Abstract
Neural circuits underlie our ability to interact in the world and to learn adaptively from experience. Understanding neural circuits and how circuit structure gives rise to neural firing patterns or computations is fundamental to our understanding of human experience and behavior. Fear conditioning is a powerful model system in which to study neural circuits and information processing and relate them to learning and behavior. Until recently technological limitations have made it difficult to study the causal role of specific circuit elements during fear conditioning. However, newly developed optogenetic tools allow researchers to manipulate individual circuit components such as anatomically or molecularly defined cell populations, with high temporal precision. Applying these tools to the study of fear conditioning to control specific neural subpopulations in the fear circuit will facilitate a causal analysis of the role of these circuit elements in fear learning and memory. By combining this approach with in vivo electrophysiological recordings in awake, behaving animals, it will also be possible to determine the functional contribution of specific cell populations to neural processing in the fear circuit. As a result, the application of optogenetics to fear conditioning could shed light on how specific circuit elements contribute to neural coding and to fear learning and memory. Furthermore, this approach may reveal general rules for how circuit structure and neural coding within circuits gives rise to sensory experience and behavior.
Keywords: optogenetics, fear conditioning, neural circuits, learning and memory, neural plasticity, electrophysiology
Neural circuits are anatomically and functionally interconnected networks of neurons which mediate specific aspects of experience and behavior. Many neural circuits control behavior by integrating sensory signals from the environment, memories acquired from previous experience, and information about the current state of the organism. Specific circuits mediate a range of adaptive functions, from feeding and mating, to visual and other forms of sensory processing, to emotional learning, to working memory, attention, and other higher cognitive functions. A central goal in neuroscience research is to define the functional anatomy and the neural computations occurring within these circuits.
Fear conditioning is a powerful system in which to study neural circuits, neural coding in these circuits and the influence of learning, memory and plasticity on circuit processes (1–8), as well as being an important model for studying fear and anxiety (4, 9, 10). Fear conditioning occurs when a sensory conditioned stimulus (CS, usually an auditory tone) is paired with an aversive unconditioned stimulus (US, usually a mild electric shock) during a training phase. Following training, the presentation of the CS alone produces behavioral and visceral fear conditioned responses (CRs), demonstrating that a long term memory has been formed (1). One major advantage in using fear conditioning to study neural circuits is that it is a relatively simple procedure in which easily quantifiable behaviors are elicited by stimuli that are under the control of the experimenter (1–8). This relative simplicity facilitates the mapping of functional circuits and the identification of sites of neural plasticity in these circuits.
Over the last 30 years, studies using lesion, electrophysiological, pharmacological, and biochemical/molecular techniques have revealed a great deal about the neural mechanisms of fear learning (LeDoux et al, in press) (1–7, 11, 12). In spite of this progress, much remains to be understood about the fundamental principles by which fear conditioning is implemented at the level of defined neural circuits. In addition, information processing by neurons in these brain regions and particularly how circuit mechanisms give rise to these computations is largely unknown. While traditional techniques have been valuable in defining the fear circuit, they lack the temporal and spatial specificity needed to make further progress on many of these issues. In order to address these questions, techniques for manipulating specific circuit elements (i.e. subpopulations of neurons and specific axonal projections) with high temporal precision are needed.
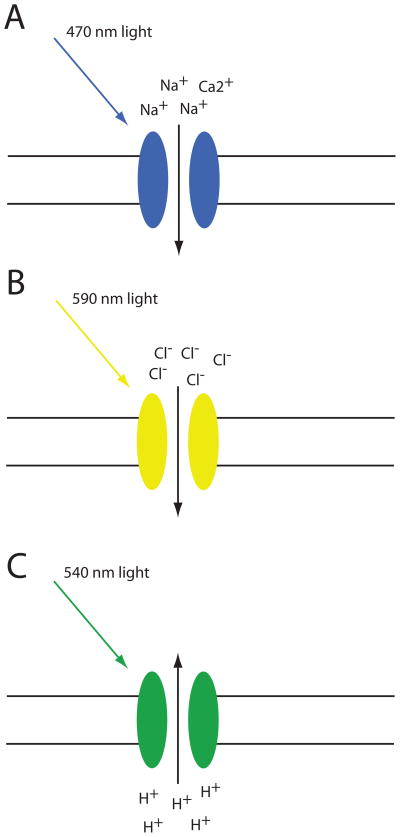
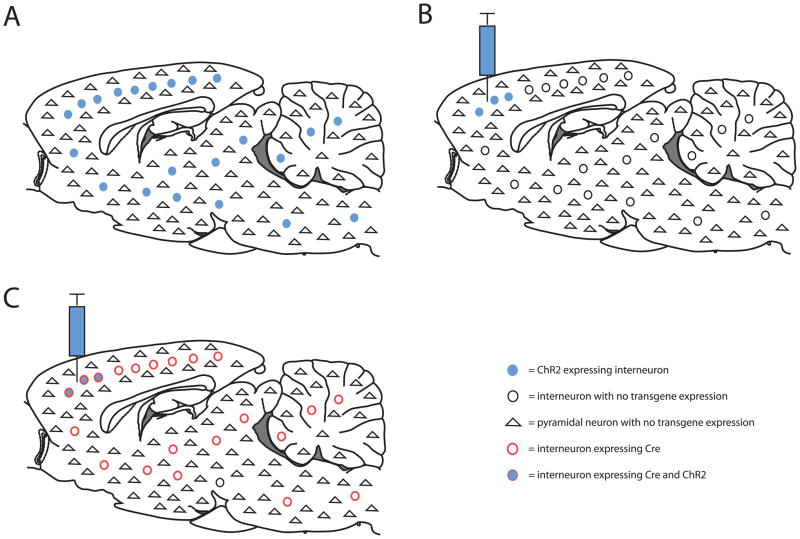
The recent development of optogenetics –the combined use of optical and genetic technologies to control cells in intact neural circuits (13)- provides a tool to ask important and previously unaddressable questions as it provides the ability to modulate specific circuit elements with high temporal precision (see below for detailed discussion of some of these questions in fear conditioning) (13–16). An important step in the development of optogenetics was the discovery of the algael light activated Channelrhodopsin-2 (ChR2) and the functional expression of ChR2 in neurons to control neural activity (17–19). ChR2 is a blue light activated, non-specific cation channel which can be expressed heterologously in neurons and used to depolarize and excite cells using light (see Figure 1A and (20, 21) for review). Other ion channels and pumps activated by different wavelengths of light have since been developed, including two which inhibit neural activity, Halorhodopsin (Figure 1B) and Archaerhodopsin (Figure 1C) (22–25). Throughout the rest of this review ChR2 (and the other modified ChR2 variants) will be referred to as excitatory opsins and Halo- and Archaerhodopsin (and their variants) will be referred to as inhibitory opsins. Opsins can be expressed globally or in specific subpopulations of neurons in distinct brain regions using transgenic animals, local viral infection, or combinations of Cre-recombinase (Cre) expressing mouse lines with Cre-dependent viral mediated opsin expression (see Figure 2 for description of these different approaches and (20) for review of this topic). Lasers or LEDs can then be employed to deliver light to the brain to control the activity of opsin expressing cells. The use of both excitatory and inhibitory opsins in this way can unambiguously demonstrate both necessity and sufficiency of defined circuit elements. This approach has been used to control behavior and has been reviewed previously in (16, 26).
Figure 1.
Prototypic opsin proteins for bidirectional manipulation of neuronal activity. A) Channelrhodopsin-2 (ChR2) is a light-gated, non-specific cation channel (with low Ca2+ permeability, (96)), which is activated by blue light (~470 nm). In ChR2-expressing neurons illumination with blue light causes depolarization and spiking of the cell. While traditional ChR2 variants produce reliable spiking up to about 20 Hz, modified versions are capable of producing much higher spiking frequencies (see (97) for review). B) Halorhodopsin is an inward chloride pump, which causes hyperpolarization of expressing neurons, inhibiting their activity, upon illumination with yellow light (~590 nm). C) Archaerhodopsin is an outward proton pump that also hyperpolarizes expressing neurons upon illumination with green or yellow light (~540–590 nm).
Figure 2.
Strategies for opsin expression. A) Opsins can be expressed using a transgenic approach in specific subpopulations of neurons with tissue specific promoters such as the interneuron cell specific promoter parvalbumin (PV, circular cells are interneurons and triangles are pyramidal cells). Illustrated here is hypothetical ChR2 expression (filled blue cells) in all PV interneurons in the brain driven by the PV promoter. B) Specific cell populations can also be targeted using a virus only approach in which a virus can be injected into specific brain regions and produces expression of opsins in specific cells types using cell type specific promoters such as the PV promoter to target PV interneurons neurons (as illustrated here). This approach has not been demonstrated for PV interneurons, however, and can be non-optimal for targeting specific cell populations because most viruses have limited packaging capacity making it necessary to use truncated versions of tissue specific promoters which can reduce cell type specificity. Furthermore, only few promoters can be appropriately truncated, which limits the number of cell populations which can be targeted using this approach. C) Specific cell populations can also be targeted using a combined transgenic and virus based approach. In this method, transgenic animals can be constructed which express Cre-recombinase (see (20) for review and (98, 99) for recent application) under the control of tissue specific promoters such as the PV promoter (pictured here as red outlined circular cells). Viruses whose expression is dependent on Cre-recombinase can then be injected into the specific brain region in which opsin expression is desired. Since the opsins will only be expressed in Cre-recombinase expressing neurons (blue filled and red outlined cells), this approach adds cell type-specificity to the spatial selectivity. Overall, this approach offers increased cell type specificity due to the use of endogenous, full length promoters driving Cre expression and allows better spatial and temporal control of opsin expression than transgenic opsin-expressing animals. Fiber optic cables attached to a light source can then be inserted into the brain region in which opsins are expressed and optogenetic control is desired.
The optogenetic approach provides the capability to control neural activity in the fear circuit with millisecond precision and to manipulate specific cell populations and afferent inputs to a given brain region. In this article we will first provide a brief introduction to the functional anatomy of the fear circuit and the computations performed by neurons in this circuit during fear conditioning. We will then discuss the potential applications of optogenetics to the study of the neural circuits of fear.
Fear Conditioning Circuits
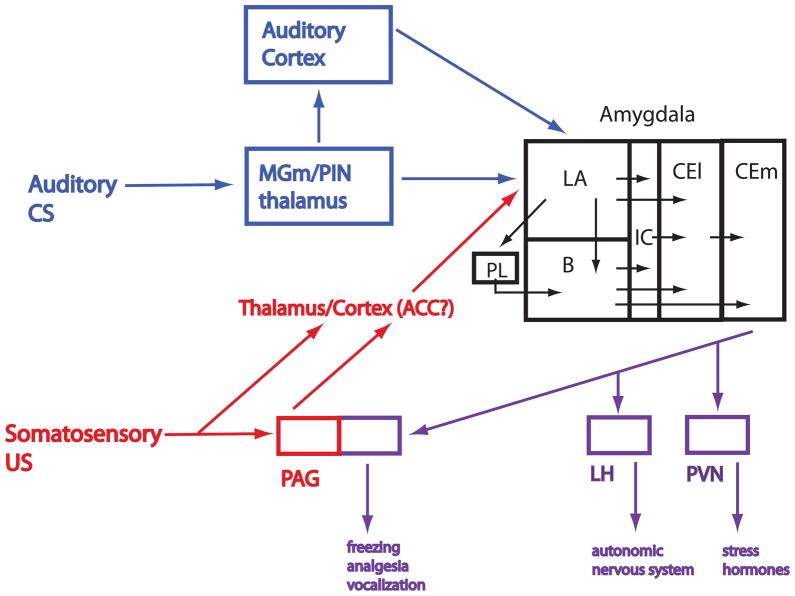
A rough working model of the fear circuit (Figure 3) has been developed through a variety of approaches including brain lesion and pharmacological manipulations as well as electrophysiological measurements. Studies examining the circuit architecture of fear conditioning have focused on pathways which transmit auditory CS information, aversive somatosensory US information, those which integrate CS and US information and those involved in producing fear CRs.
Figure 3. Working Model of the Fear Conditioning Circuit.
Auditory conditioned stimulus (CS) information is conveyed through medial geniculate (MGm) and posterior intralaminar nucleus (PIN) of the thalamus and cortical relays (temporal association area, TeA) to the lateral nucleus of the amygdala (LA). Unconditioned stimulus (US) information is conveyed through a pathway which includes the periaqueductal gray (PAG) and other, relays possibly in the thalamus and/or anterior cingulate cortex (ACC), to the LA. Single LA neurons receive convergent CS and US information and undergo associative synaptic plasticity during fear conditioning. Plasticity may also occur in the central nucleus of the amygdala and in the MGm/PIN. LA connects with the central lateral nucleus of the amygdala (CEl) directly and indirectly by way of amygdala connections in the prelimbic (PL), basal (B) and intercalated (ic) amygdala subregions. The central medial nucleus of the amygdala (CEm) receives input from the B and CEl and is an output nucleus which projects to other regions including the PAG, lateral hypothalamus (LH) and paraventricular nucleus of the hypothalamus (PVN) that control the expression of conditioned fear responses, including freezing, autonomic and hormonal responses. Neural processing of CS and US information has been examined in several of these regions and is described in the text.
Auditory CS pathways for fear conditioning
The auditory CS pathways involved in simple forms of fear conditioning (those in which a pure tone or other acoustic stimuli with simple features is used) require the medial geniculate (MGm) and the posterior intralaminar thalamic nuclei (PIN)(27–30) (but see (29, 31)) while fear conditioning to more complex CSs recruits both thalamic and auditory cortical pathways (30, 32). Neurons in both the MGm/PIN and primary auditory cortex and auditory association cortex (temporal association cortex, TeA) are responsive to auditory CSs and some neurons in the MGm/PIN and in TeA also respond to somatosensory stimuli (33–37). Many MGm/PIN neurons do not exhibit precise frequency tuning to auditory stimuli prior to learning (36, 38, 39), but auditory cortical neurons are more finely tuned to frequency (37). Neurons in both auditory thalamus and cortex increase their CS-evoked responses following fear conditioning (33–37, 40, 41) and tuning in both of these regions is sharpened to the specific tone frequencies which are paired with the aversive US (36, 37).
Lateral Amygdala is a critical site of associative plasticity for fear conditioning
The lateral nucleus of the amygdala (LA) is known to be a critical site of CS-US convergence and associative plasticity and memory storage during auditory fear conditioning (thugh it is likely not the only site of plasticity in the fear circuit, see (36, 42) and amygdala neurons may participate in fear conditioning induced by a wide range of sensory stimuli, see (43–45)). Both MGm/PIN and TeA project to and form synapses with neurons in the LA (see (1) for review). LA neurons receive convergent input from auditory CSs and somatosensory USs (46, 47) and prior to fear conditioning LA neurons code auditory frequency crudely, exhibiting large auditory frequency receptive fields (48, 49). Importantly, fear conditioning induces an enhancement of CS-evoked responding as measured by electrophysiological recordings (35, 47, 50–54). Central to our understanding of the fear circuit is the idea that the LA is a key site of associative plasticity during fear conditioning. According to this model, CS-US convergence in LA pyramidal cells induces associative plasticity such that the CS more effectively drives postsynaptic neurons in the LA after pairing with the US. Supporting this, both fear conditioning at the level of behavior and associative plasticity of auditory CS inputs to postsynaptic LA neurons requires activation of various intracellular signaling molecules or processes which are thought to be important for synaptic plasticity in LA (see LeDoux et al in press, (5, 6) for review).
The LA is made up of pyramidal cells and interneurons and because a larger percentage of LA neurons are pyramidal cells it is possible that this cell population was preferentially sampled in most of the in vivo recording studies. However, the specific contribution of LA interneurons and pyramidal cells to neural coding and behavior during fear conditioning is largely unknown.
Though the US pathways which trigger LA plasticity are not clearly defined (55–59), it does appear that the Periaqueductal Gray (PAG) may be a part of the circuit which transmits US information to the LA (11, 47). Like CS coding, US information processing in LA neurons is also modulated by learning, but in the opposite direction from learning related modulation of CS-evoked responding (11, 47, 60). Namely, US-evoked responding is reduced as animals learn that the CS predicts the US. This type of expectancy modulated coding of US information is also seen in the PAG and PAG inactivation attenuates this US signal in LA neurons (Johansen, et al., 2010c). This suggests that the expectancy modulated US signal in the LA is encoded in (or prior to) the PAG and then directly or indirectly transmitted to the LA.
Output circuits for the production of fear behaviors
Following fear conditioning, the CS gains access to the output circuits responsible for producing fear responses. Projections from the LA to the the central nucleus of the amygdala (CE), directly and indirectly (possibly through the basal nucleus of the amygdala (B) (61, 62), but see also(63)), the prelimbic (PL) cortex (for review see(64) and through the amygdala intercalated cells (IC) (see (62, 65) for review) may provide an output pathway from the LA for the elicitation of fear CRs (66, 67). Recent studies (68, 69) suggest that a pathway from the lateral division of the CE (CEl) to the medial division of the CE (CEm) transmits CS information through the CE. The CEm is then thought to project to the PAG, hypothalamus and directly or indirectly to other brainstem effector sites to control specific components of the concerted fear response (for review see (1–7)).
A number of studies have reported that fear conditioning produces changes in CS processing by CE neurons (68–72). Several recent papers demonstrate that subpopulations of CEl neurons (which are mainly inhibitory) are altered differentially by fear conditioning (68, 69, 72). CE “on” cells exhibit fear conditioned enhancement of CS-evoked excitatory responding while “off” neurons show conditioned enhancement of CS-evoked inhibitory responding. In addition to different electrophysiological subtypes of CEl neurons there are also many molecularly defined subclasses of CEl neurons(69, 73, 74). One study (69) identified a molecular marker for CEloff cells, opening the possibility for genetic and optogenetic manipulation of these neurons (see below). In contrast to CEl neurons, CEm neurons (which are known to receive input from CEl) were primarily excited by a fear conditioned CS. To date there have been no in-vivo physiological recordings of CS processing during fear conditioning in any CE projection targets involved in producing the individual fear responses.
Optogenetics and Fear conditioning
While a rough outline of the fear circuit has been delineated using traditional techniques, there is still much to be discovered. For example, neurons within particular areas of the fear circuit are known to be activated during specific time periods of fear conditioning (ex. CS or US periods), but in most cases their temporally limited, functional role in behavior and neural processing is unknown. In addition, within specific areas of the fear circuit there are neuronal subpopulations (some of which are discussed above). These subpopulations may be distinguishable based on their unique molecular identity or anatomical projection patterns, but before the advent of optogenetics it was difficult to target these specific neural elements. Optogenetics offers a means to surmount these issues by providing the ability to 1) manipulate neural firing rate with high temporal precision during specific time epochs of fear conditioning, 2) target manipulations to particular subclasses of neurons, specific afferent input terminals to a given brain region or specific cell types based on their projection patterns or molecular markers (see Figure 4), 3) identify specific cell types during extracellular recordings (see Figure 5) and 4) map the detailed connectivity of defined inputs to cells in a given brain region (see Figure 6).
Figure 4. Virus Mediated Targeting of LA Pyramidal Neurons and not Interneurons.
Lateral Amygdala (LA) pyramidal neurons can be targeted (as in Johansen et al., 2010b) using a minimal Ca2+/Calmodulin (Cam) dependent protein kinase II (CAMKII) promoter which can be used to preferentially drive expression in CaMKII+ (illustrated here as blue cells), as opposed to GABA+, neurons. Laser light (473 nm wavelength) can then be shone into the LA (blue sphere in figure) through a fiber optic cable to manipulate fear learning and behavior. This technique could also be used in conjunction with in vivo physiology to record single neurons or field potential responses. It would then be possible to manipulate activity specifically in LA pyramidal cells (blue cells) and examine the effects of these manipulations on neural processing and associative plasticity in the LA. Other populations of LA neurons, such as interneurons (black cells), could also be targeted. However, this would likely require a transgenic approach or the combination of transgenic mouse or rat lines expressing Cre-recombinase in defined neuronal populations with Cre-dependent viruses for opsin expression. (as has been done previously, see (98, 99) for example).
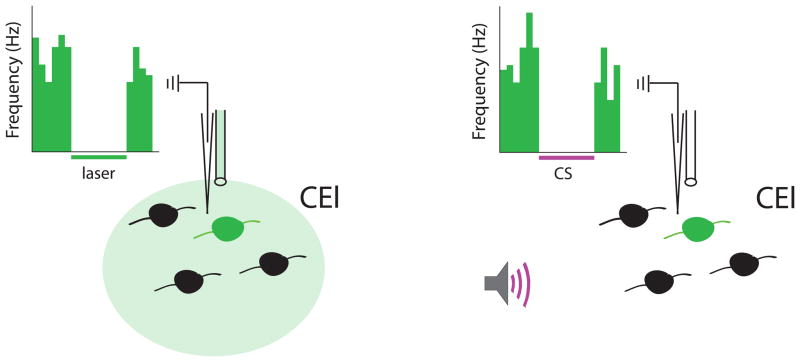
Figure 5. Optogenetic Identification and Characterization of Molecularly Defined Cell Types in the central lateral nucleus of the amygdala (CEl).
Molecularly or anatomically defined cell populations can be identified through optogenetic manipulations and their neural coding can be assayed. In this example, protein kinase Cδ positive (PKCδ+) cells in CEl are targeted (green cells) with an inhibitory opsin using a transgenic mouse expressing Cre-recombinase driven by a PKCδ promoter combined with injection of a Cre-dependent virus encoding an inhibitory opsin into the CEl. Once a single cell has been isolated, light can be shone onto it (left panel). Inhibition of neural activity by green or yellow light (green sphere in figure) identifies the cell as PKCδ+. This is shown in the perievent time histogram of hypothetical data in which firing rate (y-axis) is reduced during the laser on period (green bar under x-axis). The neural response to various experimental manipulations can then be assayed (right panel). In this illustrative case, conditioned stimulus (CS) presentation (purple line under x-axis) inhibits neural activity in the cell. A non-optogenetic technique has been used previously to identify PKCδ cells in CEl as being CEloff cells (Haubensak et al., 2010) and it is shown here to illustrate the potential for optogenetic identification of specific cell populations. Thus this technique allows on-line identification of individual cell populations while recording in the awake, behaving animal, which will then facilitate the study of how these specific cell populations encode sensory (in this case) or other types of information.
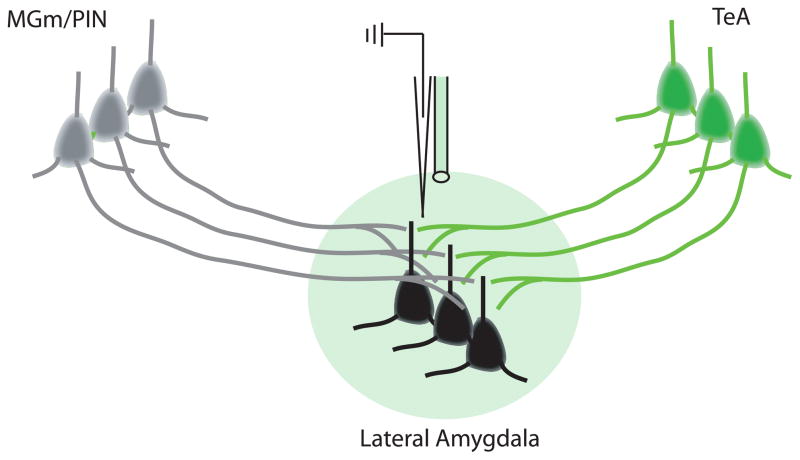
Figure 6. Optogenetic Control of Specific Afferent Inputs to the lateral nucleus of the amygdala (LA).
Infection of temporal association cortex (TeA) cells with an opsin expressing virus (an inhibitory opsin in this case) will produce opsin expression in TeA terminals in the LA. Green or yellow light (green sphere in figure) delivered through an in-dwelling fiber optic cable can then be used to inhibit the release of neurotransmitter specifically from TeA terminals while not affecting other inputs such as those from medial geniculate/posterior intralaminar thalamic nuclei (MGm/PIN, grey cells). When combined with in vivo neural recording in awake, behaving animals it would be possible to determine the contribution of TeA inputs to neural coding (for example of conditioned stimulus (CS) information) in LA neurons (black cells) and to fear behavior.
Cell type specific manipulations with precise temporal control
As mentioned previously, lesion, pharmacological inhibition and electrical/pharmacological techniques have a number of limitations. Lesion and pharmacological manipulations affect cell processing permanently or throughout the entire behavioral training or testing session and commonly modulate activity across all cell populations in a given region. Electrical stimulation, while more temporally precise stimulates all cell types and fibers of passage. Optogenetics provides the ability to manipulate defined cell types (using tissue specific promoters or conditional viruses in combination with Cre lines to drive expression of opsins, see Figures 2 & 4) during specific temporal epochs of fear conditioning.
While the use of this technique is in its early stages, it has already been exploited successfully in a few fear conditioning papers. Using a minimal version of the Ca2+/Calmodulin (Cam) dependent protein kinase II (CaMKII) promoter, one set of studies preferentially targeted LA pyramidal cells (as opposed to LA inhibitory interneurons) using a viral approach in rats during fear conditioning to examine the mechanisms by which the aversive US triggers learning (75, 76). It has generally been assumed that LA plasticity and fear learning involve associative Hebbian mechanisms in LA pyramidal cells. Thus it is thought that US-evoked depolarization in LA pyramidal neurons triggers plasticity of coactive CS inputs onto the same cells resulting in fear memory formation. If this were true, then pairing an auditory CS with direct depolarization of LA pyramidal neurons, in place of an actual footshock US, should produce learning. By targeting expression of ChR2 to LA pyramidal neurons and delivering laser light to this population of cells, this study (75) showed that large numbers of CS-laser US pairings produced some fear learning and memory. However, this learning was weak and more recent preliminary work (76) has found that under normal training conditions (i.e. lower numbers of CS-US pairings) US-evoked depolarization of LA pyramidal cells is not sufficient to produce normal levels of fear learning and that a multi-process mechanism involving US-evoked depolarization and activation of the noradrenergic system is important in triggering fear memory formation. The use of optogenetics in these studies to manipulate cell activity in a specific subpopulation of neurons (LA pyramidal cells) during a temporally defined period made it possible to test a question that was not addressable previously (Figure 4).
Another recent experiment which elucidated the functional microcircuitry of CE (as discussed above in (68)) used a viral based optogenetic approach in mice to determine whether stimulation of CEm neurons is sufficient to produce freezing behavior (the most well studied response to fear inducing stimuli). As discussed, CEm is thought to be an important output nucleus of the amygdala for producing conditioned fear responses, but it was not clear, prior to this study, whether excitation or inhibition of CEm cells produced fear responses such as freezing. Previous work did find evidence that stimulation of CEm drives freezing behavior, but these studies used electrical stimulation which excites fibers of passage in addition to cell bodies. Furthermore, one previous experiment found that putative projection neurons in the CE were inhibited by CSs, suggesting that inhibition of CE neurons may produce freezing behaviors. The recent study demonstrated that CEm projection neurons were robustly excited by CSs following fear conditioning and that direct, optical stimulation of CEm neurons was sufficient to produce freezing responses.
These are just the first few studies using optogenetics to study fear, but the potential for its use in understanding the function of specific cell populations during temporally defined periods of fear conditioning is impressive. For example, one potential application would be to elucidate the functional and temporal contribution of the many different subpopulations of neurons in the CEl (69, 74, 77, 78) to the learning and performance of fear conditioning by using Cre lines specific for these subpopulations. The recent genetic engineering of PKCδ and CRF promoter driven Cre mice (69, 79) (and other Cre lines are available through commercial suppliers) could be used to optogenetically target specific cell populations in the CE. It will also be possible to combine recordings from single neurons in the awake, behaving animal with optogenetics (80, 81) (82, 83) and examine the contribution of these different populations of CEl neurons to coding in CEm neurons and cells in areas which receive CE projections such as the PAG. Furthermore, optogenetics allows for identifying cell types during extracellular single unit recordings (84). Using this approach, molecularly or anatomically defined subtypes of neurons can be recorded from awake animals during fear conditioning and their information coding capabilities can be assayed (Figure 5).
Subtype and afferent specific control of neural circuits based on anatomical connectivity
Another advantage that the optogenetic approach affords is the ability to control the activation of specific afferent inputs in a given brain nucleus. Expressing excitatory or inhibitory opsins in neurons in one brain region, results in expression of the corresponding opsins throughout the cell including in the axons and synaptic terminals in brain structures distant from the region which was originally infected/transduced. Synaptic release can then be controlled by shining light onto the terminals of these neurons (85–93). Optogenetic control of afferent terminals expressing opsins has been used to map circuit structure (see below) and to manipulate behavior. For example, one study found, using a viral approach in rats, that optical excitation of basal amygdala projections to CEm reduced and inhibition of these same terminals enhanced anxiety-like behavior (89). Interestingly, this effect was not seen when the cell bodies of these neurons were manipulated, demonstrating afferent specific modulation of behavior.
Other viral based approaches have recently been utilized which allow control of specific cell populations in a given brain region based on their projection patterns to other brain regions. For example, several recent studies have taken advantage of certain viruses that are taken up preferentially by synaptic terminals and transported retrogradely to the cell bodies of these terminals in other brain regions (24, 84). This makes it possible to express opsins and control neural activity in cells which project to the brain region in which a virus is introduced. Another similar approach uses transsynaptic rabies viruses to express opsins in retrogradely transduced cell populations which project to a specific subpopulation of target neurons (94). To date no one has used either of these approaches to manipulate behavior. However, they could allow light control of particular subpopulations of neurons in a given brain region based on their anatomical connectivity with other brain regions or with specific postsynaptic neurons.
Combined with the temporal control that optogenetics allows, these strategies have obvious advantages for studying the circuits and computations mediating fear conditioning. For example, the MGm/PIN and TeA both project to the LA and likely provide different types of information to LA neurons (for review see (1, 36, 37)) during specific temporal epochs. However, both of these regions contain heterogeneous subpopulations of cells which project to brain regions other than the LA making it difficult to interpret the results of manipulations that target all subpopulations of neurons in these regions. To examine the specific role of the thalamic and cortical projections to the LA, a virus encoding excitatory or inhibitory opsins could be injected into the MGm/PIN or TeA. This would allow control of the terminals of these neurons in the LA and make it possible to determine the functional/temporal contribution of these inputs to fear behaviors and to neural coding in LA (Figure 6). Alternatively, a virus which is taken up by synaptic terminals could be injected into the LA where it would travel retrogradely to the MGm/PIN and TeA neurons which project there. This technique has not been used to control behavior (24, 84), but it could allow light control of the specific MGm/PIN or TeA neural subpopulations which project to the LA. Though in early stages of development, these two complementary approaches could be widely utilized in the fear circuit to determine the functional involvement of anatomically defined cell populations and their synaptic inputs in specific brain regions to fear conditioning and to neural processing.
Mapping circuit connectivity
Optogenetic control of specific synaptic afferents to a given brain region has also been used to map circuit connectivity. For example, ChR2 has been expressed in various thalamic and cortical regions as well as in basal ganglia circuits and the afferent axons of these cells were stimulated in projection regions to determine the distinct connectivity of these inputs in target neurons (85–88, 90, 91). Using this approach combined with imaging of cortical neurons, one study mapped out both the laminar specificity of different inputs to the barrel cortex as well as the sub-cellular specificity of these inputs onto different regions of the dendritic arbor (85).
This technique has also been applied to the amygdala. One study (88) used a viral approach in mice and infected TeA or anterior cingulate cortex (ACC, which may convey US information to the LA) neurons with ChR2 and strongly stimulated TeA or ACC inputs in the LA to produce synaptic plasticity. The authors found that high frequency stimulation induced long term potentiation (LTP, a cellular model of synaptic plasticity) only occurs in the TeA-LA pathway if feedforward inhibition is blocked, but that ACC-LA LTP does not recruit feedforward inhibitory circuits in the LA. This suggests that synaptic plasticity in TeA-LA CS input pathway may be modulated by feedforward inhibitory circuits. This approach has also been used to reveal the connectivity between a particular subclass of CEl neurons and CEm output neurons and to elucidate a specific intra-amygdalar pathway which includes B-CEl-CEm connections (69, 89). These types of approaches along with traditional techniques (95) can be used in future studies to, for example, map out the detailed connectivity of different afferent inputs to the LA (and to other parts of the fear circuit) and reveal how postsynaptic LA neurons integrate information from these input pathways. This approach could also be used to study how the local LA circuits and integrative properties of the postsynaptic cells together contribute to synaptic plasticity at particular input pathways.
Future Directions
We have limited our discussion here to a few optogenetic applications which we believe will be most advantageous for studying the circuits and computations underlying behavioral fear conditioning. While there are some caveats to consider (see Supplemental Information) advances in molecular biology will help to refine and expand this technology and will likely offer new unexplored avenues of study to researchers from a broad range of disciplines. Using optogenetic manipulations in combination with behavior and physiology it will be possible to reveal, in much greater detail, the temporal contribution of specific inputs and cell types to fear behavior and to neural coding. Eventually, this will provide an avenue toward the ultimate goal of understanding how brain circuits and computations within these circuits mediate fear behavior and may suggest general mechanisms of circuit and computational coding that are shared by many neural systems.
Supplementary Material
Acknowledgments
This work was supported by a F32-MH082505 to J.P.J. and R01-MH046516 to J.E.L. AL is supported by grants from the Swiss National Science Foundation, the European Commission (Eurospin Project, Contract HEALTH-F2-2009-241498), and the Novartis Research Foundation. SBEW is supported by a grant from the Schering Foundation. We thank Linnaea Ostroff and Tamás Madarász for valuable comments on the manuscript.
Footnotes
Financial Disclosure: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 3.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 4.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 5.Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- 6.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- 9.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 15.Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol Rep. 2011;3:11. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 24.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antunes R, Moita MA. Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. J Neurosci. 2010;30:9782–9787. doi: 10.1523/JNEUROSCI.1037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- 32.Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav Neurosci. 2008;122:1178–1185. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- 33.Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. J Neurosci. 2007;27:12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 36.Weinberger NM. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res. 2010 doi: 10.1016/j.heares.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- 39.Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- 40.Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J Neurosci. 2001;21:270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- 44.Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishijo H, Ono T, Nishino H. Topographic distribution of modality-specific amygdalar neurons in alert monkey. J Neurosci. 1988;8:3556–3569. doi: 10.1523/JNEUROSCI.08-10-03556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 47.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci. 1993;107:757–769. doi: 10.1037/0735-7044.107.5.757. [DOI] [PubMed] [Google Scholar]
- 49.Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 51.Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 53.Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 54.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 55.Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunzell DH, Kim JJ. Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behav Neurosci. 2001;115:365–375. [PubMed] [Google Scholar]
- 57.Lanuza E, Moncho-Bogani J, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 59.Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain. 2005;1:6. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 63.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 64.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 66.Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- 67.Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- 68.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 69.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 71.Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238:457–462. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- 72.Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci. 2011;31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 74.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 75.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johansen JP, Hamanaka H, Diaz-Mataix L, Le Doux JE. Hebbian and neuromodulatory mechanisms act synergistically to instruct associative memory formation. Society for Neuroscience Abstract. 2010:914.15. [Google Scholar]
- 77.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 78.Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- 79.Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, et al. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsaki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279–2291. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kravitz AV, Kreitzer AC. Optogenetic manipulation of neural circuitry in vivo. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 87.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morozov A, Sukato D, Ito W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. J Neurosci. 2011;31:339–345. doi: 10.1523/JNEUROSCI.5537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, et al. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 93.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011 doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- 95.Humeau Y, Herry C, Kemp N, Shaban H, Fourcaudot E, Bissiere S, et al. Dendritic spine heterogeneity determines afferent-specific Hebbian plasticity in the amygdala. Neuron. 2005;45:119–131. doi: 10.1016/j.neuron.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 96.Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods. 2007;4:139–141. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- 97.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.