Abstract
CD4+CD25+Foxp3+ regulatory T (Treg) cells migrate into both inflammatory sites and draining lymph nodes (LNs) during an immune response, and have unique and overlaping functions in each location. Current studies suggest that Treg cells in draining LNs and inflamatory sites may not simply be a division of labor, rather Treg migrate in a coordinated fashion between peripheral tissues and draining LNs. Trafficking between inflammatory sites and draining LNs is not only critical for Treg to act, but also for them to acquire optimal immune regulatory activities. Furthermore, recent work has revealed that T helper (Th) 1, Th2, and Th17 master transcription factors, control Treg function by regulating genes important for Treg migration and suppression, and consequently affecting disease pathogenesis.
Immune regulation by regulatory T (Treg) cells
CD4+CD25+Foxp3+ Treg cells are important regulators of almost all immune responses, and they mediate suppressive functions both through the production of cytokines, including TGFβ, IL-10 and IL-35, and direct cell-cell contacts [1–4]. Foxp3 is the major transcription factor that determines the fate and identity of regulatory CD4+ T (Treg) cells, and its induction in CD4+Foxp3− single positive thymocytes results in natural Treg (nTreg) cell development [2, 5]. nTreg cells exit the thymus, circulate and migrate to secondary lymphoid tissues, as well as to inflammatory sites, to exert supressor activities. Foxp3 expression can also be directly induced in peripheral naïve CD4+CD25− T cells forming adaptive Treg (aTreg) cells that also suppress immune responses. TGFβ and IL-2 signal cascades play a major role in aTreg cell development [6], although other factors such as cytokines and vitamins are also involved [7–11].
Diminished Treg cell development and function links to autoimmunity, allergy and infectious diseases, whereas elevated Treg cell responses are observed in tumor immunity. In mouse models, Treg cells can be utilized to inhibit immune responses in autoimmunity, allograft rejection, graft versus host disease and allergic diseases; and blocking their suppressive activity has been used in fighting tumors and developing vaccines. Thus the ability to manuipulate Treg cell activity has huge therapeutic potential [12–15].
Treg cell-mediated suppression has been reported in peripheral tissues [16–22], in draining LN [23–25], or in both [26–29]. A recent study suggested [30] that Treg cell suppressive activity is more important at the site of tissue inflammation than in draining LN, whereas the initial interactions of Treg cell precursors with antigen in draining LN are important for induction of aTreg cells and activation of both nTreg and aTreg cells for their suppressive function [31]. This suggests that Treg cells interact with DC first in the LN, become primed or activated, concomitantly suppress effector T cell priming, and then subsequently migrate to non-lymphoid tissues to suppress inflammation [32]. Thus, Treg cells appear similar to effector T cells in that priming takes place within lymphoid organs, with subsequent migration to inflamed tissue. Understanding how Treg cells migrate during an immune response and their importance at different locations might provide new strategies for targeting Treg cells in disease. In this review we examine how a nTreg cell migratory phenoptype develops, trafficking at stady state, both nTreg and aTreg cell trafficking during an immune reaction and the importance of trafficking of Treg cells between draining LNs and inflammatory sites for their immune suppression. We also discuss how Th1, Th2, and Th17 transcription factors affect Treg cell migration and suppressive function and Treg cell trafficking in various disease models.
A critical role of gut in shaping nTreg cell homing receptor, phenotype and migration
After leaving the thymus, naïve T cells circulate in the blood from which they migrate into secondary lymphoid tissues due to expression of L-selectin (CD62L) and the chemokine receptor CCR7 [33]. Naïve T cells migrate across high endothelial venules (HEV), which reside exclusively in secondary lymphoid organs such as lymph nodes, through specific interactions between CD62L and CCR7 with the HEV-expressed ligands PNAd and CCL21. During an inflammatory response, naïve T cells interact with antigen-presenting cells (APCs) that display antigen-derived peptides in the context of MHC class I or II molecules, leading to proliferation, chromatin remodeling at lymphoid specific genes and differentiation into antigen-specific effector cells. Effector cells do not express CD62L or CCR7, thus enabling them to egress from lymphoid organs, and migrate into inflammatory sites via the selective expression of tissue-specific intergrins and chemokine receptors, such as α4β7 and CCR9 for the endothelium of small intestinal blood vessels, and E- and P-selectins, CCL17 (TARC) and CCL27 (cTACK) for inflamed skin [34].
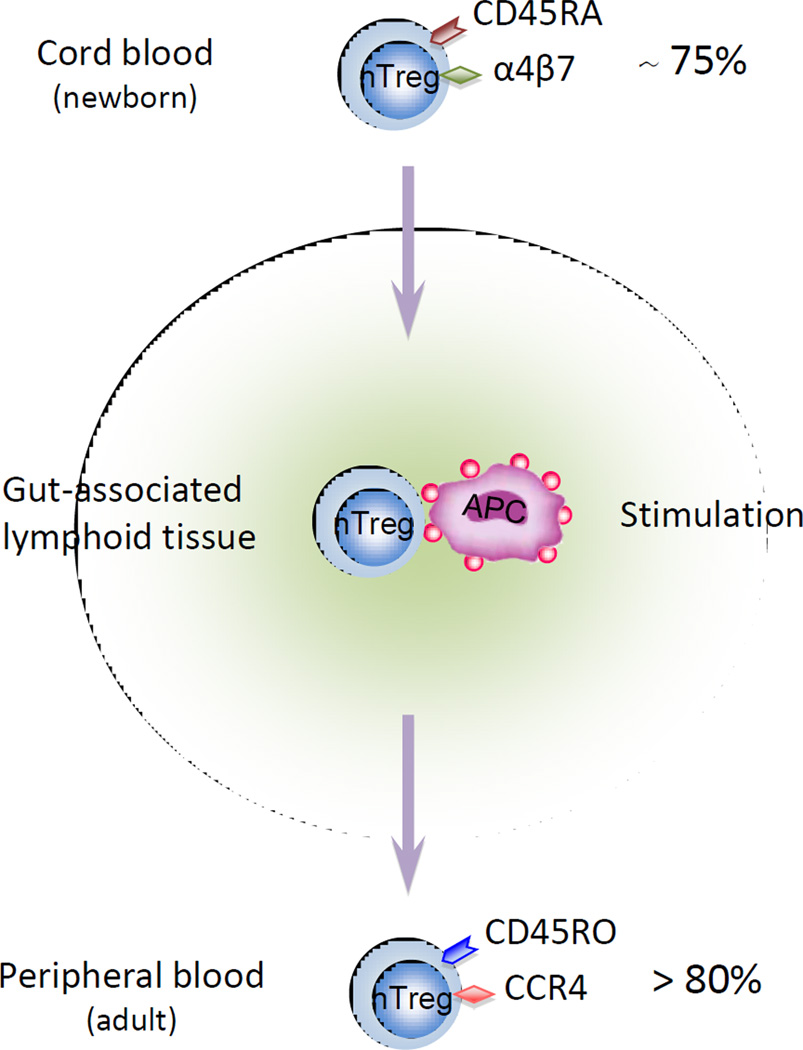
In contrast to conventional T cells that must be activated for acquisition of effector functions, CD4+CD25+Foxp3+ nTreg cells have suppressive abilities in the absence of antigen exposure, even when they are isolated in thymus or from cord blood [35]. Although nTreg and aTreg cells can exert immune regulatory functions through cytokine production (mainly TGFβ and IL-10), similar to convential T effector cells, they require close contact with target cells to exert full suppressive activity and can suppress independent of cytokine production. nTreg cells expressing the naïve T cell molecule CD45RA+ are more prevalent in early life and 75% of nTreg cells are CD45RA+ in full-term cord blood, whereas more than 80% of circulating nTreg cells in the peripheral blood of adult humans express the activation and memory molecule CD45RO+ [36]. Recently, homing receptor expression on the surface of human nTregs cells in cord blood, and peripheral blood at 18 months, 3 years and in adults was analyzed [37]. The majority of the nTreg cells in cord blood expressed the gut-homing integrin α4β7 and only a minor population of cells expressed CCR4, whereas in adult nTreg cells the majority expressed CCR4 but not α4β7. Altered expression of nTreg cell expression of homing receptors from α4β7 to CCR4 occured between 1.5 and 3 years of age, along with a switch from the CD45RA+ naïve to the CD45RO+ memory phenotype. These changes indicate a crucial role for the gut in nTreg cell stimulation by exogenous antigens in early life, and in shaping nTreg cell homing receptor expression patterns, phenotype and abilities for migration and circulation (Figure 1).
Figure 1. A concept for nTreg cell phenotype switch.
Treg cell homing receptor switching from α4β7 to CCR4 is associated with a change to a memory phenotype. The majority of Treg cells at birth express the gut homing receptor α4β7, favoring migration to intestinal secondary tissues. The majority of Treg cells in adults express CCR4, enabling migration to non-gastrointestinal tissues, such as the skin and the lung.
After exiting gut-associated lymphoid tissues during early life, nTreg cells display effector/memory phenotypes similar to conventional T cells such as E- and P-selectins expression [36, 37], and they can migrate from blood to reside in different peripheral tissues. These rapid phenotypic and homing receptor switches by nTreg cells may enable them to be easily mobilized and migrate to tissues, and may serve important roles to avoid tissue pathology during intial immune responses [37].
nTreg cell trafficking at steady state
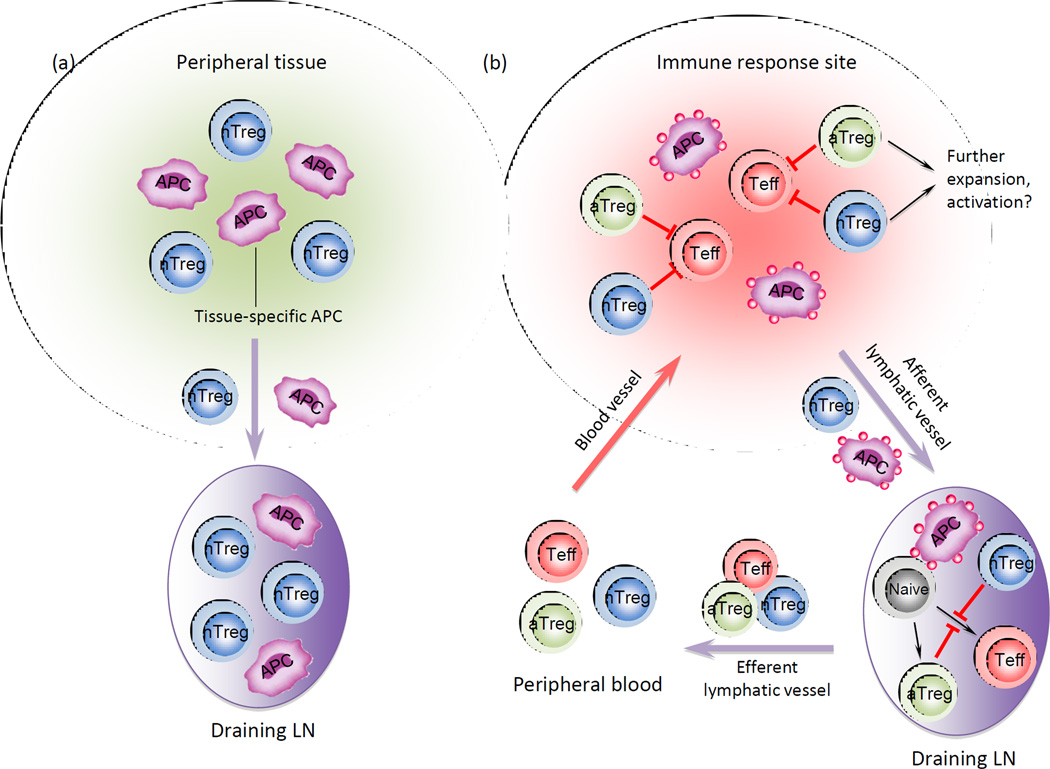
The high percentage of nTreg cells with effector/memory phenotype and tissue homing receptor expression in adults enable them to migrate from blood into peripheral tissues. However, whether nTreg cells migrate through normal peripheral tissues as part of their routine recirculation route from blood, tissue then into afferent lymphatics and lymphoid organs is not certain. Using adoptively transferred fluorescent-labeled lymphocytes, it was demonstrated that CD4+ T cell exit from the skin into afferent lymphatics, even at steady state, is a selective and active process dependent on CCR7, and is not a simple passive or random diffusion [38]. Using transgenic mice that express Kaede protein that rapidly converts from green to red after exposure to ultraviolet or violet light, both CD4+CD25− non-Treg cells and CD4+CD25+ nTreg cells were found to move from peripheral tissue (in this case skin) into draining LNs but not in non-draining LNs at steady state (Figure 2A) [39, 40]. nTreg cell entry into tissues at the steady state may enable a quick and effective suppressive response against unwanted local immune responses, and their exit from tissues prevents accumulation which could lead to impaired immune responses against pathogens or tumor.
Figure 2. Treg cell migration in steady state and during an immune response.
(a) Treg cells constitute one of the major populations migrating from peripheral tissues to draining LNs in the steady state. (b) During inflammatory responses, Treg cells migrate from peripheral tissues into draining LNs, and together with LN resident Treg cells, suppress immune responses. Treg cells also interact with tissue specific DCs and gain tissue homing specificity, migrate back to tissues and suppress inflammation. Treg cells may also expand and be further activated locally to acquire optimal capacity for immune suppression.
Treg cell trafficking during an immune reaction
Using the Kaede transgenic mice, during a cutaneous immune reaction the frequency of UV-exposed migratory CD4+ T cells increased in draining LNs by more than 10-fold compared to the steady state, and about half of the migrating CD4+ T cells were Treg cells [39], indicating that during an immune responses Treg cell migration from peripheral tissues to draining LNs is accelerated compared to steady state. Although these results did not distinguish between nTreg cells and aTreg cells, they suggest that rapid movement of Treg cells to the LN may enable them to effectively interfere with interactions between APCs and effector T cells by modulating the activation state and function of APCs, thus regulating T cell activation and controlling immune responses within draining LNs.
During an immune response, CD4+ Treg cell and CD4+ non-Treg cell numbers are also elevated in inflamed tissues and the ratio of Treg cell to non-Treg CD4+ T cells is higher than at steady state, further suggesting that Treg cells are rapidly mobilized to migrate from blood to the inflammatory site. When Treg cell movement from draining LNs and inflammatory tissue was tracked using Kaede transgenic mice, a substantial fraction of Treg cells were immigrants (Kaede-red) during immune reactions, and with a more memory-related phenotype [39]. The rapid trafficking of Treg cells from blood and draining LNs to peripheral tissues further indicates that mobilizing Treg cells into inflammatory sites plays an important role for Treg cell-mediated immune suppression.
Importance of draining LN versus tissue homing for Treg cell-mediated immune suppression
During an immune response, tissue-specific APCs, including DCs, take up antigen, migrate to draining LNs, and present antigen to CD4+ T cells and drive their differentiation into T helper (Th) effector subsets, including Th1 Th2, Th17, Tr1, Th22, and Tfh cells. Treg cells are also generate under these conditions, Th1 and Th2 express different chemokine receptors, so that Th1 express mostly CCR5, CXCR3 and CXCR6; while Th2 express CCR3, CCR4, CCR8 and the prostaglandin D2 receptor CRTh2. Considering that inflammatory Th17 are involved in both Th1 and Th2 diseases, it is not surprising that Th17 express both Th1- and Th2-associated chemokine receptors. Treg cells have been shown to have similar migration properties to Th17 cells [41–44] (Table 1), and CCR6 in particular is important for both Th17 and Treg cell migration [45]. Draining LNs are crucial in configuring conventional T cells, as well as Treg cells, with organ-selective homing properties to efficiently traffic into distinct tissues [19, 46]. Activation of nTreg cells within mesenteric LNs leads to induction of the integrin α4β7, which is required for migration into mucosa during an immune responses, whereas activation of nTreg cells in peripheral LNs leads to the expression of selectin ligands, which facilitate migration into inflamed skin.
Table 1.
Chemokine receptor expression
| Cell type | Chemokine receptors | Function |
|---|---|---|
| Th1 | CCR5, CXCR3, CXCR6 | Host defense against intracellular pathogens; Autoimmunity, Type 1 diseases |
| Th2 | CCR3, CCR4, CCR8, CRTh2 | Host defense against parasites; Allergy, Asthma |
| Th17 | CCR2, CCR4, CCR6, CCR9, CXCR3, CXCR6 | Host defense against extracellular pathogens; Inflammation, Autoimmunity |
| Th22 | CCR4, CCR6, CCR10 | Tissue immunity and remodeling |
| Treg | CCR2, CCR4, CCR5, CCR6, CCR7, CXCR4 | Immunosuppression, Tolerance, Tumor |
| Tr1 | CCR3, CCR4, CCR5, CCR8, CCR9, CXCR3 | Immunosuppression |
| TFH | CXCR5 | B cell immunity |
Treg cells are detected in peripheral tissues in inflamed organs, transplanted grafts, tumors and infection, demonstrating that these cells control effector T cells in peripheral tissues at sites of ongoing immune responses [23–25]. Naive Treg cells efficiently migrate into LNs, but lack suppressive capacity under acute inflammatory conditions [5, 20]. In an islet allograft model, Treg cell migration to grafts is essential for their in vivo suppressive function. Treg cell protective effects for graft survival were abrogated if they failed to migrate to the graft due to CCR2, CCR4, CCR5, or P- and E-selectin ligand deficiency, and protection was enhanced when Treg cells were delivered locally into the grafts [26].
Although aTreg cell development is negatively regulated by high expression of Th1-, Th2-, and Th17-associated transcription factors [47–51], recent data suggests that Treg cells may utilize pathogenic T helper cell developmental machinery to suppress immune responses. T-bet is a Th1-specific transcription factor crucial for IFNγ production, Th1 development and type 1 immune disease pathogenesis [52]. Thus, inhibition of T-bet may be beneficial for controlling Th1 differentiation and related inflammatory responses. However, T-bet is also required for Treg cells to effectively suppress type 1 inflammation. Although both wild type and T-bet-deficient nTreg cells are immune suppressive in vitro, only wild type are immune suppressive during Th1 immune responses in vivo [47]. This is due to lack of T-bet-mediated CXCR3 expression and failure of T-bet-deficient Treg cells to migrate and accumulate at sites of Th1 cell dominated inflammation. T-bet-deficient Treg cells also expressed less IL-10 and TGFβ [47]. Similarly, IRF4, a key transcription factor for Th2 differentiation, is essential for nTreg cells to selectively control Th2 responses. IRF4-deficient nTreg cell express less of the chemokine receptor CCR8 and have lower expression of several genes important for suppression including Il10, Gzmb and Fgl2, In vivo, IRF4-deficient nTreg cells lead to elevated IL-4-dependent Th2 autoimmune lymphoproliferative diseases [53]. Similarly, Treg-mediated suppression of Th17 inflammatory responses is lost when Stat3 (a Th17 associated cytokine) is ablated in nTreg cells. The Stat3-deficient nTreg cells are immunosuppressive in vitro and are able to control Th1 and Th2 responses but not Th17 responses in vivo [49]. This is accompanied by reduced CCR6 expression, and lower expression of the suppressive Il10, Ebi3, Gzmb and prf1 genes [49]. These data show that specific transcription factors important for Th1 Th2, and Th17 differentiation and pathologic responses, also control Treg cell functions. These transcription factors seem important for expression of genes that enable Treg cells to migrate to sites of inflammation for interaction with effector T cells and expression of suppressive molecules. FoxP3 interacts with and regulates Th1-, Th2-, and Th17-associated transcription factor expression and function [47–51, 53]. Thus FoxP3 might partially impair these transcription factors signaling cascades in Treg cells, preventing effector cytokine expression while maintaining chemokines and other molecules important for migration and suppression to effectively control immune responses. These data also further demonstrate the importance of Treg cell migration into and accumulate at sites of inflammation.
Early studies showed that CD62Lhigh nTreg cells, which are able to migrate into secondary lymphoid organs, are superior compared to CD62Llow nTreg cells in preventing the development of autoimmunity or in suppressing graft-versus-host disease (GVHD) [54]. This suggests that LN homing of nTreg cells is important for suppression. Supporting this, in an autoimmune diabetes model, nTreg cells control T cell priming within the LNs [55]. In a cardiac allotransplant model, tolerance induction depends on CD62L-mediated LN homing, and generation of alloantigen-specific aTreg cells occurs only in the LNs, but not in the spleen or graft [56, 57]. Furthermore, LN migration is necessary for Treg cell function [25, 58], and Treg cell-deficient in CCR7 have impaired migration into LNs and reduced suppressive effects [28]. Together, the unique environment of the LNs has a dual role in establishing Treg cell-mediated immune suppression: in the LN Treg cells acquire the capacity and specificity to home to the inflammatory site and they also suppress effector T cell priming, migration, proliferation, and accumulation within the LNs.
Importance of Treg cell trafficking between inflammatory sites and draining LNs
A recent study examined how Treg cells traffic between tissues and draining LNs during an immune response and how migration between these two locations contributes to Treg cell suppressive. In an islet allograft model [59], both nTreg and aTreg cells were activated in the allograft, and subsequently migrated to the draining LNs in a CCR2-, CCR5- and CCR7-dependent manner. Sequential migration from tissue to draining LN was a key component of Treg cell function, because migration was found to be coupled to Treg cell generation and function which is essential for optimal suppression. This data is consistent with the inflamed skin model, in which few Treg cells are present in the skin at steady state [39], supported by our findings that nTreg cells migrated from blood to tissue, and subsequently migrated into draining LNs in the islet allograft model. The nTreg cells that had migrated to LNs showed much stronger suppressive function and expressed higher amounts of IL-10, TGFβ and cytotoxic T lymphocyte antigen 4 (CTLA4) in the inflamed skin model [39], again demonstrating that activation of Treg cells in inflammatory sites and subsequent movement into draining LNs are critical for Treg cells to fully acquire immune suppressive capacities.
In an oral tolerance model, although FoxP3-expressing cells are generated in LNs in integrin beta 7 (ITGB7) and mucosal vascular addressin cell adhesion molecule 1 (MADCAM1)-deficient mice, oral tolerance is abrogated due to gut-homing defects of these cells. Thus aTreg cells generated in the LNs need to home to the gut to undergo local expansion to mediate oral tolerance [60]. Together, these results demonstrate that the movement of nTreg and aTreg cells between LNs and inflammatory sites is coordinated, and crucial for their suppressive activities (Figure 2B).
Treg cell migration during disease development
Treg cells are central in restraining autoimmunity, suppressing inflammation, preventing transplant graft rejection while also affecting immune responses to infection and tumor in growth. Understanding the migratory properties of Tregs cells in various diseases models might facilitate development of novel approaches for therapeutic manipulation of these cells.
Colitis
In experimental models, nTreg cells can prevent and cure colitis [61, 62]. In a model for preventing colitis, Treg cell migration into the intestine was not necessary for nTreg cells to prevent naïve T cell-induced colitis [16]. However, when nTreg cells are used to cure colitis, nTreg cells divide rapidly and accumulate both in the secondary lymphoid organs and in the inflamed tissue, and a significant numbers of nTreg cells exit the lymphoid tissue and migrate into the lamina propria in close contact with pathogenic cells [63]. Thus nTreg cells control immune responses in both draining LNs and lamina propria.
Multiple sclerosis
Autoimmune diseases, including multiple sclerosis (MS), are characterized by T effector cell migration and accumulation at the pathological site. Treg cells also accumulate within the murine central nervous system (CNS) during experimental autoimmune encephalomyelitis (EAE) [64], an animal model for multiple sclerosis. Both murine and human nTreg cells migrate more rapidly across brain endothelium compared to conventional T cells, and nTreg cell migratory abilities are significiantly impaired in patients with stable relapsing-remitting MS under non-inflammatory conditions compared to healthy donors [65, 66]. Impaired nTreg cell migration across the intact blood-brain barrier may contribute to early CNS lesion development or phases of MS relapse.
Allergic diseases
Models of allergic inflammation in mice and studies in human suggest an important role for Treg cells in control of allergic disease [67]. The ability of Treg cells to control airway Th2 proliferation and inflammation in allergen-presensitized mice depends on the activation status [68]. Activation up-regulates CCR4, enhances Treg migration to, and accumulation within, the lung, and consequently suppresses airway inflammation. This indicates that CCR4-dependent Treg cell migration plays a critical role for Treg cells to efficiently control Th2-type airway allergic inflammation.
Tumors
Treg cells interfere with anti-tumor immunity, and a recent report showed that Treg cells induce perforin-dependent dendritic cell death in tumor-draining LNs [69]. Hodgkin’s lymphoma (HL) cells in LNs are surrounded by a large number of lymphocytes expressing both CCR4 and FoxP3 [70]. CCR4 expression may be important in Treg cells in HL because a chimeric anti-CCR4 monoclonal antibody (mAb) can deplete CCR4+ T cells and inhibit the migration of CD4+CD25+ T cells in vitro. Increased numbers of Treg cells were detected in the cerebrospinal fluid (CSF) of patients with lymphomatous/carcinomatous neoplastic meningitis and the CSF had greater Treg cell-specific chemotactic activity than from control patients. Elevated numbers of CCR4+ Treg cells attracted by the chemokines CCL17 and CCL22 correlate with numbers of atypical cells in CSF [71], suggesting that CCR4-mediated Treg cell migration to the subarachnoid space generates a microenvironment that favors survival and growth of malignant cells. Studies of pancreatic human pancreatic adenocarcinoma and a murine pancreatic tumor model show that tumor cells produce increased amounts CCR5 ligand, and reciprocally, that Treg cells reside in tumors express more CCR5 than in normal tissues. When CCR5–CCL5 signaling is disrupted, Treg cell migration to tumors is reduced and tumors are smaller than in control mice [72], indicating a possible role of CCR5 in the homing of tumor-associated Treg cells. Analysis of cytokine and chemokine production by tumor cell lines including five lung cancers, a malignant mesothelioma, and a malignant melanoma showed increased IL-8 production by all tumors and increased by IL-6 production by one lung cancer cell line, the malignant mesothelioma, and the malignant melanoma. Migration of peripheral blood mononuclear cells (PBMCs) to these tumor cells using transwell migration assays showed that Treg cells were enriched at both IL-6- and IL-8-producing tumor cell lines. Marked induction of CXCR1 expression on Treg cells by IL-6 followed by IL-8-mediated migration is responsible for enhanced migration [73]. Together, these results suggest that preventing Treg cell migration and accumulation in tumor sites is an avenue for cancer therapy.
Transplantation
Treg cells play an important role in transplant tolerance [15]. When Treg cells are adoptively transferred from spleen and LNs of tolerant mice to naïve mice tolerance is achieved. Treg cells are found in the draining LNs as well as transplanted kidney, skin, and cardiac tissues [74–76]. Treg cells are present within cardiac allografts and their homing to allografts is CCR4 dependent [74], while CCR5 is essential for the recruitment of Treg cells to both lymphoid tissues and GVHD target organs including liver, lung, and spleen [77]. LN homing is crucial for Treg cell generation and tolerance induction, because depleting or blocking CD62L and thus preventing Treg cells from entering LNs abolishes tolerance induction [56, 57]. During tolerization, naïve T cells migrate to LNs, where they are stimulated in the cortical area by specific alloantigen presenting plasmacytoid dendritic cells (pDC), to generate aTreg cells [57]. Although locally delivering nTreg or aTreg cells prolongs allograft survival, the optimal suppression by Treg cells requires migration from grafts to draining LNs [59].
Concluding remarks
Many aspects of the Treg cell migration and trafficking mechanisms that are essential for priming at one site and effector function in another remain incompletely understood. The presence of Treg cells in both the draining LNs and at the inflammatory site is important in many settings to control immune responses. Recent work indicates that Treg cells in draining LNs and inflamatory sites may not be a division of labor between distinct Treg cell subsets, but rather a component of a closely coordinated, rapid movement of Treg cell subsets between these two sites. Thus, Treg cells traffic from peripheral tissue to draining LNs in the steady state, while immune responses accelerate this movement and trigger egress from draining LNs to inflammatory sites. Importantly, Treg cell migration into local inflamatory sites is not only necessary for suppression, but is also required for acquisition of full functional capacity for suppressive activities in both the draining LNs and at the inflammatory site. Recent findings that transcription factors essential for pathogenic Th1, Th2 and Th17 development are also required for Treg cell migration and function, further demonstrate the importance of Treg cell trafficking during an immune response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner JH. Mechanisms of impaired regulation by CD4(+) CD25(+) FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 5.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson MJ, et al. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffery LE, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay S, et al. Regulatory T cells and tumor immunity. Cancer Immunol. Immunother. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 16.Denning TL, et al. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 17.Ring S, et al. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur. J. Immunol. 2006;36:2981–2992. doi: 10.1002/eji.200636207. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz A, et al. Alteration of the migratory behavior of UV-induced regulatory T cells by tissue-specific dendritic cells. J. Immunol. 2007;178:877–886. doi: 10.4049/jimmunol.178.2.877. [DOI] [PubMed] [Google Scholar]
- 19.Siewert C, et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 20.Sather BD, et al. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Q, et al. CCR-4-dependent regulatory T cell function in inflammatory bowel disease. J. Exp. Med. 2007;204:1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa H, et al. Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4+CD25+ regulatory T cells in MRL/lpr mice. Arthritis Res. Ther. 2007;9 doi: 10.1186/ar2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MK, IV, et al. Treg mediated suppression of the allograft response in the draining lymph node. Transplantation. 2006;81:1063–1066. doi: 10.1097/01.tp.0000168365.80771.5a. [DOI] [PubMed] [Google Scholar]
- 24.Schneider MA, et al. CCR7 is required for the in vivo function of CD4+CD25+ regulatory T cells. J. Exp. Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kocks JR, et al. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J. Exp. Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, et al. Regulatory T cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen VH, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 28.Menning A, et al. Distinctive role of CCR7 in migration and functional activity of naïve- and effector/memory-like Treg subsets. Eur. J. Immunol. 2007;37:1575–1583. doi: 10.1002/eji.200737201. [DOI] [PubMed] [Google Scholar]
- 29.Siegmund K, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, et al. Where CD4+CD25+ Treg cells impinge on autoimmune diabetes. J. Exp. Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samy ET, et al. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of Treg cell-mediated suppression. J. Exp. Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Förster R, et al. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 34.Campbell DJ. Targeting T cell responses by selective chemokine receptor expression. Semin. Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Wing K, et al. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–525. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booth NJ, et al. Different Proliferative Potential and Migratory Characteristics of Human CD4+ Regulatory T Cells That Express either CD45RA or CD45RO. J. Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 37.Grindebacke H, et al. Dynamic Development of Homing Receptor Expression and Memory Cell Differentiation of Infant CD4+ CD25high Regulatory T Cells. J. Immunol. 2009;183:4360–4370. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 38.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomura M, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 2010;120:883–893. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushima H, Takashima A. Bidirectional homing of Tregs between the skin and lymph nodes. J. Clin. Invest. 2010;120:653–656. doi: 10.1172/JCI42280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol. 2005;26:632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Kroetz DN, Deepe GS., Jr CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J. Immunol. 2010;184:5224–5231. doi: 10.4049/jimmunol.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szanya V, et al. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 44.Trifari S, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudda JC, Martin SF. Tissue targeting of T cells by DCs and microenvironments. Trends Immunol. 2004;25:417–421. doi: 10.1016/j.it.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pallandre JR, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J. Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 52.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 53.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ermann J, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 55.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochando JC, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J. Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 57.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 58.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang N, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadis U, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 61.Powrie F, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 62.Annacker O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 63.Mottet C, et al. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 64.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connor RA, et al. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J. Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 66.Gärtner D, et al. CD25 regulatory T cells determine secondary but not primary remission in EAE: impact on long-term disease progression. J. Neuroimmunol. 2006;172:73–84. doi: 10.1016/j.jneuroim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Palomares O, et al. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 68.Saito K, et al. Differential Regulatory Function of Resting and Preactivated Allergen-Specific CD4+CD25+ Regulatory T Cells in Th2-Type Airway. J. Immunol. 2008;181:6889–6897. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- 69.Boissonnas A, et al. Foxp3+ T Cells Induce Perforin-Dependent Dendritic Cell Death in Tumor-Draining Lymph Nodes. Immunity. 2009;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Ishida T, et al. Specific Recruitment of CC Chemokine Receptor 4–Positive Regulatory T Cells in Hodgkin Lymphoma Fosters Immune Privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 71.Haas J, et al. Specific recruitment of regulatory T cells into the CSF in lymphomatous and carcinomatous meningitis. Blood. 2008;111:761–766. doi: 10.1182/blood-2007-08-104877. [DOI] [PubMed] [Google Scholar]
- 72.Tan MC, et al. Disruption of CCR5-Dependent Homing of Regulatory T Cells Inhibits Tumor Growth in a Murine Model of Pancreatic Cancer. J. Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eikawa S, et al. Enrichment of Foxp3+ CD4 Regulatory T Cells in Migrated T Cells to IL-6- and IL-8-Expressing Tumors through Predominant Induction of CXCR1 by IL-6. J. Immunol. 2010;185:6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- 74.Lee I, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sawitzki B, et al. Regulatory tolerance-mediating T cells in transplantation tolerance. Transplant. Proc. 2001;33:2092–2093. doi: 10.1016/s0041-1345(01)01960-1. [DOI] [PubMed] [Google Scholar]
- 76.Graca L, et al. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wysocki CA, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]