Abstract
STAT5 plays a critical role in B and T lymphocyte development. However, whether STAT5 primarily plays a role as a permissive factor, involved in lymphocyte survival, or an instructive factor, involved in lymphocyte differentiation, has been unclear. In addition, while STAT5 has been suggested to act as a transcriptional repressor, the mechanism by which it represses transcription was undefined. Recent reports have begun to shed new light on these roles for STAT5 in lymphocyte development, transcriptional repression, and leukemic transformation.
Introduction
The transcription factor STAT5 consists of two isoforms, STAT5a and STAT5b. These two proteins exhibit 95% sequence homology and appear to be largely redundant in the lymphoid system. STAT5 is a key target of multiple cytokine receptors that are known to play critical roles in lymphocyte development, including those for interleukin-2 (IL2) and -7 (IL7). The availability of mice expressing either STAT5 gain-of-function or loss-of-function mutants has provided strong evidence that STAT5 plays a key role downstream of these receptors in entraining the early stages of B and T cell development (Figure 1) [1]. However, the molecular mechanisms by which STAT5 affects lymphocyte development are still being elucidated.
Figure 1.
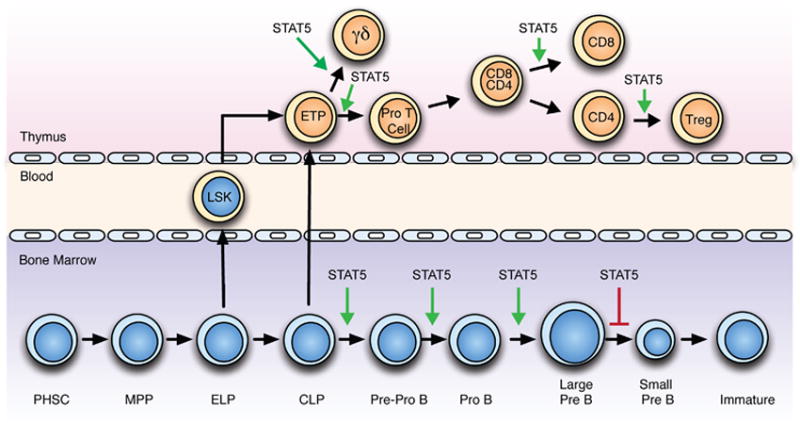
Overview of lymphocyte development with an emphasis on the role of STAT5. The green arrows mark the different stages of development where STAT5 plays a positive role in development of different lymphocyte subsets. The red arrow marks where STAT5-dependent inhibition is required for normal development.
In this review we address the ongoing controversy of whether STAT5 acts primarily as a permissive factor that allows for survival of developing lymphocytes, or as an instructive factor that plays an active role in inducing the expression of factors required for lymphocyte differentiation. In addition, we describe recent advances in our understanding of how STAT5 can act as both a transcriptional activator and repressor. Finally, we review recent studies supporting the key role that STAT5 plays in lymphocyte transformation, focusing specifically on how it initiates B cell acute lymphoblastic leukemia.
T cell development
The cytokine receptors for IL7 and IL2 play important roles in distinct aspects of thymocyte development. Activation of the STAT5 signaling pathway downstream of these receptors has been shown to play a critical role in mediating the biological effects of IL2 and IL7. For example, IL7-dependent STAT5 activation has been shown to both initiate γδ T cell differentiation and promote double negative thymocyte survival [2,3]. These initial studies have been complemented by more recent reports that highlight both the instructive and the permissive roles of STAT5 in later stages of lymphocyte development. The first of these studies focused on the process of CD4 versus CD8 lineage commitment and how this is influenced by TCR signal strength and cytokine signals. Specifically, increased TCR signal strength during positive selection was shown to result in reduced IL7R expression, which leads to reduced CD8 expression. The end result of this process of “co-receptor tuning” is that thymocytes expressing TCRs that signal more strongly are preferentially selected into the CD4+ T cell lineage [4]. Until recently the mechanism that accounts for this remained unclear. However, studies from Singer and colleagues have demonstrated that co-receptor tuning is dependent on STAT5 signaling and that STAT5 acts in both a permissive manner, by promoting CD8+ T cell survival, and in an instructive manner, by inducing expression of Runx3, a transcription factor that is typically required for CD8 expression [5]. These studies also raised several points that are important to consider when studying the role of STAT5 in lymphocyte development. First, deletion of STAT5 may allow other STAT proteins to aberrantly substitute for STAT5. For example, Singer and colleagues found that STAT5 deletion allowed the IL7R to unexpectedly activate STAT6. STAT6, which is not normally activated by IL7, effectively substituted for STAT5 function in CD8 lineage commitment; only when both STAT5 and STAT6 were deleted could the actual biological role of STAT5 be revealed. Second, care must be taken when interpreting results of studies involving overexpression of pro-survival genes. In this study, overexpression of Bcl2 rescued CD8+ T cells in Il7r-/- mice; however, these CD8+ T cells did not express Runx3 and resembled an atypical CD8+ T cell lineage rather than conventional CD8+ thymocytes. Third, neither Bcl2 nor Runx3 expression alone was able to recapitulate the effects of STAT5 signaling. This result suggests that both the permissive/pro-survival and the instructive/differentiation functions of STAT5 are required to drive appropriate CD8+ T cell lineage commitment. Finally, continuous STAT5 signaling, as opposed to a transient STAT5 signal, was required to maintain CD8 expression. Thus, STAT5 plays a critical role as both a permissive and instructive factor required for CD8+ T cell differentiation.
Although STAT5 is not required for differentiation of conventional CD4+ thymocytes, it does play a key role in the development of CD4+Foxp3+ regulatory T cells (Tregs). Mice lacking STAT5 are strikingly devoid of CD4+Foxp3+ Tregs. In this case STAT5 functions as part of a signaling pathway emanating primarily from the IL2R. This observation is consistent with a newly proposed two-step model for Treg development [6,7]. In this model, TCR/CD28 dependent signals leads to the generation of CD4+ Treg progenitors that express high levels of the IL2R complex but do not express FOXP3. In the second step, IL2 stimulation, via a STAT5-dependent process, rapidly converts these Treg progenitors into CD4+Foxp3+ mature Tregs. The exact mechanism by which this occurs remains unclear. However, STAT5 has been shown by several groups to bind to the promoter and first intron of the Foxp3 gene and thus may play a role in initiating Foxp3 expression [8-10]. Once FOXP3 protein is made, it has been shown to participate in a positive feedback loop that promotes it’s own expression and likely limits the need for STAT5 to maintain Foxp3 expression [11]. In this model STAT5 likely plays a role as a switch that helps initiate Foxp3 transcription but is not required to maintain Foxp3 expression once the gene has been induced.
B Cell Development
Mice lacking the IL7 receptor (IL7R) have almost no pro B cells and lack more mature B cell subsets; furthermore, the few remaining B cells exhibit aberrant immunoglobulin gene rearrangement [12,13]. STAT5 plays an especially important role in this process as expression of a constitutively active form of STAT5b (Stat5b-CA) largely restores B cell development in Il7r-/- mice [14]. Subsequent studies using mice in which STAT5 could be selectively deleted in developing B cells confirmed the key role of STAT5 in B cell development [2,3]. This led to a series of studies suggesting that STAT5 plays a critical instructive role in B cell development. Specifically, STAT5 was shown to bind to VH gene promoters and thereby promote IgH gene rearrangement [15]. Consistent with this study, other reports observed prolonged contraction and pericentromeric localization of the IgH locus in Stat5b-CA mice; this latter finding supported a role for STAT5 in promoting accessibility of the IgH region to the recombination machinery [16]. Finally, IL7 and STAT5-dependent signals influence expression of Ebf1, although it is unclear whether this reflects a direct or indirect role for STAT5 in governing Ebf1 expression [17-19]. Consistent with this last report, we observed that Stat5b-CA mice exhibit modestly increased expression of both Ebf1 and Pax5 [20,21]; once again whether this reflects a direct or indirect effect of STAT5 on transcription of these genes remains unclear. Taken together these findings suggested that STAT5 plays an important instructive role in initiating B cell differentiation.
STAT5 plays an important permissive role in B cell development as well. It has long been appreciated that STAT5 can bind to regulatory regions of two pro-survival genes Bcl2 and Bcl2l1 and promote their transcription [22]. More recent work by Busslinger and colleagues using Rag1-Cre × Stat5FL/FL mice demonstrated that STAT5 is required for the expression of the pro-survival gene Mcl1 but not for Ebf1 or Pax5 in pro-B cells; moreover, they demonstrated that Rag1-Cre × Mcl1Fl/FL mice exhibit a similar phenotype to that seen in Rag1-Cre × Stat5FL/FL mice. Finally, this report demonstrated that expression of a pan hematopoietic Vav-Bcl2 transgene could partially restore pro-B cell development in Rag1-Cre × Stat5FL/FL mice [23]. In contrast, forced expression of Ebf1 did not rescue the developmental block observed in Rag1-Cre × Stat5FL/FL mice. Based on these findings Busslinger and colleagues concluded that STAT5 only plays a permissive role in B cell development by regulating cell survival and does not regulate B cell differentiation in an instructive manner as previously proposed.
The explanation for these contradictory findings is not completely clear, although several explanations are possible. First, as suggested by Sigvardsson and colleagues [24], the study by Busslinger and colleagues made use of a Cre construct driven by the Rag1 promoter, which may delete STAT5 too late. Other studies have shown that though Rag1-Cre is transcribed in CLPs, it is likely confined to the more mature Ly6D+ CLP stages that express much higher levels of Rag1 [25,26]. Thus, it is possible that in Rag1-Cre × Stat5FL/FL mice B cell progenitors may receive an IL-7/STAT5 signal prior to deletion of STAT5. In that scenario, early IL-7/STAT5 signaling may be sufficient to initiate Ebf1 expression allowing the cells to overcome the developmental block in B cell development. In such a model, STAT5 would function as a transient switch that initiates Ebf1 expression; EBF1 then establishes a positive feedback loop via induction of Pax5 that acts to maintain both Ebf1 and Pax5 in the absence of continued STAT5 signaling. In contrast, the pro-survival/permissive function of STAT5 clearly requires continuous STAT5 function throughout early B cell development (Fig. 2). A second potential explanation is that other STAT factors may substitute for STAT5 as was described for CD8+ T cell lineage commitment. Future studies will be required to fully define whether STAT5 acts solely as a pro-survival, permissive factor in B cell development or whether it has both permissive and instructive roles.
Figure 2.
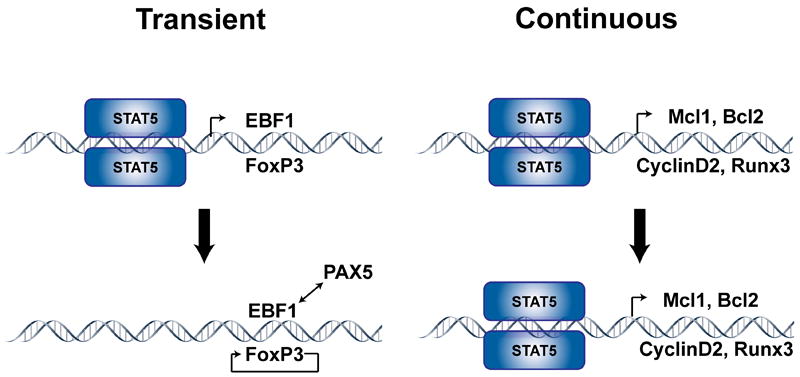
Model for how STAT5 might regulate gene transcription in T and B cell development. In some instances, transient expression of STAT5 correlates with an instructive role in development. As illustrated for Ebf1 and Foxp3, STAT5 activation may initiate transcription but continued expression of genes is maintained by positive feedback loops that are STAT5-independent. Therefore, these genes would only require an initial threshold level of STAT5 signaling without a need for continuous STAT5 signaling. In contrast, continuous STAT5 signaling is required for permissive genes such as Mcl1, Bcl2, and Cyclin D2, although it can be required for instructive factors, such as Runx3, as well.
STAT5 as a transcriptional inhibitor
Most studies of STAT5 have focused on its ability to activate gene transcription. However, recent research has emphasized the role of STAT5 as an important repressor of gene transcription. This is perhaps most clearly demonstrated by the observations made by several groups that IL7 signaling prevents rearrangement at the immunoglobulin kappa light chain locus [23,27,28]. These studies have been extended to show that STAT5 is the critical IL7-dependent factor involved, as Rag1-Cre × Stat5FL/FL mice exhibit premature kappa light chain germline transcripts [23]. In these initial studies they show STAT5 bound to the intronic kappa enhancer (Eki) in a way that might prevent binding of a second transcription factor E2A, which is required for initiating kappa germline transcription. However, a recent report from Clark and colleagues demonstrated that STAT5 still acted as a repressor even when the STAT5 and E2A binding sites were physically separated [29]. Instead, they observed that STAT5 acted to recruit EZH2, a histone H3K27 methylase. This resulted in H3K27-tri-methylation of the kappa light chain locus, an epigenetic change associated with repressed chromatin (Fig. 3). These studies point toward a novel mechanism by which STAT5 can actively repress transcription [29].
Figure 3.
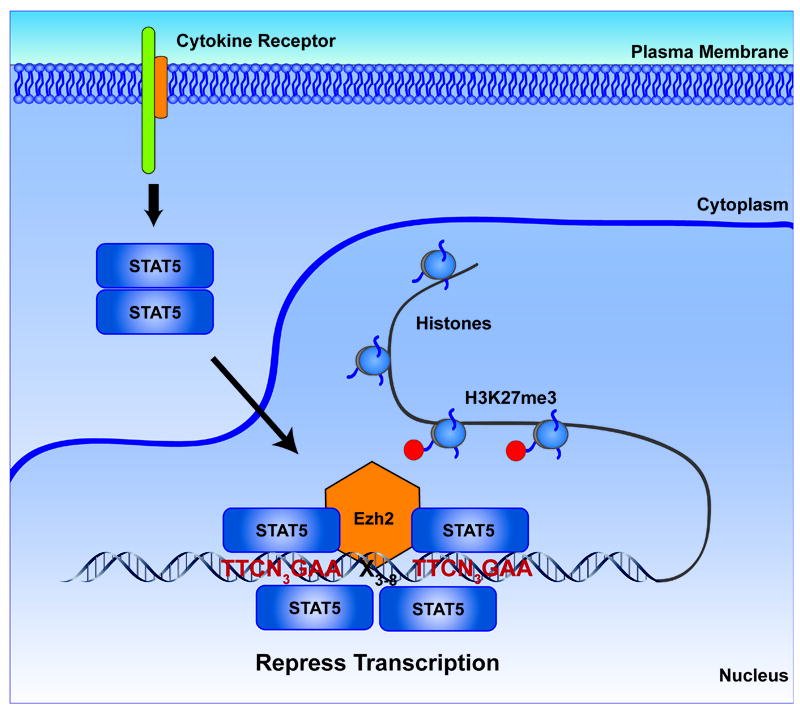
STAT5 repressive mechanism at the light chain immunoglobulin locus. STAT5 homodimers translocate to the nucleus following cytokine activation. In the nucleus, STAT5 may bind as a tetramer to tandemly repeated STAT5 DNA-binding sites. These tetrameric complexes are capable of recruiting EZH2 and other members of the polycomb repressor family. The resulting complex generates repressive chromatin via H3K27 trimethylation (H3K27me3).
In addition to repressing kappa gene rearrangement, STAT5 also represses many other gene targets. One target of particular interest is the transcriptional repressor Bcl6 [30,31]. BCL6 represses pre-B cell proliferation, in part by inhibiting c-Myc transcription [31]. Thus, STAT5 repression of Bcl6 in large pre-B cells allows for appropriate expansion of this developing cell population. Upon downregulation of the IL7R and STAT5 inactivation, Bcl6 levels increase and cause pre-B cells to exit cell cycle. Importantly, BCL6 also exhibits a pro-survival role in later stages of B cell development. Intriguingly, this represents an important mechanism by which BCR-ABL+ leukemias escape death following treatment with imatinib [32]. Imatinib treatment blocks STAT5 activation which is normally important for survival of the transformed pre-B cell; paradoxically, however, this inhibition of STAT5 results in re-expression of Bcl6 which acts to protect leukemia cells from death. Thus strategies that inhibit both BCR-ABL and BCL6 are more effective at treating BCR-ABL+ leukemias [32]. Future studies of gene targets repressed by STAT5 are likely to provide additional important insights into STAT5 biology.
A major question that remained from these studies is why STAT5 acts as a transcriptional activator when bound to some STAT5 binding sites but a transcriptional repressor when bound to other sites. To address this question, Clark and colleagues carried out STAT5 ChIP-Seq studies in progenitor B cells. Subsequent analysis of these studies found that STAT5 appears to function as a transcriptional activator when to bound to sites that bind STAT5 as a STAT5 homodimer but functions as a transcriptional repressor when bound to sites that bind STAT5 as a larger tetrameric complex [29]. These results suggest that the nature of the STAT5 binding complex determines whether it interacts with transcriptional repressors such as EZH2. This is a very appealing model, although future studies will be required to address the mechanism by which this occurs in more detail.
STAT5 and transformation
STAT5 is involved in both proliferation and survival of lymphoid cells. Therefore, it is perhaps not surprising that STAT5 activation has been correlated with lymphocyte transformation. For example, STAT5 is a known downstream target of the BCR-ABL fusion gene [33-35]. Consistent with this observation, Weber-Nordt and colleagues found that ~50% of human acute lymphoblastic leukemia (ALL) samples displayed elevated levels of activated STAT5, although only 3 fresh and 15 frozen primary samples were studied [36]. Using a much larger pool of 128 patients with acute lymphoblastic leukemia (ALL), we observed elevated levels of STAT5 phosphorylation (and hence activation) in >35% of these samples. This varied by subset with BCR-ABL-positive patients expressing the highest levels of STAT5 phosphorylation. Importantly, we observed that the level of STAT5 phosphorylation prior to therapy predicted the subsequent outcome to treatment of BCR-ABL-positive leukemia with high STAT5 phosphorylation correlating with poor overall survival [20]. These findings strongly suggest that STAT5 plays an important role in progenitor B cell transformation.
Studies of human patients with ALL indicate that STAT5 phosphorylation is increased in ALL and correlates with outcome. Such studies do not demonstrate that STAT5 is actually a major driver of transformation. However, work in mouse models of ALL clearly demonstrates the key role of STAT5 in transformation. For example, expression of a BCR-ABL cDNA in B cell progenitors leads to B-ALL in mouse models; transformation fails to occur in mice lacking STAT5 [37]. We were able to demonstrate a role for STAT5 in ALL utilizing a Stat5b-CA transgenic mouse model. Approximately 1-2% of Stat5b-CA mice develop a disease that resembles human ALL [38]. When these same mice were crossed to mice lacking the adaptor protein BLNK, the mice developed a highly penetrant form (80-90% penetrance) of the disease [38]. Interestingly, Blnk mutations have been noted in a small subset of human patients with B-ALL [39,40]. These studies suggest that STAT5 activation in concert with defective Blnk signaling plays an important role in initiating some forms of B-ALL.
More recent studies have indicated that STAT5 plays an important role in cooperating with defects in Ebf1 or Pax5 to initiate transformation. EBF1 and PAX5 are transcription factors critical for B cell development; surprisingly, deletions in Ebf1 and Pax5 were found in over 30% of patients with B-ALL [39]. Whether these mutations were important drivers of leukemia or merely passengers in the transformation process remained unclear. To address this issue, we generated Stat5b-CA × Pax5+/- and Stat5b-CA × Ebf1+/- mice; these mice rapidly developed fatal progenitor B-ALL. The role of STAT5 was not due solely to effects on survival as Ebf1+/- mice crossed to transgenic mice expressing the pro-survival factor Bcl2l1 failed to develop leukemia. Likewise, no difference in the rate of proliferation was seen between WT progenitor B cells and transformed B-ALL cells, suggesting that STAT5 did not initiate transformation by enhancing pro-B cell proliferation. However, transformation required the expression of the IL7R and correlated with increased expression of the TSLPR. This latter observation is interesting for three reasons: first, STAT5 can be activated downstream of TSLP signaling [41], second, elevated levels of TSLPR have been identified in human ALL [42,43], and third, gain-of-function mutations in the IL7Rα chain, one of the two receptor chains required for TSLP binding, have been found in human ALL [44]. The mechanism by which STAT5 initiates transformation is not entirely clear. However, it may involve synergistic effects of STAT5 activation coupled with defects in the EBF1/PAX5 signaling network. For example, one of the most highly expressed genes in Stat5b-CA × Ebf1+/- leukemia’s is RANKL; this is consistent with the suggestion that RANKL promotes pro-B cell expansion, as Rankl-/- mice have reduced numbers of progenitor B cells. However, although STAT5 induces RANKL expression in breast epithelial cells, it fails to do so in progenitor B cells; only in cells with reduced expression of EBF1 and PAX5, which both repress RANKL [45,46], is increased STAT5 activation capable of driving RANKL expression. Thus, cooperative effects between STAT5 activation and defects in the EBF1/PAX5 transcription factor network that orchestrates normal B cell development appear to be a potent combination that initiates progenitor B cell transformation
Summary and perspective
These studies have begun to shed light on the molecular mechanisms by which STAT5 affects lymphocyte development and leukemia. However, important questions remain to be answered. First, the mechanisms by which STAT5 initiates gene transcription remain poorly defined. Likewise, while we have gained some insights as to how STAT5 represses gene transcription, the precise mechanism by which STAT5 recruits EZH2 remain unclear. Second, although we now know that STAT5 can act as a transcription repressor, the important biological settings in which this occurs are just beginning to be identified. Third, whether STAT5 acts as an instructive factor in initiating B cell development remains unresolved. Finally, the mechanisms by which STAT5 drives progenitor B cell leukemia are just beginning to be understood. A better understanding of how STAT5 initiates leukemia and maintains the transformed state should suggest targets for future pharmaceutical intervention.
Highlights.
STAT5 plays a permissive role as a pro-survival factor in lymphocyte development
STAT5 regulates lymphocyte differentiation
STAT5 functions as both an activator and a repressor of transcription
STAT5 plays an important role in initiating acute lymphoblastic leukemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Farrar MA. Design and use of constitutively active STAT5 constructs. Methods Enzymol. 2010;485:583–596. doi: 10.1016/B978-0-12-381296-4.00030-0. [DOI] [PubMed] [Google Scholar]
- 2.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, Beug H, Hennighausen L, Moriggl R, Sexl V. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood. 2006;107:4898–4906. doi: 10.1182/blood-2005-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- **5.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. This paper demonstrates that STAT5 plays a critical role in the process of co-receptor tuning, and hence CD8+ lineage commitment via effects on Runx3 and pro-survival genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 9.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CA, Harmon IR, O’Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol. 2004;172:4770–4778. doi: 10.4049/jimmunol.172.8.4770. [DOI] [PubMed] [Google Scholar]
- 15.Bertolino E, Reddy K, Medina KL, Parganas E, Ihle J, Singh H. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt SL, Yin B, Ji Y, Chaumeil J, Marszalek K, Tenthorey J, Salvagiotto G, Steinel N, Ramsey LB, Ghysdael J, et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat Immunol. 2009;10:655–664. doi: 10.1038/ni.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roessler S, Gyory I, Imhof S, Spivakov M, Williams RR, Busslinger M, Fisher AG, Grosschedl R. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsapogas P, Zandi S, Ahsberg J, Zetterblad J, Welinder E, Jonsson JI, Mansson R, Qian H, Sigvardsson M. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- **20.Heltemes-Harris LM, Willette MJ, Ramsey LB, Qiu YH, Neeley ES, Zhang N, Thomas DA, Koeuth T, Baechler EC, Kornblau SM, et al. Ebf1 or Pax5 haploinsufficiency synergizes with STAT5 activation to initiate acute lymphoblastic leukemia. J Exp Med. 2011;208:1135–1149. doi: 10.1084/jem.20101947. STAT5 activation is found in human ALL with elevated levels of pSTAT5 prior to treatment coorelating with poor disease outcome following treatment with imatinab. Constitutively active STAT5 cooperates with genes critical to B cell development (Ebf1 or Pax5) to induce highly penetrant ALL identifying a new mouse model of human B-ALL. The induction of leukemia was not due solely to a block in B cell development or increased proliferation but did require expression of the IL7R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz CA, Harmon IR, O’Neil JJ, Burchill MA, Johanns TM, Farrar MA. Restricted STAT5 activation dictates appropriate thymic B versus T cell lineage commitment. J Immunol. 2005;174:7753–7763. doi: 10.4049/jimmunol.174.12.7753. [DOI] [PubMed] [Google Scholar]
- 22.Lord JD, McIntosh BC, Greenberg PD, Nelson BH. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- **23.Malin S, McManus S, Cobaleda C, Novatchkova M, Delogu A, Bouillet P, Strasser A, Busslinger M. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. This paper identified Mcl1 as a key downstream target of STAT5 in pro-B cell development. Also the authors show that STAT5 activity is responsible for supressing premature expression of IgK rearrangements in pro-cells, pointing to a critical repressive role for STAT5 in B cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandi S, Bryder D, Sigvardsson M. Load and lock: the molecular mechanisms of B-lymphocyte commitment. Immunol Rev. 2010;238:47–62. doi: 10.1111/j.1600-065X.2010.00950.x. [DOI] [PubMed] [Google Scholar]
- 25.Mansson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 26.Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, Fehling HJ, Kincade PW. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K, Hashimshony T, Sawai CM, Pongubala JM, Skok JA, Aifantis I, Singh H. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335–345. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Mandal M, Powers SE, Ochiai K, Georgopoulos K, Kee BL, Singh H, Clark MR. Ras orchestrates exit from the cell cycle and light-chain recombination during early B cell development. Nat Immunol. 2009;10:1110–1117. doi: 10.1038/ni.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Mandal M, Powers SE, Maienschein-Cline M, Bartom ET, Hamel KM, Kee BL, Dinner AR, Clark MR. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat Immunol. 2011;12:1212–1220. doi: 10.1038/ni.2136. This paper demonstrates that STAT5 repression of the Igκ locus is not solely due to a block of E2A binding to the kappa enhancer but rather involves epigenetic repression of the locus. STAT5 complexes are able to uniquely interact with EZH2 which results in the formation of H3K27-trimethylated repressive chromatin. Further, it was shown that the STAT5 complexes bind tandemly repeated STAT5 binding sites as tetramers suggesting that tetramer complexes are involved in repression while the more well characterized dimeric complexes are involved in gene activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker SR, Nelson EA, Frank DA. STAT5 represses BCL6 expression by binding to a regulatory region frequently mutated in lymphomas. Oncogene. 2007;26:224–233. doi: 10.1038/sj.onc.1209775. [DOI] [PubMed] [Google Scholar]
- 31.Duy C, Yu JJ, Nahar R, Swaminathan S, Kweon SM, Polo JM, Valls E, Klemm L, Shojaee S, Cerchietti L, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med. 2010;207:1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S, Klemm L, Kweon SM, Nahar R, Braig M, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473:384–388. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- 34.Sillaber C, Gesbert F, Frank DA, Sattler M, Griffin JD. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–2125. [PubMed] [Google Scholar]
- 35.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 36.Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R, Finke J. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- *37.Hoelbl A, Schuster C, Kovacic B, Zhu B, Wickre M, Hoelzl MA, Fajmann S, Grebien F, Warsch W, Stengl G, et al. Stat5 is indispensable for the maintenance of bcr/abl-positive leukaemia. EMBO Mol Med. 2010;2:98–110. doi: 10.1002/emmm.201000062. STAT5 is required continuously in order to maintain the transformed state in BCR-ABL1 mouse model of leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama J, Yamamoto M, Hayashi K, Satoh H, Bundo K, Kubo M, Goitsuka R, Farrar MA, Kitamura D. BLNK suppresses pre-B-cell leukemogenesis through inhibition of JAK3. Blood. 2009;113:1483–1492. doi: 10.1182/blood-2008-07-166355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 40.Jumaa H, Bossaller L, Portugal K, Storch B, Lotz M, Flemming A, Schrappe M, Postila V, Riikonen P, Pelkonen J, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423:452–456. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 41.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- *42.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, West N, Xiao Y, Brown JR, Mitsiades C, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;107:252–257. doi: 10.1073/pnas.0911726107. Identified CRLF2 overexpression in adult precursor B-ALL and this overexpression of CRLF2 can substitute for IL3 expression and results in a cell signature similar to that seen in BCR-ABL patient samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, Zhang J, Ma J, Coustan-Smith E, Harvey Harvey, Willman CL, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. Identify a rearrangement between P2RY8 and CRLF2 that results in constitutive STAT5 activation and JAK mutations with results in cytokine independent growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, Te Kronnie G, Cario G, Cazzaniga G, Kulozik AE, Stanulla M, et al. Gain-of-function mutations in interleukin-7 receptor-{alpha} (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011 doi: 10.1084/jem.20110580. Identify mutations in the extracellular and transmembrane domain of the IL7R protein in human B and T ALL which confers cytokine independent growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, Grosschedl R, Allman D, Singh H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]


