Abstract
Identifying effective interventions is vitalin preventing slip-induced fall accidents in older adults. The purpose of the current study was to evaluate the efficacy of moveable platform training in improving recovery reactions and reducing fall frequency in older adults. Twenty-four older adults were recruited and randomly assigned to two groups (training and control). Both groups underwent three sessions including baseline slip, training, and transfer of training on a slippery surface. Both groups experienced two slips on a slippery surface, one during the baseline and the other (after two weeks) during the transfer of training session. In the training session, the training group underwent twelve simulated slips using a moveable platform while the control group performed normal walking trials. Kinematic, kinetic, and EMG data were collected during all the sessions. Results indicated a reduced incidence of falls in the training group during the transfer of training trial as compared to the control group. The training group was able to transfer proactive and reactive control strategies learned during training to the second slip trial. The proactive adjustments include increased center-of-mass velocity and transitional acceleration after training. Reactive adjustments include reduction in muscle onset and time to peak activations of knee flexors and ankle plantarflexors, reduced ankle and knee coactivation, reduced slip displacement, and reduced time to peak knee flexion, trunk flexion, and hip flexion velocities. In general, the results indicated a beneficial effect of perturbation training in reducing slip severity and recovery kinematics in healthy older adults.
Keywords: Falls, elderly, biomechanics, locomotion, fall prevention training
INTRODUCTION
Fall accidents are associated with considerable medical cost and suffering in older adults. Annually, 33% of older adults (> 65 years) experience a fall and many of these falls are recurrent [1]. Slip-induced falls account for 87% of all hip fractures, which often results in immobility and may require admission to a nursing home facility [2]. As the size of the older population (> 65 years) is growing and fall injuries remain prevalent in this age group, there is a need for prevention strategies to reduce the risks associated with falls. Numerous exercise interventions based on strength training[3], balance training[4], and Tai Chi [5]have been proposed to prevent falls. However, efficacy of these interventions in reducing fall rates have produced mixed results [6–8]. The differences may be because most of the training programs are general in nature and not designed to improve specific motor skills related to recovering from a slip-induced fall.
A training program that may help older adults learn movements directly related to recovery responses may improve their sensory and muscle co-ordination and thus their ability to recover from a postural perturbation (i.e., slip). Perturbation-based training using a moveable platform has shown to evoke biomechanical and neuromuscular reactions similar to slip-induced fall[9, 10]. The perturbation training in general follows the principle that the central nervous system will continuously adapt and adjust to postural disturbances induced to maintain balance[11, 12]. Numerous studies have used repeated perturbations to observe improvements in adaptive responses [12–15]. These adaptive changes were believed to be caused by the modulation of feedforward and feedback motor control systems. Similarly, there is evidence for long-lasting modifications in the inter-limb co-ordination after a period of walking on a rotating disk [13, 15] or a split-belt treadmill [16]. The presence of aftereffects following a period of training under new conditions implies the process of “re-learning” the motor output for a given task.
Recently, Bhatt et al. [17] demonstrated improved recovery in young adults after repeated exposures to a simulated slip-perturbation. Slips were induced using a moveable platform (free to slide when unlocked) that shifted unexpectedly when the participants walked over it. This created an overall sensory conflict (similar to a slip) by perturbing the somatosensory system. Improvements were seen both during pre-slip and post-slip center-of-mass (COM) stability, with participants reaching a steady-state after a few trials. Additionally, Wang et al. [50] demonstrated generalization of motor adaptation by transferring adaptive control acquired from sit-to-stand-slip to improved recovery in an unexpected novel slip in walking. Furthermore,Bieryla et al. [11] demonstrated improvements in trip recovery following repeated trip perturbations on a treadmill in older adults. Pavol and Pai [10]found a decreased incidence of falls in older adults with repeated slip exposure during a sit to stand task. These studies provide evidence that slip-perturbation training can be generalized to other movement tasks, and older adults have the capability to adapt their movements to recover from a perturbation through training.
Although previous studies examined the adaptation of individuals to the simulated slip-perturbation training, none of the studies validated the training effects on an actual slippery surface. Additionally, there is a need to assess the extent to which such training effects can be reproduced in older adults. Further, little is known about the various biomechanical and neuromuscular responses in older adults that may govern the recovery process during postural maintenance when exposed to perturbation training. The objective of the current study was to evaluate the effects of moveable platform training in improving recovery in older adults and reducing fall frequency. Additionally, the purpose of the study was to identify the various biomechanical and neuromuscular changes that occur during the moveable platform training (MPT). It was hypothesized that the MPT group would be able to transfer strategies learned during the training to an actual slip.
METHOD
Participants
Twenty-four healthy older adults (> 65 years, 12 males, and 12 females) were recruited for the study (Table 1).Participants signed a written consent formapproved by the Institutional Review Board (IRB) of Virginia Tech before participation. The participants were screened by a physician for general physical fitness and balance problems.Exclusionary criteria included cardiovascular, respiratory, neurological, and musculoskeletal abnormalities as well as any other difficulties hindering normal gait.Participants were randomly divided into a control group (n = 12), and a moveable platform training group (n = 12). No significant differences were found in the demographics of participants between groups (Table 1).
Table 1.
Participants demographics (Mean ± SD)
| Group |
|||
|---|---|---|---|
| Control (n = 12) | Training (n =12) | P value | |
| Age (yrs) | 74.18 ± 5.82 | 71.24 ± 6.82 | 0.91 |
| Mass (kg) | 69.63 ± 9.45 | 68.24 ± 8.04 | 0.78 |
| Stature(cm) | 169.41 ± 9.16 | 167.45 ± 11.52 | 0.11 |
Note. The p value represents the results of a t test comparing two-groups
Apparatus
Slip-perturbation set-up
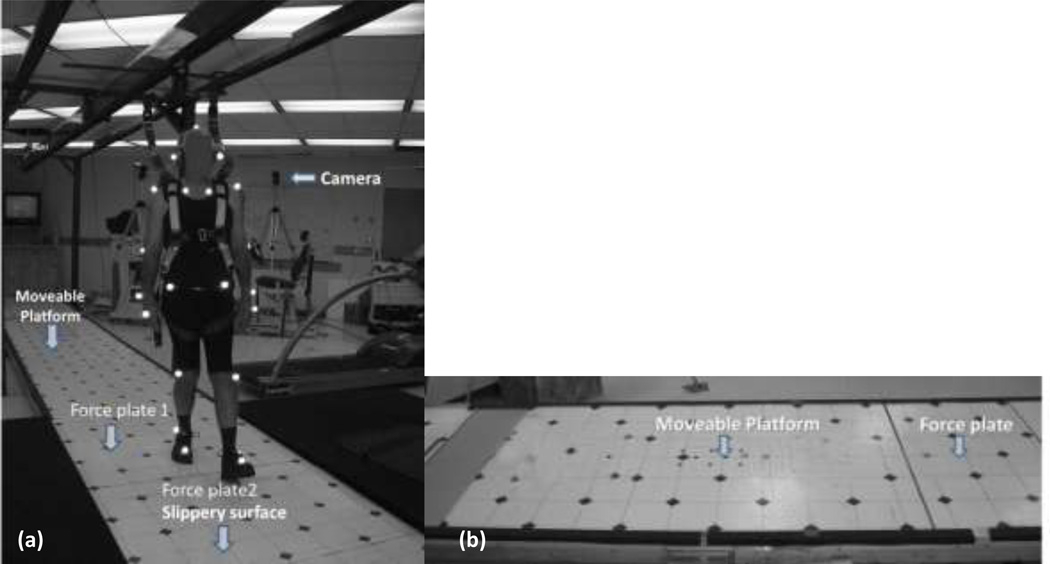
The slip trials were conducted on a 15 m long walkway. The walkway was embedded with two force plates (Type 45550-08, Bertec Corporation, USA) which were used to record gait characteristics and induce an actual slip (Fig1a). The slippery surface (i.e., top of one the force plates) was covered with a water and clear jelly mixture (1:1) to reduce the coefficient of friction (COF) (dynamic COF = 0.12) of the floor surface. In order to provide a consistent floor COF, same person applied the same amount of mixture using a sponge roller.Participants were unaware of the position of this surface as the force plates are covered with the same vinyl as the walkway and, the slippery substance was not visible on the walkway. This is a standardized approach used in previous slip and fall studies [18, 19]. The experimental layout is shown in Figure 1(a).
Figure 1.
a. Experimental set-up for the baseline and transfer of training session including the walkway, harness, markers, two force plates (F1 and F2), and motion capture system,b. Experimental lay-out of moveable platform training set-up with the motorized platform and force plate.
Moveable platform set-up
The slip-perturbation training was conducted by inducing slips using a custom built sliding device consisting of a low friction, motorized moveable platform (40×120cm). The moveable platform was embedded into an existing 15 m walkway and was covered with the same vinyl floor material as that of the walkway (Fig 1a). One force plate (BP400600-1000, AMTI, MA) was placed before the moveable platform so that the ground reaction force of the step prior to contacting the platform could be recorded. Slips were induced by a computer-controlled program that moved the platform right after the heel contact of the slipping limb (right), when the vertical ground reaction force of the trailing limb (left) dropped below a threshold (i.e., 40% of body weight was lifted off the force plate) (Fig 1b). This platform movement simulated a backward fall when slipping over a slippery surface. The computer program, written in LabVIEW 6.2 (National Instruments, Austin, TX), was used for the real-time monitoring of the force and required individual’s weight (in kg) as an input parameter.
Measurement
Full-body kinematics were recorded at 100 Hz using a six-camera motion capture system (Qualisys). Twenty-four reflective markers were attached to various bony landmarks of the body. The marker configuration was similar to previous studies [20, 21]. Kinetic data were collected at 1000 Hz from the force plates. An eight-channel EMG telemetry Myosystem 2000 (Noraxon, USA) was used to record temporal activations of various muscles in the lower extremity during all the sessions. Bipolar surface electrodes were placed bilaterally over vastus lateralis (VL), medial hamstring (MH), tibialis anterior (TA), and medial gastrocnemius (MG) muscles. The EMG data were sampled at 1000Hz. Uniform clothes and shoes were provided to all participants to minimize loose clothing and shoe-sole differences. Participants wore a full body fall-arresting harness throughout the experiment (Fig 1a)[20]
Protocol
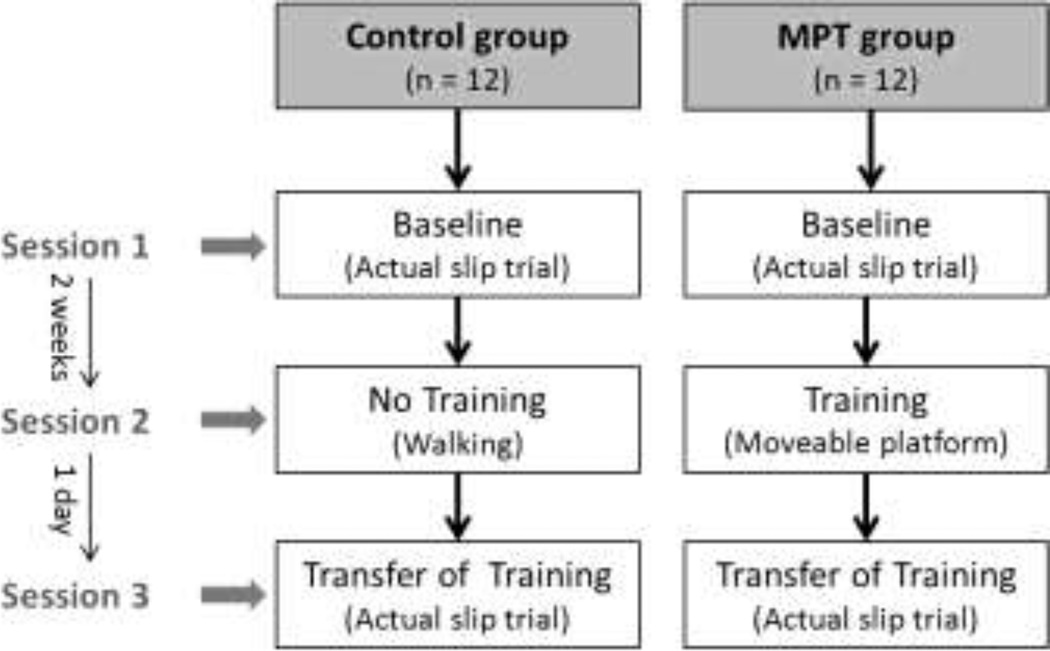
The experiments were divided into three sessions: baseline measure, training acquisition, and transfer of training, on three separate days (Fig 2). During the first session, all participants underwent a slip trial on a slippery floor surface that served as a baseline measure (Slip1). After two weeks, the training group performed the slip training and the control group performed normal walking trials. The third session was on the following day of the training, where both groups were exposed to a slippery floor surface similar to the baseline session (Slip2).
Figure 2.
Experimental sessions for the control and the moveable platform training groups
Baseline Measure and Transfer of Training
Participants were instructed to walk on the walkway for 10 minutes at a self-selected pace to become familiarized with the harness and the laboratory environment. A metronome was used to record participants’ pace. The starting point of their walking was adjusted so that their right foot landed on the force plate at the center of the walkway, which was later switched to a slippery surface (Fig1(a)). The baseline kinematic, kinetic, and EMG data were recorded from five walking trials before inducing the slip. After collecting the normal walking trial, an actual slippery surface was introduced without participants’ knowledge and the data were collected (Slip1). Based on the group assignment, participants were called for their next session (moveable platform training or control walking session) (Fig 2). After the training acquisition session, participants returned to the laboratory the following day for the transfer of training session that was similar to the baseline session.
Training Acquisition
Although the control group did not undergo any training, they were brought to the lab for the second session which was similar to the baseline session to maintain consistency in the number of lab sessions experienced by both groups. The control group was instructed to walk on the walkway for 15–20 min at their self-selected pace which was matched to their baseline session. Simple filing tasks were provided to the participants at the end of the walkway. The training group underwent moveable platform training in their second session. Participants were instructed to walk on the walkway at their self-selected pace which was matched to their pace recorded from the first session. The starting point of their walking was adjusted such that their trailing limb (left) landed on the force plate placed prior to the moveable platform. While participants walked on the walkway, a simulated slip was induced by moving the platform 0.3 m at a speed of 1.2 m/s (acceleration at 20 m/s2). After the first exposure to the simulated slip, participants were instructed to continue walking at the same speed as that of the previous trial and that they may or may not be slipped again.
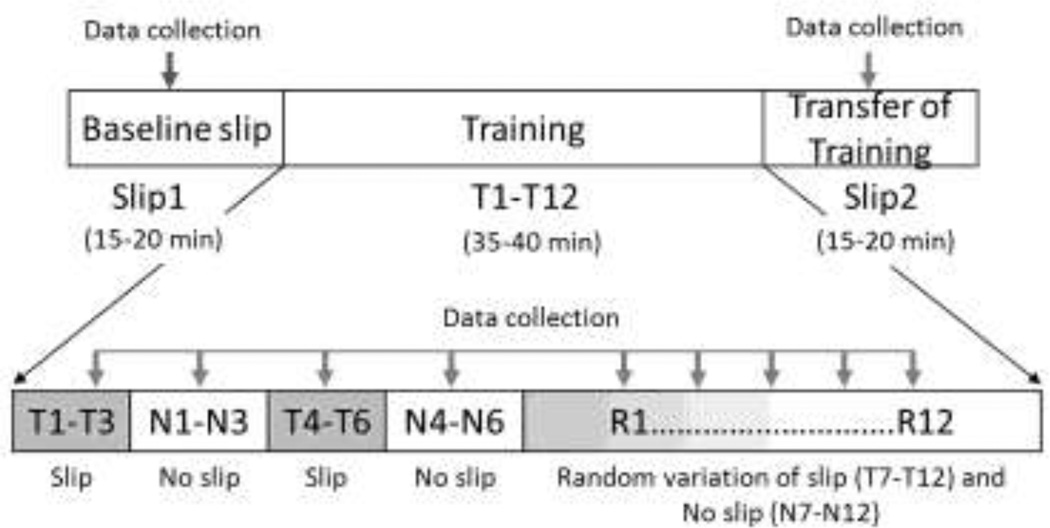
The training session consisted of 24 trials, consisting of a block of three repeated slips (T1-T3), then a block of three no slips (N1-N3), followed by a second block of three repeated slips (T4-T6), another block of three no slips (N4-N6), followed by 12 trials of random variations of slips and no slips (R1-R12) (Fig 3). A combination of blocked and randomized practice sessions have been shown to enhance motor leaning[22]. The structure of the training session is similar to the protocol adopted by Bhatt et al. [17]. However, after the first block of repeated slips, the speed of the moveable platform was increased or decreased by 0.24 m/s (20% of the initial velocity) for the next block of slip trials based on whether the participants successfully recovered from the perturbation (by observation). The decrease in velocity was believed to provide a better opportunity for successful recovery in cases where failed recoveries were observed, whereas an increase in speed was believed to provide greater challenge, if successful recoveries are observed; both of which has been shown to improve motor learning [23]. The last 12 trials included two slip speeds from block 1 and 2 trials, and no slip presented in a random order. Whole body kinematics, kinetic, and EMG data were recorded during all the trials.
Figure 3.
Experimental protocol for moveable platform training consisting of 24 trials of blocked slipand no slip trials (12), and randomized slip and no slip trials (12).
This training adheres to the principles of progressive overload, as progressions were made by increasing the magnitude of perturbation whenever the participants were able to recover, thus increasing challenge to the motor control system [24]. Progressions were matched to the individual’s rate of adaptation, that is the magnitude of perturbation was either increased or decreased based onparticipants’ ability to recover balance, promoting individualization[25]. Variability (i.e., speed of platform) and randomization (i.e., order of slip/no slip trials)of the practice conditions helped in the transfer of the learned recovery mechanisms (motor skill) to different situations (i.e., slippery floor surface), thereby promoting generalizability[26, 27].
Data Analyses
The converted coordinate kinematic (marker data) and kinetic (force plate) data were low-pass filtered using a fourth order, zero lag, Butterworth filter at a cut off frequency of 7 Hz. The EMG data were digitally band pass filtered at 10–450 Hz following data collection [28]. They were then rectified and low-pass filtered using a fourth order, zero lag, Butterworth filter with a 7 Hz cut off frequency to create a linear envelope [28, 29]. Heel-contact (HC) and Toe-off (TO) instances were identified from the ground reaction forces. The analyses were performed during the stance phase (HC to TO) of the slipping foot. The dependent variables are divided into two categories. 1) Variables that describe the responses after the slip is initiated (reactive adjustments) and, 2) Variables that describe characteristics at heel contact before the slip is initiated (proactive adjustments).
Reactive Adjustments
Slip distances (SDI & SDII) and peak sliding heel velocity (PSHV)were used as measures of slip severity as described by previous studies[20, 30]. The outcome of the slip (i.e., fall or recovery) was measured using the fall frequency. For a slip to be considered a fall, the slip distance must exceed 10 cm and the peak sliding heel velocity must exceed the center of mass velocity while slipping [20].Additionally, videos for each of the participants were analyzed to detect a fall along with the trunk marker (fall to vertical minimum).
Muscle activity onsets and durations of the slipping limb were determined using a threshold of two standard deviations above activity during a quiet period of gait cycle[29, 31]. The onset and time to peak activation of MG, TA, MH and TA of the slipping limb after the slip is initiated were used in the statistical analyses.Co-contraction index (CCI) or coactivity was calculated based on the ratio of the EMG activity of the antagonist/agonist muscle pairs (TA/MG and VL/MH) using the method proposed by Rudolph et al. [32]. The peak ankle and knee coactivity and the time to peak coactivity (ankle and knee) of the slipping limb after the slip is initiated were utilized for the statistical analyses.
The lower extremity 2D joint angles (ankle, knee and hip) and angular velocities were calculated using methods described previously [18]. Trunk angle was defined as angle between the trunk segment (mid-point between shoulder and mid-point between ASIS) and vertical. Peak angles, peak angular velocity, time to peak angle, and time to peak angular velocities of the slipping limb and the trunk were calculated after the slip was induced. All analyses were performed in the sagittal plane. The foot onset, foot down, and unperturbed foot reaction time in mswere analysed to reveal how fast the non-slipping foot could substantiate its role in the recovery process after a slip[33].
Proactive Adjustments
The proactive adjustments were defined as the changes in gait measures, angular kinematics, and EMG measures at heel contact before the slip was initiated. These variables were measured to quantify any anticipatory changes participants may have at heel contact between Slip1 to Slip2 session.Center-of-mass velocity (COMvel) was calculated as average of all the COMs from the 14 segments as described by Lockhart et al. [20]. Transitional acceleration of the whole body COM (TA) was defined as the change in horizontal COMvel between heel contact and shortly after (~ 50ms) heel contact [20, 31].Required coefficient of friction (RCOF) was defined as the minimum ratio of horizontal to vertical ground reaction force[34]. Ankle, knee, and hip angles of the slipping limb along with the trunk angle at the heel contact were used to quantify any proactive angular adjustments. The muscle (MG, TA, MH, and VL) onsets along with ankle and knee coactivity of the slipping limb at the heel contact were used to quantify any proactive muscular adjustments.
Statistical analyses
The experiment employed a two-group pretest-posttest design. To determine the effect of moveable platform training on recovery performance, difference values were calculated between the two slips (Slip2 – Slip1), and a one-way multivariate analysis of variance (MANOVA) was conducted between the two groups including all the dependent measures. If a statistically significant main effect of training was found, subsequent univariate analysis of variance (ANOVA) was conducted to elucidate the effect of training on each of the dependent measures (reactive and proactive measures). The frequency of falls was analyzed within the groups before and after the training, and between the groups (training and control) for Slip1 using the chi square (χ2) test statistic. To determine if the groups had similar slipping characteristics during Slip1, a between group one-way ANOVA was performed on slip distances (SDI & SDII) and PSHV. To determine if the gait characteristics prior to slipping during Slip1 were similar in both the groups, a between group one-way ANOVA was performed on COMvel, TA, and RCOF at heel contact. All statistical analyses were conducted using SPSS 11.5.0 (Chicago, IL) with a significance level of p < 0.05 for all the tests.
RESULT
The training group was able to reduce the frequency of falls from 42% upon the first unexpected slip (Slip1) during the baseline session to 0% upon the second unexpected slip (Slip2) during the transfer of training session (χ2= 12.67, df = 1, p = 0.007). Although, the frequency of falls in the control group reduced from 50% upon Slip1 to 25% uponSlip2, the results were not statistically significant (χ2= 1.67, df = 1, p = 0.216). Both groups were at a similar fall rate during Slip1 (χ2= 0.57, df = 1, p = 0.862). The MANOVA on the difference values (Slip2 – Slip1) for all the dependent variables indicated a significant effect of training (Wilk’s lambda: F (1, 18) = 6.01, p = 0.009).
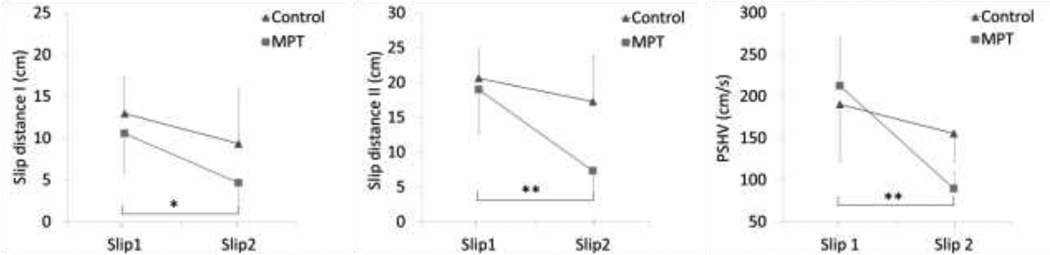
The ANOVA indicated that SDI and SDII decreased more from Slip1 to Slip2 in the training group compared to control (SDI: F (1, 18) =12.34, p =0.002, SDII: F (1, 18) = 18.34, p = 0.001) (Fig 4). The decrease in the peak sliding heel velocity was greater for the training group (Table 2.2) compared to control (F (1, 18) = 9.008, p = 0.008) (Fig 4). No significant differences were found in the mean slip distances and peak sliding heel between the groups during Slip1 (F (1, 18) = 2.008, p = 0.22), indicating no group differences at the baseline.
Figure 4.
Changes in slip severity measures from Slip1 to Slip2 between control and moveable platform training group. Note. Statistics were performed on the difference values (Slip2 – Slip1). *p < 0.05, **p < 0.01.
Table 2.
Mean ± SD of joint angles and angular velocities during Slip1 and Slip2 trials between control and training group
| Variable | Group |
|||
|---|---|---|---|---|
| Training | Control | |||
| Slip1 | Slip2 | Slip1 | Slip2 | |
| Joint angles (deg) | ||||
| Ankle angle at HC (+ = plantar) | 98.23 ± 3.66 | 100.52 ± 4.67 | 95.56 ± 4.29 | 98.56 ± 5.29 |
| Knee angle at HC (+ = flex)† | −5.24 ± 1.23 | −3.64 ± 2.89 | −2.46 ± 1.23 | −1.53 ± 0.98 |
| Hip angle at HC (+ = flex) | 10.86 ± 4.23 | 9.54 ± 5.29 | 16.32 ± 5.28 | 18.42 ± 6.39 |
| Trunk angle at HC (+ = flex) | 9.86 ± 3.54 | 10.34 ± 5.56 | 10.34 ± 5.76 | 9.34 ± 3.56 |
| Peak Ankle angle (+ = plantar) | 108.60 ± 5.34 | 103.38 ± 4.23 | 110.32 ±4.55 | 108.87 ± 6.78 |
| Peak Knee angle (+ = flex)** | 25.63 ± 5.50 | 18.04 ± 3.68 | 24.59 ± 5.39 | 21.24 ± 4.38 |
| Peak Hip angle (+ = flex)* | 12.44 ± 3.96 | 7.61 ± 2.45 | 18.70 ± 3.47 | 16.42 ± 2.53 |
| Peak Trunk angle (+ = ext) | 31.44 ± 13.96 | 29.61 ± 10.45 | 38.70 ± 13.47 | 39.42 ± 12.53 |
| Joint angular velocity (deg/s) | ||||
| Peak Ankle velocity | 85.66 ± 15.96 | 75.66 ± 16.47 | 102.56 ± 22.4 | 95.78 ± 10.45 |
| Peak Knee velocity* | 244.34 ± 25.9 | 189.34 ± 16.4 | 255.45 ± 32.4 | 210.29 ± 31.6 |
| Peak Hip velocity† | 150.44 ± 22.61 | 75.45 ± 12.55 | 150.4 ± 28.65 | 75.45 ± 10.53 |
| Peak Trunk velocity† | 135.32 ± 13.21 | 115.32 ± 33.81 | 135.32 ± 23.2 | 145.32 ± 16.2 |
Note.
p< 0.05,
p < 0.01,
p < 0.1, p-value represent the statistics on the difference value (Slip2 – Slip1) between groups
In terms of slipping limb kinematics, the peak knee flexion (F (1, 18) = 8.26, p = 0.01) and peak hip flexion (F (1, 18) = 15.46, p = 0.001) decreased more from Slip1 to Slip2 in the training group compared to control (Table 2). The peak knee angular velocity decreased more from Slip1 to Slip2 in the training group compared to control (F (1, 18) = 9.46, p = 0.01). A decrease in the peak angular velocity of hip, trunk, and ankle was observed but the differences were not significant between groups. The peak trunk angular velocity increased in the control group from Slip1 to Slip2 trial (Table 2). Further analysis revealed a significant effect of group on time to peak angular velocities. The time to peak trunk angular velocity (F (1, 18) = 11.46, p = 0.01) and hip angular velocity (F (1, 18) = 7.45, p = 0.03) decreased more in the training group compared to control (Table 2).
Muscle responses of the slipping limb to Slip1 were similar in both training and control groups, with activation of medial hamstrings (MH) (~ 160 ms), followed by medial gastrocnemius (MG) (~ 180 ms), tibialis anterior (TA)(~ 188 ms), and vastus lateralis (VL) (~ 240 ms). There was an early onset of MH (F (1, 18) = 14.97, p = 0.001) and TA (F (1, 18) = 10.46, p = 0.01) from Slip1 to Slip2 trial in the training group compared to control. An early onset of MG and VL muscles were also observed after training, but the differences between the groups were not significant (Table 3). The time to peak activation of MH (F (1, 18) = 15.55, p= 0.001) and TA (F (1, 18) = 16.52, p = 0.001) muscles decreased more in the training group compared to control. Peak knee coactivity decreased from Slip1 to Slip2 in the training group (F (1, 18) = 31.31, p = 0.0001). Similarly, peak ankle coactivity decreased from Slip1 to Slip2 in the training group (F (1, 18) = 19.46, p = 0.001) (Table 3). The time to peak knee coactivity decreased more in the training group from Slip1 to Slip2 compared to control (F (1, 18) = 10.46, p = 0.01) (Table 3). The non-slipping foot timing analysis indicated no differences in the toe-off and foot-onset between the groups. The unperturbed foot reaction time decreased more from Slip1 to Slip2 in the training group compared to control (F (1, 18) = 10.46, p = 0.02) (Table 4).
Table 3.
Mean ± SD of onset of muscle activity after slip-start and the time to peak activations (recovery trials only)
| Variable | Group |
|||
|---|---|---|---|---|
| Training | Control | |||
| Slip1 | Slip2 | Slip1 | Slip2 | |
| Muscle activation onset (ms) | ||||
| Medial gastrocnemius | 188 ± 33.66 | 185 ± 14.67 | 189 ± 24.29 | 179 ± 25.29 |
| Tibialis anterior* | 197 ± 22.23 | 165 ± 12.89 | 188 ± 21.23 | 178 ±12.98 |
| Medial hamstrings* | 155 ± 11.76 | 133 ± 10.33 | 168 ± 15.28 | 156 ± 16.39 |
| Vastus lateralis | 238 ± 23.54 | 220 ± 15.56 | 245 ± 25.76 | 255 ± 15.99 |
| Time to peak activations (ms) | ||||
| Medial gastrocnemius | 335 ± 25.50 | 321± 23.68 | 364 ± 15.39 | 377 ± 34.38 |
| Tibialis anterior** | 312 ± 33.96 | 277 ± 22.45 | 378 ± 23.47 | 362 ± 32.53 |
| Medial hamstrings** | 250 ± 13.96 | 215 ± 17.45 | 290 ± 23.47 | 278 ± 22.53 |
| Vastus Lateralis† | 365 ± 25.35 | 340 ± 16.68 | 369 ± 33.12 | 354 ± 20.73 |
| Coactivations | ||||
| Peak knee coactivity ** | 2.45 ± 1.12 | 1.77 ± 0.94 | 2.23 ± 1.39 | 2.44 ± 1.44 |
| Peak ankle coactivity* | 1.88 ± 0.96 | 1.32 ± 0.45 | 1.95 ± 1.11 | 2.1 ± 0.99 |
| Time to peak knee coactivity** | 310 ± 43.96 | 250 ± 37.15 | 320 ± 44.47 | 310 ± 29.66 |
| Time to peak ankle coactivity† | 290 ± 25.35 | 240 ± 36.68 | 319 ± 53.12 | 330 ± 20.55 |
Note.
p< 0.05,
p < 0.01,
p <0.1, p-value represent the statistics on the difference value (Slip2 – Slip1) between groups
Table 4.
Mean ± SD of the non-slipping foot response time after the slip was initiated
| Variable | Group |
|||
|---|---|---|---|---|
| Training | Control | |||
| Slip1 | Slip2 | Slip1 | Slip2 | |
| Non-slipping foot response time (ms) | ||||
| Toe off | 156 ± 29.23 | 149 ± 15.73 | 160 ± 18.66 | 155 ± 25.12 |
| Foot onset | 270 ± 16.24 | 260 ± 15.22 | 278 ± 20.56 | 285 ± 23.16 |
| Foot down † | 395 ± 25.22 | 368 ± 22.34 | 400 ± 28.34 | 410 ± 26.34 |
| Unperturbed foot reaction time * | 128 ± 15.22 | 100 ± 18.16 | 122 ± 20.76 | 126 ± 28.76 |
Note.
p< 0.05,
p < 0.01,
p <0.1, p-value represent the statistics on the difference value (Slip2 – Slip1) between groups
The results indicated few proactive adjustments in the training group before the slip during the transfer of training trial (Slip2). The COMvel at heel contact before slip-start increased more from Slip1 to Slip2 in the training group as compared to control (F (1, 18) = 10.76, p = 0.004). Similarly, the transitional acceleration of the whole body COM increased more in the training group compared to control (F (1, 18) = 10.34, p = 0.004). No significant differences were observed in the friction demand characteristics (RCOF) between Slip1 and Slip2 trials in either group. No significant differences were observed in the ankle, knee, hip and trunk angle at the heel contact before the slip onset in both groups. In terms of muscle activation, participants in the training group had an early onset of MH activity around heel contact compared to the control group during Slip2 trial (F (1, 18) = 5.34, p = 0.03). No significant differences were found in the ankle and knee coactivity at heel contact between the groups.
DISCUSSION
The aim of the study was to examine the effects of moveable platform training in reducing fall frequency and improving recovery strategies in older adults. The overall findings of the study indicated that older adults were able to learn movements related to recovery during the moveable platform training and transfer themto an actual slippery surface. As hypothesized, the frequency of falls reduced significantly in the training group from the initial slip during the baseline to the slip after training as compared to the control group. The reduced fall rate may have been achieved throughthe combination of training-induced improvements in various kinematic, slip-related, and neuromuscular parameters.
Reactive strategies after the training
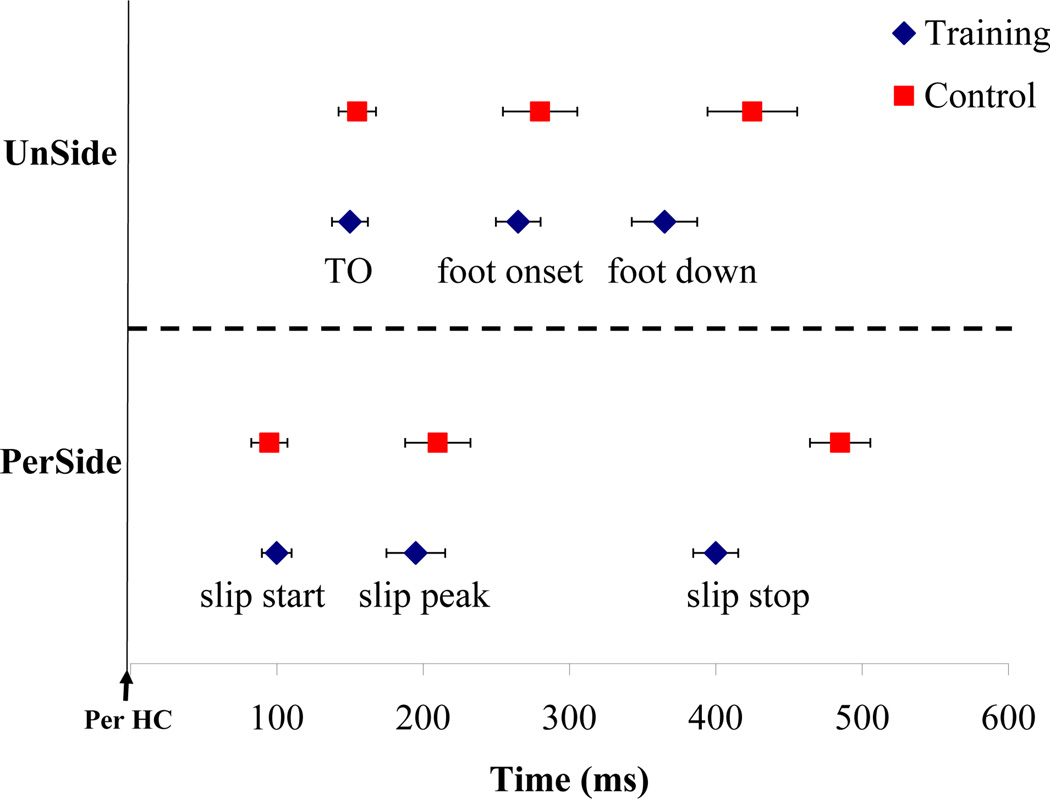
Reactive or feedback responses refer to strategies employed by individuals after a slip has been initiated. It is known that decreasing the slip displacement of the slipping foot helps in reducing the severity of slip and hence improve chances of recovery[30, 34, 35]. Our resultsindicated decreased slip distances and peak sliding heel velocity in the training group. Additionally, greater differences were seen in the reduction of slip distance II in the training group, indicating training-induced improvements in the slip recovery phase. Slips were initiated at similar time intervals in both groups during Slip1 and Slip2 trials (Fig 5). However, the time required for slip-stop was reduced in the training group as compared to the control group during the Slip2 trial. Further analysis revealed a reduction in the touch-down time of the non-slipping foot after the slip initiation in the training group (Fig 5). The unperturbed foot reaction time was 110 ± 19.9 ms for the training group and 150 ± 29.8 ms for the control group. A quick stepping response of the non-slipping/trailing foot after a slip is initiatedaids in the recovery process by widening the base of support and thus reducing slip displacement [33, 36].
Figure 5.
Slip events of the perturbed (PerSide) foot and the unperturbed (UnSide) foot during Slip2 trial for training and control group. The graph only contains data from successful recovery. (TO- unperturbed foot toe off, PerHC- heel contact of the slipping foot).
In terms of neuromuscular responses, during the Slip2 trial, an early onset and reduced time to peak MH and TA muscle of the slipping limb were observed in the training group. After a slip is initiated, faster recovery reactions within 100 to 200 ms will help in stabilizing the slipping limb and avoiding a fall. The initial muscular response to a slip consists of activation of MH followed by other muscles of the slipping limb[28]. Therefore, an early activation of MH may help in stabilizing the knee joint and assistin slip recovery process. During the training session (T1-T12), similar patterns of early onset and reduced time to peak muscle activations were observed, indicating a positive transfer to Slip2. Reactive muscle activation can be attributed to the feedback process of the motor control that uses reflex pathways to modify motor-unit recruitment and continually adjust ongoing muscle activity [37, 38]. Both proactive and reactive motor control can improve stability by necessary frequent stimulation of sensory and motor pathways. As a particular signal passes through a sequence of synapses (e.g. in this study, signals to the CNS related to the perturbation); the synapses become more capable of transmitting the same signal the next time [39, 40]. After repeated exposure to simulated slips during the training, it may be possible that older adults were able to achieve an optimal strategy to quickly activate muscles necessary for stabilization during Slip2.
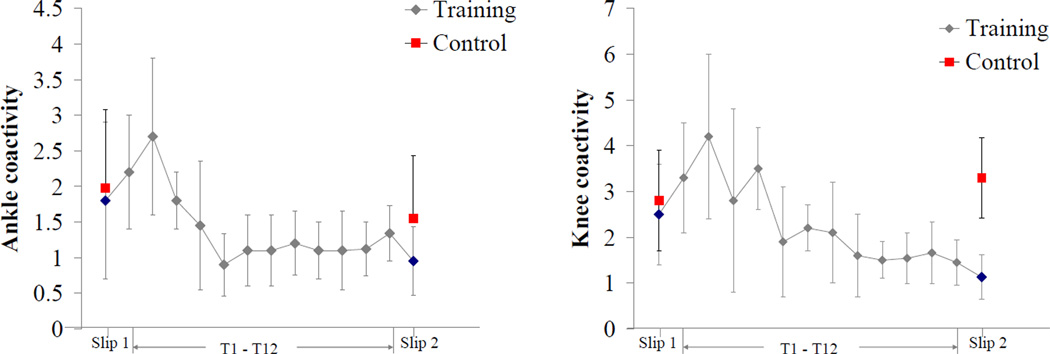
Further neuromuscular responses such as decreased peak knee and ankle coactivity of the slipping limb were observed in the training group during Slip2 trial. In general, the integrated muscle activity of all the muscles of slipping limb increased during Slip2. Such patterns of coactivity were also observed during the training, with an initial increase and subsequent decrease in the coactivity, which reached a plateau after 6–7 training trials (Fig 6). Coactivation of agonist and antagonist muscles is important for the regulation of joint stiffness [41, 42]. Empirical evidence suggests that training induces a decrease in coactivation, which may increase net joint torque and reduce energy expenditure [43, 44]. Apart from the magnitude, the time to peak knee coactivation was also reduced in the training group during the Slip2 trial. This may be attributed to the early onset and reduced time to peak MH activity after training. It may be possible that with repeated exposure to simulated slips, the CNS chose the most effective muscle synergy organization to achieve a common goal (i.e., recovery) with the least energy expenditure.
Figure 6.
Mean ± 1 SD of peak ankle and knee coactivity from T1- T12 slip training trials (training group), and from Slip1 and Slip2 trials (control and training group).
In terms of angular kinematics, successful recoveries relied on increased peak ankle plantarflexion, knee flexion, hip flexion, and decreased peak trunk extension angles. These results are consistent with previous studies investigating slips, i.e., primary knee flexion response followed by a secondary knee extension response [18, 45]. Significant training-induced improvements were found in the knee and hip angles between the groups. During training, similar kinematic changes were observed, with a plateau by 6–7 training trials. The trunk angular velocity was an important predictor of a successful recovery as compared to the peak trunk angle. The training group was able to quickly reverse trunk extension as compared to the control. Reducing forward trunk rotations are believed to have a significant effect in bringing the COM of the body within stability limits [46].
Proactive strategies after the training
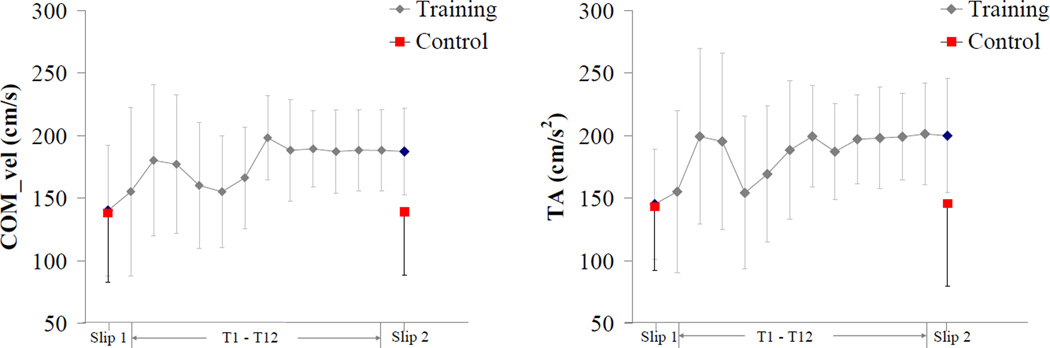
Study findings indicated presence of proactive or feedforward strategies during training and transfer of training trials. Proactive adjustments were more prevalent during the training trials (T1-T12) as compared to the transfer of training trial (Slip2). An increased COMvel and TA were observed at the heel contact in the training group during Slip2 (Fig 7). Increases in the COMvel aids in maintaining balance when experienced with a slip [20, 47, 48]. During training, participants walked with an increased COMvel after the first exposure to the simulated slip. Bhatt et al. [17]found similar results in their study, where younger adults improved their pre-slip stability during training trials by increasing the COMvel with respect to base of support. However, in this study older adults took longer to achieve stability (~7–8 trials) as compared to the younger adults (~ 3–5 trials) in previous studies [10, 17](Fig 7).Further proactive changes were observed in early onset of MH activity at heel contact in the training group during Slip2. Similar responses were observed during training trials. Preactivation of muscles can provide quick compensation for external loads by increasing joint stiffness and are critical for dynamic joint stability [49]. No differences were found in the ankle, knee, hip, and trunk angles at the heel contact between Slip1 and Slip2, indicating a reduced reliance on proactive kinematic strategies. In summary, training-induced improvements were observed in both proactive and reactive strategies leading to reduced fall frequency in the training group.
Figure 7.
Mean ± 1 SD of center-of-mass velocity and transitional acceleration of whole body COM (TA) at heel contact from T1- T12 slip training trials (training group), and from Slip1 and Slip2 trials(control and training group).
Although beneficial effects of training were observed in the study, it is important to address the existing limitations. First, only healthy older adults were recruited in the study and it is unclear how these results may change with a different population (i.e., fall prone older adults).Second, it is difficult to generalize the results outside of the laboratory environment as the sample size is relatively low and there is a need to test with a larger population group. Third, the retention of this training has not been examined and therefore it is difficult to interpret how long the improvements will be retained in the older adults. Based on the drawbacks of this study, futureresearchshould explore factors such asretention of the training, transferability of training to community and care facilities, and a longitudinal study to record fall frequencies of the individuals in the study after training.
In conclusion, one of the major findings from this study was that healthy older participants were capable of learning specific motor skills during training on the platform and transfer them to a different situation (i.e., an actual slip).Findings from the current study contributed to the knowledge of various biomechanical and neuromuscular parameters that were sensitive to training (i.e., trainability of some mechanisms). This information may be used as a preliminary data to improve the existing perturbation training methods to further refine the recovery reactions in older adults.The ultimate goal of training interventions is for older adults to transfer the learned motor task to different context outside the laboratory setting.
Acknowledgement
This research was supported by the NSF(grant #CBET-0756058) and NIOSH (grant #CDC/NIOSH-R01-OH009222).
REFERENCE
- 1.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of physical medicine and rehabilitation. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 2.Sterling DA, O'Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50(1):116–119. doi: 10.1097/00005373-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Buchner DM, Cress ME, de Lateur BJ, Esselman PC, Margherita AJ, Price R, et al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1997;52(4):M218–M224. doi: 10.1093/gerona/52a.4.m218. [DOI] [PubMed] [Google Scholar]
- 4.Steadman J, Donaldson N, Kalra L. A Randomized Controlled Trial of an Enhanced Balance Training Program to Improve Mobility and Reduce Falls in Elderly Patients. Journal of American Geriatric Society. 2003;51(6):847–852. doi: 10.1046/j.1365-2389.2003.51268.x. [DOI] [PubMed] [Google Scholar]
- 5.Woo J, Hong A, Lau E, Lynn H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age and Ageing. 2007;36(3):262–268. doi: 10.1093/ageing/afm005. [DOI] [PubMed] [Google Scholar]
- 6.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ. 1997;315(7115):1065–1069. doi: 10.1136/bmj.315.7115.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. The Lancet. 2005;366(9500):1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 8.Mansfield A, Peters A, Liu B, Maki B. A perturbation-based balance training program for older adults: study protocol for a randomised controlled trial. BMC Geriatrics. 2007;7(1):12. doi: 10.1186/1471-2318-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt T, Pai YC. Generalization of Gait Adaptation for Fall Prevention: From Moveable Platform to Slippery Floor. J Neurophysiol. 2009;101(2):948–957. doi: 10.1152/jn.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for a potential loss of balance. Experimental Brain Research. 2002;145(4):528–538. doi: 10.1007/s00221-002-1143-4. [DOI] [PubMed] [Google Scholar]
- 11.Bieryla KA, Madigan ML, Nussbaum MA. Practicing recovery from a simulated trip improves recovery kinematics after an actual trip. Gait & Posture. 2007;26(2):208–213. doi: 10.1016/j.gaitpost.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Lam T, Anderschitz M, Dietz V. Contribution of Feedback and Feedforward Strategies to Locomotor Adaptations. J Neurophysiol. 2006;95(2):766–773. doi: 10.1152/jn.00473.2005. [DOI] [PubMed] [Google Scholar]
- 13.Gordon CR, Fletcher WA, Melvill JG, Edgerton VR. Adaptive plasticity in the control of locomotor trajectory. Experimental Brain Research. 1995;102:540–545. doi: 10.1007/BF00230658. [DOI] [PubMed] [Google Scholar]
- 14.Tjernstrom F, Fransson PA, Hafstrom A, Magnusson M. Adaptation of postural control to perturbations - a process that initiates long-term motor memory. Gait & Posture. 2002;15(1):75–82. doi: 10.1016/s0966-6362(01)00175-8. [DOI] [PubMed] [Google Scholar]
- 15.Weber KD, Fletcher WA, Gordon CR, Jones GM, Block EW. Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Experimental Brain Research. 1998;120(3):377–385. doi: 10.1007/s002210050411. [DOI] [PubMed] [Google Scholar]
- 16.Jensen L, Prokop T, Dietz V. Adaptational effects during human split-belt walking: influence of afferent input. Experimental Brain Research. 1998;118(1):126–130. doi: 10.1007/s002210050262. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt T, Wang E, Pai YC. Retention of adaptive control over varying intervals: Prevention of slip-induced backward balance loss during gait. Journal of Neurophysiology. 2006;95(5):2913–2922. doi: 10.1152/jn.01211.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lockhart T, Liu J. Effects of aging on lower extremity joint torque and muscle activation patterns during slip-induced falls. Journal of Biomechanics. 2006;39(Supplement 1):S87–S87. [Google Scholar]
- 19.Lockhart T, Smith J. Effects of aging on the biomechanics of slips and falls. Human Factors. 2005;47(4):708–729. doi: 10.1518/001872005775571014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart T, Wolstad J, Smith J. Effects of age-related gait changes on biomechanics of slips and falls. Ergonomics. 2003;46(12):1136–1140. doi: 10.1080/0014013031000139491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parijat P, Lockhart TE. Effects of quadriceps fatigue on the biomechanics of gait and slip propensity. Gait & Posture. 2008;28(4):568–573. doi: 10.1016/j.gaitpost.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TD, Swanson LR, Hall AL. What Is Repeated in a Repetition? Effects of Practice Conditions on Motor Skill Acquisition. PHYS THER. 1991;71(2):150–156. doi: 10.1093/ptj/71.2.150. [DOI] [PubMed] [Google Scholar]
- 23.Kottke FJ, Halpern D, Easton JKM, Ozel AT, Burrill CA. The training of coordination. Arch Phys Med Rehabil. 1978;59:567–572. [PubMed] [Google Scholar]
- 24.Drowatzky KL, Drowatzky JN. Physical training programs for the elderly. Clinical Kinesiology. 1999;53:52–62. [Google Scholar]
- 25.Briggs J. Sports therapy: theoretical and practical thoughts and considerations. Chichester: Corpus Publishing; 2001. [Google Scholar]
- 26.Dick MB, Hsieh S, Dick-Muehlke C, Davis DS, Cotman CW. The Variability of Practice Hypothesis in Motor Learning: Does It Apply to Alzheimer's Disease? Brain and Cognition. 2000;44:470–489. doi: 10.1006/brcg.2000.1206. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt RA. A schema theory of discrete motor skill learning. Psychological Review. 1975;82(4):225–260. [Google Scholar]
- 28.Chambers AJ, Cham R. Slip-related muscle activation patterns in the stance leg during walking. Gait & Posture. 2007;25(4):565–572. doi: 10.1016/j.gaitpost.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Tang P, Woollacott MH, Chong RKY. Control of reactive balance adjustments in perturbed human walking: roles of proximal and distal postural muscle activity. Experimental Brain Research. 1998;119(2):141–152. doi: 10.1007/s002210050327. [DOI] [PubMed] [Google Scholar]
- 30.Brady R, Pavol M, Owings T, Grabiner M. Foot displacement but not velocity predicts the outcome of a slip induced in young subjects while walking. Journal of Biomechanics. 2000;33(7):803–808. doi: 10.1016/s0021-9290(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart T, Kim S. Relationship between hamstring activation rate and heel contact velocity: Factors influencing age-related slip-induced falls. Gait & Posture. 2006;24(1):23–34. doi: 10.1016/j.gaitpost.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surgery, Sports Traumatology, Arthroscopy. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart T. An integrated approach towards identifying age-related mechanisms of slip initiated falls. Journal of Electromyography and Kinesiology. 2008;18(2):205–217. doi: 10.1016/j.jelekin.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins PJ. Measurement of slip between the shoe and ground during walking American Society of Testing and Materials. Special Technical Publication. 1978;649:71–87. [Google Scholar]
- 35.Strandberg L, Lanshammar H. The dynamics of slipping accidents. Journal of Occupational Accidents. 1981;3(3):153–162. [Google Scholar]
- 36.Marigold DS, Bethune AJ, Patla AE. Role of the Unperturbed Limb and Arms in the Reactive Recovery Response to an Unexpected Slip During Locomotion. Journal of Neurophysiology. 2003;89(4):1727–1737. doi: 10.1152/jn.00683.2002. [DOI] [PubMed] [Google Scholar]
- 37.Brener J. Sensory and perceptual determinants of voluntary visceral control. In: Schwartz GE, Beatty J, editors. Bio-feedback: Theory and Research. New York: Academic Press; 1977. pp. 29–66. [Google Scholar]
- 38.Dunn TG, Gillig SE, Ponser SE, Weil N. The learning process in biofeedback: Is it feed-forward or feedback? Biofeedback Self Regul. 1986;11(2):143–155. doi: 10.1007/BF00999982. [DOI] [PubMed] [Google Scholar]
- 39.Guyton AC. Textbook of Medical Physiology. Philadelphia: Saunders; 1981. pp. 534–536.pp. 562–564. [Google Scholar]
- 40.Hodgson JA, Roy RR, DeLeon R, Dobkin B, Edgerton RV. Can the mammalian lumbar spinal cord learn a motor task? Med. Sci. Sports Exerc. 1994;26(12):1491–1497. [PubMed] [Google Scholar]
- 41.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D'Ambrosia R. Muscular coactivation. The American Journal of Sports Medicine. 1988;16(2):113–122. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 42.Osternig LR, Caster BL, James CR. Contralateral hamstring (biceps femoris) coactivation patterns and anterior cruciate ligament dysfunction. Medicine & Science in Sports & Exercise. 1995;27(6):805–808. [PubMed] [Google Scholar]
- 43.Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. J Appl Physiol. 1992;73(3):911–917. doi: 10.1152/jappl.1992.73.3.911. [DOI] [PubMed] [Google Scholar]
- 44.Enoka RM. Neuromechanics of human movement. Champaign, IL: Human Kinetics; 2008. pp. 298–299. [Google Scholar]
- 45.Cham R, Redfern MS. Changes in gait when anticipating slippery floors. Gait & Posture. 2002;15(2):159–171. doi: 10.1016/s0966-6362(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 46.Grabiner MD, Donovan S, Bareither ML, Marone JR, Hamstra-Wright K, Gatts S, et al. Trunk kinematics and fall risk of older adults: Translating biomechanical results to the clinic. Journal of Electromyography and Kinesiology. 2008;18(2):197–204. doi: 10.1016/j.jelekin.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Pai Y-C, Patton J. Center of mass velocity-position predictions for balance control. Journal of Biomechanics. 1997;30(4):347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- 48.You J-Y, Chou Y-L, Lin C-J, Su F-C. Effect of slip on movement of body center of mass relative to base of support. Clinical Biomechanics. 2001;16(2):167–173. doi: 10.1016/s0268-0033(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 49.Grillner S. The Role of Muscle Stiffness in Meeting the Changing Postural and Locomotor Requirements for Force Development by the Ankle Extensors. Acta Physiologica Scandinavica. 1972;82(1):92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang TY, Bhatt T, Yang F, Pai YC. Generalization of motor adaptation to repeated-slip perturbation across tasks. Neuroscience. 2011;180:85–95. doi: 10.1016/j.neuroscience.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]