Abstract
Rationale
A crucial step in atherogenesis is the infiltration of the sub-endothelial space of large arteries by monocytes where they differentiate into macrophages and transform into lipid-loaded foam cells. Macrophages are heterogeneous cells which adapt their response to environmental cytokines. Th1 cytokines promote monocyte differentiation into M1 macrophages, while Th2 cytokines trigger an “alternative” M2 phenotype.
Objective
We previously reported the presence of CD68+MR+ M2 macrophages in human atherosclerotic plaques. However, the function of these plaque CD68+MR+ macrophages is still unknown.
Methods and Results
Histological analysis revealed that CD68+MR+ locate far from the lipid core of the plaque and contain smaller lipid droplets compared to CD68+MR− macrophages. IL-4 polarized CD68+MR+ display a reduced capacity to handle and efflux cellular cholesterol due to low expression levels of the nuclear receptor Liver X Receptor (LXR)α and its target genes, ABCA1 and ApoE, caused by the high 15-lipoxygenase activity in CD68+MR+ macrophages. By contrast, CD68+MR+ highly express opsonins and receptors involved in phagocytosis resulting in high phagocytic activity. In M2 macrophages, Peroxisome Proliferator-Activated receptor (PPAR)γ activation enhances the phagocytic, but not the cholesterol trafficking pathways.
Conclusions
These data identify a distinct macrophage sub-population with a low susceptibility to become foam cells, but high phagocytic activity due to different regulatory activities of the PPARγ-LXRα pathways.
Keywords: Cell Differentiation; physiology; Cells, Cultured; Cholesterol; metabolism; Genetic Predisposition to Disease; Humans; Leukocytes, Mononuclear; metabolism; pathology; Macrophages; metabolism; pathology; Orphan Nuclear Receptors; metabolism; physiology; PPAR gamma; metabolism; Phagocytosis; physiology; Plaque, Atherosclerotic; metabolism; pathology
Keywords: Atherosclerosis, macrophages, nuclear receptors, cholesterol
INTRODUCTION
A crucial step in atherogenesis is the infiltration of monocytes within the sub-endothelial space of large arteries and their differentiation into macrophages. In early plaques, recruited macrophages play reparatory roles via the phagocytosis of oxidized lipids and apoptotic cells. However, during atherosclerosis progression, macrophages contribute to foam cell formation, lesion growth, plaque rupture and thrombosis by secreting immune-inflammatory factors, growth factors, proteolytic enzymes and tissue factor 1.
One of the most important functions of macrophages in the context of atherosclerosis is the handling of lipids, especially cholesterol. The maintenance of macrophage cholesterol homeostasis is of critical importance in the pathogenesis of atherosclerosis, since an imbalance between cholesterol influx and efflux leads to an excessive accumulation of cholesterol in macrophages and their transformation into foam cells 2,3. Macrophage scavenger receptors, including scavenger receptor A (SR-A), CD36 and lectin-like oxidized LDL receptor-1 (LOX-1), mediate the uptake of modified LDL lipoproteins, including oxidized (Ox)LDL. Within the macrophages, modified LDL-derived cholesteryl esters are hydrolysed in lysosomes by the lysosomal acid lipase (LAL). The released unesterified cholesterol traffics to and integrates in the plasma membrane. The excess of cholesterol is transported back to the endoplasmic reticulum where it is re-esterified by acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) and stored as lipid droplets 4. Cellular cholesterol at the plasma membrane is available for efflux, a highly regulated process mediated by specific proteins, including ATP-binding cassette (ABC) transporters (ABCA1 and ABCG1/ABCG4) 5 and apolipoprotein (apo) E 6.
Besides their role in lipid handling and the immune-inflammatory response, macrophages are also involved in phagocytosis of opsonized bacteria and pathogens and efferocytosis, the clearance of apoptotic cells and debris, since they express receptors and adaptors which recognize the “eat-me” signals at the surface of dying cells 7. Ingestion of apoptotic cells generally triggers an anti-inflammatory response 8. Hence, atherosclerosis may be caused not only by a sustained pro-inflammatory reaction, but also by the failure of anti-inflammatory control mechanisms.
Macrophages are heterogeneous cells, which adapt their response to environmental cytokines and microbial products. While Th1 cytokines (IFNγ and IL1-β) or bacterial LPS induce a “classical” activation profile (M1), the Th2 cytokines IL-4 and IL-13 induce an “alternative” activation (M2) of macrophages. Macrophages are plastic cells because they can switch from the M1 to M2 state and vice versa, upon specific signals 9. M1 macrophages produce pro-inflammatory cytokines, such as tumor necrosis factor α (TNF), IL-6 and IL-12 9, whereas M2 macrophages dampen this inflammatory Th1 response by producing anti-inflammatory factors (IL-10, TGF-β, IL-1 receptor antagonist (IL-1Ra)), thus promoting angiogenesis and tissue repair 9,10.
Macrophage functions are under the control of several transcription factors, among which the Liver X Receptors (LXR) and the Peroxisome Proliferator-Activated Receptors (PPAR). LXRα, LXRβ and PPARγ are ligand-activated nuclear receptors controlling cholesterol distribution and efflux, the inflammatory response 11 and, in the case of PPARγ, macrophage polarization state 12,13.
We have previously shown that, besides classically activated M1 macrophages, human atherosclerotic plaques contain also macrophages expressing the mannose receptor (MR), an alternative macrophage marker, suggesting heterogeneity of plaque macrophage populations 12. However, the phenotypic characteristics and functions nor the regulatory pathways operative in these alternative M2 macrophages are still unknown. The objective of this study was to characterize the macrophage sub-populations in human atherosclerotic plaques and to study their functions.
METHODS
Endarterectomy tissue samples
Human atherosclerotic plaques were removed from 30 patients eligible for surgical carotid endarterectomy according to the European Carotid Trialists’ Collaborative Group 14, recruited at the Cardiovascular Surgery Department, Hospital of Lille, France 15. Informed consent was obtained from all patients.
Cell culture
Human peripheral blood mononuclear cells were isolated from healthy donors by Ficoll density gradient centrifugation. Monocytes were differentiated into resting (RM), alternative (M2) or classical (M1) macrophages (see online supplemental file). Where indicated, T0901317 (T09, 1 μmol/L), 22(R)-hydroxycholesterol (22OH, 10 μmol/L), GW1929 (600 nmol/L), cinnamyl-3,4-dihydroxy-a-cyanocinnamate (CDC, 5 μg/ml) and R04508159 (10 μmol/L) compounds were added for 24h.
Immunohistochemical analysis and laser capture microdissection (LCM)
Immunohistochemical analysis and laser capture micro-dissection were performed on frozen sections (see the online supplemental file).
RNA extraction and analysis
Total cellular RNA was extracted from macrophages using Trizol (Life Technologies, France). RNA extraction from LCM-isolated samples was performed using the Picopure RNA extraction kit (MDS Analytical Technologies). Gene expression was analysed by quantitative PCR (Q-PCR) (see the online supplemental file).
Protein extraction and western blot analysis
Cells were harvested in lysis buffer containing PBS, 4% Triton X100, 10% Na Deoxycholate and protease inhibitors. Proteins (20 μg) were separated by SDS-PAGE, transferred to Hybond-C Extra membranes (Amersham) and immunoblotted using antibodies against human LXRα (abcam), human LOX-1 or β-actin (SantaCruz Biotechnology).
Cellular triglyceride and cholesterol loading
Macrophages were lipid-loaded by incubation with AcLDL or VLDL (50 μg/ml) for 48h or with native LDL (1 mg/ml) or OxLDL (50 μg/ml) for 24h. Intracellular lipids were extracted with hexane/isopropanol (3v:2v) and triglycerides and total cholesterol measured using an enzymatic assay (Boehringer) and cholesterol distribution were measured (see the online supplemental file).
In certain experiments, cholesterol-loaded macrophages were stained with Oil red O. Where indicated cells were incubated with fluorescent DiI-LDL (0.2 mg/ml) for 24h 16.
Measurement of cholesteryl ester formation
Cholesteryl ester formation was assessed by measuring the incorporation of [14C]-oleate into cholesteryl esters. Human macrophages were cholesterol-loaded by incubation with AcLDL (50 μg/ml) for 48 hours. After the cholesterol-loading period, cholesteryl ester formation was measured (see the online supplemental file).
Cholesterol efflux
Macrophages were cholesterol-loaded by incubation with [3H]cholesterol-AcLDL (50 μg/mL) for 48h. HDL3 and ApoAI-mediated cholesterol efflux was measured (see the online supplemental file).
Small interfering (si)RNA-mediated macrophage RNA interference
siRNA oligonucleotides corresponding to human LXRα and LXRβ (Dharmacon), PPARγ (Ambion) sequences and scrambled control RNA oligonucleotides (Ambion) were used. RM and M2 macrophages were transfected using Dharmafect4 reagent (Dharmacon) for 16h and then treated for a further 24h with T0901317 (1 μmol/L) or GW1929 (600 nmol/L).
Adenovirus generation
The recombinant adenovirus (Ad)-GFP (Green Fluorescent Protein) and Ad-PPARγ were obtained as described 17. Viral titers were determined on HEK 293 cells and defined as plaque-forming units/ml. 2×106 macrophages were infected for 16h at a multiplicity of 100 viral particles/cell and subsequently incubated for 24h in the absence or presence of GW1929 (600 nmol/L).
In vitro phagocytosis assay
Phagocytosis tests were performed using fluorescent apoptotic cells or latex beads (see the online supplemental file).
Statistical analysis
Statistical differences between groups were analyzed by Student t-test and considered significant when p <0.05.
RESULTS
Identification of distinct macrophage sub-populations in human atherosclerotic plaques
We previously reported the presence of MR-expressing macrophages in human atherosclerotic lesions 12. However, their morphology, phenotype and tissue localization has not been investigated yet. Therefore, macrophage-rich areas were identified by immunostaining with the pan-macrophage marker CD68, a glycoprotein located on the lysosomal membrane 18 (fig. 1A). Distinct macrophage sub-populations were then discriminated on the basis of co-expression of MR, an endocytic and phagocytic receptor whose expression is strongly enhanced during alternative M2 macrophage differentiation 19. As expected, CD68+MR− macrophages were abundantly found in the lipid core of the plaque and very rarely in the intimal space. By contrast, CD68+MR+ macrophages were predominantly observed in the area of the plaque overlying the lipid core (fig. 1A). Q-PCR analysis of areas infiltrated by CD68+MR+ macrophages and isolated by laser capture-microdissection (LCM) showed a higher expression of IL-4, a cytokine primarily produced by Th2 lymphocytes and inducer of macrophage alternative differentiation, compared to CD68+MR− rich areas (fig. 1B).
Figure 1. Identification of distinct macrophage sub-populations in human atherosclerotic plaques.
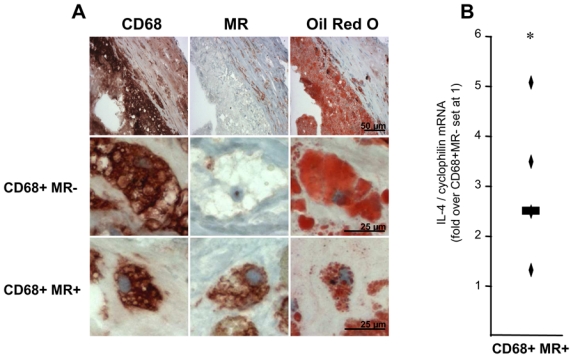
Panel A. Immunostaining (top row) and higher magnification (bottom rows) of representative stainings for CD68, MR and Oil red O in human carotid atherosclerotic lesions. Scale bars are shown.
Panel B. Q-PCR analysis of IL-4 performed on RNA from LCM isolated CD68+MR− and CD68+MR+ macrophage-rich areas. mRNA levels were normalized to cyclophilin mRNA and expressed relative to the levels in CD68+MR− area set at 1. Each point corresponds to a single atherosclerotic plaque. The median value is shown. Statistically significant differences are indicated (t-test; *p< 0.05).
Thus, based on morphology, localization and differential expression of the MR, human atherosclerotic lesions contain at least two macrophage sub-populations present in distinct, discrete zones of the plaque.
Alternative macrophages are less susceptible to transform into foam cells
Interestingly, Oil Red O staining revealed a different pattern of lipid droplet accumulation in CD68+MR− and CD68+MR+ macrophages in vivo (fig. 1A). Morphological comparison revealed that while CD68+MR+ macrophages are smaller and their cytoplasm contain several small lipid droplets (mean diameter of the lipid droplet: 1–1.5 μm), CD68+MR− macrophages contain fewer and bigger lipid droplets (mean diameter approximately 15 μm) (fig. 1A).
To investigate the mechanism behind the lower lipid accumulation observed in CD68+MR+ macrophages, in vitro studies were performed using human primary monocytes polarized to the M2 alternative phenotype with IL-4. As expected, the expression of the M2 macrophage markers MR, AMAC-1 and IL-1Ra was strongly induced by IL-4, compared to resting macrophages (RM) (data not shown). By contrast, the expression of the specific dendritic cell marker CD1a was almost undetectable in M2 macrophages, showing that dendritic cell differentiation is not induced in our model (data not shown). Expression of SR-A and CD36, two scavenger receptors, was similar in RM and M2 macrophages (fig. 2A,B). In line, alternative differentiation of macrophages did not modify AcLDL or VLDL-induced cholesterol or triglyceride accumulation (fig. 2C,D).
Figure 2.
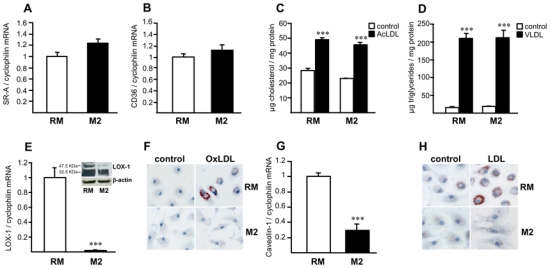
Alternative macrophage differentiation decreases native and oxidized LDL accumulation.
Panels A,B – E,G. Q-PCR analysis of SR-A (A), CD36 (B), LOX-1 (E) and caveolin-1 (G) mRNA in primary human resting (RM) or alternative macrophages (M2). mRNA levels were normalized to cyclophilin mRNA and results expressed as mean ± SD of triplicate determinations relative to the levels in RM set at 1.
Panels C,D. RM or M2 macrophages were loaded with AcLDL (C) or with VLDL (D) and cellular cholesterol or triglycerides determined, respectively. Results are the mean ± SD of triplicate determinations. Statistically significant differences between RM and M2 are indicated (t-test; ***p< 0.001).
Panel E. LOX-1 protein expression was determined in RM and M2 macrophages by western blot analysis.
Panels F,H. RM or M2 macrophages were incubated with oxidized LDL (OxLDL) (F) or native LDL (LDL) (H) and Oil red O staining performed. Results are representative of 3 independent experiments.
Interestingly, M2 macrophages displayed a lower gene expression level of LOX-1 and caveolin-1 compared to RM (fig. 2E,G). Both the pro- (approx. 50KDa) and mature (approx. 30KDa) LOX-1 protein forms were lower expressed in M2 than in RM macrophages (fig. 2E). In line, M2 macrophages exhibited a reduced accumulation of oxidized and native LDL (fig. 2F,H, online fig. I), as measured by ORO staining and by a reduced accumulation of DiI-native LDL (online fig. I). In line, cholesterol content was lower in M2 macrophages loaded with native LDL compared to RM (online fig. I).
Macrophage foam cell formation induced by native LDL might occur through receptor-independent fluid phase pinocytosis, a process highly stimulated by the PKA activator PMA 20. However, LDL accumulation in M2 macrophages was similar as in RM upon PMA activation, thus excluding a defective fluid phase pinocytosis (online fig. II). Altogether, M2 alternative macrophages accumulate less oxidized and native LDL, an effect likely contributing to the lower accumulation of cytoplasmic lipid droplets in CD68+MR+ macrophages observed in vivo.
Human alternative macrophages display a lower cholesterol efflux capacity
To determine whether alternative differentiation may influence the reverse cholesterol transport pathway, the impact of M2 differentiation on the expression of key genes involved in cholesterol efflux such as ABCA1 and ApoE, was studied (fig. 3A,B). The expression of both genes was significantly lower in M2 macrophages compared to RM and pro-inflammatory M1 macrophages (online fig. II), suggesting that M2 macrophages may have lower cholesterol efflux capacities. CD68+MR+ macrophage-rich areas of human atherosclerotic plaques exhibit lower levels of ABCA1 mRNA (fig. 3C), protein (fig. 3E) and ApoE mRNA (fig. 3D) compared to CD68+MR− rich zones.
Figure 3. Alternative macrophages display a lower cholesterol efflux capacity.
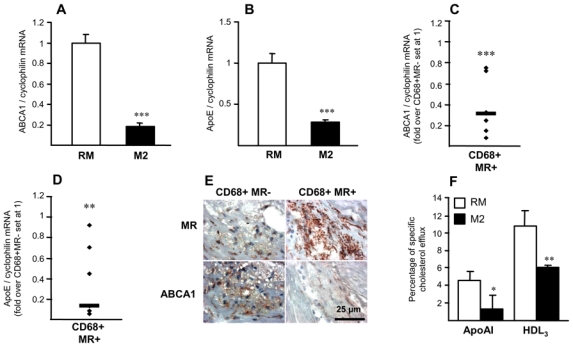
Panels A–D. Q-PCR analysis of ABCA1 (A), ApoE (B) mRNA in RM or M2 macrophages. mRNA levels were normalized to cyclophilin mRNA and results expressed as mean ± SD of triplicate determinations relative to the levels in RM set at 1. Statistically significant differences are indicated (t-test; ***p< 0.001). Q-PCR analysis of ABCA1 (C) and ApoE (D) performed on RNA from LCM-isolated CD68+MR− and CD68+MR+ macrophage-rich areas isolated from 7 samples. mRNA levels were normalized to cyclophilin mRNA and expressed relative to the levels in CD68+MR- area set at 1. Each point corresponds to a single atherosclerotic plaque. The median value is shown. Statistically significant differences are indicated (t-test; **p< 0.01, ***p< 0.001).
Panel E. MR and ABCA1 immunostaining performed in human carotid atherosclerotic lesions. Scale bar is shown.
Panel F. [3H]-cholesterol-loaded macrophages were incubated with medium with or without apoAI or HDL3 to measure cholesterol efflux Values are expressed as percentage of specific cholesterol efflux and are mean ± SD of 3 independent experiments. Statistically significant differences are indicated (t-test; *p< 0.05, **p< 0.01, ***p< 0.001).
In line, apoAI- and HDL3-mediated cholesterol efflux was significantly lower in [3H]cholesterol-AcLDL-loaded M2 macrophages compared to RM macrophages (fig. 3F), indicating that alternative differentiated macrophages display impaired cholesterol efflux capacities.
Alternative macrophage differentiation leads to an increased cholesteryl ester content and cholesteryl ester formation
Increased macrophage cholesterol esterification capacity is a protective mechanism to control free cholesterol toxicity in conditions in which the cholesterol efflux pathway is defective 21. We sought to determine whether the reduced cholesterol efflux capacity in M2 macrophages can, in turn, affect cholesterol esterification. Expression of LAL was higher in M2 macrophages compared to RM or TNFα and IL-1β-activated macrophages (fig. 4A, online fig. III,B), suggesting enhanced lysosomal cholesteryl ester hydrolysis in M2 macrophages and higher unesterified cholesterol availability for intracellular trafficking. In line, the expression of the cholesterol transporter Niemann Pick type C 1 (NPC-1) 22 was higher in M2 compared to RM (data not shown). Moreover, intracellular cholesterol esterification (fig. 4D,E) was higher in M2 macrophages, which correlated with increased ACAT-1 mRNA levels in M2 macrophages, both in vivo and in vitro (fig. 4B,C, online fig. III,C). By contrast, CPT-1, a key enzyme in mitochondrial fatty acid catabolism and the neutral cholesteryl ester hydrolase (NCEH) were expressed at similar levels in RM and M2 macrophages (fig. 4F and data not shown).
Figure 4. Alternative macrophages display enhanced cholesteryl ester formation capacities.
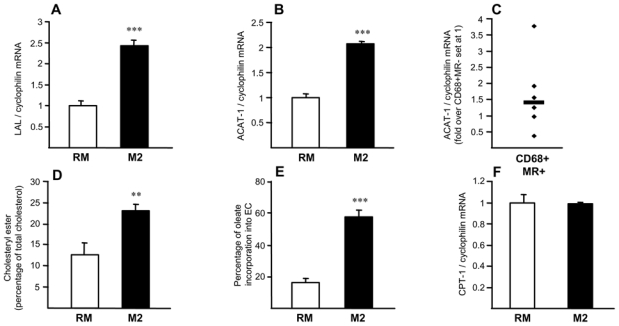
Panels A,B,F. Q-PCR analysis of LAL (A), ACAT-1 (B) and CPT-1 (F) mRNA in RM and M2 macrophages, normalized to cyclophilin mRNA and expressed as mean ± SD relative to RM set at 1 from three independent experiments. Statistically significant differences are indicated (t-test; ***p< 0.001).
Panel C. Q-PCR analysis of ACAT-1 performed on RNA from LCM isolated CD68+MR− and CD68+MR+ macrophage-rich areas isolated from 7 samples. mRNA levels were normalized to cyclophilin mRNA and expressed relative to the levels in CD68+MR− area set at 1. Each point corresponds to a single atherosclerotic plaque. Median value is shown.
Panel D. RM or M2 macrophages were loaded with [3H]-cholesterol AcLDL and lipids extracted and separated by TLC. Spots corresponding to CE and FC were scraped and radioactivity measured. Results are expressed relative to untreated cells set as 1 as mean ± SD of triplicate determinations obtained from 4 independent macrophage preparations.
Panel E. RM or M2 macrophages were cholesterol-loaded with AcLDL and cholesteryl ester formation measured by incubation with [14C]oleic acid. Intracellular lipids were extracted and separated by TLC. Spots corresponding to cholesteryl oleate and oleic acid were scraped and radioactivity measured. Cholesteryl ester formation was calculated as percentage of [C14]oleate incorporated into cholesteryl esters. Results are expressed relative to untreated cells set as 1 as mean ± SD of triplicate determinations obtained from 4 independent macrophage preparations.
LXRα expression and activity is decreased in alternative macrophages
Since ABCA1 and ApoE are well-known LXR target genes 11, we investigated the expression of LXRα and LXRβ. LXRα mRNA (fig. 5A) and protein (fig. 5C), but not LXRβ expression (fig. 5B) were significantly lower in M2 macrophages compared to RM or IL-1β and TNFα pro-inflammatory M1 macrophages (online fig. III,F). A lower expression level of LXRα mRNA and protein was also observed in vivo in CD68+MR+ macrophage-rich areas of human atherosclerotic plaques (fig. 5D,E). To determine the capacity of M2 macrophages to respond to LXR activation, differentiated macrophages were incubated with the highly active synthetic dual LXRα/β agonist T0901317 23. Pharmacological LXR activation in M2 macrophages enhanced LXRα, ABCA1 and ApoE gene expression to levels comparable to untreated RM macrophages, but much lower than in T0901317-activated RM macrophages (fig. 6). In line, M2 macrophages were less sensitive than RM to the activation by the natural LXR agonist 22-hydroxycholesterol (22OH) 24 (online fig. V). LXR activation did not affect the macrophage alternative phenotype, since the expression of MR was unchanged (online fig. IV). To determine the role of LXRα and LXRβ in M2 macrophage function, their expression was silenced by siRNA in M2 macrophages resulting in about 70% reduction (in comparison with control siRNA-transfected cells; fig. 6E,L). LXRβ-silencing did not affect T0901317-induced expression of LXRα, ABCA1 or ApoE, indicating that the observed responses are LXRα-dependent (fig. 6F,G,H). LXRα-silencing affected the basal as well as the T0901317-induced expression of LXRα itself, ABCA1 and ApoE, without affecting the LXRβ expression (fig. 6I,L,M,N). These results indicate that LXRα is the LXR isoform controlling M2 macrophage cholesterol metabolism and that its low expression in these cells is involved in the decreased cholesterol handling properties.
Figure 5. Decreased LXRα expression in alternatively differentiated macrophages.
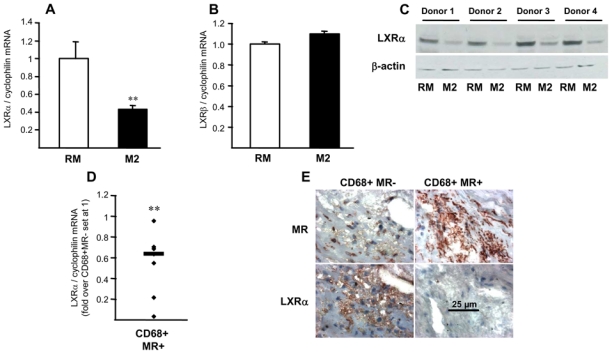
Panels A,B. Q-PCR analysis of LXRα (A) and LXRβ (B) in RM and M2 macrophages. mRNA levels were normalized to cyclophilin mRNA and expressed as means ± SD relative to RM set at 1 from three independent experiments. Statistically significant differences are indicated (t-test; *p< 0.05, **p< 0.01).
Panel C. LXRα protein expression analyzed by western blot in RM and M2 macrophages isolated from 4 different donors.
Panel D. Q-PCR analysis of LXRα performed on RNA from LCM-isolated CD68+MR− and CD68+MR+ macrophage-rich areas isolated from 7 samples. mRNA levels were normalized to cyclophilin mRNA and expressed relative to the levels in CD68+MR− area set at 1. Each point corresponds to a single atherosclerotic plaque. The median value is shown. Statistically significant differences are indicated (t-test; **p< 0.01).
Panel E. MR and LXRα immunostaining performed in human carotid atherosclerotic lesions. Scale bar is shown.
Figure 6. Decreased LXRα activity in M2 macrophages.
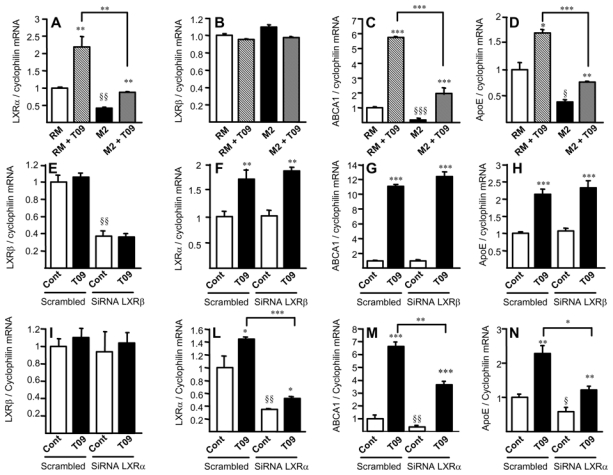
Panels A–D. Q-PCR analysis of LXRα (A), LXRβ (B), ABCA1 (C) and ApoE (D) in RM or M2 macrophages treated or not with T0901317 (T09). mRNA levels were normalized to cyclophilin mRNA and expressed as means ± SD relative to RM set at 1 from three independent experiments. Statistically significant differences are indicated (t-test; RM vs M2 § p< 0.05, §§ p< 0.01, §§§ p< 0.001; and T09 treated vs control **p< 0.01, ***p< 0.001). Panels E–N. Q-PCR analysis of LXRβ, LXRα, ABCA1 and ApoE in M2 macrophages transfected with non-silencing control (scrambled), LXRβ siRNA (E–H) or LXRα siRNA (I–N) and treated or not with T0901317 (T09). Results were normalized to cyclophilin mRNA and expressed relative to the levels in control-siRNA transfected cells set at 1 (mean ± SD of two independent experiments). Statistically significant differences are indicated (t-test; siRNA vs scrambled §p<0.05, §§p<0.01, T09-treated vs control *p<0.05, **p<0.01, ***p<0.001).
IL-4 decreases LXRα expression via a mechanism involving 15-lipoxygenase
15-lipoxygenase (15-LOX) is a lipid peroxidating enzyme responsible for the conversion of arachidonic and linoleic acid into 13-hydroxyoctadecadienoic (13-HODE). 15-LOX expression is strongly induced by IL-4 25. Since 12/15-lipoxygenase transgenic mouse macrophages display reduced ABCA1 expression and cholesterol efflux 26, a phenotype similar to the one observed in human M2 macrophages, the potential involvement of human 15-LOX in the regulation of LXRα signalling in M2 macrophages was assessed.
Interestingly, 15-LOX gene expression was elevated in M2 macrophages in vitro (online fig. VI) and 15-LOX protein was higher in CD68+MR+ macrophages compared to CD68+MR− macrophage-rich areas in atherosclerotic plaques (fig. 7A). Expression of 15-LOX (blue) colocalized with MR (red), leading to purple staining (fig. 7A). Inhibition of 15-LOX activity in M2 macrophages with two different chemical inhibitors, CDC and R04508159 27,28, resulted in an increased expression of LXRα and its target genes ABCA1 and ApoE (fig. 7B–G). Taken together, these results indicate that the decreased LXRα expression and activity in M2 macrophages is likely related to an enhanced 15-LOX activity.
Figure 7. 15-LOX inhibition restores the expression of LXRα and its target genes in M2 macrophages.
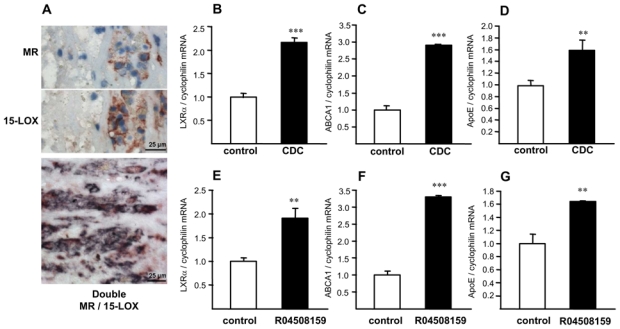
Panel A. MR and 15-LOX immunostaining in human carotid atherosclerotic lesions. Bottom: Double staining of MR (red) and 15-LOX (blue). Most cells present double staining (purple). Scale bar is shown.
Panels B–G. Q-PCR analysis of LXRα (B & E), ABCA1 (C & F) and ApoE (D & G) performed in M2 macrophages in the absence or in the presence of the CDC or the R04508159 compounds. mRNA levels were normalized to cyclophilin mRNA and expressed as means ± SD relative to untreated cells set at 1 from three independent experiments. Statistically significant differences are indicated (t-test; **p< 0.01, ***p< 0.001).
Expression of genes involved in phagocytosis is altered in alternative macrophages
To determine whether other macrophage functions, such as phagocytosis, are operative in CD68+MR+ macrophages, the expression of several key genes involved in this pathway was analyzed both in vivo and in vitro. The expression of C1qa, C1qb, C1qc, GAS-6, and TSP-1 was higher in vivo when comparing CD68+MR+ macrophages to CD68+MR− macrophage-rich areas of atherosclerotic plaques (online fig. VII,A–F). Interestingly, this gene expression profile is comparable to the one observed in vitro in M2 macrophages, compared to RM, with the exception of MERTK, which is drastically lower upon M2 polarization (online fig. VII,G–M). Amongst these, TSP-1 gene was the highest expressed in CD68+MR+ macrophage-rich areas in vivo (online fig. VII,F). These results suggest that CD68+MR+ macrophages likely have enhanced phagocytic activities.
PPARγ activation enhances phagocytic activity of alternative macrophages
To assess functional phagocytic activities, engulfment of fluorescent apoptotic cells or beads was determined. M2 macrophages displayed an increased capacity of phagogytosis compared to RM macrophages (fig. 8A,B) and this effect was enhanced by PPARγ activation (fig. 8C,D). Since TSP-1 is highest expressed in vivo (median value=3.25), and given its role in phagocytosis 29,30, we investigated whether it is regulated by LXRα and PPARγ. Whereas TSP-1 expression was not altered upon LXR activation of IL-4 driven-alternative macrophages in vitro (data not shown), its expression was enhanced by PPARγ activation with GW1929 (fig. 8E), an effect which was abolished upon PPARγ siRNA-silencing (fig. 8F). Moreover, infection of macrophages with an adenovirus coding for PPARγ (Ad-PPARγ) increased TSP-1 expression 3.5-fold, which was further enhanced by GW1929 (fig. 8G). Expression of the TSP-1 receptor CD47 was higher in alternative macrophages, but not regulated by PPARγ (fig. 8H). Inhibition of TSP-1 in M2 macrophages using a blocking antibody inhibited the PPARγ-enhanced phagocytosis activity (online fig. VIII).
Figure 8. Alternative macrophage phagocytic activity is enhanced by PPARγ activation.
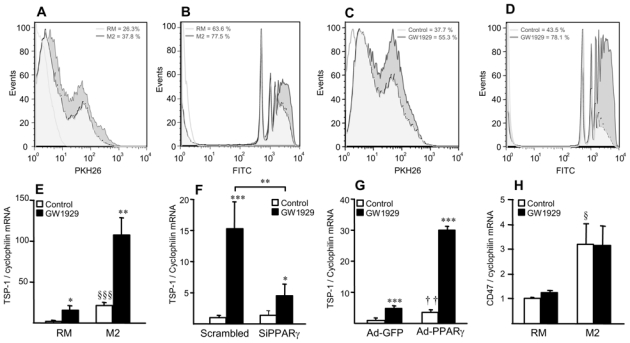
Panels A,B. Phagocytosis of apoptotic cells (A) and fluorescent beads (B) in RM and M2 macrophages.
Panels C,D. Phagocytosis of apoptotic cells (C) and fluorescent beads (D) in M2 macrophages in the absence or in the presence of GW1929 (600 nmol/L) for 24h. White histogram: isotype control.
Panels E–H. Q-PCR analysis of TSP-1 (E–G) and CD47 mRNA (H) in RM and M2 macrophages 24h-tretaed with GW1929 (600 nmol/L) (E,H), or in RM macrophages transfected with control or human PPARγ siRNA (F) or infected with Ad-GFP or Ad-PPARγ (G) and subsequently treated or not with GW1929 (600 nmol/L) during 24h. TSP-1 and CD47 mRNA levels were normalized to cyclophilin mRNA and expressed as means ± SD relative to control set at 1, from three independent experiments. Statistically significant differences are indicated (t-test; M2 vs RM, §p<0.05, §§§p<0.001; Ad-PPARγ vs Ad-GFP, ††p<0.01; GW1929-treated vs control, *p<0.05, **p<0.01, ***p<0.001).
Altogether, these results indicate that alternative macrophages display enhanced phagocytic capacity compared to RM macrophages, an effect amplified by PPARγ activation.
DISCUSSION
Human atherosclerotic lesions are highly heterogeneous structures containing a variety of cell types contributing to a complex inflammatory environment. The local cytokine environment can promote differentiation of infiltrated monocytes to alternative or to classical macrophage sub-populations. We have been the first to report the presence of alternative macrophages expressing the MR within human carotid atherosclerotic plaques 12. However, the phenotypic characterization of macrophage sub-populations within the plaques and their role in the pathogenesis of atherosclerosis has not yet been elucidated.
Immunohistological analysis shows that the macrophage sub-populations positive for CD68 and MR expression are abundant in stable cell-rich areas of the atherosclerotic plaque. In contrast, CD68+MR− macrophages predominate in the lipid core of the atherosclerotic plaque and can occasionally be detected in the intimal space. We also found that CD68+MR+ macrophage-rich areas express higher levels of IL-4 than CD68+MR− rich zones, thus providing the appropriate cytokine environment necessary to orient macrophage polarization. Considering that the cytokine milieu is complex and might vary during the different stages of atherosclerotic plaque progression, it is likely that intermediate spectra of differentiation states exist, with CD68+MR− and CD68+MR+ being the extreme situations, similar as reported for human adipose tissue macrophages 31. However, it is of note that CD68+MR− macrophages are filled more abundantly with lipid droplets which are larger than those observed in CD68+MR+ macrophages. Our results using in vitro differentiated M2 macrophages indicate that these cells are less competent to take up native and oxidized lipoproteins, indicating the existence of cell-intrinsic functional differences in terms of lipid accumulation and storage.
The macrophage CD68+MR+ sub-population identified in vivo shares the same features with in vitro differentiated primary monocytes in the presence of the Th2 cytokine IL-4. Moreover, these IL-4 driven alternative macrophages display a lower capacity of cholesterol efflux to extra-cellular acceptors, associated with reduced expression of ABCA1 and ApoE. Interestingly, the reduced cholesterol efflux capacity of alternative macrophages is probably independent of ABCG1 expression, since alternative macrophages express 2.5-fold more ABCG1 mRNA than RM macrophages (data not shown). These data are in agreement with a previous report showing that cholesterol efflux from human primary macrophage-foam cells to HDL is independent of ABCG1, but specifically requires ABCA1 expression 32.
Our results also show that, as a consequence of a defective cholesterol efflux, IL-4-induced alternative human macrophages instore a protective mechanism against free cholesterol excess by increasing cholesterol esterification capacities, due to an induction of LAL and ACAT-1 gene expression. This latter observation is in agreement with previous results obtained in IL-4-stimulated mouse macrophages 33.
Our results show that LXRα, but not LXRβ expression, is much lower in alternative macrophages, both in vitro and in vivo in CD68+MR+ macrophages. Previously, we reported that LXR activation in RM macrophages increases cholesterol trafficking to the plasma membrane leading to a reduced cholesteryl ester formation 34. The increased esterification in alternative macrophages could be the consequence of the low LXRα activity. Indeed, pharmacological treatment of alternative macrophages in vitro with the highly active synthetic LXR ligand T0901317 and the natural ligand 22-OH cholesterol restored the expression of LXRα and its target genes to a level comparable to those observed in untreated RM macrophages, albeit still much lower than in T0901317-activated RM macrophages indicating that endogenous LXRα is limiting. Interestingly, siRNA mediated LXRα and LXRβ silencing demonstrated that the responses to T091317 stimulation are mediated by LXRα, thus identifying a specific regulatory role of the LXRα isoform in alternative human macrophages. Silencing of LXRα expression in alternative macrophages further supports this conclusion.
These results are in agreement with a recent publication 35 reporting a distinct non-redundant role for LXRα and LXRβ in atherosclerosis susceptibility in mice and that LXRα is required for a robust response to LXR ligands, such as T0901317. Of note, these effects of LXRα occur without affecting the alternative phenotype, since MR expression did not change upon T091317 treatment.
This phenotype, characterized by reduced expression of ABCA1 and cholesterol efflux, resembles the one of macrophages isolated from 12/15-LOX transgenic mice. 12/15 LOX is the homolog of human 15-LOX. However, human 15-LOX is a unique lipoxygenase, since it can oxygenate polyenoic fatty acids esterified to membrane lipids and hence may have biological roles distinct from its action on free arachidonic acid. The expression of 15-LOX is highly induced by Th2 cytokines such as IL-4 in vitro 36. Moreover, we show that in vivo CD68+MR+ macrophages express higher levels of 15-LOX compared to CD68+MR− macrophages. Using two distinct chemical inhibitors we demonstrate that inhibition of 15-LOX in M2 macrophages restores the expression of LXRα and its target genes. Further studies are required to determine how and which 15-LOX-derived lipid mediators inhibit LXRα.
Whereas CD68+MR− macrophages display a lower cholesterol handling capacity, they appear competent for phagocytosis. In line, they express higher levels of genes coding for opsonins and receptors that bridge apoptotic cells to macrophages. Notably, alternative macrophages express higher levels of C1qa, C1qb, C1qc, GAS-6 and TSP-1 than RM macrophages. Since C1q-deficiency leads to defective clearance of apoptotic cells 37, the high levels of several opsonins in alternative macrophages likely provides the molecular basis for their high phagocytotic capacity. Interestingly, while the cholesterol handling properties are likely due to the reduced expression of LXRα, the phagocytosis appears to be directly controlled by PPARγ, whose expression is induced by IL-4 in macrophages 38. Moreover, TSP-1, which is involved in phagocytosis, is induced by PPARγ in macrophages. By contrast, treatment of alternative macrophages with GW1929 did not result in the induction of the expression of ABCA1 (data not shown), whose expression is indirectly induced via LXRα induction in RM 39. Thus, in alternative macrophages PPARγ activities mediated via LXRα induction are deactivated, whereas the phagocytosis pathway is activated, at least in part, through the regulation of TSP-1.
In conclusion, we identified a macrophage sub-population in human atherosclerotic plaques which presents a different morphology and localization, a functional heterogeneity related to the deactivation of the LXRα signalling pathway, being less susceptible to handle lipids and more competent for phagocytosis, an activity enhanced by PPARγ activation. It is tempting to speculate that these alternative macrophages may exert beneficial activities in atherosclerotic lesion development since they are less prone to transform into foam cells and more competent for cell engulfment, in addition to their anti-inflammatory properties.
Novelty and Significance.
What is known?
Monocytes differentiate in functionally distinct pro-inflammatory “classical” M1 macrophages and anti-inflammatory “alternative” M2 macrophages depending on the cytokine-environment.
M1 macrophages contribute to atherogenesis by generating inflammatory foam cells.
M2 macrophages have only recently been identified in human atherosclerotic lesions.
What new information does this article contribute?
M2 macrophages in human atherosclerotic plaques display lower lipid-handling capacities and reduced foam cell transformation capacity.
This phenotype is due to low expression levels of the nuclear receptor Liver X Receptor alpha (LXRα) resulting in decreased Peroxisome Proliferator-Activated Receptor (PPAR)γ-LXRα regulation of cholesterol metabolism.
By contrast, M2 macrophages display an enhanced apoptotic cell phagocytosis capacity which is enhanced by PPARγ activation.
During atherogenesis, monocytes infiltrate the sub-endothelial space of large arteries where they differentiate into macrophages which turn into lipid-loaded foam cells. Macrophages are functionally heterogeneous cell populations which adapt their phenotype depending on the cytokine environment. Th1 cytokines promote M1 macrophage differentiation, while Th2 cytokines trigger an “alternative” M2 phenotype.
We previously reported the presence of M2 macrophages in human atherosclerotic plaques. However, their function was still unknown. We now show that atherosclerotic lesion M2 macrophages contain smaller lipid droplets. In vitro IL-4 polarized M2 macrophages display a reduced capacity to handle and efflux cellular cholesterol due to low expression levels of the nuclear receptor LXRα and its target genes. By contrast, M2 macrophages express opsonins and receptors involved in phagocytosis resulting in high phagocytic activity, which is enhanced by PPARγ activation. We thus identified a distinct macrophage sub-population with a low susceptibility to become foam cells, but high phagocytic activity due to de-activation of the PPARγ-LXRα and activation of alternative PPARγ-regulatory pathways. We speculate that these alternative M2 macrophages may exert beneficial activities in atherosclerotic lesion development since they are less prone to transform into foam cells and more competent to clear apoptotic cells.
Supplementary Material
Acknowledgments
A. Blondy, C. Duhem and A. D’huysser are acknowledged for technical contribution. We thank Genfit SA (Loos, France) for providing the T0901317 and R04508159 compounds.
SOURCES OF FUNDING
Grants from the Région Nord-Pas de Calais/FEDER (CPER N. 1449), COST action BM0904, the Agence Nationale de la Recherche, France (AlMHA project), the transatlantic Leducq HDL Network, and the Fondation Coeur et Artères are acknowledged.
Footnotes
DISCLOSURES
None
Non-standard Abbreviations and Acronyms: None
References
- 1.Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol. 2009;7:234–43. doi: 10.2174/157016109787455635. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Aikawa M, Schonbeck U. Cholesterol and atherosclerosis. Biochimica Biophysica Acta. 2000;1529:299–309. doi: 10.1016/s1388-1981(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 4.Buhman KF, Accad M, Farese RV. Mammalian acyl-CoA:cholesterol acyltransferases. Biochimica Biophysica Acta. 2000;1529:142–154. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 5.Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, Duverger N, Funke H, Assman G, Dinger M, Dean M, Chimini G, Santamarina-Fojo S, Fredrickson DS, Denefle P, Brewer HB., Jr Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original tangier disease kindred. Proceedings of the National Academy of Sciences USA. 1999;96:12685–12690. doi: 10.1073/pnas.96.22.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WY, Gaynor PM, Kruth HS. Apolipoprotein E produced by human monocyte-derived macrophages mediates cholesterol efflux that occurs in the absence of added cholesterol acceptors. J Biol Chem. 1996;271:28641–6. doi: 10.1074/jbc.271.45.28641. [DOI] [PubMed] [Google Scholar]
- 7.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–95. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–81. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 11.Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28:1050–9. doi: 10.1161/ATVBAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 12.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadski C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARg activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1991;337:1235–43. [PubMed] [Google Scholar]
- 15.Zawadzki C, Susen S, Richard F, Haulon S, Corseaux D, Jeanpierre E, Vincentelli A, Lucas C, Torpier G, Martin A, Van Belle E, Staels B, Jude B. Dyslipidemia shifts the tissue factor/tissue factor pathway inhibitor balance toward increased thrombogenicity in atherosclerotic plaques: evidence for a corrective effect of statins. Atherosclerosis. 2007;195:e117–25. doi: 10.1016/j.atherosclerosis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Pitas RE, Innerarity TL, Weinstein JN, Mahley RW. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981;1:177–85. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- 17.Rigamonti E, Fontaine C, Lefebvre B, Duhem C, Lefebvre P, Marx N, Staels B, Chinetti-Gbaguidi G. Induction of CXCR2 Receptor by Peroxisome Proliferator-Activated Receptor {gamma} in Human Macrophages. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Pomares L, Platt N, McKnight AJ, da Silva RP, Gordon S. Macrophage membrane molecules: markers of tissue differentiation and heterogeneity. Immunobiology. 1996;195:407–16. doi: 10.1016/S0171-2985(96)80012-X. [DOI] [PubMed] [Google Scholar]
- 19.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruth HS, Huang W, Ishii I, Zhang WY. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–80. doi: 10.1074/jbc.M205059200. [DOI] [PubMed] [Google Scholar]
- 21.Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A:cholesterol acyltransferase and accumulation of unesterified cholesterol. J Biol Chem. 1995;270:5772–8. doi: 10.1074/jbc.270.11.5772. [DOI] [PubMed] [Google Scholar]
- 22.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 23.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lala DS, Syka PM, Lazarchik SB, Mangelsdorf DJ, Parker KL, Heyman RA. Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci U S A. 1997;94:4895–900. doi: 10.1073/pnas.94.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigal E, Sloane DL, Conrad DJ. Human 15-lipoxygenase: induction by interleukin-4 and insights into positional specificity. J Lipid Mediat. 1993;6:75–88. [PubMed] [Google Scholar]
- 26.Nagelin MH, Srinivasan S, Nadler JL, Hedrick CC. Murine 12/15-lipoxygenase regulates ATP-binding cassette transporter G1 protein degradation through p38- and JNK2-dependent pathways. J Biol Chem. 2009;284:31303–14. doi: 10.1074/jbc.M109.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, Inoue T, Ogino R, Tatsuoka T, Ishihara T, et al. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34:1503–5. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- 28.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–32. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- 29.Brechot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Brechot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moodley Y, Rigby P, Bundell C, Bunt S, Hayashi H, Misso N, McAnulty R, Laurent G, Scaffidi A, Thompson P, Knight D. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol. 2003;162:771–9. doi: 10.1016/S0002-9440(10)63874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger G, Stulnig T. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 32.Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, Kim MJ, Van Eck M, Couvert P, Carrie A, Giral P, Chapman MJ, Guerin M, Le Goff W. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol. 2009;29:1930–6. doi: 10.1161/ATVBAHA.109.194548. [DOI] [PubMed] [Google Scholar]
- 33.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, Bouhlel MA, Bultel S, Fruchart JC, Ikonen E, Clavey V, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res. 2005;97:682–9. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXR{alpha} and LXR? in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89:217–21. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15 lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 39.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Pineda Torra I, Teissier E, Minnich A, Jaye M, Duverger N, Brewer BH, Fruchart JC, Clavey V, Staels B. PPARα and PPARγ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nature Medicine. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


