Abstract
Two-component systems are widely used by bacteria to mediate adaptive responses to a variety of environmental stimuli. The CusR/CusS two-component system in Escherichia coli induces expression of genes involved in metal efflux under conditions of elevated Cu(I) and Ag(I) concentrations. As seen in most prototypical two-component systems, signal recognition and transmission is expected to occur by ligand binding in the periplasmic sensor domain of the histidine kinase CusS. Although discussed in the extant literature, little experimental evidence is available to establish the role of CusS in metal homeostasis. In this study, we show that the cusS gene is required for Cu(I) and Ag(I) resistance in E. coli and that CusS is linked to the expression of the cusCFBA genes. These results show a metal dependent mechanism of CusS activation and suggest an absolute requirement for CusS in Cu(I) and Ag(I)-dependent upregulation of cusCFBA expression in E. coli.
Introduction
Metals such as copper and silver have been used as antimicrobial agents in clinical and non-clinical settings for centuries due to their effectiveness in limiting the growth of a broad range of organisms. Silver (Ag(I)) is reported to be lethal to bacteria in sub-millimolar concentrations for a wide range of bacterial species (Silver et al., 2006; Holt & Bard, 2005). The mechanism of silver ion toxicity mainly lies in its ability to bind to sulfhydryl groups of proteins and inhibit key functions such as phosphate uptake and respiration (Bragg & Rainnie, 1974; Schreurs & Rosenberg, 1982). These properties make silver ions very potent biocides.
While copper is a micronutrient used as a catalyst in key biochemical reactions and its deficiency can lead to disintegration of a variety of cellular processes, excess copper can be lethal (Peña et al., 1999). This makes copper an extremely effective antimicrobial agent, accounting for its extensive use in agricultural and non-clinical settings (Brown et al., 1992). The unique redox chemistry of copper allows it to readily shuttle between the cuprous (Cu(I)) and cupric (Cu(II)) states under constantly changing physiological conditions, making it ideal for many fundamental biological processes involving electron transfer reactions. However, the same redox property of copper can lead to Fenton-type reactions that generate reactive oxygen species that can lead to cellular damage (Macomber et al., 2007; Peña et al., 1999). Copper, in either the Cu(I) or Cu(II) states, has strong affinity for sulfhydryl groups and the binding of copper to thiol or nitrogen-containing groups in proteins could inhibit protein function (Kershaw et al., 2005; Gerba & Thurman, 1989). However, copper is more toxic to bacterial cells under anaerobic conditions, where there is a greater proportion of Cu(I) (Beswick et al., 1976; Outten et al., 2001). Early work on copper suggested that Cu(I) is more toxic due to increased binding to amino acids and nucleosides (Cramp, 1967). Cu(I) displaces the iron in iron-sulfur clusters and bind to the thiol groups in important metabolic enzymes (Macomber & Imlay, 2009). In order to avoid the toxicity exerted by copper, bacteria utilize intricate mechanisms to reduce free intracellular concentrations of the metal (Osman & Cavet, 2008).
Although there are distinct differences between copper and silver in their role in and effects on biological systems, these metals share very similar chemical and ligand binding properties. Cu(I) and Ag(I) belong to the group of soft Lewis acids that have high polarizability and form bonds with nitrogen and sulfur containing molecules, which are soft Lewis bases (Housecroft & Sharpe, 2005). Silver can actively compete for copper sites in biomolecules, thus disrupting their function and key interactions (Dibrov et al., 2002). It has been observed that systems that aid in copper homeostasis can also actively detoxify silver (Rensing et al., 2000; Stoyanov et al., 2003).
Regulatory control of metal concentrations in living organisms is vital to prevent cellular damage due to toxic effects exerted by these elements. Due to the toxic nature of the metals, bacteria have developed sophisticated mechanisms conferring silver and copper resistance (Grass & Rensing, 2001b; Grass et al., 2011; Rensing & Grass, 2003). In Escherichia coli, the Cue and the Cus systems detoxify/remove excess silver and copper from the cells. The Cue response system consists of CopA, a P-type ATPase that exports intracellular Cu(I) into the periplasm (Rensing et al., 2000) and CueO, a periplasmic multicopper oxidase that oxidizes Cu(I) to Cu(II) (Grass & Rensing, 2001a). The Cus response system consists of the chemiosmotic CusCFBA efflux system (Franke et al., 2003; Grass & Rensing, 2001b). The Cus system is activated when the Cue system is overwhelmed with copper or under anaerobic conditions, when the oxidase CueO is inactive (Outten et al., 2001). The Cus system is particularly important to confer periplasmic Ag(I) tolerance to the cell, as CueO is inhibited by Ag(I) (Singh et al., 2011). The Cus system, although originally identified for its role in Ag(I) detoxification, is also involved in copper homeostasis; it is known that this system is activated in the presence of sub-millimolar concentrations of this transition metal (Franke et al., 2001; Yamamoto & Ishihama, 2005)
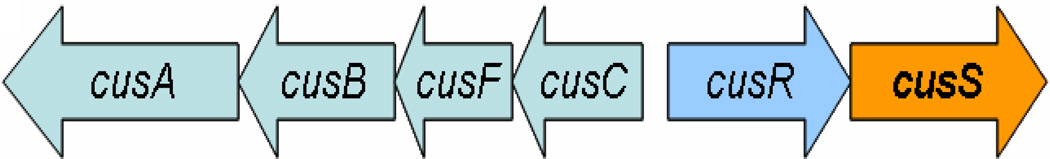
The E. coli Cus system consists of two operons, one of which encodes the proteins of the CusCFBA efflux pump. The second operon is divergently transcribed from the cusCFBA genes and encodes the CusR/CusS two-component system (TCS) (Figure 1). The CusR/CusS TCS is involved in regulation of transcription from the cusCFBA genes upon the onset of silver or copper stress (Franke et al., 2001; Munson et al., 2000). There is at least a two-fold increase in transcription from cusR and cusS genes upon induction by Ag(I) or Cu(I) ions (Yamamoto & Ishihama, 2005). The central role of CusS is seen in its occurrence in association with metal efflux genes in different species of Gram negative bacteria (Pontel & Soncini, 2009). In Pseudomonas putida, the CusS homolog CinS activates the transcription of the cinR and cinS genes in response to both Cu(I) and Ag(I) (Quaranta et al., 2009).
Figure 1. Open Reading Frames within the cus locus.
The cusRS locus encodes the two-component system and cusC, cusF, cusB and cusA form the transmembrane efflux channel in E. coli. The operons are divergently transcribed.
Based on sequence homology to other histidine kinases of two-component systems, E. coli CusS is predicted to be a membrane-bound kinase, which forms a two-component system with the response regulator CusR (Munson et al., 2000; Yamamoto et al., 2005). Under conditions of elevated concentrations of Cu(I)/Ag(I), CusS and CusR are essential for the induction of the copper efflux genes cusCFBA (Franke et al., 2003; Munson et al., 2000). Signal recognition by ligand binding in the periplasmic sensor domain of CusS is expected to elicit downstream transmembrane and cytoplasmic signaling events, and thus CusS is predicted to play an important role in cell adaptation to changes in extracytoplasmic levels of copper and silver ions.
This study establishes the role of the cusS gene in Cu(I) and Ag(I) resistance in E. coli. Additionally, we report that the presence of the cusS gene is essential for upregulation of the cusCFBA genes in the bacterium.
Materials and Methods
Bacterial strains and growth conditions
All strains were grown at 37 °C in modified Luria broth (MLB) (1% tryptone, 0.5% yeast extract), MLB-agar plates or modified M9 broth (MM9) (0.1% ammonium sulfate as the source of nitrogen and no sodium chloride) or MM9-agar plates. Antibiotics (ampicillin 100 µg /mL and kanamycin 30 µg/ml) were added to the growth media for purposes of strain selection. All overnight cultures containing the pBAD24 vectors were grown in the presence of 0.02% D-glucose to prevent the expression from the arabinose promoter. To promote expression from the genes on the pBAD24 vector, 0.2% L-arabinose was added to the growth media. Reagents and chemicals were obtained from Sigma and MLB components were obtained from Difco.
Strain and Plasmid Construction
Bacterial strains and plasmids used in this study are listed in Table 1. Knockout strains were made using the lambda-Red mediated gene recombination technique as detailed by Datsenko and Wanner (Datsenko & Wanner, 2000). Antibiotic resistance cassettes from the knockout strains were removed using the temperature sensitive pCP20 plasmid as described previously (Datsenko & Wanner, 2000). For gene complementation assays all strains were transformed with the pBAD24 (Guzman et al., 1995) vector containing the necessary gene.
Table 1.
Strains and Plasmids
| Strain | Relevant Genotype | Source/Reference |
|---|---|---|
| BW25113 | Wild-type, lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | (Datsenko & Wanner, 2000) |
| E. coli wild-type | BW25113/pBAD24 | This work |
| E. coli ΔcusS | BW25113/ΔcusS::kanR/pBAD24 | This work |
| E. coli ΔcusS ΔcusCFBA | BW25113/ΔcusS ΔcusCFBA/pBAD24 | This work |
| Plasmid | ||
| pBAD24 | pBR322 ori, BAD promoter, ampR | (Guzman et al., 1995) |
| pBADcusS | E. coli cusS cloned into pBAD24 | This work |
The plasmids and oligonucleotide sequences used in this present study are listed in Table S1 and were obtained from Integrated DNA Technologies. For construction of pBADcusS plasmid the pBADcusS-F and the pBADcusS-R primers were used. To amplify the cusS gene from E. coli W3110 genomic DNA, the PCR product digested with HindIII/EcoRI restriction enzymes and ligated into the HindIII/EcoRI sites in vector pBAD24. All plasmids were purified and sequenced for accuracy.
Determination of MIC of Ag(I), and growth and copper accumulation in CuSO4 containing media
Overnight cultures were grown aerobically in MLB to an OD600 of 2.05 – 2.10, then diluted 1:200 into MM9 medium containing 100 µg/mL ampicillin. Growth was continued until the OD reached 0.6 – 0.8, then the cells were induced with 0.2% arabinose. To study the effect of increasing copper on growth of wild-type and mutant E. coli BW25113 strains in liquid medium, 30 minutes after induction with arabinose the cultures were diluted again 1:200 in MM9 medium containing various concentrations of CuSO4 and incubated at 37 °C under anaerobic conditions. Cell growth was measured after 15 hours and cell densities were normalized before plotting as a function of copper concentrations.
To study the effect of AgNO3, wild-type and mutant E. coli BW25113 strains were grown as mentioned above. The cell density was allowed to reach OD600 0.9 – 1.0. The cultures were diluted in sterile phosphate buffered saline pH 7.4 (PBS) 1:200 and spotted on MM9-agar plates containing different concentrations of AgNO3. The plates were incubated aerobically at 37 °C for 20 hours in the dark. The MIC values were determined as the minimum concentration of AgNO3 with no growth observed.
To determine metal accumulation in cells, E. coli wild-type, E. coli ΔcusS and E. coli ΔcusS/pBADcusS were grown as described above. After induction of genes on the pBAD24 vectors with L-arabinose, 7.5 µM CuSO4 was added to the medium and cell aliquots were taken at 0, 2 and 4 hours after addition of copper. All cultures were normalized to 3 × 108 cells/ml and centrifuged to obtain the cell pellet. The pellets were washed three times with MM9 containing 1 mM EDTA and dried at 75 °C for 3 hours. 50 µL nitric acid (10% v/v) was added to the pellet and the samples were incubated at 75 °C for 30 min. The copper concentrations in the sample were measured using inductively coupled plasma mass spectrometry (ICP-MS) on a Elan DRC II instrument (Perkin Elmer). The instrument was initially monitored for background noise and metal contaminants and then calibrated using an ICP multi-element stock solution (AccuStandard) prepared in 1% nitric acid. Samples were also diluted with 1% nitric acid until signal was within the calibration range. All glassware used for this experiment were washed with 10% nitric acid. The concentration of copper was determined as an average of three independent experiments. Statistical significance of this data was analyzed using ANOVA with multiple comparisons.
Cell growth, RNA extraction, cDNA synthesis for qRT-PCR
E. coli cells were grown in MM9 as described above. At an OD600 of 0.6 – 0.8, 0.2% L-arabinose was added to the cells and when the OD600 reached 0.9 – 10, 5 µM AgNO3 was added. Cell aliquots were taken 0.25, 2.25 and 4.25 hours post silver stress and were normalized to 3 × 108 cells/ml and were frozen.
RNA was extracted by resuspending 3 × 108 cells in 300 µL TRIZOL Reagent, phase separated using chloroform, and total RNA was precipitated by using isopropanol followed by centrifugation. The RNA pellet was resuspended in nuclease free water (Bioexpress). Quality and purity of RNA preparations were assessed by electrophoresis and via spectrophotometric determination of the ratio of absorbance at 260/280 nm.
Total-RNA extracted from the previous step was treated with RNase free DNaseI (Fermentas). First strand cDNA was prepared from 2 µg of total RNA using the Superscript III cDNA synthesis kit (Quanta Biosciences). The cDNA was then diluted with SYBR green qPCR master mix. Reactions were amplified using the Applied Biosystems 7300 Real-time PCR system. Each cDNA sample was assessed in triplicate using 16S-rRNA as an internal control. Thermal cycle conditions consisted of an initial denaturation step at 95°C for 60 sec followed by 40 cycles of 95 °C for 15 s, 60 °C for 60 sec. Fluorescence was measured at the beginning of each annealing/extension step. Amplicon size was also determined by electrophoresis on an agarose gel (1% w/v). The quantity of cDNA measured by Real Time PCR was normalized to the abundance of 16S cDNA. Primers used for qRT-PCR are listed in supplementary Table S1. To check specificity of each primer, the predicted amplicon melting temperature was confirmed via dissociation curve analysis. Relative expression from the cusC gene was calculated using the ΔΔCt quantification method (Livak & Schmittgen, 2001) and values are averages of three independent experiments. Statistical significance of this data was analyzed using ANOVA with multiple comparisons.
Results and Discussion
The role of copper ions in bacterial growth and survival is well documented. Due to the toxic nature of copper ions, bacteria such as E. coli and Salmonella have molecular systems that tightly control the copper concentration within the cells (Pontel & Soncini, 2009). In E. coli the Cus system was first identified as a silver resistance system and was shown to be inducible by copper ions as well. Upon further investigation it was revealed that the chemiosmotic CusCFBA system in E. coli confers anaerobic copper and silver resistance and is regulated by the CusR/CusS two-component system. The sensor kinase CusS and the response regulator CusR are activated by copper (Munson et al., 2000) and silver (Franke et al., 2001) ions and these proteins are required for regulation of the cusCFBA operon.
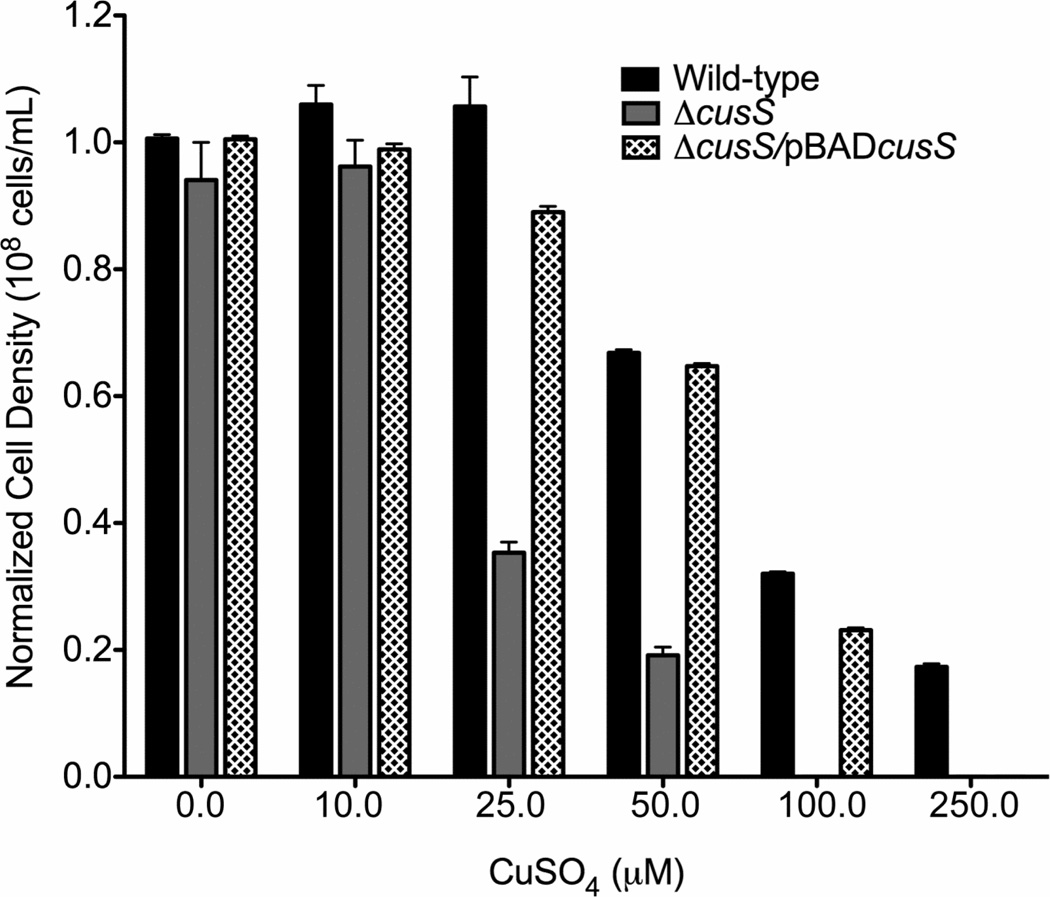
To define the role of CusS in copper resistance in E. coli, an E. coli strain was created in which the chromosomal copy of cusS was disrupted (Table 1). Since the Cus system is the primary copper response system in the absence of oxygen (Outten et al., 2001), the sensitivity of these cells to different concentration of copper was tested in the absence of oxygen. Disruption of cusS led to an increase in the toxicity of copper in the strain E. coli ΔcusS (Figure 2). Upon exposure to copper concentrations above 10 µM, E. coli ΔcusS showed a significant inhibition of growth as observed by the cell density measurements. No growth was seen in the ΔcusS strain above 50 µM CuSO4. However, resistance could be restored through the addition of cusS on the pBADcusS plasmid which has cusS under the control of the arabinose promoter (Figure 2). No significant differences in growth were seen between the strain ΔcusS/pBADcusS and the wild-type strain up to 100 µM CuSO4.
Figure 2. Copper sensitivity of E. coli wild-type, E. coli ΔcusS and E. coli ΔcusS/pBADcusS to copper.
The normalized cell densities of the different strains of E. coli were compared after 15 hours of growth under anaerobic conditions in media containing different concentrations of CuSO4. The data are plotted as an average of three independent experiments including their standard error of mean (SEM).
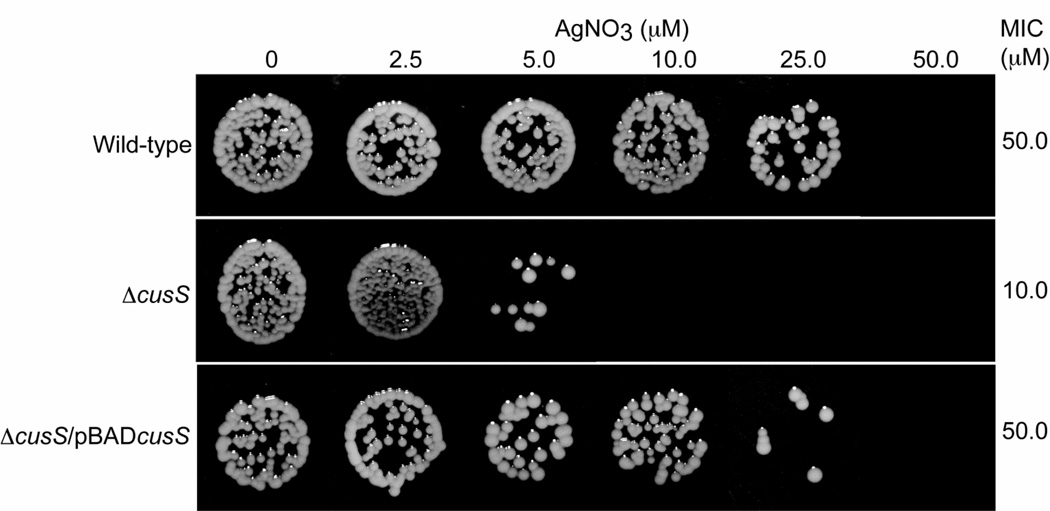
To address the role of CusS in silver tolerance, E. coli ΔcusS and E. coli ΔcusS/pBADcusS (Table 1) were tested for sensitivity to media containing Ag(I). The MIC of Ag(I) for E. coli strains containing the cusS gene either on the genome (wild-type) or on a plasmid (pBADcusS) was 50 µM (Figure 3 and Table 2). In comparison the disruption of the cusS gene had a potent effect on Ag(I) sensitivity, where the strain E. coli ΔcusS showed Ag(I) sensitivity at 10 µM metal concentrations.
Figure 3. Minimum inhibitory concentration (MIC) of silver for E. coli wild-type, E. coli ΔcusS and E. coli ΔcusS/pBADcusS.
E. coli cells were on MM9 plates containing different concentration of AgNO3 for 16 hours. The MIC values are shown on the right and are an average of three independent experiments.
Table 2.
Minimal inhibitory concentrations of AgNO3 for different E. coli strains
| Strain | MIC of AgNO3 (µM) |
|---|---|
| Wild-type | 50 |
| ΔcusS/pBAD24 | 10 |
| ΔcusS/pBADcusS | 50 |
| ΔcusS ΔcusCFBA/pBAD24 | 2.5 |
| ΔcusS ΔcusCFBA/pBADcusS | 2.5 |
The above data establishes that the gene encoding histidine kinase CusS responds to elevated levels of copper and silver in E. coli. Mutants that lack the cusS gene have higher susceptibility to silver compared to the wild type or cusS complemented strain of E. coli. The cusS gene is also required for anaerobic copper resistance as indicated by slower growth of E.coli ΔcusS cells in medium containing copper.
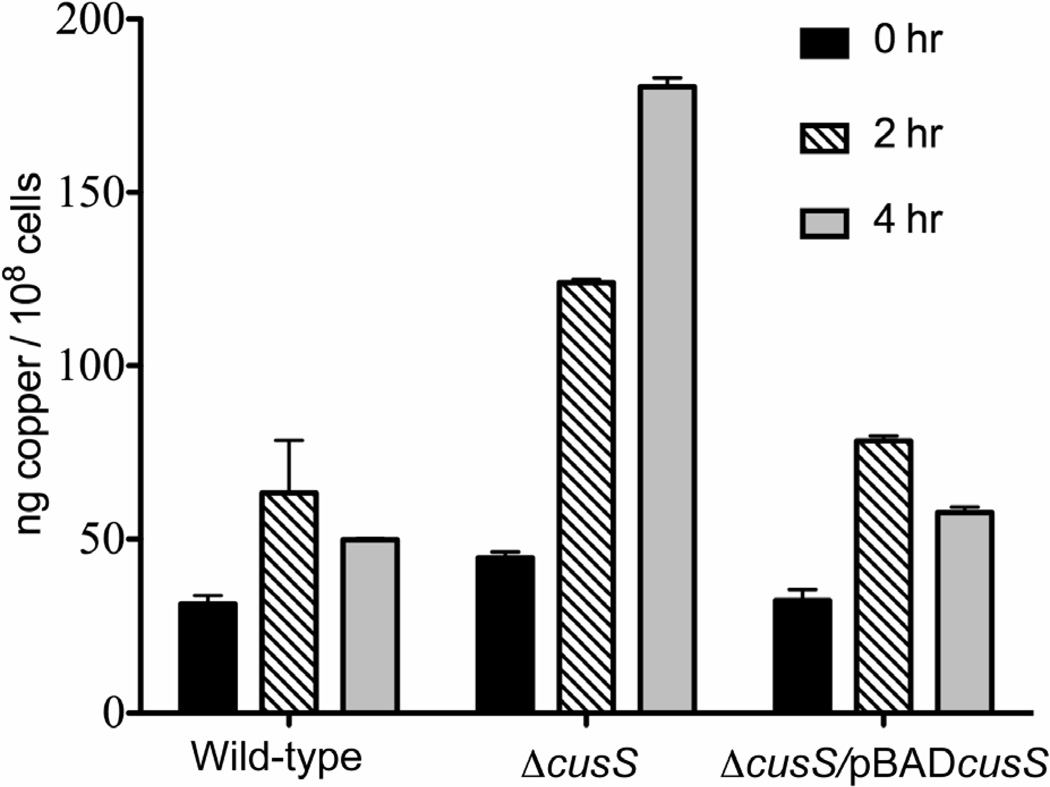
Previous work has shown that E. coli and yeast cells undergo increased copper accumulation under anaerobic conditions (Outten et al., 2001; Strain & Culotta, 1996; Weissman et al., 2000). If the role of CusS is to activate the cus efflux genes under elevated copper concentrations, in the absence of CusS, no expression from the cusCFBA genes would occur and therefore, no efflux of copper is expected from the cells. To test this hypothesis, the levels of copper were examined in E. coli wild-type, E. coli ΔcusS and E. coli ΔcusS/pBADcusS by growing the cells anaerobically in copper-containing medium and determining copper content by ICP-MS. E. coli ΔcusS, which lacks the cusS gene, showed a steady increase in copper accumulation with a four-fold increase in copper concentration as compared to the wild-type strain after four hours. Supplying cusS on a plasmid rescued this phenotype, as the copper concentration in E. coli ΔcusS/pBADcusS was similar to that of E. coli wild-type. The copper concentrations in E. coli ΔcusS/pBADcusS reached about 76 ng/108 cells after 2 hours and decreased to 60 ng/108 cells after 4 hours (Figure 4).
Figure 4. Metal accumulation in E. coli cells grown in medium containing copper.
The graph represents copper accumulation in cells at 0 (black), 2 (diagonal lines) and 4 hours (gray) after addition of 7.5 µM CuSO4 respectively. The table below the graph represents the actual concentration of copper measured using ICP-MS. Each bar represents the average of three independent experiments and their SEM. ANOVA analysis with multiple comparisons shows that in the absence of cusS, E. coli cells accumulate higher concentrations of copper (P < 0.001).
The deficiency of CusS within the cell leads to copper accumulation, as E. coli ΔcusS was observed to accumulate copper when grown in medium containing the metal under anaerobic conditions. The copper accumulation phenotype was not seen when cusS was either present on the genome or provided to the mutant externally on a plasmid (Figure 4). It has been previously established that the Cus system mediates copper homeostasis primarily under anaerobic conditions (Franke et al., 2003; Outten et al., 2001) and upon increase in cellular levels of copper, cusS is expected to upregulate the cusCFBA genes, ultimately leading to copper export. Anaerobic copper accumulation in the absence of cusS suggests an alteration in copper export, most likely due to the absence or delayed expression of components of the CusCFBA efflux pump. These results show that E. coli utilizes CusS under anaerobic conditions to prevent overaccumulation of metal.
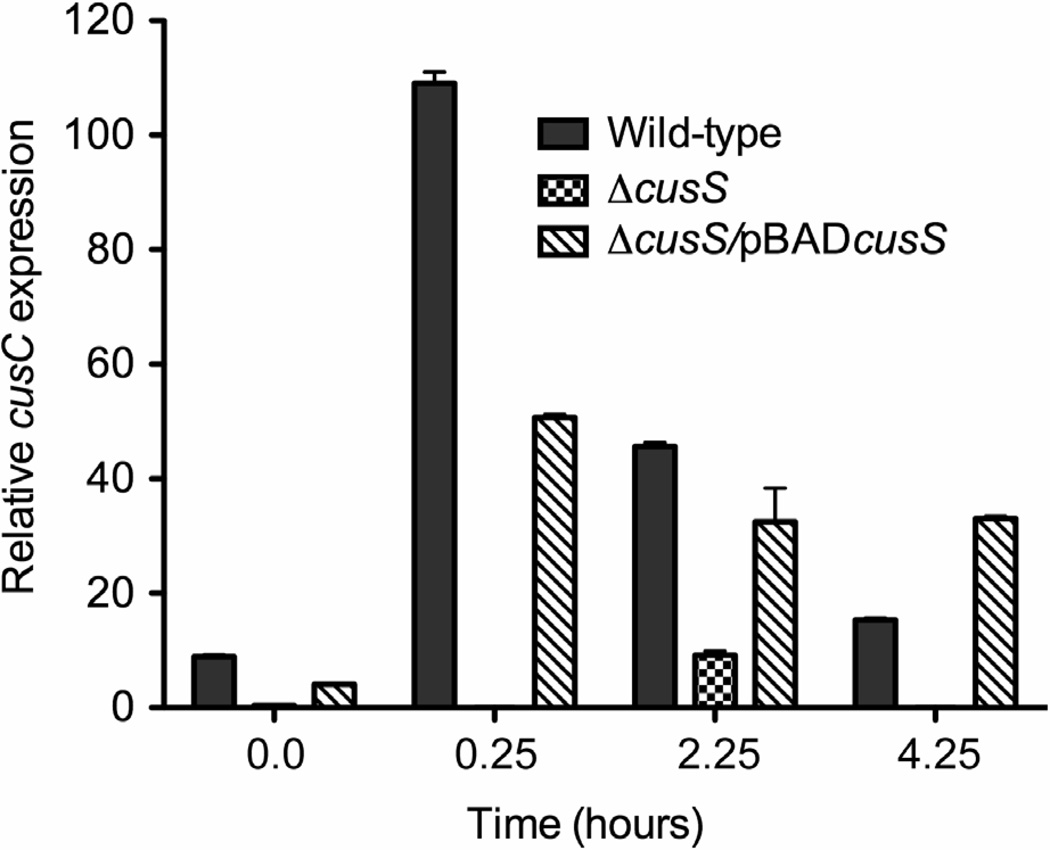
Ag(I) is very similar to Cu(I) in its chemical properties and is also known to activate the Cus system. In order to investigate the regulatory effects of CusS on the cusCFBA system, we used qRT-PCR to examine the changes in the expression levels of cusC mRNA upon addition of Ag(I). Total RNA was isolated from exponential phase cultures containing AgNO3 of E. coli wild-type, E. coli ΔcusS and E. coli ΔcusS/pBADcusS and cDNA was synthesized. The expression level of cusC was compared in the presence and absence of cusS gene (Figure 5). No expression from cusC was detected immediately after Ag(I) addition and appeared in very minimal quantities after two hours in strains lacking cusS. The expression from cusC in wild-type cells is greatest immediately after addition of Ag(I). Expression from cusC in strain E. coli ΔcusS/pBADcusS was seen to be higher than E. coli ΔcusS in which the cusS gene is deleted but was not as responsive as compared to the wild-type strain. By 4 hours post silver treatment, all strains had very slow growth rate and E. coli ΔcusS which lacks the cusS gene was most affected.
Figure 5. CusS is required for expression from cusC.
Data shows the relative expression from the cusC gene +/− SEM, as determined in E. coli wild-type (gray), E. coli ΔcusS (checkered) and E. coli ΔcusS/pBADcusS (diagonal lines) after exposure to 5 µM AgNO3 for 0, 2 and 4 hours. Total RNA from cells was extracted, reverse transcribed and cDNA was subjected to real-time PCR using primers specific for cusC. ANOVA analysis with multiple comparisons showed that transcription from cusC is absent when cusS is disrupted (P < 0.0001).
It is evident from the above results that transcription from cusC is negligible in the absence of cusS. However, in order to link the cusS-mediated phenotypes to CusCFBA activity, we created a strain of BW25113 that lacks both cusS and cusCFBA. The strain that lacks cusCFBA failed to grow in concentrations of silver of 2.5 µM (Table 2). Under anaerobic conditions, these cells also failed to grow in medium containing as low as 10 µM copper. Supplementing the strain with cusS externally on a plasmid did not change the Cu(I)/Ag(I) sensitive phenotype. These results, in addition to the observation that cusC is minimally expressed in the absence of CusS, suggests that the metal sensitive phenotype observed in the ΔcusS strain is due to the loss of CusCFBA.
The cusS gene is located on an operon that is transcribed in the opposite direction to the cusCFBA structural genes (Figure 1). It has been shown that Ag(I) exposure leads to polycistronic transcriptional activation of the cusCFBA genes (Franke et al., 2001). Previous studies on the Cus system have offered evidence for transcription from the cusR/cusS region and cusC regions upon exposure to silver (Franke et al., 2001; Franke et al., 2003) and copper ions (Munson et al., 2000). The transcriptional levels from the cusC gene therefore serve as an indicator of expression from the structural cus genes. Our results show that expression from cusC is reduced at least two-fold in the absence of cusS (Figure 5). This decrease indicates that CusS is the primary activator for Ag(I)-activated expression from cusC. The presence of cusC transcript in E. coli ΔcusS two hours after addition of silver may indicate the presence of another signaling system that is responsive to silver ions.
Two candidates for other two-component systems that may be responsible for this effect are CpxA/CpxR and YedV/YedW, which have been implicated in copper-facilitated signaling events (Kershaw et al., 2005). The histidine kinase CpxA is activated by denatured membrane proteins and therefore its activation by copper-induced cellular stress is not surprising, since copper toxicity may lead to loss of integrity of protein structure and/or protein degradation, either by oxidative stress (Macomber et al., 2007) or by displacement of the parent ligand in proteins (Macomber & Imlay, 2009). Transcription from the histidine kinase encoding yedV increases two-fold after induction by copper and its role in copper response is not fully understood (Yamamoto & Ishihama, 2005). Comparison of the amino acid sequence in the predicted sensor domains of these histidine kinases does not reveal any information about how CpxA and YedV may be involved in metal-regulated gene expression. Also, the involvement of another histidine kinase or a different signaling mechanism is a tangible possibility, since in the presence of low levels of silver or copper the same OD600 is achieved in cells in which cusS is disrupted (Figure 2). Alternative mechanisms by which the cells could protect themselves from metal toxicity, allowing growth to continue, may include removal of metal ions from the cytoplasm to the periplasm by CopA, or sequestration of ions by other cellular components.
Based on our results, we have demonstrated that cusS plays a central role in copper and silver resistance in E. coli. Through direct or indirect mechanisms, CusS senses increased periplasmic copper or silver and mediates the expression of the cusCFBA genes. Periplasmic detoxification of copper is expected to occur through the CusCFBA chemiosmotic transmembrane efflux pump. The mechanism by which CusS senses elevated metal concentration and transmits the signal to the cytoplasmic response regulator CusR still remains unclear and will be an important area for future investigation.
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr. Jun Isoe (University of Arizona) for assistance with qRT-PCR and Dr. Jonathan Beckwith (Harvard Medical School) for the pBAD24 plasmid. This work was supported by the National Institute of Health (GM079192 to M.M.M and C.R).
Footnotes
The authors declare no conflict of interest.
References
- Beswick PH, Hall GH, Hook AJ, Little K, McBrien DC, Lott KA. Copper toxicity: evidence for the conversion of cupric to cuprous copper in vivo under anaerobic conditions. Chem. Biol. Interact. 1976;14:347–356. doi: 10.1016/0009-2797(76)90113-7. [DOI] [PubMed] [Google Scholar]
- Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- Brown NL, Rouch DA, Lee BT. Copper resistance determinants in bacteria. Plasmid. 1992;27:41–51. doi: 10.1016/0147-619x(92)90005-u. [DOI] [PubMed] [Google Scholar]
- Cramp WA. The Toxic Action on Bacteria of Irradiated Solutions of Copper Compounds. Radiation Research. 1967;30:221–236. [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibrov P, Dzioba J, Gosink KK, Häse CC. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob. Agents Chemother. 2002;46:2668–2670. doi: 10.1128/AAC.46.8.2668-2670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke S, Grass G, Nies DH. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology (Reading, Engl.) 2001;147:965–972. doi: 10.1099/00221287-147-4-965. [DOI] [PubMed] [Google Scholar]
- Franke Sylvia, Grass Gregor, Rensing Christopher, Nies Dietrich H. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 2003;185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C, Thurman R. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Critical Reviews in Environmental Science and Technology. 1989;18:295–315. [Google Scholar]
- Grass G, Rensing C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001a;286:902–908. doi: 10.1006/bbrc.2001.5474. [DOI] [PubMed] [Google Scholar]
- Grass G, Rensing C. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 2001b;183:2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Rensing L, Rensing C. Metal toxicity. Metallomics. 2011;11:1095–1097. doi: 10.1039/c1mt90048j. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt KB, Bard AJ. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ Biochemistry. 2005;44:13214–13223. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- Housecroft CE, Sharpe AG. Inorganic chemistry. Pearson Prentice Hall; 2005. [Google Scholar]
- Kershaw CJ, Brown Nigel L, Constantinidou C, Patel MD, Hobman JL. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology (Reading, Engl.) 2005;151:1187–1198. doi: 10.1099/mic.0.27650-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macomber L, Rensing Christopher, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson GP, Lam DL, Outten FW, O’Halloran TV. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 2000;182:5864–5871. doi: 10.1128/jb.182.20.5864-5871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman D, Cavet JS. Copper homeostasis in bacteria. Adv. Appl. Microbiol. 2008;65:217–247. doi: 10.1016/S0065-2164(08)00608-4. [DOI] [PubMed] [Google Scholar]
- Outten FW, Huffman DL, Hale JA, O’Halloran TV. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001;276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- Peña MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J. Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- Pontel LB, Soncini FC. Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mol. Microbiol. 2009;73:212–225. doi: 10.1111/j.1365-2958.2009.06763.x. [DOI] [PubMed] [Google Scholar]
- Quaranta D, McEvoy MM, Rensing Christopher. Site-directed mutagenesis identifies a molecular switch involved in copper sensing by the histidine kinase CinS in Pseudomonas putida KT2440. J. Bacteriol. 2009;191:5304–5311. doi: 10.1128/JB.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. CopA: An Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U.S.A. 2000;97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003;27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Schreurs WJ, Rosenberg H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982;152:7–13. doi: 10.1128/jb.152.1.7-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006;33:627–634. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- Singh SK, Roberts SA, McDevitt SF, Weichsel A, Wildner GF, Grass GB, Rensing Christopher, Montfort WR. Crystal structures of multicopper oxidase cueo bound to copper(I) and silver(I): Functional role of a methionine-rich sequence. 2011 doi: 10.1074/jbc.M111.293589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanov JV, Magnani D, Solioz M. Measurement of cytoplasmic copper, silver, and gold with a lux biosensor shows copper and silver, but not gold, efflux by the CopA ATPase of Escherichia coli. FEBS Lett. 2003;546:391–394. doi: 10.1016/s0014-5793(03)00640-9. [DOI] [PubMed] [Google Scholar]
- Strain J, Culotta VC. Copper ions and the regulation of Saccharomyces cerevisiae metallothionein genes under aerobic and anaerobic conditions. Mol. Gen. Genet. 1996;251:139–145. doi: 10.1007/BF02172911. [DOI] [PubMed] [Google Scholar]
- Weissman Z, Berdicevsky I, Cavari BZ, Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3520–3525. doi: 10.1073/pnas.97.7.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 2005;56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J. Biol. Chem. 2005;280:1448–1456. doi: 10.1074/jbc.M410104200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.