delayed endotoxin clearance from the blood (Urbaschek et al., 2001); oxidative stress and alcohol-induced generation of nitric oxide (Tang et al., 2009, Banan et al., 2000, Keshavarzian et al., 1996). Translocation of LPS across the gut epithelial barrier has recently been attributed to the disruption of intestinal barrier function (Parlesak et al., 2000, Banan et al., 1999, Keshavarzian et al., 1994). LPS stimulates different cells in the liver to release cytokines/chemokines, and reactive oxygen species (ROS) via Toll-like receptor 4 (TLR4)-mediated mechanisms (Hritz et al., 2008, Mandrekar and Szabo, 2009). In addition to TLR4, other TLRs may play a role in liver hypersensitivity to LPS and other gut-derived pathogen-associated molecules (Gustot et al., 2006, Petrasek et al., 2010).
There is considerable evidence that the amount and type of fat in the diet is an important determinant of the liver injury in ALD. It has been shown that diets enriched in saturated fatty acids or medium chain triglycerides (MCT) protect against development of ALD in rodents, whereas diets containing polyunsaturated fatty acids (PUFA) promote alcohol-induced liver damage (Nanji et al., 1995b, Nanji et al., 2001, Ronis et al., 2004). Moreover, it has been demonstrated in an epidemiological study (Nanji and French, 1986), that expected mortality from cirrhosis was correlated with intake of saturated and unsaturated fatty acids. However, the underlying molecular mechanisms by which different types of fats potentiate or prevent ALD are not fully understood.
In the present study we investigated the effects of saturated fat (MCT enriched) and unsaturated fat (corn oil/linoleic acid enriched) diets on intestinal tight junction (TJ) integrity and permeability, regulation of multiple hepatic TLRs and consequent liver injury in a mouse model of ALD.
MATERIALS AND METHODS
Animals and Ethanol Treatment
Eight-week-old male C57BL/6N mice were obtained from Harlan (Indianapolis, IN). All mice were housed in a pathogen-free, temperature-controlled animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care with 12 hour light/12 hour dark cycles. All experiments were carried out according to the criteria outlined in the Guide for Care and Use of Laboratory Animals and with approval of the University of Louisville Animal Care and Use Committee.
Animals were fed a modified Lieber-DeCarli liquid diet containing EtOH (35% of total calories) and enriched in unsaturated fat (USF, corn oil, 55-60% of LA) or saturated fat (SF, medium-chain triglyceride:beef tallow, 82:18 ratio, Research Diet, New Brunswick, NJ, Fig. 1). Soybean oil was used in both diets to provide essential free fatty acids. Control liquid maltose-dextrin diets provided 40% of energy from fat, 43% - from carbohydrate, 17% - from protein. Initially, all mice were given the control liquid maltose dextrin diets (SF or USF, no EtOH) ad libitum for one week. Afterwards, mice were fed either the liquid ethanol diet or the control liquid maltose-dextrin diet. Ethanol was gradually increased every 3-4 days from 11.2% to 35% of total calories (5.0% [vol/vol]). The mice were fed with the EtOH diet (5% EtOH vol/vol) ad libitum for 8 weeks. The control mice were pair-fed with SF or USF maltose-dextrin diets on an isocaloric basis.
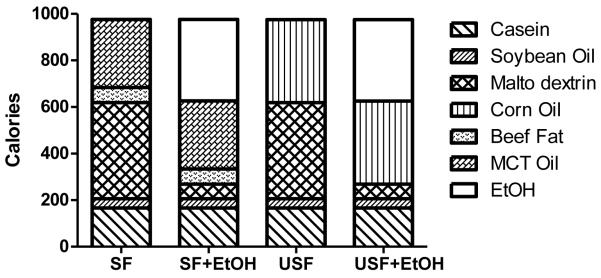
Fig. 1.
Composition of the experimental Lieber-DeCarli liquid diets. Saturated fat (SF) diet was enriched with medium chain triglyceride (MCT) oil:beef tallow fat, 82:18 ratio. Unsaturated fat (USF) diet was enriched with corn oil. Soybean oil was used in both diets to provide essential free fatty acids. The control (SF and USF) diets contained 43% of calories from carbohydrate, 17% from protein, 40% from fat. SF+EtOH and USF+EtOH diets contained 35% of calories from EtOH to replace the calories from carbohydrate.
At the end of the feeding experiment, mice were fasted overnight, anesthetized with sodium-pentobarbital (nembutal, 80 mg/kg, intraperitonially), and blood, liver, and intestinal samples were collected for assays. The killing sequence was randomized in order to eliminate any time dependent variation due to length of fasting. Blood samples were collected from the inferior vena cava using heparinized syringes and were then centrifuged at 300 g for 15 minutes at 4°C. Whole livers and intestines were removed and their weights were measured. Part of the liver from left lobe was harvested and fixed in 10% neutral-buffered formalin, while the remaining liver tissue was snap frozen in liquid N2 and stored at −80°C. Freshly isolated intestinal segments (duodenum, jejunum, ileum) were used for ex vivo intestinal permeability assay.
Assessment of Liver Injury
Alanine aminotransferase (ALT) activity was measured as a marker of liver injury using commercially available reagents from Thermo Fisher Scientific Inc. (Middletown, VA).
Liver Histological Examination
For histological analysis, liver sections were fixed in 10% buffered formalin for 24 hours and embedded in paraffin. Tissue sections were stained with hematoxylin-eosin (H&E) and examined under light microscopy at 200x magnification.
Liver Triglycerides (TG), Nonesterified-Fatty Acid (NEFA) Assay
Hepatic TG were determined as previously described (Kirpich et al., 2010) using Triglyceride Reagent (Thermo Fisher Scientific Inc., Middletown, VA). Liver NEFA were assayed using a commercially available kit from Wako Chemical USA (Richmond, VA).
Liver Reactive Oxygen Species (ROS) Detection
Dihydroethidium (DHE, Sigma, St.Louis, MO) staining was used to evaluate intracellular ROS. Nonfluorescent DHE is oxidized by ROS to yield a red fluorescent product that binds to nucleic acids, staining the nucleus a bright fluorescent red. Liver tissue cryosections were cut (10 um) on a cryostat, thaw-mounted on glass slides, and stained with 10 uM DHE for 15 min. The red fluorescence was detected using a Nicon 2000S fluorescence microscope.
Blood Endotoxin Assay
Plasma endotoxin levels were measured with Limulus Amebocyte Lysate kit (Lonza, Walkersville, MD) according to the manufacturer’s instructions.
Ex Vivo Intestinal Permeability Assay
The intestinal segments (duodenum, jejunum, ileum) were freshly isolated and placed in modified Krebs-Henseleit bicarbonate buffer (KHBB). The small intestine segment from the pyloric sphincter to the ligament of Treitz was defined as duodenum. The small intestine segment from the ligament of Treitz to ileocecal junction was divided into equal lengths as jejunum and ileum, respectively. One end of the gut segment was first ligated with suture, and 200 λl fluorescent dextran-FITC (M.W. 4,000, FD-4, 40 mg/ml) was injected into the lumen using a gavage needle to avoid mucosal injury. Then the other end of the gut segment was ligated to form a gut sac. After rinsing in the KHBB buffer, the gut sac was placed in 4 ml of KHBB and incubated at 37°C for 20 min. The FD-4 that penetrated from the lumen into the incubation buffer was measured spectrofluorometrically with an excitation wave length of 485 nm and an emission wave length of 530 nm.
RNA isolation and Real Time Reverse Transcription Polymerase Chain Reaction Assay
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Reverse transcription was performed with qScript cDNA Supermix (Quanta Biosciences) and RT-PCR with Perfecta SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD) with an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). The reverse and forward specific primers for Cyp2e1, MCP1, tight junction protein and TLR genes were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) and are listed in Table 1. TNF-α primer was purchased from SA Biosciences (Frederick, MD). All primer pairs were validated by demonstrating high amplification efficiency, consistent single peak dissociation patterns and the presence of single products of the expected size on agarose gels. The relative gene expression was normalized with 18s mRNA as the internal control.
Table 1.
Primer sequences for the targeted mouse genes (qRT-PCR assay)
| Primer set | Forward | Reverse |
|---|---|---|
| 18s | ctcaacacgggaaacctcac | cgctccaccaactaagaacg |
| mmOccludin | acccgaagaaagatggatcg | catagtcagatgggggtgga |
| mmClaudin-1 | cgggcagatacagtgcaaag | acttcatgccaatggtggac |
| mmZO-1 | tgggaacagcacacagtgac | gctggccctccttttaacac |
| mmFodrin | cgcatctttttcctcagcag | ccaggacttgctgtcgtctc |
| mmSymplekin | tgagggctgagaaggctgta | cagcacctctgccttgaatc |
| mmTjap1 (7H6) | ctccagagcaccgagagcta | gcgtttgcgaagttcttcat |
| mmp130 (Angiomotin) |
ctggatgctgctgcaactgt | gggcatggagggtcttaatc |
| mmMCP1 | ggctcagccagatgcagt | tgagcttggtgacaaaaactacag |
| mmCyp2e1 | aggggacattcctgtgttcc | ttaccctgtttccccattcc |
| mmTlr1 | gacacccctacagaaacgtccta | tgagggaatttggtttagtcattg |
| mmTlr2 | aaacctcagacaaagcgtcaa | gcgtttgctgaagaggactg |
| mmTlr3 | cagggattgcacccataatct | gacaaaagtcccccaaagga |
| mmTlr4 | gatctgagcttcaaccccttg | tgccatgccttgtcttcaat |
| mmTlr5 | ctccagacgcctcatctcac | tggcatatgttccaagcgta |
| mmTlr7 | cttggctcccttctcaggat | tccgtgtccacatcgaaaac |
| mmTlr8 | caaacgttttaccttcctttgtc | cactggttccagaggacagc |
| mmTlr9 | tccatctcccaacatggttc | ccagagtctcagccagcact |
Western Blotting
Western blotting was performed to evaluate the levels of CYP2E1 using commercially available antibody (Abcam, Cambridge, MA). Liver tissue lysates were separated by sodium dodecyl sulfate (SDS)-PAGE and subsequently transferred to a PVDF membrane. Blots were visualized using Amersham Enhanced chemiluminescence (ESL) Western blot detection (GE Healthcare, Hercules, CA), and quantified using ImageJ software. The results were expressed as the ratio of protein to β-actin.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.01 for Windows. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to evaluate significant differences between the four compared groups (SF, SF+EtOH, USF, USF+EtOH). A Student’s t-test (2-tailed) was performed to evaluate significant differences between alcohol-fed and pair-fed animals. Pearson’s correlation analysis was used to determine the correlation coefficient. A p value of <0.05 was considered statistically significant. Data were expressed as mean + SEM.
RESULTS
Food Intake, Body Weight, and Body Composition of Alcohol and Saturated or Unsaturated Fat Diets Fed Mice
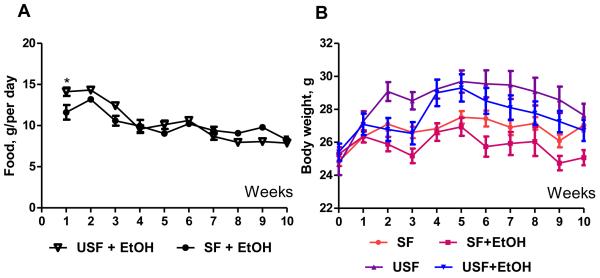
Male C57BL/6N mice were pair-fed a modified Lieber-DeCarli liquid diet enriched in corn oil (USF) or MCT:beef tallow saturated fat (SF) in the presence or absence of EtOH (35% of total calories) for 8 weeks. The amount of food consumption was significantly different between USF and SF-fed groups (14.2+0.51 vs 11.6+0.89 g/per day, p<0.05) at the beginning of the experiment when no EtOH was added to the diets (Fig. 2A). Starting from the second week of the 5% EtOH feeding, USF+EtOH and SF+EtOH groups showed significantly reduced food consumption compared to the beginning of the experiment (10.10+0.50 and 9.04+0.38 g/per day, respectively, p<0.05). There was no difference between SF and USF groups in terms of total calories consumption per day during the total period of 5% EtOH feeding. At the end of the experiment (8 weeks of feeding) USF+EtOH fed mice consumed 7.88+0.15, and SF+EtOH group consumed 8.26+0.39 g/kcal per day. According to the isocaloric pair-feeding protocol, the control animals of both SF and USF groups, received the same amount of g/kcal per day as SF+EtOH and USF+EtOH mice during all the period of experiment.
Fig. 2.
Food consumption and body weight of mice fed Lieber-DeCarli liquid diets. C57BL/6N male mice were fed Lieber-DeCarli diets for 10 weeks (1th week – SF and USF control diets, 2nd week – gradual increase in EtOH followed by 8 weeks – 5% EtOH). Control animals were pair-fed with equal amounts of isocaloric liquid diets. (A) Mice fed USF diet consumed significantly more food compared to SF diet fed animals during the 1st week (14.2+0.51 vs 11.61+0.89 g/per day, p<0.05, Student’s t-test). 5% EtOH feeding significantly reduced food consumption by both USF+EtOH and SF+EtOH groups. (B) USF diet feeding resulted in markedly increased body weight compared to SF diet feeding. Supplementation with EtOH led to gradually reduced body weight in both USF+EtOH and SF+EtOH groups.
Mice fed USF diet gained more weight than mice fed SF diet at the beginning of the experiment, when no EtOH was added. Adaptation to alcohol feeding caused a slight, but not significant, reduction of body weight in both EtOH-fed groups. USF and USF+EtOH mice had mean body weight of 29.69+0.67 g and 29.30+0.82 g, respectively (Fig. 2B), compared with a mean body weight of 27.52+0.37 g and 26.94+0.54 g in SF and SF+EtOH at the end of first month of feeding. There was a noticeable gradual reduction of body weight during second month of the experiment in both SF+EtOH and USF+EtOH groups.
Effects of Alcohol and Saturated or Unsaturated Fat Diets on Liver Injury and Steatosis
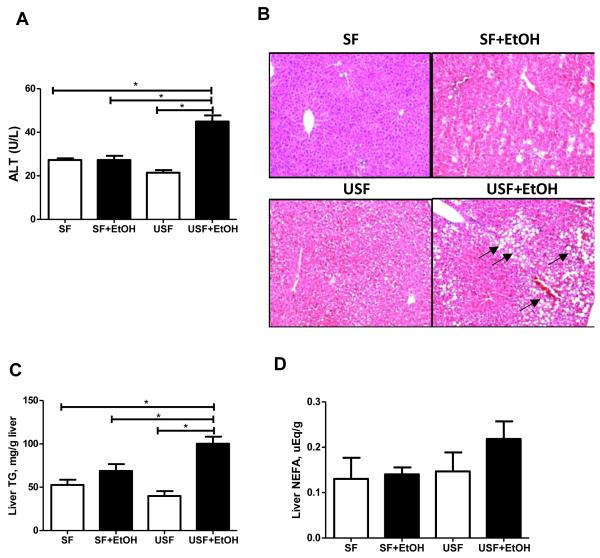
After 8 weeks of EtOH feeding serum ALT levels had increased 2.1-fold in USF+EtOH compared to the pair-fed control group. The effect of EtOH was blunted by SF containing MCT, and there was no difference in ALT levels between SF+EtOH and their pair fed littermates. Thus, liver injury was greater in USF+EtOH fed animals compared to SF+EtOH, which was confirmed by increased plasma ALT levels (44.91+2.81 vs 27.27+1.92 IU/L, respectively, p<0.05, Fig. 3A).
Fig. 3.
Effects of saturated and unsaturated diets on liver injury and steatosis in response to chronic alcohol feeding. (A) Liver injury was evaluated by plasma ALT activity. USF+EtOH feeding resulted in significant elevation of plasma ALT levels compared to control pair-fed animals as well as to SF+EtOH fed group (44.91+2.81 vs 21.43+1.19 and 27.27+1.92 IU/L, respectively, *p<0.05, one-way ANOVA with Tukey’s post hoc test). Values are mean+SEM, n=6 animals/per group. (B) Liver hematoxylin-eosin staining demonstrated severe micro- and macrovesicular steatosis in USF+EtOH fed mice compared to control pair-fed animals as well as SF+EtOH fed groups. Arrows indicate the fat droplets (x200 final magnification). (C) Significant triglyceride accumulation was observed in the livers of USF+EtOH fed mice compared to control pair-fed fed animals as well as to SF+EtOH fed group (100.2+8.1 vs 39.93+5.59 and 68.76+7.96 mg/g liver, respectively, *p<0.05, one-way ANOVA with Tukey’s post hoc test). (D) Liver nonesterified fatty acid (NEFA) measurement. NEFA levels were not significantly different between the experimental groups.
Histological examination of livers from SF+EtOH and USF+EtOH-fed mice after 8 weeks of 5% EtOH feeding demonstrated noticeable fat accumulation in the USF+EtOH-fed animals (Fig. 3B). There was extensive microvesicular and macrovesicular liver steatosis in this group compared to SF+EtOH. However, a small number of tiny lipid droplets were observed in the SF+EtOH group. Confirming the histological examination, the hepatic TG content was significantly higher in USF+EtOH compared to SF+EtOH fed animals (100.2+8.1 vs 68.76+7.96 mg/g liver, p<0.05 [one way ANOVA], Fig. 3C). To further confirm the USF+EtOH-mediated liver fat accumulation, hepatic NEFA were measured. NEFA levels in the USF+EtOH group were increased by 33% compared to control, however there was no statistically significant difference in the liver NEFA in the USF+EtOH group compared to control as well as to SF+EtOH animals (Fig. 3D).
Effects of Alcohol and Saturated or Unsaturated Fat Diets on Hepatic Inflammation, Macrophage Infiltration and Oxidative Stress
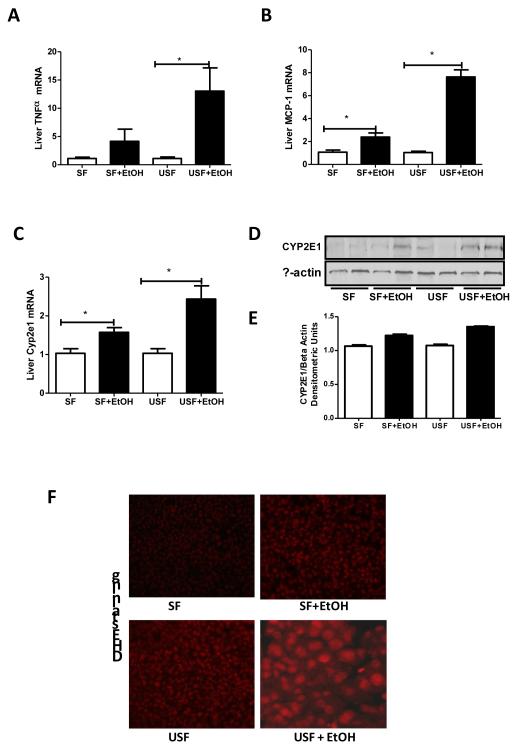
To further evaluate the effects of different types of dietary fat on the liver in association with alcohol, we measured several markers of hepatic inflammation, macrophage infiltration and oxidative stress. Expression of MCP-1, a marker of macrophage infiltration as well as TNF-α were significantly up-regulated in response to USF+EtOH compared to control animals and SF+EtOH group (Fig. 4A, 4B). There were no changes observed in the expression of these genes between the two types of fat alone.
Fig. 4.
Hepatic markers of inflammation, macrophage infiltration and oxidative stress in response to alcohol and saturated and unsaturated diets. EtOH feeding induced hepatic inflammation, macrophage infiltration and reactive oxygen species (ROS) accumulation in both SF+EtOH and USF+EtOH groups compared to control pair-fed animals with more prominent elevation in USF+EtOH group. (A) Inflammation marker: TNF-α mRNA levels. (B) Macrophage infiltration marker: MCP-1 mRNA levels. (C) Oxidative stress marker: Cyp2e1 mRNA levels. All mRNA levels were assessed by RT-PCR. The relative mRNA expression was analyzed using 2-ΔΔCt method by normalizing with 18s mRNA expression. Results are given as fold changes in EtOH fed groups compared to control pair-fed animals. Values in control groups were set as 1. Values are mean+SEM, n=6 animals/per group, *p <0.05, Student’s t-test. (D) Western blot analysis of CYP2E1 protein expression. Expression of β-actin was used as a loading control, n=2 animals/group. (E) Quantitative analysis of CYP2E1 levels demonstrated increase in CYP2E1 expression in the livers of mice fed SF+EtOH and USF+EtOH diets compared to their control pair-fed animals. The intensity of protein bands was quantified by densitometry using the NIH ImageJ software (NIH, Bethesda, MD). (F) ROS accumulation in the livers assessed by dihydroethidium staining. ROS accumulation was markedly increased in USF+EtOH group compared to control pair-fed animals and SF+EtOH fed littermates. Liver cryostat sections were incubated with 10 uM dihydroethidium for 15 min. Red fluorescence was formed upon oxidation by ROS, and intensity was detected by fluorescence microscope.
Chronic alcohol exposure induces cytochrome P450 (Cyp2e1), which plays an important role in the development of ALD (Lu et al., 2008). In addition, Cyp2e1 levels are sensitive to dietary manipulations (Yoo et al., 1991), therefore, dietary fat is important in modifying EtOH-dependent induction of Cyp2e1 (Amet et al., 1998). However, we did not observe any changes in Cyp2e1 expression in response to either SF and USF diets alone. As expected, EtOH feeding up-regulated hepatic Cyp2e1 expression at gene and protein levels in both experimental groups compared to controls, with a more profound induction in USF+EtOH group (Fig. 4C, 4D, 4E).
ROS accumulation assessed by fluorescent ethidium, a marker of intracellular superoxide anion generation, was markedly increased in USF+EtOH group compared to control pair-fed animals and SF+EtOH fed littermates (Fig. 4F).
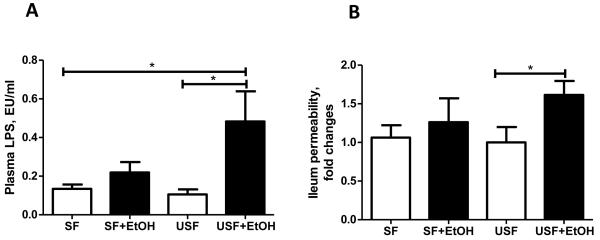
Unsaturated But Not Saturated Fat Fed Animals Demonstrated Significantly Increased Levels of Blood Endotoxin and Altered Intestinal Tight Junction in Response to Ethanol Feeding
Feeding of USF+EtOH for 8 weeks resulted in 4.5-fold (p<0.05) increase of blood LPS compared to control, pair-fed animals (Fig. 5A). A moderate, but not significant, increase of LPS levels was also observed in SF+EtOH compared to pair-fed group. Because of high variability between animals in USF+EtOH group, there was also no statistically significant difference in LPS levels between USF+EtOH and SF+EtOH even though a two-fold difference was noted. We did not observe any differences in blood LPS levels between the two different diets (SF and USF).
Fig. 5.
Plasma endotoxin levels and intestinal permeability in response to alcohol and saturated and unsaturated fat diets. (A) Plasma endotoxin levels assessed by plasma LPS (lipopolysaccharides) measurement. LPS levels were significantly increased in USF+EtOH compared to USF pair-fed control animals (0.48+0.15 vs 0.10+0.02, *p<0.05). LPS levels in SF+EtOH group appeared to be increased compared to control SF pair-fed control animals (0.21+0.05 vs 0.13+0.02, p=0.18), but did not reach statistical significance. Values are mean+SEM, n=6 animals/per group, one-way ANOVA with Tukey’s post hoc test. (B) Intestinal permeability of the ileum segment. Significantly increased intestinal permeability was observed in mice fed USF+EtOH compared to USF pair-fed control group. There were no differences in ileum permeability in SF+EtOH compared to SF pair-fed control animals. Ileum permeability was determined ex vivo using fluorescent dextran FD-4. Data are expressed as fold changes in EtOH fed groups compared to control pair-fed animals. FD-4 permeability in control groups was set as 1. Values are mean+SEM, n=6-8 animals/per group, *p <0.05, Student’s t-test.
The plasma LPS levels reflect intestinal barrier integrity. Therefore, we evaluated the effects of EtOH in association with the different types of fat on intestinal integrity by ex vivo measuring intestinal permeability to fluorescent dextran-FITC (FD-4). A trend to increased permeability was noticed in the duodenum segments in both experimental groups, but there was no difference in the jejunum segment (data not shown). Significantly increased permeability was observed in the ileum in mice fed USF+EtOH (Fig. 5B), which was consistent with the results of a previous study from our group, showing increased ileal permeability in response to chronic EtOH feeding for 4 weeks (Zhong et al., 2010).
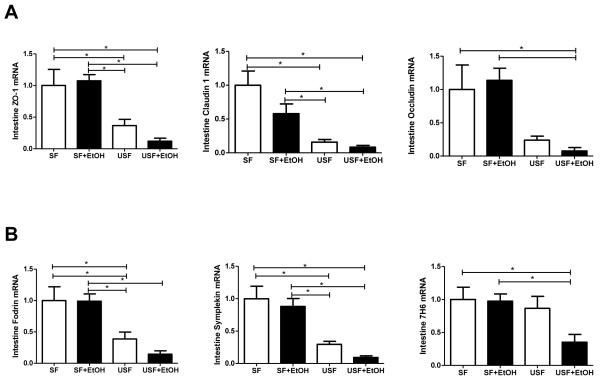
Since intestinal integrity is controlled by multiple tight junction (TJ) proteins, we evaluated expression of key markers of TJ integrity (ZO-1, claudin-1 and occludin), as well as several protein-adaptors which may contribute to intestinal barrier dysfunction in response to dietary fat and EtOH. Analysis of mRNA expression revealed significant (p<0.05) down regulation of ZO-1, claudin-1, and occludin in response to USF diet (corn oil/LA) compared to SF diet (MCT:beef tallow). Similarly, expression of several protein-adaptors, including symplekin and fodrin, was also found to be down regulated (Fig. 6). Importantly, USF+EtOH diet further suppressed expression of these proteins. A strong positive correlation (r=0.82, p=0.045) was seen between symplekin and liver injury, as assessed by ALT, suggesting that this protein may play an important role in EtOH-induced gut-liver interactions. A positive correlation was also observed between liver injury and claudin-1 (r=0.88, p=0.019).
Fig. 6.
Effects of saturated and unsaturated diets on ileum tight junction protein and protein-adaptor mRNA levels in response to chronic alcohol feeding. USF diet significantly down-regulated mRNA levels of (A) ileum tight junction proteins and (B) protein-adaptors compared to SF diet. EtOH supplemented to the USF diet resulted in further suppression of these proteins. Intestinal tight junction protein mRNA expression was assessed by RT-PCR. The relative mRNA levels were analyzed using 2-ΔΔCt method by normalizing with 18s mRNA expression. Results are presented as fold changes over SF group set as 1. Values are mean+SEM, n=6 animals/per group, *p <0.05, one-way ANOVA with Tukey’s post hoc test.
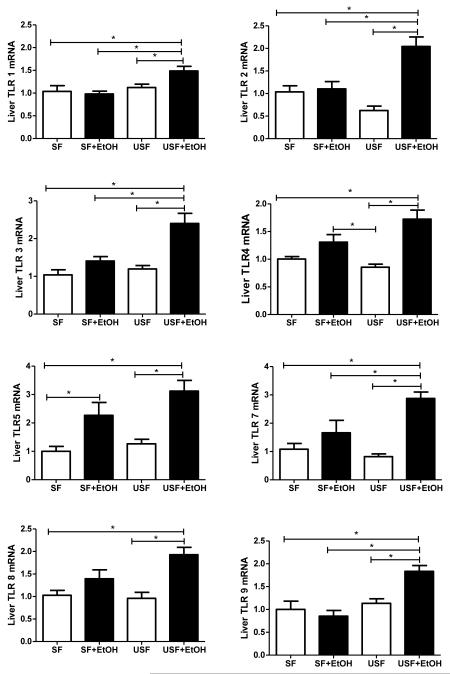
Up Regulation of the Hepatic Toll-Like Receptors in Response to EtOH and Unsaturated but not to EtOH and Saturated Fat Diets
Toll-like receptors (TLRs) are a group of evolutionarily conserved pattern recognition receptors involved in the activation of the immune system in response to various pathogens. The healthy liver expresses low mRNA levels of TLRs in comparison to other organs (Zarember and Godowski, 2002), which may contribute to the high tolerance of the liver to the intestinal pathogen-associated molecules to which the liver is constantly exposed (Mencin et al., 2009). In our study, RT-PCR analyses showed that after 8 weeks of alcohol feeding, hepatic TLR mRNA expression was significantly up-regulated in the USF+EtOH group compared to control and SF+EtOH fed animals. Interestingly, TLR-5 was the only TLR which had up-regulated mRNA expression levels in both SF+EtOH and USF+EtOH treated groups (Fig. 7). Notably, the type of fat in the diet alone did not affect hepatic TLR expression. To determine the possible link between induction of the hepatic TLR system and other mechanisms of EtOH-induced liver injury, we investigated the relation between hepatic Cyp2e1, MCP-1, TNF-α and TLRs. The most significant associations were seen between levels of Cyp2e1 and TLR5 gene expression. Interestingly, a strong correlation was observed in both experimental groups, SF+EtOH (r=0.83, p=0.039) and USF+EtOH (r=0.81, p=0.043).
Fig. 7.
Effects of saturated and unsaturated diets on hepatic TLR mRNA levels in response to chronic alcohol feeding. No significant differences in hepatic TLR mRNA expression were observed between the SF or USF diet groups. After 8 weeks of alcohol feeding, hepatic TLR 1, 2, 3, 4, 7, 8, 9 mRNA levels were significantly up-regulated in the USF+EtOH fed mice compared to USF pair-fed control animals. Only TLR5 mRNA was up-regulated in both SF+EtOH and USF+EtOH groups compared to their respective pair-fed groups. Hepatic TLR mRNA levels were assessed by RT-PCR. The relative mRNA expression was analyzed using 2-ΔΔCt method by normalizing with 18s mRNA expression. Results are presented as fold changes over SF group set as 1. Values are mean+SEM, n=6 animals/per group, *p <0.05, one-way ANOVA with Tukey’s post hoc test.
DISCUSSION
The present study further documents the important role of different types of dietary fat in the gut-liver pathology associated with chronic alcohol consumption. We demonstrate the protective effect of SF, mainly represented as MCT, on EtOH-induced liver injury through maintaining intestinal TJ integrity and consequently decreasing gut leakiness and endotoxemia. The ability of the USF diet (containing high amounts of corn oil) to down-regulate intestinal TJ protein expression may prime intestinal epithelial cells to EtOH, resulting in further suppression and dysfunction of intestinal TJ integrity, increasing intestinal permeability and blood endotoxin levels, all of which were correlated with liver pathology. Several studies address the role of different types of fat in alcohol-induced gut permeability, endotoxemia and liver injury. The protective effect of MCT on the liver and gut permeability was also demonstrated in rats administrated endotoxin (LPS) intravenously (Kono et al., 2003). Our data are in agreement with previously published observations showing increased plasma endotoxin levels in corn oil+EtOH-fed rats and more severe liver injury compared to animals fed SF+EtOH (Nanji et al., 1993). In the rat model of enteral alcohol feeding, MCT (32% of total calories) compared to animals fed corn oil, inhibited formation of TNF-α by Kupffer cells and free radical formation caused by elevated blood endotoxemia (Kono et al., 2000). However, it was also shown that the PUFA (primarily from corn oil)+EtOH did not cause a significant increase of plasma endotoxin levels in mice compared with the control diet (You et al., 2005). This discrepancy with our results and earlier published studies from our group (Zhong et al., 2010), might be explained by different animal strains and the duration of feeding.
Although disruption of intestinal integrity is considered to be a major cause of alcoholic endotoxemia (Parlesak et al., 2000), the exact mechanism(s) by which alcohol and the different types of fat in a diet contribute to increased intestinal permeability and endotoxemia are not fully elucidated. Alcohol (Ma et al., 1999, Zhong et al., 2010) and its metabolite, acetaldehyde (Rao, 1998) induce increased intestinal permeability via alterations of intestinal TJ in vivo and in vitro. Decreased ZO-1 protein levels were found in sigmoid colon biopsies of alcoholics (Tang et al., 2008). It was also shown that EtOH-mediated oxidative stress can lead to disruption of TJ integrity (Farhadi et al., 2006). Our data and results from previously published studies in NAFLD (Cani et al., 2008) clearly demonstrate that the amount of fat in a diet and its composition can also significantly modulate the intestinal TJ. For example, a high fat diet containing 72% fat (corn oil and lard) decreased mRNA expression of ZO-1 and occludin in an animal model of NAFLD (Cani et al., 2008). We have also observed that USF diet (40% fat from corn oil) down-regulates mRNA expression of intestinal TJ proteins compared to SFD (MCT:beef tallow). Importantly, alcohol further suppressed the expression of TJ proteins in USF+EtOH fed animals and did not affect intestinal TJ in SF+EtOH group. In addition to down-regulation of well studied TJ proteins (ZO-1, occludin, claudin-1) in response to USF and EtOH, we observed significant suppression of several protein-adaptors involved in TJ integrity, including symplekin, and 7H6. A strong positive correlation between symplekin and liver injury suggests that symplekin may play an important role in EtOH-induced gut-liver interactions. Although little is known to date about the functional importance of these proteins in the TJ integrity, their down-regulation in response to USF but not SF and EtOH requires further investigation.
One possible mechanism of the disruption of intestinal integrity in response to the USF diet (corn oil/linoleic acid (LA) enriched) could be intestinal inflammation-mediated alterations of TJ. LA is known to induce pro-inflammatory effects in human vascular endothelial cells (Hennig et al., 2006), and to activate NF-kB in hepatic Kupffer cells (Rusyn et al., 1999). Similarly, LA may play a role in modulation of the inflammatory response in intestinal epithelial cells by NF-kB activation and increased pro-inflammatory cytokine production. Recently, the important role of pro-inflammatory mediators in altered intestinal epithelial TJ permeability has been demonstrated (Ma et al., 2004, Ye et al., 2006, Amin et al., 2009). Furthermore, other diet-associated mechanisms could be involved in the increased gut permeability observed in our study in response to EtOH and LA enriched diet. For example, LPS can translocate across the gut epithelium in association with chylomicrons (Ghoshal et al., 2009). We did not observe increased LPS levels in the USF diet fed group, possibly because the animals were fasted overnight. Therefore, the mechanisms contributing to alcohol-induced endotoxemia need additional study and are currently under investigation in our laboratory.
The most significant observation of this study addressing the roles of different types of dietary fat on alcohol-induced liver injury is that USF (LA enriched diet) activates the hepatic TLR signaling pathways in response to EtOH. While SF+EtOH did not cause significant changes in TLR mRNA expression with the exception of TLR5, USF+EtOH up-regulated TLR1, 2, 3, 4, 5, 7, 8, and 9. Induction of the hepatic TLR system paralleled the increased blood endotoxin levels. Our data are consistent with a recently published study demonstrating increased liver TLR mRNA expression in response to 10 days of EtOH feeding (Gustot et al., 2006). Interestingly, up-regulation of hepatic TLRs in the study by Gustot et al. was observed even though plasma endotoxin levels were not elevated. Antibiotic treatment reduced the severity of alcohol-induced fatty liver. However, the expression of hepatic TLRs remained significantly higher in EtOH-fed mice compared to control animals, suggesting that hepatic TLR mRNA expression was not directly dependent on gut bacteria. The investigators demonstrated that increased TLR 2, 4, 6, and 9 mRNA expression was dependent on NADPH oxidase-induced oxidative stress.
Although our study was not designed to address the question of the potential contribution of the hepatic TLR/ligands interaction to the liver pathology associated with chronic alcohol consumption, it is likely that other bacterial components rather than LPS are responsible for some pathological changes observed in ALD. In research by Gustot and coworkers, specific ligands to different TLRs were administerred to experimental animals, resulting in increased liver TNF-α expression in EtOH-fed but not control animals (Gustot et al., 2006). Interestingly, the authors also obseved that the livers of EtOH-fed animals were more sensitive to flagellin challenge compared to control animals even in the absence of up-regulation of TLR5 (flagellin receptor). Up-regulation of hepatic TLR5 mRNA expression observed in our study may be due to a more extended period of alcohol feeding (8 weeks in our experiments compared to 10 days in the above mentioned study). Furthermore, TLR5 over-expression in response to EtOH was observed regardless the type of fat in a diet, and it was not associated with the liver injury in SF+EtOH group compared to USF+EtOH. Since a strong positive correlation was observed between Cyp2e1 and TLR5, increased TLR5 expression may be dependent not only on gut-derived pathogens but also, at least in part, on the degree of oxidative stress in the liver, as was demonstrated by Gustot and coworkers (Gustot et al., 2006).
One of the mechanisms which primes or sensitizes animals to LPS-induced liver injury is induction of Cyp2e1 (Lu et al., 2005). EtOH-induced Cyp2e1 plays an important role in the liver pathology, leading to increased lipid peroxidation, mitochondrial dysfunction and hepatotoxicity. In spite of some controversy about the role of Cyp2e1 in ALD, a strong correlation between the degree of steatosis and EtOH-induced Cyp2e1 was demonstrated in a rat model of ALD, and inhibition of Cyp2e1 decreased liver fat accumulation (Jarvelainen et al., 2000). No liver steatosis was observed in Cyp2e1-knockout mice after 2, 3, or 4 weeks of EtOH feeding (Lu et al., 2008). It has also been demonstrated that dietary fat is an important factor in modifying EtOH-dependent induction of Cyp2e1 (Amet et al., 1998, Takahashi et al., 1992, Nanji et al., 1995a, Nanji et al., 1994a). In our study, USF diet caused higher induction of hepatic Cyp2e1 gene expression compared to SF, which was correlated with the levels of endotoxemia and severity of liver injury. Our observation is in agreement with previous studies in a rat model of intragastric EtOH feeding, where Cyp2e1 protein content was higher in rats fed corn oil compared to MCT diet (Amet et al., 1998). Importantly, the MCT/LCT ratio plays an important role in modulating Cyp2e1 expression. MCT as partial replacement of LCT was not beneficial, whereas all alcohol effects were attenuated by a diet containing MCT as a total replacement for LCT (Lieber et al., 2007).
Several mechanisms of either harmful or beneficial effects of different types of dietary fat on alcohol-associated liver injury have been proposed. The deleterious effects of PUFAs are thought to be mediated through induction of oxidative stress, potentiated by inducing cytochrome P450 2E1 (Nanji et al., 1994b, Nanji et al., 1994a, Nanji et al., 1995a), elevated endotoxin levels, and increased production of proinflammatory cytokines (Nanji, 2004). It was shown that dietary linoleic acid (LA) was required for the development of experimental alcoholic liver injury in the rat intragastric EtOH feeding model (Nanji and French, 1989). Nanji’s group demonstrated that the conversion of LA to arachidonic acid and its subsequent metabolism was responsible for some of the observed pathological changes. Feeding of unsaturated fatty acids (corn oil, 25% of calories) along with EtOH was associated with increased concentrations of cyclooxygenase-2, thromboxane B2, and leukotriene B4, as important mediators of ALD (Nanji et al., 1993).
The postulated protective effects of SF against the development of alcoholic fatty liver are multifactorial and include down-regulation of lipid peroxidation (Nanji et al., 1995b), increased liver membrane resistance to oxidative stress (Ronis et al., 2004), induction of adiponectin (You et al., 2005), and regulation of the hepatic SIRT1-SREBP-1-histone H3 axis, resulting in suppression of genes encoding lipogenic enzymes (You et al., 2008). The beneficial effect of MCT was also attributed to the reduction of EtOH-induced mitochondrial Cyp2e1 levels, normalization of mitochondrial glutathione (Lieber et al., 2007), inhibiting free radical formation, and reducing TNF-α production via inhibition of endotoxin-mediated activation of Kupffer cells (Kono et al., 2000).
There are some limitations in the study. Our study was not designed to identify the potential contribution of the hepatic TLR/ligands interactions, however, it will be of a great interest to evaluate the ethanol-mediated response of the liver to the challenges of gut-derived pathogens other than LPS.
In summary, our overall data demonstrate that USF, enriched in linoleic acid, is an important cofactor in alcohol-mediated liver injury and its role in gut-liver interactions, is at least, twofold. In the intestine USF by itself induces dysregulation of intestinal TJ integrity and alcohol further exacerbates these alterations. In the liver USF and alcohol up-regulate hepatic TLRs, pattern recognition receptors of gut-derived endotoxins, contributing to liver damage.
ACKNOWLEDGMENTS
The authors thank Marion McClain for manuscript proofreading.
The work presented in this study was supported by NIH grants R01 AAO14371 (SB), P01 AA017103 (CJM), R01 AA0015970 (CJM), R01 AA018016 (CJM, SB), R01 DK071765 (CJM), R37 AA010762 (CJM), R01 AA018869 (CJM), P30 AA019360 (CJM), RC2AA019385 (CJM), and the Department of Veterans Affairs (CJM).
REFERENCES
- Amet Y, Adas F, Nanji AA. Fatty acid omega- and (omega-1)-hydroxylation in experimental alcoholic liver disease: relationship to different dietary fatty acids. Alcohol Clin Exp Res. 1998;22:1493–1500. [PubMed] [Google Scholar]
- Amin PB, Diebel LN, Liberati DM. Dose-dependent effects of ethanol and E. coli on gut permeability and cytokine production. J Surg Res. 2009;157:187–192. doi: 10.1016/j.jss.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- Bode C, Kolepke R, Schafer K, Bode JC. Breath hydrogen excretion in patients with alcoholic liver disease--evidence of small intestinal bacterial overgrowth. Z Gastroenterol. 1993;31:3–7. [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17:766–772. doi: 10.1016/j.jnutbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89:2205–2211. [PubMed] [Google Scholar]
- Keshavarzian A, Jacyno M, Urban G, Winship D, Fields JZ. The role of nitric oxide in ethanol-induced gastrointestinal dysfunction. Alcohol Clin Exp Res. 1996;20:1618–1624. doi: 10.1111/j.1530-0277.1996.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Gobejishvili LN, Homme MB, Waigel S, Cave M, Arteel G, Barve SS, McClain CJ, Deaciuc IV. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Enomoto N, Connor HD, Wheeler MD, Bradford BU, Rivera CA, Kadiiska MB, Mason RP, Thurman RG. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. Am J Physiol Gastrointest Liver Physiol. 2000;278:G467–476. doi: 10.1152/ajpgi.2000.278.3.G467. [DOI] [PubMed] [Google Scholar]
- Kono H, Fujii H, Asakawa M, Yamamoto M, Matsuda M, Maki A, Matsumoto Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann Surg. 2003;237:246–255. doi: 10.1097/01.SLA.0000048450.44868.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, Cao Q, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol Clin Exp Res. 2007;31:1660–1668. doi: 10.1111/j.1530-0277.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol. 2005;289:G308–319. doi: 10.1152/ajpgi.00054.2005. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW. Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res. 1986;10:271–273. doi: 10.1111/j.1530-0277.1986.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44:223–227. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Griniuviene B, Sadrzadeh SM, Levitsky S, McCully JD. Effect of type of dietary fat and ethanol on antioxidant enzyme mRNA induction in rat liver. J Lipid Res. 1995a;36:736–744. [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:638–644. [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Sadrzadeh SM, Yang EK, Fogt F, Meydani M, Dannenberg AJ. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology. 1995b;109:547–554. doi: 10.1016/0016-5085(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Zhao S, Lamb RG, Dannenberg AJ, Sadrzadeh SM, Waxman DJ. Changes in cytochromes P-450, 2E1, 2B1, and 4A, and phospholipases A and C in the intragastric feeding rat model for alcoholic liver disease: relationship to dietary fats and pathologic liver injury. Alcohol Clin Exp Res. 1994a;18:902–908. doi: 10.1111/j.1530-0277.1994.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994b;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract 2010. 2010 doi: 10.1155/2010/710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724–1730. [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Bradham CA, Cohn L, Schoonhoven R, Swenberg JA, Brenner DA, Thurman RG. Corn oil rapidly activates nuclear factor-kappaB in hepatic Kupffer cells by oxidant-dependent mechanisms. Carcinogenesis. 1999;20:2095–2100. doi: 10.1093/carcin/20.11.2095. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Johansson I, French SW, Ingelman-Sundberg M. Effects of dietary fat composition on activities of the microsomal ethanol oxidizing system and ethanol-inducible cytochrome P450 (CYP2E1) in the liver of rats chronically fed ethanol. Pharmacol Toxicol. 1992;70:347–351. doi: 10.1111/j.1600-0773.1992.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- Yoo JS, Ning SM, Pantuck CB, Pantuck EJ, Yang CS. Regulation of hepatic microsomal cytochrome P450IIE1 level by dietary lipids and carbohydrates in rats. J Nutr. 1991;121:959–965. doi: 10.1093/jn/121.7.959. [DOI] [PubMed] [Google Scholar]
- You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr. 2008;138:497–501. doi: 10.1093/jn/138.3.497. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]