Abstract
Objective
To identify factors associated with severity of postpartum hemorrhage (PPH) among characteristics of women and their delivery, the components of initial PPH management, and the organizational characteristics of maternity units.
Methods
This population-based cohort study included women with PPH due to uterine atony after vaginal delivery in 106 French hospitals between November 2004 and October 2006 (N=4,550). Severe PPH was defined by a peripartum change in hemoglobin (Δ[Hgb]) of 4 g/dL or more. A multivariable logistic model was used to identify factors independently associated with PPH severity.
Results
Severe PPH occurred in 952 women (20.9%). In women with PPH, factors independently associated with severity were: primiparity, previous PPH, previous cesarean delivery, cervical ripening, prolonged labor, and episiotomy; delay in initial care for PPH and specifically, administration of oxytocin more than 10 minutes after PPH diagnosis (10–20 minutes after, adjusted OR 1.38, 95% CI 1.03–1.85; more than 20 minutes after, 1.86, CI 1.45–2.38), manual examination of the uterine cavity more than 20 minutes after (adjusted OR 1.83, 95% CI 1.42–2.35), call for additional assistance more than 10 minutes after (adjusted OR 1.61, 95% CI 1.23–2.12 for an obstetrician and 1.51, 95% CI 1.14–2.00 for an anesthesiologist); and delivery in a public non-university hospital. Epidural analgesia was found to be a protective factor against severe blood loss in women with PPH.
Conclusion
Aspects of labor, delivery, and their management, delay in initial care, and place of delivery are independent risk factors for severe blood loss in women with PPH caused by atony.
INTRODUCTION
Postpartum hemorrhage (PPH) remains the leading cause of maternal mortality worldwide and the main component of severe maternal morbidity in western countries.1–4 Most PPHs are due to uterine atony. Although pharmacological prevention of uterine atony in the third stage of labor significantly decreases the incidence of PPH5 and is now recommended in international and national guidelines,6–11 reports from developed countries indicate a recent rise in the PPH rate.12–15 This increase is especially troubling because severe PPH, even when not fatal, jeopardizes the woman’s fertility, exposes her to the risks of transfusion and intensive care, and incurs costs. In this context, decreasing the prevalence of severe PPH constitutes a major current obstetrical challenge.
The likelihood of a continuum of morbidity between simple and severe PPH makes the identification of factors that modulate the course of PPH from excessive bleeding to severe hemorrhage an important approach for increasing our understanding of the women and situations most at risk of severe PPH. To our knowledge, no previous study has specifically addressed this question.
Two categories of explanatory factors can be considered: the individual characteristics of women and deliveries, and factors related to medical care, that is, both the content of care and the organization of healthcare services. Various characteristics of women and deliveries have been reported to be risk factors for PPH,16–18 but whether they are associated with an increased risk of severe PPH once early PPH has occurred is not known. On the other hand, focusing on prevention requires identifying the potential risk factors associated with medical care because they are most amenable to change. Clinical guidelines for management of early PPH are based mainly on expert consensus, a low level of evidence. Data documenting the components of initial care that significantly influence the course of PPH would be useful, making it possible to define the most relevant recommendations and thus perhaps increase their translation into practice.
The Pithagore6 trial, because it ascertained all cases of PPH in 106 French maternity units during one year and collected detailed data on them, provides unique data for studying the various factors modulating the continuum of severity in PPH-related maternal morbidity.
The aim of this study was to identify factors associated with PPH severity among characteristics of women and deliveries, components of initial PPH management, and organizational characteristics of maternity units, in women with PPH due to uterine atony after vaginal delivery.
MATERIALS AND METHODS
Population
The study population was a cohort of women with PPH selected from the Pithagore6 trial population. The Pithagore 6 trial was a cluster randomized controlled trial in 106 French maternity units operating as 6 perinatal networks. The main objective of this trial was to evaluate a multifaceted educational intervention for reducing the rate of severe PPH. No significant difference in the rate of severe PPH was found between the two groups of hospitals (details of this trial available elsewhere19).
A 1998 French statute aimed at optimizing the organization of obstetric care made it mandatory for all maternity units to belong to a perinatal network,20 organized around one or more level 3 units (reference centers with an onsite neonatal intensive care unit) and including units rated as level 1 (no facilities for non-routine neonatal care) and 2 (with a neonatal care unit), both public and private. The six perinatal networks involved in the Pithagore 6 trial were the Perinat Centre network around Tours (23 units), the Port-Royal St Vincent de Paul network in Paris (22 units), and the 4 networks of the Rhône-Alpes region: the Aurore network around Lyon (33 units), the Savoie network around Chambery (14 units), the Grenoble network (5 units), and the St-Etienne network (9 units). The 106 Pithagore6 maternity units represented 17% of all French maternity units and covered 20% of deliveries nationwide. Data were collected from December 2004 through November 2005 in the Aurore network, and from December 2005 through November 2006 in the other five. PPH was clinically assessed by the caregivers if the estimated postpartum blood loss was greater than 500 ml or defined by a peripartum change in hemoglobin (Hb) greater than 2 g/dL (considered equivalent to the loss of more than 500 mL of blood). Prepartum hemoglobin was collected as part of routine prenatal care during the last weeks of pregnancy; postpartum hemoglobin was the lowest hemoglobin level found in the three days after delivery. Birth attendants in each unit prospectively identified all deliveries with PPH and reported them to the research team. In addition, a research assistant reviewed the delivery suite logbook of each unit monthly, as well as computerized patient charts when available. For every delivery with a mention of PPH, uterine cavity examination, or manual removal of the placenta, the patient’s obstetric file was further checked to verify the PPH diagnosis. During the one-year data collection period, 9,365 cases of PPH (defined either by estimated blood loss or drop in Hb) occurred among 146,876 deliveries in the 106 Pithagore6 units, for a total PPH incidence of 6.4% of deliveries.
For the present analysis, a specific definition of PPH cases was used. We excluded cases of PPH where no excessive bleeding was clinically identified and that were identified only by a decreased hemoglobin level, because, by definition, these cases did not receive any specific care for PPH, and one major objective was to study the association between components of initial care for PPH and the risk of severe PPH within this cohort of PPH. The cohort was further restricted to PPH due to uterine atony after vaginal delivery, a more homogeneous situation that is the leading cause of PPH and the main target of clinical guidelines. Finally, the study population included 4,550 women. Figure 1 shows the process of selection of the study population.
Figure 1.
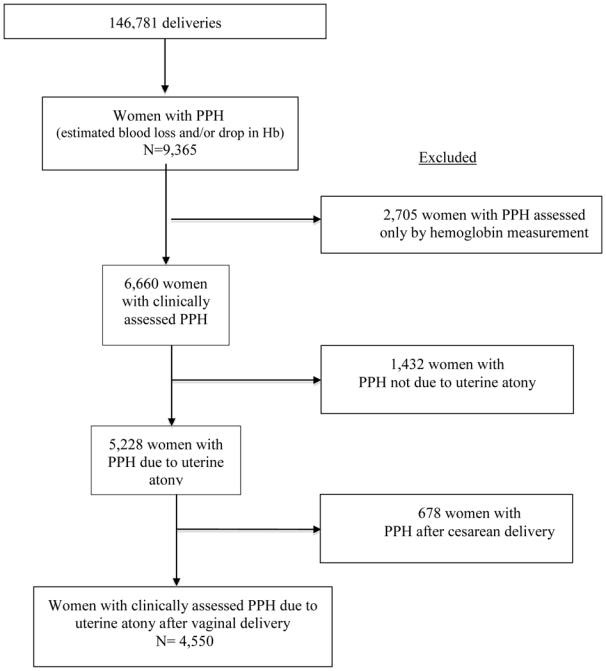
Study population
Data collection
Characteristics of the patient, pregnancy, labor, delivery, and PPH management were collected on a case report form from the chart of every delivery with confirmed PPH. The procedures for PPH management were considered to have been performed only if they were specifically mentioned in the chart.
Study variables
The outcome was severe PPH, defined by a peripartum change in hemoglobin of 4 g/dL or more (considered equivalent to the loss of 1000 mL or more of blood).
Three groups of potential risk factors for severe PPH were examined: characteristics of the women and aspects of labor and delivery before PPH; components of initial PPH management; and organizational characteristics of the units. The individual preexisting characteristics were as follows: age in years at delivery in 3 categories: <25, 25–35, >35; body mass index (BMI) at conception in 4 categories: ≤18, >18–25, >25–30, and >30; parity and previous cesarean delivery, categorized as: primiparous, multiparous without previous cesarean delivery, and multiparous with previous cesarean delivery (one or more). History of PPH, multiple pregnancy, hydramnios, epidural analgesia, prolonged labor (defined as an active phase of labor > 6 h without expulsive efforts), prolonged expulsive efforts (defined as a duration of pushing > 30 minutes), and prophylactic oxytocin after delivery were analyzed as dummy variables. Onset of labor was categorized as spontaneous, induced by oxytocin, and induced cervical ripening. Gestational age at delivery in weeks was categorized as preterm (<37), term (37–41), or post-term delivery (>41). Episiotomy and delivery were categorized as spontaneous delivery without episiotomy, spontaneous delivery with episiotomy, operative delivery without episiotomy, and operative delivery with episiotomy. Birth weight in grams was studied in 3 categories: < 2,500, 2,501–3,999, and 4,000 or more. Data were missing for no more than 3% of women for all variables, except BMI (13.2%) and prolonged expulsive efforts (14.3%); a specific missing data class was added for them.
Four components of initial care for PPH were studied. For all of them, the reference category was the performance within 10 minutes of PPH diagnosis, as recommended by the 2004 French national guidelines.11 Oxytocin administration and manual examination of the uterine cavity were both classified as performed in ≤10 minutes, >10–20 minutes, >20 minutes, done but delay unknown, and not done. The calls for assistance from a senior obstetrician and an anesthesiologist were classified as: present or called in ≤10 minutes, >10 minutes, called but delay unknown, and not called.
The organizational characteristics of the units included: status, classified as university public, other public, or private; number of annual deliveries, categorized as <1,500, 1,500–2,500, or >2,500; level of neonatal care, categorized into 1, 2, or 3; 24/24 onsite presence of an obstetrician, and of an anesthesiologist, studied as dummy variables.
Analysis
The characteristics of women, labor, delivery, and initial PPH management were described as proportions in all PPH deliveries meeting the study case definition. The percentage of PPH deliveries meeting severity criteria was calculated overall and by pregnancy characteristics. The crude associations of severe PPH with these variables were tested with χ2 statistics and quantified with unadjusted odds ratios (ORs) and their 95% confidence intervals. Multivariable logistic regression modeling was used to assess the independent effect of each variable. Given the hierarchical structure of our data, level 1: women, level 2: centers (“clusters”), we took into account the intraclass (or intra-cluster) correlation for outcomes of women cared for at a given center by using random-intercept hierarchical logistic regression models 21. Such modeling provides a more accurate estimation of associations and makes it possible to study explanatory variables at both levels. In a first step, a logistic regression analysis including all relevant characteristics of women, labor, and delivery before PPH was performed to determine whether these characteristics were independently associated with PPH severity. Then, separate multilevel models tested the association of each component of initial PPH care with PPH severity, after adjustment for the significant characteristics of women, labor, and delivery. Finally, the association of each organizational characteristic with PPH severity was examined after adjustment for characteristics of women, labor, delivery, and components of initial care.
Cases with one or more missing value among the characteristics of women, labor, and delivery were not included in the multivariate analyses (n= 151 women, 3.3% of total). Cases with missing data for the timing of procedures were included in a specific category “done but unknown delay”. Organizational characteristics were available for all units.
Based on a sample size of 4,500 women with PPH delivery that met the study definition and an expected 20% prevalence of severe PPH in this group, we estimated that the power of the study would be more than 80% to detect a relative risk of 2.0 between exposed and unexposed women for variables with a prevalence of 2% or more of deliveries and to detect a relative risk of 1.5 for variables with a prevalence of 6% or more of deliveries.
Statistical analysis used Stata v.10 software (Stata Corporation, College Station, TX).
Approval for the study was obtained from the Sud Est III Institutional Review Board and from the French Data Protection Agency (CNIL).
RESULTS
Among 4,550 women with PPH in the study population, 952 (20.9%) had severe PPH.
Characteristics of women, labor, and delivery
Table 1 reports the distributions of the characteristics of women, labor, and delivery in the cohort of women with PPH and their association with severe PPH. After adjustment for other individual potential risk factors, the risk of severe PPH for women with PPH was significantly higher in primiparas, multiparas with previous cesarean delivery, women with previous PPH, women who had induced cervical ripening, prolonged labor, episiotomy (for both spontaneous and instrumental delivery), and women who received prophylactic uterotonics.
Table 1.
Characteristics of women, labor, and delivery: distribution in the cohort of PPH and risk of severe PPH, univariable and multivariable analyses
| n | % | Proportion with severe PPH (%) | OR* | 95% CI | aOR§ | 95% CI | |
|---|---|---|---|---|---|---|---|
| Women and pregnancy | |||||||
| Age (yrs) | |||||||
| <25 | 876 | 19.3 | 22.0 | 1.05 | 0.87–1.26 | 0.95 | 0.77–1.17 |
| 25–35 | 2 970 | 65.3 | 21.3 | 1.00 | - | 1.00 | - |
| >35 | 700 | 15.4 | 18.3 | 0.83 | 0.67–1.02 | 0.98 | 0.77–1.24 |
| BMI | |||||||
| ≤ 18 | 215 | 5.4 | 24.2 | 1.18 | 0.86–1.64 | 1.10 | 0.78–1.55 |
| >18–25 | 2 864 | 72.5 | 21.1 | 1.00 | - | 1.00 | - |
| >25–30 | 595 | 15.1 | 18.7 | 0.85 | 0.68–1.06 | 0.85 | 0.67–1.08 |
| > 30 | 275 | 7.0 | 19.3 | 0.89 | 0.65–1.20 | 0.89 | 0.63–1.24 |
| Previous PPH | 249 | 5.5 | 20.9 | 0.99 | 0.73–1.37 | 1.47 | 1.02–2.13 |
| Fibroma | 33 | 0.7 | 21.2 | 1.02 | 0.44–2.35 | 0.74 | 0.29–1.98 |
| Hydramnios | 44 | 1.0 | 25.0 | 1.26 | 0.64–2.51 | 0.94 | 0.42–2.11 |
| Parity | |||||||
| primiparous | 2 268 | 49.9 | 26.2 | 1.99 | 1.70–2.32 | 1.88 | 1.51–2.33 |
| multiparous with no previous cesarean delivery | 2 036 | 44.8 | 15.1 | 1.00 | - | 1.00 | - |
| multiparous with previous cesarean delivery | 245 | 5.4 | 20.7 | 1.47 | 1.05–2.04 | 1.66 | 1.15–2.41 |
| Multiple pregnancy | 119 | 2.6 | 26.9 | 1.40 | 0.93–2.12 | 1.17 | 0.70–1.96 |
| Labor | |||||||
| Onset of labor | |||||||
| spontaneous | 3 457 | 76.0 | 20.3 | 1.00 | - | 1.00 | - |
| induction | 571 | 12.5 | 21.2 | 1.06 | 0.86–1.32 | 1.20 | 0.93–1.55 |
| induced cervical ripening | 522 | 11.5 | 25.7 | 1.37 | 1.10–1.69 | 1.45 | 1.13–1.85 |
| Epidural analgesia | 3 552 | 78.2 | 20.2 | 0.83 | 0.70–0.98 | 0.53 | 0.43–0.67 |
| Prolonged labor | 1 376 | 31.2 | 24.5 | 1.38 | 1.19–1.61 | 1.27 | 1.06–1.53 |
| Oxytocin during labor | 3 029 | 66.7 | 21.7 | 1.15 | 0.98–1.34 | 1.04 | 0.85–1.28 |
| Prolonged expulsive efforts | 534 | 13.7 | 27.0 | 1.45 | 1.18–1.79 | 0.97 | 0.77–1.24 |
| Delivery | |||||||
| Gestationnal age (wks) | |||||||
| < 37 | 218 | 4.8 | 23.9 | 1.25 | 0.90–1.72 | 1.14 | 0.70–1.85 |
| 37–41 | 3 565 | 78.5 | 20.1 | 1.00 | - | 1.00 | - |
| > 41 | 759 | 16.7 | 24.1 | 1.26 | 1.05–1.52 | 1.10 | 0.89–1.37 |
| Delivery | |||||||
| spontaneous without episiotomy | 2 444 | 53.7 | 16.3 | 1.00 | - | 1.00 | - |
| spontaneous with episiotomy | 1 230 | 27.0 | 25.2 | 1.73 | 1.46–2.05 | 1.55 | 1.27–2.87 |
| operative without episiotomy | 176 | 3.9 | 19.3 | 1.23 | 0.83–1.81 | 1.05 | 0.69–1.62 |
| operative with episiotomy | 698 | 15.4 | 30.1 | 2.21 | 1.82–2.69 | 1.70 | 1.33–2.18 |
| Prophylactic uterotonics | 2 486 | 54.6 | 21.7 | 1.11 | 0.96–1.28 | 1.22 | 1.03–1.43 |
| Birth weight (g) | |||||||
| ≤2,500 | 179 | 3.9 | 23.5 | 1.18 | 0.83–1.68 | 0.97 | 0.56–1.69 |
| 2,501–3,999 | 3 880 | 85.3 | 20.6 | 1.00 | - | 1.00 | - |
| ≥4,000 | 486 | 10.7 | 22.4 | 1.11 | 0.89–1.40 | 1.21 | 0.95–1.58 |
| Total | 4,550 | 100.0 | 20.9 | NA | |||
Simple logistic regression
Multivariable logistic regression including all variables
Epidural analgesia was associated with a significantly reduced risk of severe PPH.
Initial PPH management
The distribution of the components of initial PPH management in the cohort is shown in Table 2, as well as their crude associations with severe PPH.
Table 2.
Initial management of PPH and characteristics of the units: distribution in the cohort of PPH and risk of severe PPH, univariable analysis
| n | % | Proportion with severe PPH (%) | OR | 95%CI | |
|---|---|---|---|---|---|
| Initial management of PPH* | |||||
| Oxytocin administration | |||||
| ≤10 min | 2,208 | 48.5 | 20.5 | 1.00 | - |
| >10–20 min | 329 | 7.2 | 24.6 | 1.27 | 0.97–1.66 |
| >20 min | 447 | 9.8 | 31.8 | 1.81 | 1.45–2.26 |
| done but delay unknown | 1,224 | 27.0 | 17.7 | 0.83 | 0.63–1.13 |
| not done | 342 | 7.5 | 17.8 | 0.8 | 0.70–1.00 |
| Manual examination of the uterine cavity | |||||
| ≤10 min | 2,114 | 46.5 | 20.7 | 1.00 | - |
| >10–20 min | 326 | 7.2 | 23.9 | 1.21 | 0.92–1.59 |
| >20 min | 490 | 10.8 | 28.2 | 1.50 | 1.20–1.88 |
| done but delay unknown | 929 | 20.4 | 15.6 | 0.71 | 0.58–0.87 |
| not done | 691 | 15.2 | 22.3 | 1.10 | 0.89–1.35 |
| Call for obstetrician | |||||
| present/call ≤10min | 2,050 | 45.1 | 24.8 | 1.00 | - |
| call > 10 min | 362 | 8.0 | 29.8 | 1.29 | 1.01–1.65 |
| called but delay unknown | 294 | 6.5 | 24.8 | 1.00 | 0.76–1.33 |
| not called. not present | 1,844 | 40.6 | 14.3 | 0.50 | 0.43–0.6 |
| Call for anesthesiologist | |||||
| present/call ≤10 min | 999 | 22.0 | 29.9 | 1.00 | - |
| call > 10 min | 356 | 7.8 | 35.1 | 1.27 | 0.98–1.64 |
| called but delay unknown | 318 | 7.0 | 28.3 | 0.92 | 0.70–1.22 |
| not called. not present | 2,877 | 63.2 | 15.2 | 0.42 | 0.35–0.50 |
| Characteristics of the units** | |||||
| Status | |||||
| University public | 1,423 | 31.3 | 17.6 | 1.00 | - |
| Other public | 2,219 | 48.8 | 23.8 | 1.45 | 1.09–1.92 |
| Private | 908 | 19.9 | 19.2 | 1.07 | 0.77–1.50 |
| Level of care | |||||
| 1 | 1,369 | 30.1 | 21.4 | 0.97 | 0.76–1.23 |
| 2 | 2,219 | 48.8 | 21.1 | 1.00 | - |
| 3 | 962 | 21.1 | 19.8 | 0.92 | 0.64–1.33 |
| Number of annual of deliveries | |||||
| <1,500 | 1,483 | 32.6 | 23.3 | 1.18 | 0.93–1.51 |
| 1,500–2,500 | 1,922 | 42.2 | 19.6 | 1.00 | - |
| >2,500 | 1,145 | 25.2 | 20.2 | 1.02 | 0.73–1.43 |
| 24h/24 presence of obstetrician | 3,318 | 73.0 | 19.7 | 0.80 | 0.64–1.00 |
| 24 h/24 presence of anesthesiologist | 4,084 | 89.8 | 20.8 | 1.07 | 0.78–1.47 |
| Total | 4,550 | 100.0 | 20.9 | NA | |
Logistic regression
Multilevel logistic regression
Oxytocin was administered late or not at all to 24.5% of women with PPH, who therefore did not receive the recommended care. Manual examination of the uterine cavity was inappropriate (late or not done) for 33.2%. In this cohort, 40.6% of women with PPH were managed with no senior obstetrician called or present, and 63.2% with no anesthesiologist called or present.
Delayed care, compared with the recommended management, was associated with an increased risk of severe PPH (Table 2), and the associations remained significant when controlling for characteristics of women, labor, and delivery before PPH (Table 3). After adjustment for preexisting factors, the risk of severe PPH was 1.4 times higher in women who received oxytocin between 10 and 20 minutes after PPH diagnosis, and 1.9 times higher when it was administered more than 20 minutes after diagnosis compared with those who received it within the first 10 minutes (Table 3 model 1), and 1.8 times higher in women who had a manual examination of the uterine cavity more than 20 minutes after diagnosis compared to the first 10 minutes (Table 3 model 2). Similarly, a delayed call for obstetric assistance was associated with a 1.6 times higher risk of severe PPH, compared with cases where a senior obstetrician was present or called within 10 minutes (Table 3 model 3). The same was true for a delayed call for an anesthesiologist (Table 3 model 4). Associations between delayed management and severe PPH remained when several components of care were included in the same model (models 5 and 6 in Table 3), except for the obstetrician call. When all four components of care were included, only delayed administration of oxytocin remained significantly associated with severe PPH (table 3, model 7).
Table 3.
Initial management of PPH and risk of severe PPH, multivariable analysis
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Oxytocin administration | ||||||||||||||
| ≤10 min | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | ||||||
| >10–20 min | 1.38 | 1.03–1.85 | 1.39 | 1.02–1.89 | 1.38 | 1.01–1.88 | 1.33 | 0.97–1.83 | ||||||
| >20 min | 1.86 | 1.45–2.38 | 1.63 | 1.26–2.11 | 1.60 | 1.23–2.08 | 1.49 | 1.14–1.94 | ||||||
| done but delay unknown | 0.75 | 0.61–0.91 | 0.95 | 0.74–1.21 | 0.97 | 0.76–1.25 | 1.00 | 0.78–1.30 | ||||||
| not done | 0.82 | 0.60–1.13 | 0.96 | 0.68–1.33 | 0.97 | 0.69–1.36 | 1.02 | 0.72–1.43 | ||||||
| Manual examination of uterine cavity | ||||||||||||||
| ≤10 min | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | ||||||
| >10–20 min | 1.30 | 0.97–1.76 | 1.12 | 0.82–1.55 | 1.04 | 0.75–1.43 | 0.87 | 0.62–1.20 | ||||||
| > 20 min | 1.83 | 1.42–2.35 | 1.60 | 1.23–2.10 | 1.35 | 1.01–1.80 | 1.05 | 0.78–1.41 | ||||||
| done but delay | 0.62 | 0.50–0.78 | 0.69 | 0.52–0.92 | 0.67 | 0.50–0.89 | 0.68 | 0.50–0.91 | ||||||
| not done | 0.96 | 0.76–1.21 | 1.01 | 0.79–1.27 | 1.10 | 0.86–1.40 | 1.30 | 1.01–1.66 | ||||||
| Call for obstetrician | ||||||||||||||
| present/call ≤10min | 1.00 | - | 1.00 | - | 1.00 | - | ||||||||
| call > 10 min | 1.61 | 1.23–2.12 | 1.29 | 0.96–1.73 | 1.10 | 0.80–1.52 | ||||||||
| called but unknown delay | 1.14 | 0.84–1.56 | 1.32 | 0.96–1.82 | 1.24 | 0.87–1.76 | ||||||||
| not called. not present | 0.54 | 0.44–0.65 | 0.56 | 0.46–0.68 | 0.70 | 0.57–0.85 | ||||||||
| Call for anaesthesiologist | ||||||||||||||
| present/call ≤10 min | 1.00 | - | 1.00 | - | ||||||||||
| call > 10 min | 1.51 | 1.14–2.00 | 1.33 | 0.96–1.84 | ||||||||||
| called but delay unknown | 0.99 | 0.73–1.35 | 1.06 | 0.75–1.50 | ||||||||||
| not called. not present | 0.37 | 0.31–0.46 | 0.43 | 0.35–0.54 | ||||||||||
Models 1–7: multilevel logistic regression models adjusted for characteristics of women before PPH: previous PPH, parity/previous cesarean delivery, multiple pregnancy, onset of labor, epidural analgesia, prolonged labor, gestational age, prophylactic uterotonics, delivery/episiotomy, birth weight (N= 4 399 PPH)
Women in the category “done but delay unknown” were at lower risk of severe PPH than those for whom the procedure was performed within 10 minutes for oxytocin administration and manual examination (Table 3 Model 1 and 2).
The risk of severe PPH was lower when the obstetrician or the anesthesiologist was absent and not called than when they were called promptly (Table 3 Model 3 and 4). We performed a secondary analysis restricted to the population of PPH women who received sulprostone (second-line pharmacological treatment) to test the hypothesis that an indication bias might explain these associations, given that midwives and junior doctors manage the most minor cases of postpartum bleeding alone. In this population, the obstetrician “not called, not present” category was associated with an increased risk of severe PPH (adjusted OR 2.01 95%CI 1.44–2.84); no significant association was found between the presence of an anesthesiologist and the risk of severe PPH (data not shown).
Characteristics of the units
The distribution of hospital-of-birth characteristics among the PPH deliveries is shown in Table 2. The risk of severe PPH was 1.5 times higher for PPH in non-teaching public hospitals compared with university hospitals, and this significant association remained after adjustment for the characteristics of the women, labor, delivery, and components of early PPH management (Table 4). When we controlled for the characteristics of women, labor, and delivery, severe PPH was not significantly associated with the annual number of deliveries, the level of care, or the onsite presence of an obstetrician or an anesthesiologist (Table 4).
Table 4.
Characteristics of the units and risk of severe PPH, multivariable analysis
| Variables | Adjusted OR | 95% CI |
|---|---|---|
| Status | ||
| University public | 1.00 | - |
| Other public | 1.46 | 1.10–1.87 |
| Private | 1.00 | 0.73–1.37 |
| Level of care | ||
| 1 | 0.92 | 0.72–1.17 |
| 2 | 1.00 | - |
| 3 | 0.94 | 0.66–1.34 |
| Number of annual of deliveries | ||
| <1,500 | 1.11 | 0.81–1.53 |
| 1,500–2,500 | 1.00 | - |
| >2,500 | 1.06 | 0.76–1.46 |
| 24 h/24 presence of obstetrician | 0.83 | 0.66–1.04 |
| 24 h/24 presence of anesthesiologist | 1.05 | 0.78–1.42 |
Multilevel logistic regression adjusted for characteristics of women before PPH (previous PPH, parity/previous cesarean delivery, multiple pregnancy, onset of labor, epidural analgesia, prolonged labor, gestational age, prophylactic uterotonics, delivery/episiotomy, birth weight) and early management of PPH (oxytocin administration. manual examination of the uterine cavity) (N= 4 399 PPH)
DISCUSSION
This study is the first, to our knowledge, to document the factors that modulate the course from simple to severe PPH. Our results suggest that various specific characteristics are independent determinants of PPH aggravation. These include the woman’s obstetric history, aspects of delivery before PPH, delay in initial care for PPH, and hospital status.
We found that several characteristics of the woman and her pregnancy, previously described as risk factors for PPH,16–18 also are associated with a higher risk of severity once PPH has occurred. Although any PPH requires immediate management, for women with a history of PPH or cesarean, or having a first baby, or whose labor was managed with induced cervical ripening or was prolonged, or who had an episiotomy, excessive but not severe postpartum bleeding requires even more careful attention because they are at higher risk of severe hemorrhage. Interestingly, several of these characteristics -previous cesarean, cervical ripening, and episiotomy- are related to the management of labor and delivery, and the associations found here provide additional evidence to be considered in balancing the risks and benefits of those procedures. In our cohort of 4550 women with PPH, 2314 (51%) had at least one of these 3 characteristics; efforts to decrease the rate of these procedures may actually reduce the importance of this group and possibly the incidence of severe PPH.
Interestingly, episiotomy is associated with a higher risk of severe PPH, although the study population was restricted to PPH due to uterine atony, with PPH due to bleeding episiotomy excluded. This finding suggests that the existence of multiple sources of blood loss, even in physiological range, increases the risk of severe PPH and reinforces the relevance of policies for limited use of episiotomy at vaginal birth.
An unexpected result was the increased risk of severe PPH in women who received preventive oxytocin, as compared to women with PPH who had no prophylaxis. This may reflect an indication bias, prophylaxis being more likely in women with risk factors, although these risk factors were taken into account in our analysis. Alternatively, excessive postpartum bleeding occurring after and despite prevention may be more likely to be severe, since, by definition, prophylactic oxytocin was not able to prevent it. Another possible explanation is that the surveillance of postpartum blood loss may be less intense when prophylaxis has been done, leading to delayed diagnosis.
Epidural anesthesia had a protective effect here. It has previously been discussed as a risk factor for PPH,17, 22 presumably by lengthening labor or negatively affecting the endogenous oxytocin level or both, but evidence for such an effect is weak so far. Our results suggest that regardless of the effect of the epidural on the occurrence of PPH, women diagnosed with PPH who already have an epidural are at a smaller risk of severe bleeding. This unexpected result illustrates the importance of analyzing the role of risk factors at different levels of the continuum of severity. The presence of the epidural catheter likely facilitates immediate management of PPH since some procedures, such as examination of the uterine cavity, manual removal of the placenta, or instrumental examination of the vagina and cervix, are usually done under anesthesia. Inversely, the need for anesthesia may delay initial care for PPH and thus increase the risk of severe PPH in women who delivered without epidural: in our study population, this group had a significantly higher proportion of women with no or delayed examination of the uterine cavity than did the women with epidurals.
Delay in initial PPH care (manual examination of the uterine cavity, oxytocin administration, and call for extra help) was associated with an increased risk of severe PPH. These results might appear expected or even obvious. However, gathering evidence to support clinical practice recommendations is the principle of evidence-based medicine and an essential task, for it increases their level of proof and thus their legitimacy, both factors that may improve their translation into practice.23 The content of PPH-related guidelines for the initial steps is very similar in all countries. The present study is the first, to our knowledge, to provide evidence to support the recommendations for immediate management of excessive bleeding.
The risk of severe blood loss is higher for women with a PPH after vaginal birth in public non-university hospitals, compared with other public university or private hospitals, and this excess risk is not explained by characteristics of the women, their delivery, or the initial PPH management. We hypothesize that second-line treatment for PPH may be inappropriate or delayed in these hospitals because of limited human (e.g., available staff, surgical skills of obstetricians) or material (e.g., interventional radiology) resources. However, these further steps of PPH management are less standardized and their appropriateness is more difficult to assess because the corresponding content of guidelines is quite imprecise. That is why they were not considered in this study.
The design of the present study had several strengths. It was population-based, covering all maternity units and consequently all deliveries in a given area. This feature ensures the external validity of its results. The prospective identification of deliveries with PPH and the characterization of severe PPH within the cohort of women with identified PPH provided unbiased comparison groups with regard to the study objectives. The large number of units and deliveries provided good power for studying the independent role of multiple factors and allowed an analysis that could take the hierarchical structure of the data into account and explore the role of factors at the levels of both the women and the units. Finally, the definition of severe PPH was based on change in hemoglobin, a more objective criterion than the clinically assessed volume of blood loss, or the need for surgery, embolization, or transfusion, all dependent on practices likely to vary widely among clinicians and centers.
Our definition of severe PPH also has some limitations. Peripartum change in hemoglobin may not always accurately reflect postpartum blood loss. It may overestimate blood loss in women who received large amounts of fluids, who could then be wrongly classified as severe PPH; it may underestimate the total blood loss if not performed after 48 hours of delivery. This is however unlikely to bias our conclusions, as the consequence would actually be an underestimation of the strength of the associations we found with severe PPH. Given our study objectives and the constraints of our data, the definition of severe PPH by a maximum change in Hb appeared as the least biased option.
Selection by indication bias is common in observational studies assessing the role of procedures or treatments on health outcomes. In the present study, some women with PPH may have received more appropriate immediate management because their bleeding was considered at high risk of immediate aggravation. Conversely, in women with excessive bleeding after delivery but not considered to be at risk for heavy blood loss, delay in adequate management is more likely. The effect of this bias is to attenuate the negative impact of inadequate care. The actual associations between delayed initial care and severe PPH may therefore be stronger than we found here. As mentioned above, this bias probably also explains the apparent lower risk of severe PPH in cases where an obstetrician or anesthesiologist were not called promptly.
We cannot exclude the possibility that some procedures were performed but not recorded in the medical files, although this seems unlikely for pharmacological treatments such as oxytocin or invasive exams such as examination of the uterus. The relatively high proportion of missing data for the timing of oxytocin administration and manual examination shows that the quality of data recording in obstetrics files needs to improve. We found that the women with PPH for whom these two procedures were done, but at an unknown time, were at lower risk of severe PPH. One possible explanation for this finding is that the procedures were performed immediately after PPH diagnosis and that the specific time was not recorded because implicitly considered simultaneous with the diagnosis.
Identifying factors that influence the course of PPH from simple to severe has direct potential implications for clinicians, especially for factors related to care, which have been rarely explored so far. Our study shows that some aspects of the management of labor and delivery, as well as delayed initial care for PPH, and place of delivery, increase the risk of heavy postpartum bleeding caused by atony. More specifically, it provides evidence suggesting that reducing the use cervical ripening, episiotomy, or cesarean delivery, in particular in situations where these interventions do not provide clear benefits, as well improving the rapidity of first care once PPH has occurred, may reduce the incidence of severe PPH.
Acknowledgments
Funding
The project was funded by the French Ministry of Health under its Clinical Research Hospital Program (contract n° 27–35).
MD was supported by a student grant from the Fondation pour la Recherche Medicale
The authors want to thank staff from the participating maternity units, and the Fondation pour la Recherche Medicale for its financial support.
References
- 1.Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. Bjog. 2004 May;111(5):481–4. doi: 10.1111/j.1471-0528.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008 Aug;199(2):133, e1–8. doi: 10.1016/j.ajog.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A. Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. Bjog. 2005;112(1):89–96. doi: 10.1111/j.1471-0528.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 4.Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. Bjog. 2008 Jun;115(7):842–50. doi: 10.1111/j.1471-0528.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 5.Elbourne DR, Prendiville WJ, Carroli G, Wood J, McDonald S. Prophylactic use of oxytocin in the third stage of labour. Cochrane Database Syst Rev. 2001;(4):CD001808. doi: 10.1002/14651858.CD001808. [DOI] [PubMed] [Google Scholar]
- 6.Department of health New South Wales Government Australia. Postpartum haemorrhage- framework for prevention, early recognition and management. 2005. http://www.health.nsw.gov.au/policies/PD/2005/PD_264.html.
- 7.National Guideline Clearinghouse. Postpartum haemorrhage. American College of Obstetricians and Gynecologists; 2006. http://www.guideline.gov/summary/pdf.aspx?doc_id=10922&stat=1&string= [Google Scholar]
- 8.The Society of Obstetricians and Gynecologists of Canada (SOGC) Prevention and management of postpartum haemorrhage. 2000. http://www.sogc.org/guidelines/public/88E-CPG-April2000.pdf.
- 9.World Health Organization. WHO recommendations for the prevention of postpartum haemorrhage. Geneva: World Health Organization Department of making pregnancy safer; 2007. ( http://www.who.int/making_pregnancy_safer/publications/WHORecommendationsforPPHaemorrhage.pdf) [Google Scholar]
- 10.Lalonde A, Daviss BA, Acosta A, Herschderfer K. Postpartum hemorrhage today: ICM/FIGO initiative 2004–2006. Int J Gynaecol Obstet. 2006;94(3):243–53. doi: 10.1016/j.ijgo.2006.04.016. Epub 2006 Jul 12. [DOI] [PubMed] [Google Scholar]
- 11.Goffinet F, Mercier F, Teyssier V, Pierre F, Dreyfus M, Mignon A, et al. Postpartum haemorrhage: recommendations for clinical practice by the CNGOF (December 2004) Gynecol Obstet Fertil. 2005 Apr;33(4):268–74. doi: 10.1016/j.gyobfe.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010 Apr;202(4):353, e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF. Investigation of an increase in postpartum haemorrhage in Canada. Bjog. 2007 Jun;114(6):751–9. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 14.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts CL, Ford JB, Algert CS, Bell JC, Simpson JM, Morris JM. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy Childbirth. 2009 Feb 25;9(1):7. doi: 10.1186/1471-2393-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. Bjog. 2008 Sep;115(10):1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 17.Combs CA, Murphy EL, Laros RK., Jr Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76. [PubMed] [Google Scholar]
- 18.Sosa CG, Althabe F, Belizan JM, Buekens P. Risk factors for postpartum hemorrhage in vaginal deliveries in a Latin-American population. Obstet Gynecol. 2009 Jun;113(6):1313–9. doi: 10.1097/AOG.0b013e3181a66b05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deneux-Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J, et al. Educational multifaceted intervention to decrease the rate of severe postpartum haemorrhage- the Pithagore6 cluster-randomized controlled trial. BJOG. 2010 doi: 10.1111/j.1471-0528.2010.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French ministry of health. Décret 98–899. 1998. http://wwwsante-jeunesse-sportsgouvfr/fichiers/bo/1998/98-50/a0503153htm.
- 21.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 22.Saunders NS, Paterson CM, Wadsworth J. Neonatal and maternal morbidity in relation to the length of the second stage of labour. Br J Obstet Gynaecol. 1992;99(5):381–5. doi: 10.1111/j.1471-0528.1992.tb13753.x. [DOI] [PubMed] [Google Scholar]
- 23.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. Cmaj. 1997 Aug 15;157(4):408–16. [PMC free article] [PubMed] [Google Scholar]


