Abstract
T cell growth and function must be tightly regulated to provide protection against foreign pathogens while avoiding autoimmunity and immunodeficiency. It is now apparent that T cell metabolism is highly dynamic and has a tremendous impact on the ability of T cells to grow, activate and differentiate. Specific metabolic pathways provide energy and biosynthetic precursors that must support specific cell functions, as effector, regulatory, memory, and alloreactive T cells have distinct metabolic needs in immunity and inflammation. Here we review the signaling pathways that control metabolism and how the metabolic phenotypes of T cell subtypes integrate with T cell function. Ultimately, these metabolic differences may provide new opportunities to modulate the immune response and treat inflammatory and autoimmune diseases.
Immunometabolism: Linking metabolism and immune function
While most normal healthy cells use oxidative phosphorylation as an energy source, Otto Warburg observed almost a century ago that cancer cells are glycolytic and produce lactic acid even in the presence of oxygen [1, 2]. Although less efficient at producing ATP, this transformation from oxidative metabolism to aerobic glycolysis enables cancer cells to acquire the biosynthetic precursors necessary to support rapid growth and proliferation [3]. Based on this phenotype, the field of cancer biology is now actively seeking to inhibit aerobic glycolysis characteristic of tumor metabolism as a therapeutic treatment for cancer [4]. While fundamental to transformed cells, this metabolic phenotype is not unique to tumors. Indeed, it is now clear that this same metabolic transition to aerobic glycolysis is critical in T cell activation independent of any transformation event and that alterations in metabolism can significantly impact normal T cell function. Modulation of lymphocyte metabolism may, therefore, also be beneficial in the treatment of immune disorders.
Lymphocyte stimulation leads to an exceptionally high rate of cell growth and proliferation. To fuel the energetic and biosynthetic demands of rapid clonal expansion, activated T cells rapidly upregulate glucose uptake and glycolysis [5-8]. This process is co-stimulation dependent and failure to receive sufficient co-stimulation leads not only to functional inactivation or anergy but also to metabolic anergy, with a reduction in glucose metabolism [9]. Increased metabolism is a critical part of activation because if T cells fail to increase glucose metabolism due to inadequate nutrients or direct metabolic inhibition, activation and proliferation are suppressed [10-12]. Conversely, increased glucose uptake can enhance T cell activation and proliferation [10]. While altered metabolic needs and activity certainly follow changes in signaling and proliferation rate, evidence is now emerging that the regulation of T cell metabolism is also intimately linked to T cell function and differentiation. In each stage of a T cell's life, whether it is naïve, activated, antigen-experienced, or anergic, cell metabolism must be matched to the function of that particular T cell (Table 1). This review focuses on how metabolic pathways integrate with T cell function and the possibility that manipulation of metabolic pathways may provide a new approach to modulate the immune response.
Metabolic Dysregulation and T Cell Activation
Activation of effector T cells leads to increased glucose uptake, glycolysis, and lipid synthesis to support growth and proliferation (Figure 1). Just as altered metabolism can promote or support cancer cell growth and survival, improperly controlled T cell metabolism can lead to chronic T cell activation and inflammatory disease. Indeed, direct manipulation of glucose metabolism in vivo has been shown to modulate inflammatory disease. Overexpression of the glucose transporter Glut1 leads to increased glucose uptake and glycolysis, and transgenic expression of Glut1 specifically in T cells leads to increased T cell proliferation, survival and cytokine production [10, 13]. These cells are larger than normal T cells and less dependent on co-stimulation for IL-2 production. In addition, T cells from aged Glut1 transgenic mice exhibited elevated surface levels of several activation markers, including CD25, CD44 and CD69. They also produce more IL-2 and IFNγ than control cells. Ultimately, Glut1 transgenic mice develop lymphadenopathy and a mild generalized inflammatory disorder [10, 13].
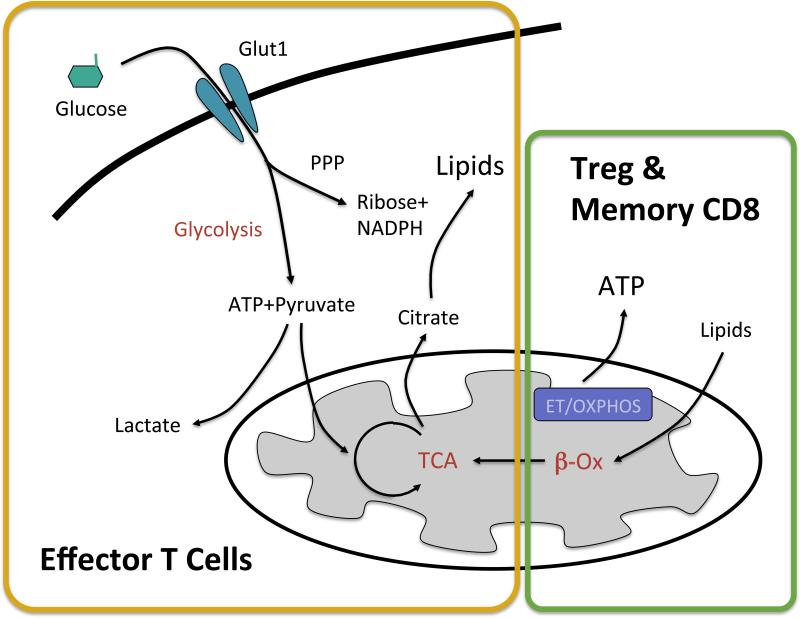
Figure 1. Metabolic differences between effector, Treg and memory T cells.
Upon stimulation, CD4+ and CD8+ effector T cells upregulate glucose uptake and glycolysis to provide biosynthetic precursors necessary for growth and proliferation. Glucose enters the pentose phosphate pathway (PPP) to generate ribose and NADPH necessary for nucleotide synthesis and is converted to citrate in the citric acid cycle (TCA) for lipid synthesis. Lactate is produced as a byproduct of the rapid rate of glycolysis in effector T cells. Treg and CD8+ memory cells instead burn lipids through beta-oxidation (β-ox) in the mitochondria and generate ATP through electron transport and oxidative phosphorylation (ET/OXPHOS).
Failure of effector T cells to properly upregulate glucose metabolism results in decreased cytokine production, proliferation, and can lead to apoptosis [10, 11, 14-16]. If T cells survive this metabolic stress, inhibition of metabolism during T cell activation can lead to anergy [9]. Glucose metabolism in particular is essential for T cells, as this glucose deprivation prevents T cell function despite the presence of other metabolic fuels [10, 11, 14]. Except in rare instances, glucose is generally available in the extracellular environment and the availability of glucose for lymphocyte metabolism is regulated instead by glucose uptake and the expression and trafficking patterns of glucose transporters (Glut) [17, 18]. Glut1 is expressed at a low level in naïve T cells and is rapidly induced by Myc following TCR activation [10, 19]. Glut1 trafficking is also highly regulated, with Glut1 protein remaining in intracellular vesicles until T cell activation. CD28 co-stimulation to further activate the PI3K/Akt/mTOR pathway, in particular, provides a signal for Glut1 expression and cell surface localization [7]. Constitutive Akt expression leads to a higher level of Glut1 protein on the surface of the cell [20] whereas PI3K inhibition or Akt-deficiency lowers glucose uptake [17, 18, 21].
Metabolism and Inflammatory Disease
Targeting glucose metabolism has been shown to be effective to reduce T cell effector function. Treatment of mice with the glycolytic inhibitor 2-deoxyglucose, for example, can suppress experimental autoimmune encephalomyelitis (EAE) [12]. In humans, chronically activated T cells in allergic asthma patients have been shown to produce high levels of lactate and overexpress pyruvate dehydrogenase kinase 1 (PDK1). PDK1 acts to inhibit pyruvate dehydrogenase and thus restrict entrance of pyruvate into the mitochondrial citric acid cycle, instead promoting aerobic glycolysis and the production of lactic acid [22]. Treatment of CD4+ T cells isolated from asthma patients with the PDK1 inhibitor dichloroacetate (DCA) promotes pyruvate oxidation in the mitochondria and prevented inflammatory cytokine production and T cell proliferation. As in human patients, T cells from mice in models of asthma produce high levels of lactate. Treatment of these mice with DCA reduced lactate production and inhibited airway inflammation in vivo. Inhibition of aerobic glycolysis with DCA also inhibited collagen-induced arthritis in female mice [23].
Mitochondria have many metabolic roles and dysregulation of mitochondrial metabolism is also associated with autoimmune and inflammatory disease. There is increasing evidence that chronically stimulated autoreactive T cells preferentially meet their metabolic demands through mitochondrial oxidative phosphorylation (OXPHOS) rather than aerobic glycolysis. Autoreactive splenocytes in mice with active lupus preferentially convert glucose to CO2 rather than lactate, increasing glucose oxidation by 40% over control splenocytes and relying on mitochondrial metabolism for ATP synthesis in vivo [24]. Similarly, chronically stimulated mouse T cells proliferating in response to alloantigen in the context of graft-versus-host disease (GVHD) greatly increase both glycolysis and OXPHOS compared to normal T cells [25]. This altered metabolic phenotype with chronic stimulation also occurs in humans with autoimmune disease, as T cells from patients with systemic lupus erythematosus (SLE) have mitochondrial abnormalities including elevated mitochondrial transmembrane potential, increased mitochondrial mass and depletion of ATP [26, 27].
This preference of chronically stimulated or alloreactive lymphocytes for OXPHOS may allow selectively targeting of mitochondrial metabolism. Accordingly, the small molecule Bz-423 that partially inhibits the mitochondrial F0F1 ATPase preferentially induced the apoptosis of alloreactive T cells with minimal impact on healthy lymphocytes [25]. While Bz-423 does not appear to significantly reduce cellular capacity for ATP generation, it does result in superoxide generation that leads to the apoptosis of alloreactive cells [28]. This sensitivity is likely due to limited antioxidant reserves in these oxidative cells. In vivo, Bz-423 treatment reduced GVHD clinical scores and improved survival compared to vehicle treatment without impairing reconstitution in the spleen or thymus after bone marrow transplant [25].
Nutrient excess or deprivation can also alter immune responses. Obesity has long been associated with low-grade inflammation, with macrophages and other immune cells infiltrating the adipose tissue of obese mice and humans [29]. Both the immune cells and the adipose tissue itself produce inflammatory cytokines, such as TNFα, that continue the inflammatory response, and obese patients have an increased risk for a wide array of inflammatory diseases [30, 31]. On the other hand, malnutrition is immunosuppressive and associated with increased mortality from infectious disease [32]. Further, anorexic patients with severe malnutrition have extremely low CD4+ T cell counts [33]. Mice fed a low protein diet also had decreased CD8+ memory T cells and these cells had defects in homeostatic proliferation and impaired recall response to a secondary infection [34]. While the root of weakened immunity in malnutrition is not entirely clear, it may be that the metabolic burden of an immune response is sufficient that it benefits animals to link and balance metabolic and immune systems. In support of this link, a variety of hormones and neuropeptides, including insulin and leptin, play a role in both metabolic and immune processes [35]. These systems are even controlled by the same tissue in some organisms, such as Drosophila, where the fat body functions similarly to the mammalian immune system, adipose tissue and liver [36].
Metabolic reprogramming in T cell activation
Mechanisms that control T cell metabolic reprogramming are now coming to light, and many of the same oncogenes important in cancer metabolism are also critical to drive T cell metabolic transformations, most notably Myc, HIF1α, ERRα, and the mTOR pathway (Box 1). The proto-oncogenic transcription factor Myc is known to promote transcription of genes for both cell cycle as well as aerobic glycolysis and glutamine metabolism [37, 38]. Recently, Myc has been shown to play an essential role to induce the expression of glycolytic and glutamine metabolism genes in the initial hours of T cell activation [19]. In a similar fashion, the transcription factor HIF1α can upregulate glycolytic genes to allow cancer cells to survive under hypoxic conditions [39]. The role for HIF1α in T cells is more complex, however, as HIF1α does not appear to play a role to promote glycolysis in initial T cell activation. Rather, HIF1α influences the balance of Th17:Treg cells at later times [12, 40]. In addition to glycolytic genes, other metabolic pathways, including mitochondrial pathways, must be upregulated during T cell activation to fully support biosynthesis and cell growth. Consistent with this diversity of metabolic demands, we recently showed a potential role for the nuclear hormone receptor Estrogen Related Receptor-α (ERRα) in T cell metabolism and activation [41]. ERRα is a well-known mitochondrial regulator that is associated with a variety of cancers, and our data support a role for ERRα in mitochondrial metabolism of the glycolytic product, pyruvate, in stimulated T cells [41-43].
In addition to transcriptional regulation, post-translational control of glucose uptake and glycolysis has also proven to be critical to support generation of T cell effector function. In particular, the phosphatidyl-inositol-3-kinase (PI3K)/Akt/mTOR pathway is important for T cell metabolism and is a potent target for T cell immunosuppression. Inhibition or deficiency in this pathway leads to T cell anergy despite the presence of co-stimulation [44] and the mTOR inhibitor rapamycin is used clinically as an immunosuppressive to treat a variety of inflammatory disorders and to prevent transplant rejection [45, 46]. Importantly, this pathway has been shown to play a key role to induce aerobic glycolysis and anabolic processes in cell growth. Activation of mTORC1 induces genes involved in glycolysis, the pentose phosphate pathway and sterol and lipid biosynthesis [47].
Glucose is selectively required for effector CD4+ T cells
After early activation events, the cytokine environment promotes CD4+ T cells to differentiate into Teff (Th1, Th2 or Th17) or regulatory (Treg) subsets, each with a distinct functional role in immunity. Th1 cells are involved with cell-mediated immunity, Th2 with humoral immunity and Th17 with mucosal immunity and inflammation [48], while Treg limit inappropriate or excessive immune responses by suppressing Teff cells [49]. Modulation of differentiation into a specific Teff or Treg subsets may, therefore, provide a way to regulate immunity in the context of inflammatory disorders [12, 13, 40, 41, 50]. Consistent with the distinct roles of CD4+ T cell subsets in immunity, the metabolic needs of CD4+ subsets differ and Teff and Treg are now known to utilize specific metabolic programs that may allow metabolic modulation of immunity (Figure 1, 2A).
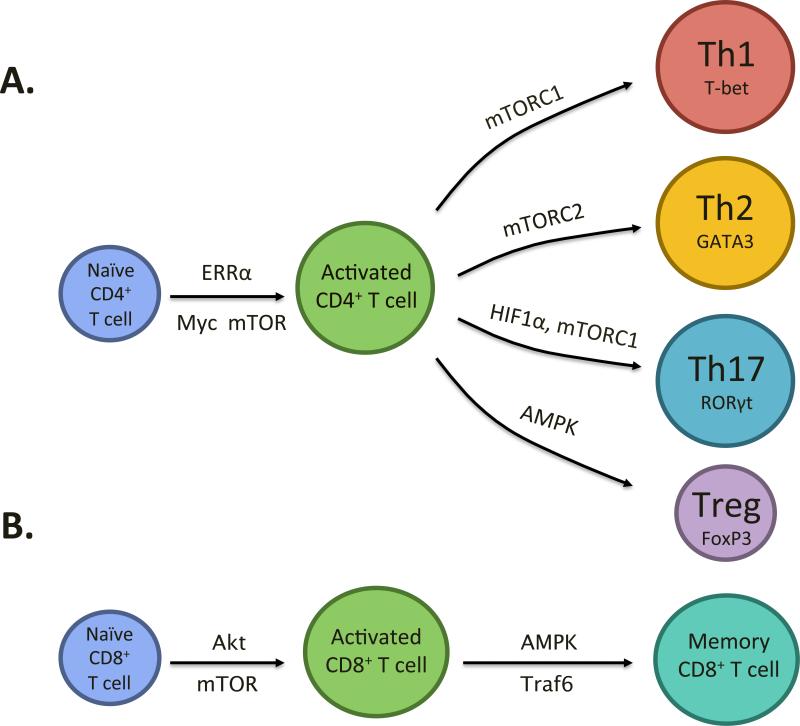
Figure 2. Signaling molecules involved in T cell metabolism and differentiation.
(A) Activation of a naïve CD4+ T cell requires appropriate TCR and co-stimulation as well as metabolic reprogramming and the signaling molecules mTOR, Myc and ERRα contribute to this metabolic transition. An activated CD4+ T cell can differentiate into an effector T cell (Th1, Th2 or Th17) or regulatory T cell (Treg) depending on the cytokine environment. mTORC1 is important for Th1 and Th17 differentiation, mTORC2 for Th2, HIF1α for Th17 and AMPK for Treg differentiation. (B) Activation of a naïve CD8+ T cell also requires appropriate TCR and co-stimulation as well as metabolic reprogramming and the signaling molecules Akt and mTOR contribute to this metabolic transition. TRAF6 and AMPK are involved in the transition from an effector to memory CD8+ T cell.
Although Teff depend on glucose to support the rapid growth and proliferation that accompanies clonal expansion, it is becoming increasingly evident that Treg do not share this same requirement. Treg express low levels of cell surface Glut1 [13] and exhibit lower levels of glucose uptake and glycolysis, instead primarily relying on lipid oxidation for energy [12, 13]. Further, the addition of exogenous fatty acids promotes Treg generation while suppressing Teff formation. While lipids can have direct roles in signaling pathways in addition to providing a metabolic fuel, the high rate of lipid oxidation and ability of Treg to persist even when glycolysis is inhibited suggest that lipid metabolism can support Treg [12, 13]. In addition, while inhibition of ERRα leads to a broad loss of glucose metabolism and inhibited T cell differentiation both in vitro and in vivo, the addition of exogenous fatty acids selectively rescued Treg, but not Teff, generation. Together these data suggest that Treg are less reliant than Teff on glucose as a fuel [41].
The PI3K/mTOR pathway plays a critical role in promoting the glucose metabolism required for effector T cell differentiation while at the same time inhibiting Treg generation [47, 51]. mTOR signals through two complexes, mTORC1 and mTORC2, with mTORC1 being downstream of Akt and regulated by PI3K while mTORC2 acts to promote Akt activation. Together these pathways integrate signals from the environment and contribute to T cell metabolism, activation and differentiation [52]. T cell specific mTOR knockouts fail to become Teff even when stimulated with the appropriate cytokines [50] and instead default to the Treg phenotype. Consistent with these findings, the mTORC1 inhibitor rapamycin promotes both fatty acid oxidation [53, 54] and the generation of Treg [55]. Conversely, constitutive activation of the mTOR pathway instead promotes glycolysis and impairs the generation of Treg [56]. This pathway has even more complicated roles in T cell metabolism and differentiation, as mTORC1 and mTORC2 have specific effects on Teff subsets. Rheb-deficient T cells, which lack mTORC1 activity, fail to become Th1 and Th17 but can still differentiate into Th2 whereas mTORC2 deficient T cells differentiate into Th1 and Th17 but not Th2 [57]. The role of cell metabolism relative to altered signaling in these cell fate decisions remains unclear, but this is a topic worth exploring given the broad impact of mTOR on metabolic pathways.
The AMP-activated kinase (AMPK) promotes catabolic processes [58] and can phosphorylate TSC2, which in turn inhibits mTORC1 activity [59]. Treg have high levels of phospho-AMPK and treatment with metformin, an indirect activator of AMPK, induces the generation of Treg in a model of allergic asthma [13]. Interestingly, although Akt is downstream of PI3K and can regulate Glut1 and glycolysis [17], there is evidence that it is not essential to coordinate metabolic changes after initial activation of CD8+ T cells [60]. In this setting, Akt instead regulated the expression of chemokine and adhesion molecules important for cell migration.
In addition to the mTOR pathway, HIF1α may also play a role in upregulating Teff glucose metabolism. Although not required in the first day of stimulation [19], HIF1α has been shown to modulate the balance between Th17 and Treg by upregulating glycolytic metabolism in Th17 cells in an mTOR dependent manner [12]. In vitro, HIF1α deficiency promotes Treg generation by stabilizing the Treg transcription factor FoxP3 and directly inhibits IL-17 production [40]. In vivo, mice with HIF1α-deficient T cells have an increased number of Treg and are resistant to EAE [40]. The picture remains complex, but together it appears that glucose metabolism is required for Teff survival and function but is dispensable and perhaps inhibitory to Treg generation.
Metabolism in CD8+ memory T cells
At the end of an immune response, the majority of effector T cells die by apoptosis while a small percentage persist as memory T cells [61]. Long-lived quiescent memory cells are unlikely to have the same metabolic requirements as effector T cells during an immune response. Pathways that control the generation of memory cells are of great interest and TRAF6 was recently found to be important in the transition from the effector to memory phenotype [62]. Importantly, TRAF6-deficient cells were shown to have defects switching from a glycolytic metabolism to oxidative fatty acid metabolism, suggesting that this metabolic transition is required for memory T cells (Figure 2B). In addition, the cytokine IL-15, which is important for memory T cells, promotes both mitochondrial biogenesis and the expression of carnitine palmitoyl transferase (CPT1a), a metabolic enzyme involved in fatty acid oxidation (FAO) [63]. Retroviral expression of CPT1A in T cells increased the survival and recall response of antigen-specific CD8+ T cells in vivo. Like CD4+ Treg, memory CD8+ T cells exhibit high levels of phospho-AMPK and treatment with metformin to activate AMPK and induce fatty acid oxidation further promoted memory T cell generation in vivo [62]. Further, treatment of mice with rapamycin during T cell contraction following viral infections can enhance the formation of memory T cells and accelerate the transition from effector to memory T cells [64]. Importantly, these memory cells had a better recall response and were more protective following re-challenge with virus. Memory cells have the ability to rapidly proliferate following rechallenge and although the metabolic phenotype during this transition has not been described, it likely involves a shift back to glycolysis.
Concluding remarks
It is now clear that T cell metabolism is intimately linked to T cell survival, function and differentiation. Metabolic differences between different types of T cells may, therefore, provide a new direction for modulation of the immune response. Just as effector, regulatory and memory T cells play unique immunologic roles, they have distinct metabolic profiles to provide the proper balance of energy and biosynthetic precursors to function properly. Effector T cells require high levels of glucose to allow for rapid growth and cell division upon activation. This increase in glucose metabolism is controlled by many of the same metabolic regulators that play an important role in cancer, including PI3K/mTOR, HIF1α, Myc and ERRα. Treg and memory CD8+ T cells instead mainly utilize fatty acids for energy. Distinct programs for fuel utilization may serve additional purposes, as glucose is also used for protein glycosylation and acetyl CoA, the product of FAO, is the precursor for acetylation of protein and DNA. The promotion of FAO in Treg may modulate lipid raft formation to alter signaling pathways, lead to the breakdown of inflammatory and lipid signaling molecules, or potentially influence epigenetic modifications. These differences in metabolism may provide an opportunity to modulate the balance between effector and regulatory T cells or to inhibit autoreactive and inflammatory T cells with minimal effect on healthy lymphocytes. Targeting T cell metabolism may, therefore, provide new directions to modulate the immune response and treat an array of inflammatory diseases or to potentially impact T cell responses to infection.
Box 1. Key players in T cell metabolism and differentiation.
Myc. Myc is a proto-oncogenic transcription factor that regulates genes involved in cell metabolism proliferation, and apoptosis. Myc regulates both glucose and glutamine metabolism to generate biosynthetic precursors necessary for cell growth and proliferation [37]. Myc has recently been shown to play a role in upregulating glucose and glutamine metabolism soon after T cell activation [38].
HIF1α. Hypoxia-inducible factor 1α (HIF1α) is a transcription factor involved in the hypoxic response. HIF1α is constitutively transcribed and translated, but under normoxic conditions it is rapidly degraded. Under hypoxic conditions, the degradation of HIF1α is inhibited and it translocates to the nucleus where it upregulates glycolytic genes [39]. Importantly, HIF1α functions to direct glucose away from the mitochondria, instead upregulating glycolysis and glucose uptake to support aerobic glycolysis. HIF1α is highly expressed in Th17 and is important in the balance between Th17 and Treg differentiation [12,40].
ERRα. Estrogen related receptor alpha (ERRα) is a hormone nuclear receptor that regulates energy metabolism pathways. There is no known endogenous ligand and there is structural evidence that ERRα is constitutively active without a ligand, instead regulated by its interactions with co-activators and co-repressors including Peroxisomal Proliferator-Activated Receptor Gamma 1 α and β (PGC1α/β) [43]. In T cells, ERRα promotes mitochondrial metabolism and is important in T cell activation. Suppression of ERRα, either genetically or pharmacologically, reduces Teff generation and alleviates EAE in vivo [41].
mTOR/AMPK. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase responsible for integrating nutrient and energy status and regulating cell survival, growth and proliferation. mTOR signals through two distinct complexes, mTORC1 and mTORC2 which have distinct functions in T cell differentiation [46,47]. Inhibition of all mTOR activity prevents differentiation into Teff and promotes the generation of Treg [50]. The AMP-activated kinase (AMPK) promotes catabolic processes and can phosphorylate TSC2, which in turn inhibits mTORC1 activity [59]. Treating mice with metformin, which results in the activation of AMPK, promotes Treg generation in a model of allergic asthma [13] and promotes memory T cell generation after L. monocytogenes infection and rechallenge [62].
Acknowledgements
We thank Drs. Andrew Macintyre and Ryan Michalek for constructive comments and suggestions. National Institutes of Health Grants R01 AI063345 and R01 HL108006 (to J.C.R.); the American Asthma Foundation (J.C.R. is the Bernard Osher Fellow of the American Asthma Foundation); the Lupus Research Institute (J.C.R); and the Leukemia and Lymphoma Society (J.C.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Koppenol WH, et al. Otto Warburg's contributions to current concepts of cancer metabolism. Nature reviews. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, et al. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nature reviews. Drug discovery. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 5.Fox CJ, et al. Fuel feeds function: energy metabolism and the T-cell response. Nature reviews. Immunology. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 6.Maciver NJ, et al. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 8.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, et al. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 12.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greiner EF, et al. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. The Journal of biological chemistry. 1994;269:31484–31490. [PubMed] [Google Scholar]
- 15.Coloff JL, et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. The Journal of biological chemistry. 2011;286:5921–5933. doi: 10.1074/jbc.M110.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alves NL, et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Wieman HL, et al. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wofford JA, et al. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathmell JC, et al. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 21.Juntilla MM, et al. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostroukhova M, et al. The Role of Low-level Lactate Production in Airway Inflammation in Asthma. American journal of physiology. Lung cellular and molecular physiology. 2011 doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian L, et al. Dichloroacetate alleviates development of collagen II-induced arthritis in female DBA/1 mice. Arthritis research & therapy. 2009;11:R132. doi: 10.1186/ar2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahl DR, et al. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus. 2010;19:1492–1501. doi: 10.1177/0961203310373109. [DOI] [PubMed] [Google Scholar]
- 25.Gatza E, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Science translational medicine. 2011;3:67ra68. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gergely P, Jr., et al. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gergely P, Jr., et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blatt NB, et al. Bz-423 superoxide signals apoptosis via selective activation of JNK, Bak, and Bax. Free radical biology & medicine. 2008;45:1232–1242. doi: 10.1016/j.freeradbiomed.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal A, et al. Emerging interface between metabolic syndrome and asthma. American journal of respiratory cell and molecular biology. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 31.Delgado J, et al. Obesity and asthma. J Investig Allergol Clin Immunol. 2008;18:420–425. [PubMed] [Google Scholar]
- 32.Shears P. Epidemiology and infection in famine and disasters. Epidemiology and infection. 1991;107:241–251. doi: 10.1017/s0950268800048895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito H, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. The International journal of eating disorders. 2007;40:575–579. doi: 10.1002/eat.20417. [DOI] [PubMed] [Google Scholar]
- 34.Iyer SS, et al. Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells. J Immunol. 2012;188:77–84. doi: 10.4049/jimmunol.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends in immunology. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nature immunology. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 37.Dang CV, et al. The interplay between MYC and HIF in cancer. Nature reviews. Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 38.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer research. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 40.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CY, et al. The metabolic regulator ERRalpha, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer cell. 2011;20:500–510. doi: 10.1016/j.ccr.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends in endocrinology and metabolism: TEM. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell JD, et al. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 45.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: an update. Current opinion in hematology. 2010;17:500–504. doi: 10.1097/MOH.0b013e32833e5b2e. [DOI] [PubMed] [Google Scholar]
- 46.Young DA, Nickerson-Nutter CL. mTOR--beyond transplantation. Current opinion in pharmacology. 2005;5:418–423. doi: 10.1016/j.coph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis research & therapy. 2009;11:257. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi S, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell JD, et al. Regulation of Immune Responses by mTOR. Annual review of immunology. 2011 doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown NF, et al. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism: clinical and experimental. 2007;56:1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Sipula IJ, et al. Rapamycin-mediated inhibition of mammalian target of rapamycin in skeletal muscle cells reduces glucose utilization and increases fatty acid oxidation. Metabolism: clinical and experimental. 2006;55:1637–1644. doi: 10.1016/j.metabol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Battaglia M, et al. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 56.Haxhinasto S, et al. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of experimental medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoki K, et al. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 60.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D'Cruz LM, et al. Surviving the crash: transitioning from effector to memory CD8+ T cell. Seminars in immunology. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Windt GJ, et al. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8(+) T Cell Memory Development. Immunity. 2011 doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]