Abstract
Measles remains one of the most important causes of child morbidity and mortality worldwide with the greatest burden in the youngest children. Most acute measles deaths are due to secondary infections that result from a poorly understood measles-induced suppression of immune responses. Young children are also vulnerable to late development of subacute sclerosing panencephalitis, a progressive, uniformly fatal neurologic disease caused by persistent measles virus (MeV) infection. During acute infection, the rash marks the appearance of the adaptive immune response and CD8+ T cell-mediated clearance of infectious virus. However, after clearance of infectious virus, MeV RNA persists and can be detected in blood, respiratory secretions, urine and lymphoid tissue for many weeks to months. This prolonged period of virus clearance may help to explain measles immunosuppression and the development of lifelong immunity to re-infection, as well as occasional infection of the nervous system. Once MeV infects neurons, the virus can spread transynaptically and the envelope proteins needed to form infectious virus are unnecessary, accumulate mutations and can establish persistent infection. Identification of the immune mechanisms required for clearance of MeV RNA from multiple sites will enlighten our understanding of the development of disease due to persistent infection.
Keywords: Subacute sclerosing panencephalitis, CD8+ T cells, rhesus macaques, virus clearance, innate immunity, adaptive immunity
Introduction
Measles remains one of the most important causes of child morbidity and mortality worldwide with the greatest burden in the youngest children (Moss & Griffin, 2006; Nandy et al., 2006; Wolfson et al., 2009). Measles is unique for childhood rash diseases in that it is associated with substantial mortality with a case fatality rate of 5–10% in Africa (Grais et al., 2007; Nandy et al., 2006) and up to 25% in refugee camps and virgin populations (Moss, 2007; Shanks et al., 2011). Mortality is highest in girls and most acute measles deaths are due to secondary infections that result from a poorly understood measles-induced suppression of immune responses (Beckford et al., 1985; Garenne, 1994; Shanks et al., 2011; Tamashiro et al., 1987). In addition to the risks of acute infection, children, particularly boys, under the age of 2 years are also vulnerable to development of subacute sclerosing panencephalitis (SSPE), a progressive, uniformly fatal neurologic disease associated with persistent measles virus (MeV) infection of the nervous system. SSPE has a long latent period and presents many years after the original MeV infection (Bellini et al., 2005; Cattaneo et al., 1986; Freeman et al., 1967).
A safe and efficacious live attenuated virus vaccine is available and recent strides have been made toward global measles control. However, logistical and financial difficulties in sustaining the current vaccination strategies in developing countries have led the World Health Organization to forecast an increase in the number of measles cases and deaths (Centers for Disease Control, 2009). Furthermore, complacency and concerns about safety, along with philosophical and religious objections to vaccination, have resulted in failure to control measles in many industrialized nations (Muscat et al., 2009; Richard & Masserey Spicher, 2009).
Measles virus and virus replication
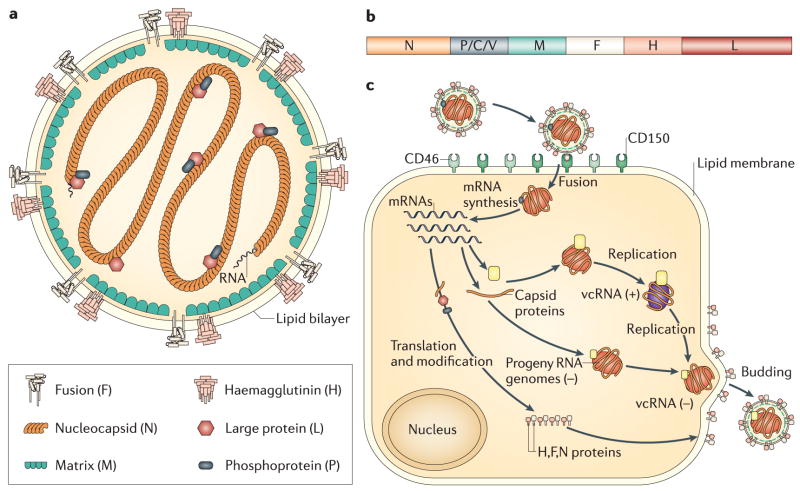
Measles virus is a negative-sense RNA virus with a non-segmented genome (Fig. 1b) and a lipid envelope that belongs to the morbillivirus genus of the family Paramyxoviridae. The 16kb genome encodes eight proteins and most likely evolved from rinderpest virus, a recently eradicated disease of cattle (Barrett, 1999; Furuse et al., 2010; Horzinek, 2011). Six proteins are found in the virion (Fig. 1a). The envelope has surface projections composed of the viral hemagglutinin (H) and fusion (F) glycoproteins with the matrix (M) protein lining the interior. The helical nucleocapsid is formed from the genomic RNA wrapped with the nucleocapsid (N) protein and is packed within the envelope in the form of a symmetrical coil with the phosphoprotein (P) and large polymerase (L) proteins attached. There are 2 nonstructural proteins, C and V, encoded within the P gene that regulate the cellular response to infection and modulate interferon (IFN) signaling (Bellini et al., 1985; Cattaneo et al., 1989). C is translated from an alternative start site by leaky scanning to produce a basic protein of 186 amino acids. V has the same N-terminus (231 amino acids) as P, but insertion of an additional guanine by RNA editing alters the reading frame to produce a unique 68 amino acid cysteine-rich zinc-binding C-terminal domain that is highly conserved amongst paramyxoviruses (Cattaneo et al., 1989; Liston & Briedis, 1994).
Figure 1.
Schematic diagrams of the measles virion (a), genome (b) and intracellular replication cycle (c). (a) The enveloped virion has 6 proteins: 2 surface glycoproteins, hemagglutinin (H) and fusion (F); a matrix (M) protein; a nucleocapsid (N) protein that surrounds the negative sense RNA and 2 replicase proteins, the phosphoprotein (P) and large (L) polymerase protein. (b) The P gene also encodes 2 host cell response regulatory proteins, V and C. (c) The H protein interacts with one of several MeV receptors resulting in F-mediated fusion with the plasma membrane. Replication occurs in the cytoplasm and assembled virions bud from the plasma membrane. (Moss & Griffin, 2006)
H interacts with the virus receptor for attachment and F interacts with H and with the same or an additional cellular protein for fusion and entry (Fig. 1c). Three receptors have been identified: membrane cofactor protein or CD46 (Dorig et al., 1993; Naniche et al., 1993), signaling lymphocyte activation molecule (SLAM) or CD150 (Tatsuo et al., 2000) and poliovirus receptor-related 4 (PVRL4) or nectin 4 (Muhlebach et al., 2011; Noyce et al., 2011). CD46 is a widely distributed human complement regulatory protein expressed on all nucleated cells (Riley-Vargas et al., 2004). It acts as a cofactor for the proteolytic inactivation of C3b/C4b by factor I (Riley-Vargas et al, 2004), but also induces proliferation and differentiation of regulatory T cells (Kemper et al., 2003). SLAM is a costimulatory molecule expressed on activated cells of the immune system (Sidorenko & Clark, 2003). The cytoplasmic domain has an immunoreceptor tyrosine-based switch motif that binds small SH-2 domain adaptor proteins important for cell signaling (Ohno et al., 2003; Yanagi et al., 2002). Nectin-4 is an adherens junction protein of the immunoglobulin superfamily expressed on epithelial cells (Shirogane et al., 2010; Sinn et al., 2002). The receptor binding regions on H are all found on the lateral surface of the head structure and are contiguous or overlapping (Colf et al, 2007; Hashiguchi et al., 2007; Hashiguchi et al., 2011; Masse et al., 2004; Santiago et al., 2002; Santiago et al., 2010; Schneider et al., 2002; Vongpunsawad et al., 2004). Both vaccine and wild type strains of MeV can use SLAM as a receptor, but wild type strains do not use CD46 efficiently (Erlenhofer et al., 2002; Ono et al., 2001; Yanagi et al., 2002). Differences in receptor usage may involve interactions with F as well as H (Kouomou & Wild, 2002; Takeuchi et al., 2002).
MeV probably uses additional receptors. In acute infections endothelial cells, as well as epithelial and immune system cells, are infected (Andres et al., 2003; Esolen et al., 1995; Oldstone et al., 2002; Takeuchi et al., 2003) and in persistent infections neurons and glial cells are important targets for infection (McQuaid & Cosby, 2002; Shingai et al., 2003). The vaccine virus was attenuated by growth in chicken cells.
H and F cooperate to induce fusion of the viral envelope and cellular plasma membrane for entry. Infected cells expressing the viral glycoproteins at the cell surface can also fuse with uninfected cells to produce multinucleated giant cells followed by cell death. However, not all types of infected cells fuse to form syncytia. In vivo, giant cells are observed in the lung, skin and lymphatic tissue, but not the central nervous system (CNS). Cellular protein synthesis is relatively unaffected by MeV infection, but specific cellular proteins (e.g. cell surface receptors) and functional responses (e.g. signal transduction, expression of transcription factors) may be altered in a cell-type-specific manner (Bazarsky et al., 1997; Fishman et al., 1997; Indoh et al., 2007).
MeV replication is interferon (IFN)-sensitive (Leopardi et al., 1992; Naniche et al., 2000) and some IFN-stimulated proteins (e.g. MxA, ADAR1) inhibit MeV replication in a cell type-specific manner (Schneider-Schaulies et al., 1994; Schnorr et al.,1993; Ward et al., 2011). However, MeV effectively inhibits both induction of IFN synthesis and IFN signaling in infected cells and this property may play an important role in the ability of MeV to establish persistent infection. The C-terminal domain of the V protein prevents induction of type I IFN synthesis both through the toll-like receptor (TLR)/MyD88 and RNA helicase pathways (He et al., 2002). V binds IKKα and inhibits TLR7/9-mediated phosphorylation of IRF7 in plasmacytoid dendritic cells (DCs) (Pfaller & Conzelmann, 2008; Schlender et al., 2005). V also binds MDA5, but not RIG-I, to prevent activation and induction of IFNβ synthesis through the RNA helicase pathway (Andrejeva et al., 2004; Childs et al., 2009). Strains of MeV differ in V sequence and transient transfection studies indicate strain-dependent differences in function (Takaki et al., 2011).
If IFN is produced by infected cells, the common N-terminal domains of the P and V proteins inhibit IFN-induced STAT1 activation (Caignard et al., 2009; Caignard et al., 2007; Palosaari et al., 2003) and the C-terminal domain of V inhibits STAT2 activation (Ramachandran & Horvath, 2010; Ramachandran et al., 2008). However, the role of type I IFN in natural MeV infection is unclear. There is little evidence that IFNα/β is induced in vivo and studies of IFN induction by MeV in vitro have been confounded by the frequent presence of defective interfering (DI) RNAs in virus stocks. DI RNAs are potent inducers of IFN and one mechanism used to establish cell lines persistently infected with MeV (Rima et al., 1977; Yount et al., 2008).
Acute disease and tissue sites of replication
MeV is efficiently spread by the respiratory route and is highly infectious. Knowledge of measles pathogenesis comes from study of naturally infected humans and experimentally infected macaques, animals that develop measles very similar to that of humans. Infection is initiated in the respiratory tract followed by rapid spread of virus to local lymphoid tissue and then to multiple other organs (Moench et al., 1988). Wild type virus replicates efficiently in activated cells of the immune system that express SLAM (Condack et al., 2007; de Swart et al., 2007; Yanagi et al., 2006) and it is likely that immature pulmonary DCs or alveolar macrophages capture and transport MeV to regional lymph nodes where the immune response is initiated and spread of infection is facilitated (Kaiserlian & Dubois, 2001; Lemon et al., 2011; Schneider-Schaulies et al., 2002).
There is a latent period of 10–14 days and a 2–3 day prodrome of fever, coryza, cough, and conjunctivitis followed by the appearance of a characteristic maculopapular rash (Lessler et al., 2009). Multiple organs (e.g. liver, lung, thymus, spleen, skin) are infected and target cells include epithelial cells, endothelial cells, B lymphocytes, T lymphocytes, monocyte/macrophages and DCs (de Swart et al., 2007; Moench et al., 1988; Plaza & Nuovo, 2005), all cells that can be replaced if eliminated by the immune response during the process of virus clearance. Neurons and glial cells are not usually targets of acute infection (McQuaid et al., 1998; Moench et al., 1988), but infected CNS endothelial cells have been observed in autopsy specimens (Esolen et al., 1995).
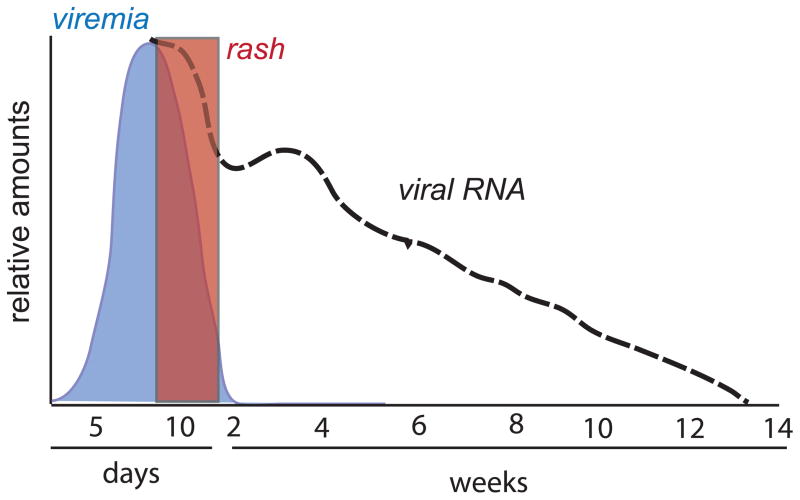
The onset of the rash coincides with the appearance of the adaptive immune response and initiation of clearance of infectious virus (Auwaerter et al., 1999). After the rash has faded, infectious virus can rarely be recovered and this correlates with decreased transmission of infection (Pan et al., 2005; Permar et al., 2001; Van Binnendijk et al., 2003). However, viral RNA persists for many weeks (Fig. 2). Mechanisms of immune-mediated clearance of infectious virus and viral RNA from different types of cells may be distinct and occur at different rates.
Figure 2.
Schematic diagram showing the time course of the clearance of infectious measles virus (blue) and viral RNA (dashed black line) from blood in relationship to the appearance and clearance of the rash (red box).
Immune response and clearance
Replication of MeV usually causes death of cells in culture, but this is not necessarily the case in vivo. Persistent non-cytopathic infection can be established In vitro and this is most easily accomplished in neuronal cells, but persistent infections in lymphoid, epithelial and glial cells have also been established (Miller & Carrigan, 1982; Rima & Duprex, 2005). Cellular factors that affect the ability of MeV to establish and maintain persistent infection include increased expression of heat shock proteins, IFN-inducible proteins and altered regulation of lipid metabolism (Miller & Carrigan, 1982; Rima & Duprex, 2005; Robinzon et al., 2009; Schnorr et al., 1993; Takahashi et al., 2007). Antisense RNA can be used to cure persistently infected cells (Koschel et al., 1995).
If the cell survives infection, virus clearance will require immune-mediated elimination of the cell or of intracellular virus. For many virus infections, factors produced by the innate immune response directly in response to virus infection (e.g. IFN-α/β, TNF, IL-1, IL-6, IL-8) inhibit virus spread and set the stage for the adaptive immune response. However, the innate response to natural measles has not been well characterized. In vitro studies have shown that innate responses triggered by interaction of MeV RNA or proteins with pathogen recognition receptors at the cell surface or in the cytoplasm to activate signaling pathways involving transcription factors NFκB and IRF 3 differ with the strain of virus, are cell type-specific and are highly regulated by the viral P, C and V proteins (Bieback et al., 2002; Duhen et al., 2010; Helin et al., 2001; Katayama et al., 2000; Sato et al., 2008; Schuhmann et al., 2011; Tenoever et al., 2002). MeV replication in vitro is sensitive to the inhibitory effects of IFNα/β. There is little evidence that type I IFN is produced in vivo during the acute phase of disease (Griffin et al., 1990; Leopardi et al., 1992; Schnorr et al., 1993; Tanabe et al., 2003; Yu et al., 2008) and this may be important for virulence as mutation of the V gene leads to virus attenuation (Devaux et al, 2011). IL-1 and IL-8 can be detected in plasma (Zilliox et al., 2007), but roles for these factors in control of MeV infection have not been identified.
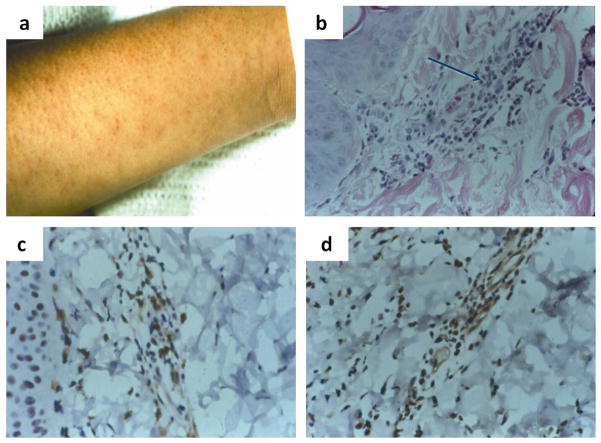
Adaptive cellular immune responses are generally regarded as most important for clearance of MeV. Children with agammaglobulinemia recover from infection while those with defects in cellular immunity (e.g. HIV infection, congenital immune deficiency, transplant immune suppression, chemotherapy, etc) are prone to develop progressive infections of the lung (giant cell pneumonia) or CNS (inclusion body encephalitis) (Albertyn et al., 2011; Enders et al., 1959; Good & Zak, 1956; McQuaid et al., 1998). MeV-specific antibody and T cell responses appear coincident with the onset of the rash and rash biopsies show infiltration of CD4+ and CD8+ T lymphocytes in regions of epithelial cell infection (Fig. 3).
Figure 3.
The measles virus rash (a) is indicative of the immune response and results from the infiltration of leukocytes (b), including CD4+ (c) and CD8+ (d) T lymphocytes into sites of virus replication in the skin. Histological examination of a biopsy of a measles skin rash lesion shows (a) an accumulation of mononuclear cells (arrow) that have infiltrated an area of infected epithelial cells (hematoxylin and eosin stain). Immunoperoxidase staining (brown) of the biopsy for CD4+ (c) and CD8+ (d) T cells shows that many of the infiltrating mononuclear cells are T lymphocytes (Polack et al, 1999).
Several lines of evidence suggest that CD8+ T lymphocytes are particularly important for control and clearance of infectious virus. MeV-specific cytotoxic T lymphocytes are found in the blood during the rash and CD4+ and CD8+ T cells infiltrate sites of virus replication (Jaye et al., 1998; Mongkolsapaya et al., 1999; Myou et al., 1993; Polack et al., 1999) (Fig. 3). In monkeys, depletion of CD8+ T cells, but not B cells, at the time of infection results in a higher and more prolonged viremia (Permar et al., 2004; Permar et al., 2003). In vitro, addition of CD8+, but not CD4+, T cells to MeV-infected B cells prevents spread to uninfected B cells (de Vries et al., 2010) and depletion of CD4+ T cells does not affect virus titers in the lungs of infected cotton rats (Pueschel et al., 2007). Both cytotoxicity and IFN-γ production have been implicated as effector mechanisms important for CD8+ T cell-mediated MeV clearance. The relative importance of each is likely to differ depending on the target cell and tissue (Finke et al., 1995; Patterson et al., 2002; Stubblefield, Sr. et al., 2011; Tishon et al., 2006). For instance, IFN-γ-induced indoleamine 2,3-dioxygenase is important for control of MeV replication in epithelial, endothelial and astroglial cells, but not in lymphoid or neuronal cells (Obojes et al., 2005).
In immunologically normal individuals, infectious virus cannot be recovered shortly after the rash fades (Fig. 2). Clearance of infectious virus and resolution of the accompanying rash are associated with clinical recovery in most children. However, clearance of infectious virus is only part of the story. Our studies of Zambian children with natural measles and of rhesus macaques experimentally infected with a wild type strain of MeV have shown that viral RNA persists in multiple locations long after infectious virus is no longer detectable (Fig. 2) (Pan et al., 2005; Permar et al., 2001; Riddell et al., 2007). In prospective studies of children hospitalized with measles, MeV RNA was detected in 62% of children from at least one site (peripheral blood mononuclear cells [PBMCs], urine or nasopharyngeal aspirates) at 1–2 months after discharge from the hospital and in 37% at 3–4 months after discharge (Permar et al., 2001; Riddell et al., 2007). These data indicate that clearance of MeV RNA after infection is a prolonged process.
Rhesus macaques infected with wild type MeV have provided additional information on clearance because they can be followed closely from the time of infection. Infectious virus appears in the blood 4–7 days after infection and is cleared by 14–18 days. However, MeV RNA can be detected in PBMCs for 4–6 months (Pan et al., 2005). Clearance of virus and viral RNA from PBMCs occurs in phases. After an initial peak of RNA coinciding with recovery of infectious virus, there is a period of rapid decline in viral RNA, followed by a rebound and then a slow decline to undetectable levels. In animals studied for longer periods of time, viral RNA may reappear in PBMCs after apparent elimination suggesting persistence in other tissues (Pan et al., 2005). The length of time required for clearance from lymphoid and other tissues is not known.
Sequencing of RNA from late samples has identified no mutations in the variable regions of either the N or H genes (Riddell et al., 2007). These data suggest slow clearance as an explanation for the prolonged presence of MeV RNA after apparent recovery rather than mutation and escape from the immune response. A switch in the type of T cell response from type 1 to type 2 with production of regulatory T cells and cytokines may play a role in slowing clearance of viral RNA (Moss et al., 2002; Ward et al., 1991; Yu et al., 2008). Prolonged presence of viral RNA is highly relevant to the development of persistent infection and could explain the immunologic abnormalities that persist after the rash fades as well as the development of life-long immunity that characterizes the recovery from measles.
Persistent infection
The frequency of failure of virus clearance from various tissues is not known, but clinically significant disease in immunologically normal individuals has only been convincingly linked to persistent infection of the CNS. Approximately 1 in 10,000 children (boys > girls) will develop SSPE as a late complication of measles (Bellini et al., 2005; Takasu et al., 2003). Both host and virus factors are likely to play a role in establishing persistence. SSPE is most likely to develop if the primary MeV infection occurs before the age of 2 years when the immune system is immature and residual maternal antibody may still be present (Bellini et al., 2005; Detels et al., 1973; Halsey et al., 1980; Jabbour et al., 1972; Miller et al., 1992; Modlin et al., 1977). In developing countries with high birth rates, measles often occurs in young infants (Grais et al., 2007; Halsey et al., 1980; Moss et al., 2008; Moss et al., 2002) and these countries appear to have a higher burden of SSPE (Saha et al., 1990; Takasu et al., 2003). This high burden is likely further exacerbated when there is a high prevalence of HIV infection because children of HIV-infected mothers are at increased risk to acquire measles at an early age (Embree et al., 1992; Moss et al., 2002) and animal models suggest that prior infection with an immunosuppressive virus increases the likelihood of persistent CNS infection (Oldstone et al., 2005).
Antibody to MeV may play a role in establishing persistent CNS infection either through alteration of the induction of the primary immune response at the time of initial infection or through modulation of infection once virus is in the nervous system (Endo et al., 2001; Fujinami & Oldstone, 1979; Rammohan et al., 1982). Passage of infected cells in the presence of antiviral antibody has been used to establish persistent infection in vitro (Rustigian, 1966). In small animals, treatment with antibody after intracerebral infection with MeV decreases acute disease, but increases the likelihood of persistent virus infection and subacute or chronic encephalitis (Liebert et al., 1990; Rammohan et al., 1981; Wear & Rapp, 1971). Cases of SSPE have been associated with passive transfer of immune globulin (Rammohan et al., 1982).
The average time to onset of SSPE after measles is 6–10 years, but ranges from 1 to 24 years (Campbell et al., 2007; Modlin et al., 1977). At the time that neurologic symptoms occur, neurons and glial cells contain nuclear and cytoplasmic MeV inclusion bodies and there is an extensive mononuclear inflammatory reaction in the CNS that includes CD4+ and CD8+ T cells, as well as monocytes and antibody-secreting B cells (Anlar et al., 2001; Brody et al., 1972; Dawson, 1934; Esiri et al., 1982; Herndon & Rubinstein, 1968). The antibody response to MeV is accentuated with significant production of MeV-specific antibody by plasma cells residing in the CNS (Burgoon et al., 2005). Thus, there is no evidence for a global defect in immune responses, but these immune responses are ineffective in clearing virus from the CNS.
Strains of MeV differ in ability to establish persistent infection in the same host cell in vitro (Fernandez-Munoz & Celma, 1992), but there is no clustering of SSPE cases to suggest that the wild type virus causing the initial infection is different from the virus causing uncomplicated disease. Sequence analysis of viral RNA from various parts of the brain shows that the virus is clonal (Baczko et al., 1993), implying that virus may have entered the brain during the original acute infection, perhaps by infecting endothelial cells (Dittmar et al., 2008; Esolen et al., 1995; Kirk et al., 1991; Ludlow et al., 2009), was not cleared and gradually spread throughout the nervous system. Once within neurons, virus can spread from neuron-to-neuron without the release of infectious particles (Ehrengruber et al., 2002) and it has been suggested that the MeV F protein interacts at the synapse with the substance P receptor neurokinin-1 to mediate trans-synaptic spread (Makhortova et al., 2007).
However, the virus that is present in cell lines persistently infected with MeV and in the CNS at the time of onset of clinically apparent SSPE differs substantially from the original wild type virus. Although viral antigen and RNA are abundant in both inclusion body encephalitis of immune compromised individuals and in SSPE, the virus is difficult, if not impossible, to culture from CNS tissue. In fact, some viruses thought to be SSPE viruses have been discovered to be laboratory contaminants (Rima et al., 1995). Variants associated with persistent infection in vitro often display properties indicative of impaired replication such as temperature-sensitivity (Rager-Zisman et al., 1984; Takahashi et al., 2007), accumulation of intranuclear and intracytoplasmic nucleocapsids and decreased release of infectious virus (Robinzon et al., 2009). Some cell lines produce no infectious virus and persistent infection is maintained by passage of encapsidated viral RNA to daughter cells during cell division (Burnstein et al., 1974).
In SSPE, no virus is seen budding from the surface of infected cells. Nuclear inclusions are filled with “smooth” nucleocapsids that lack associated RNA and P protein (Dubois-Dalcq et al., 1974; Herndon & Rubinstein, 1968). The cytoplasm contains “fuzzy” nucleocapsids of N-encapsidated RNA decorated with P that extend into neuronal processes. Thus, virus can spread within the CNS by synaptic transmission of the ribonucleoprotein from cell to cell, a process that has been observed both in vivo and in vitro (Duprex et al., 2000; Ehrengruber et al., 2002; Lawrence et al., 2000; Sawaishi et al., 1999). Limited expression of viral proteins on the surface of persistently infected cells has led to the suggestion that defects in synthesis of viral envelope proteins or processing of F may be an important determinant of persistent infection (Menna et al., 1975; Young et al., 1985). Defects in glycoprotein expression may be due in part to limited production of mRNAs for these proteins associated with steep transcriptional gradients and an increase in bicistronic messages (Cattaneo et al., 1987). However, mutations in these genes are frequent and often lead to synthesis of proteins with altered expression or function.
Frequent U to C changes suggest that mutation of viral RNA by adenosine deaminase (biased or A/I hypermutation) is occurring in persistently infected cells (Cattaneo et al., 1988; Kuhne et al., 2006; Wong et al., 1991). Failure to recover infectious virus is likely due to the mutations that accumulate in the genes for the M, F, and H envelope proteins that interfere with assembly and budding of infectious virus (Baczko et al., 1986; Cattaneo et al., 1988; Cattaneo et al., 1989; Jin et al., 2002; Roos et al., 1981). In general, expression of M protein is low (Liebert et al., 1986) due either to lack of synthesis of M or instability of the synthesized protein (Sheppard et al., 1986; Stephenson et al., 1981) and this is accompanied by low levels of antibody to M (Hall et al., 1979). In addition, defects in the M protein hinder association of N with the viral glycoproteins and facilitate persistence (Patterson et al., 2001). Studies in transgenic mice have shown that a functional M protein is not needed for virus replication and spread in the CNS (Cathomen et al., 1998; Patterson et al., 2001). Truncations, mutations, and deletions in the cytoplasmic domain of F that interfere with virus budding are almost universal (Cattaneo & Rose, 1993; Schmid et al., 1992). H proteins are often defective in intracellular transport and protein-protein interactions important for cell-cell fusion (Cattaneo & Rose, 1993). It is not known whether these mutations facilitate spread within the CNS and are necessary to establish or perpetuate CNS infection or accumulate due to lack of selective pressure to maintain envelope functions during replication in the CNS because virus spread can occur transynaptically without production of infectious virus.
Concluding remarks
The frequency of MeV RNA persistence in the absence of disease is unknown. MeV has been identified by RT-PCR or morphologic analysis in tissues from normal individuals (Haase et al., 1984; Katayama et al., 1995; Katayama et al., 1998; Schneider-Schaulies et al., 1991). In addition to SSPE, MeV antigen or RNA has been described as present and postulated to be playing an etiologic role in a large number of chronic diseases of unknown etiology (e.g. multiple sclerosis, Paget’s disease, otosclerosis, chronic active hepatitis, achalasia and Crohn’s disease) (Friedrichs et al., 2002; Haase et al., 1981; Kawashima et al., 1996; Niedermeyer et al., 2007; Wakefield et al., 1993). None of these diseases has been convincingly linked to persistent MeV infection, but a better understanding of the immune mechanisms and their regulation necessary for clearance of virus and viral RNA and of how and where the virus or viral RNA persists could help to determine if a causative role is plausible.
Acknowledgments
Work from the authors’ laboratory was funded by research grants from the National Institutes of Health (R01 AI023047) and the Bill and Melinda Gates Foundation.
Reference List
- Albertyn C, van der Plas H, Hardie D, Candy S, Tomoka T, LeePan E, Heckmann J. Silent casualties from the measles outbreak in South Africa. S Afri Med J. 2011;101:313–317. doi: 10.7196/samj.4616. [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres O, Obojes K, Kim KS, ter Meulen V, Schneider-Schaulies J. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J Gen Virol. 2003;84:1189–1197. doi: 10.1099/vir.0.18877-0. [DOI] [PubMed] [Google Scholar]
- Anlar B, Soylemezoglu F, Aysun S, Kose G, Belen D, Yalaz K. Tissue inflammatory response in subacute sclerosing panencephalitis (SSPE) J Child Neurol. 2001;16:895–900. doi: 10.1177/088307380101601206. [DOI] [PubMed] [Google Scholar]
- Auwaerter PG, Rota PA, Elkins WR, Adams RJ, DeLozier T, Shi Y, Bellini WJ, Murphy BR, Griffin DE. Measles virus infection in rhesus macaques: Altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- Baczko K, Lampe J, Liebert UG, Brinckmann U, ter Meulen V, Pardowitz I, Bucka H, Cosby SL, Isserte S, Rima BK. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993;197:188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- Baczko K, Liebert UG, Billeter M, Cattaneo R, Budka H, ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986;59:472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet Microbiol. 1999;69:3–13. doi: 10.1016/s0378-1135(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Bazarsky E, Wolfson M, Galron D, Granot Y, Argov S, Isakov N, Rager-Zisman B. Persistent measles virus infection of murine neuroblastoma cells differentially affects the expression of PKC individual isoenzymes. Virus Genes. 1997;15:227–234. doi: 10.1023/a:1007980504092. [DOI] [PubMed] [Google Scholar]
- Beckford AP, Kaschula ROC, Stephen C. Factors associated with fatal cases of measles: A retrospective autopsy study. S Afri Med J. 1985;68:858–863. [PubMed] [Google Scholar]
- Bellini WJ, Englund G, Rozenblatt S, Arnheiter H, Richardson CD. Measles virus P gene codes for two proteins. J Virol. 1985;53:908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, Shieh WJ, Rota PA. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis. 2005;192:1686–1693. doi: 10.1086/497169. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JA, Detels R, Sever JL. Measles-antibody titres in sibships of patients with subacute sclerosing panencephalitis and controls. Lancet. 1972;1:177–178. doi: 10.1016/s0140-6736(72)90572-7. [DOI] [PubMed] [Google Scholar]
- Burgoon MP, Keays KM, Owens GP, Ritchie AM, Rai PR, Cool CD, Gilden DH. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci USA. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein T, Jacobsen LB, Zeman W, Chen TT. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974;10:1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caignard G, Bourai M, Jacob Y, Tangy F, Vidalain PO. Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2. Virology. 2009;383:112–120. doi: 10.1016/j.virol.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Caignard G, Guerbois M, Labernardiere JL, Jacob Y, Jones LM, Wild F, Tangy F, Vidalain PO. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology. 2007;368:351–362. doi: 10.1016/j.virol.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Campbell H, Andrews N, Brown KE, Miller E. Review of the effect of measles vaccination on the epidemiology of SSPE. Intl J Epidemiol. 2007;36:1334–1348. doi: 10.1093/ije/dym207. [DOI] [PubMed] [Google Scholar]
- Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter MA, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Kaelin K, Baczko K, Billeter MA. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989;56:759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Rebmann G, Schmid A, Baczko K, ter Meulen V, Billeter MA. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Rose JK. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993;67:1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Billeter MA, Sheppard RD, Udem SA. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988;62:1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter MA. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Rebmann G, Baczko K, ter Meulen V, Bellini WJ, Rozenblatt S, Billeter MA. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis. Interrupted matrix protein reading frame and transcription alteration. Virology. 1986;154:97–109. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Spielhofer P, et al. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989;173:415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Global Measles Mortality, 2000–2008. MMWR. 2009;58:1321–1326. [PubMed] [Google Scholar]
- Childs KS, Andrejeva J, Randall RE, Goodbourn S. Mechanism of mda-5 Inhibition by paramyxovirus V proteins. J Virol. 2009;83:1465–1473. doi: 10.1128/JVI.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf LA, Juo ZS, Garcia KC. Structure of the measles virus hemagglutinin. Nat Struct Mol Biol. 2007;14:1227–1228. doi: 10.1038/nsmb1342. [DOI] [PubMed] [Google Scholar]
- Condack C, Grivel JC, Devaux P, Margolis L, Cattaneo R. Measles virus vaccine attenuation: suboptimal infection of lymphatic tissue and tropism alteration. J Infect Dis. 2007;196:541–549. doi: 10.1086/519689. [DOI] [PubMed] [Google Scholar]
- Dawson JR. Cellular inclusions in cerebral lesions of epidemic encephalitis. Arch Neurol Psych. 1934;31:685–700. [Google Scholar]
- de Swart RL, Ludlow M, de Witte L, Yanagi Y, van Amerongen G, McQuaid S, Yuksel S, Geijtenbeek TBH, Duprex WP, Osterhaus A. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007;3:1771–1781. doi: 10.1371/journal.ppat.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RD, Yuksel S, Osterhaus AD, de Swart RL. Specific CD8(+) T-lymphocytes control dissemination of measles virus. Eur J Immunol. 2010;40:388–395. doi: 10.1002/eji.200939949. [DOI] [PubMed] [Google Scholar]
- Detels R, Brody JA, McNew J, Edgar AH. Further epidemiological studies of subacute sclerosing panencephalitis. Lancet. 1973;2:11–14. doi: 10.1016/s0140-6736(73)91946-6. [DOI] [PubMed] [Google Scholar]
- Devaux P, Hudacek AW, Hodge G, Reyes-Del Valle J, McChesney MB, Cattaneo R. A recombinant measles virus unable to antagonize STAT1 function cannot control inflammation and is attenuated in rhesus monkeys. J Virol. 2011;85:348–356. doi: 10.1128/JVI.00802-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar S, Harms H, Runkler N, Maisner A, Kim KS, Schneider-Schaulies J. Measles virus-induced block of transendothelial migration of T lymphocytes and infection-mediated virus spread across endothelial cell barriers. J Virol. 2008;82:11273–11282. doi: 10.1128/JVI.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Coblentz JM, Pleet AB. Subacute sclerosing panencephalitis: Unusual nuclear inclusions and lengthy clinical course. Arch Neurol. 1974;31:355–363. doi: 10.1001/archneur.1974.00490420021001. [DOI] [PubMed] [Google Scholar]
- Duhen T, Herschke F, Azocar O, et al. Cellular receptors, differentiation and endocytosis requirements are key factors for type I IFN response by human epithelial, conventional and plasmacytoid dendritic infected cells by measles virus. Virus Res. 2010;152:115–125. doi: 10.1016/j.virusres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C, Rima BK. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol. 2000;74:7972–7979. doi: 10.1128/jvi.74.17.7972-7979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber MU, Ehler E, Billeter M, Naim HY. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J Virol. 2002;76:5720–5728. doi: 10.1128/JVI.76.11.5720-5728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree JE, Datta P, Stackiw W, Sekla L, Braddick M, Kreiss JK, Pamba H, Wamola I, Ndinya-Achola JO, Law BJ. Increased risk of early measles in infants of human immunodeficiency virus type 1-seropositive mothers. J Infect Dis. 1992;165:262–267. doi: 10.1093/infdis/165.2.262. [DOI] [PubMed] [Google Scholar]
- Enders JF, McCarthy K, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in cases of giant cell pneumonia without rash. N Engl J Med. 1959;261:875–881. doi: 10.1056/NEJM195910292611801. [DOI] [PubMed] [Google Scholar]
- Endo A, Izumi H, Miyashita M, Taniguchi K, Okubo O, Harada K. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. J Pediatr. 2001;138:926–928. doi: 10.1067/mpd.2001.113710. [DOI] [PubMed] [Google Scholar]
- Erlenhofer C, Duprex WP, Rima BK, ter Meulen V, Schneider-Schaulies J. Analysis of receptor (CD46, CD150) usage by measles virus. J Gen Virol. 2002;83:1431–1436. doi: 10.1099/0022-1317-83-6-1431. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Oppenheimer DR, Brownell B, Haire M. Distribution of measles antigen and immunoglobulin containing cells in the CNS in subacute sclerosing panencephalitis (SSPE) and atypical measles encephalitis. J Neurol Sci. 1982;53:29–43. doi: 10.1016/0022-510x(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Esolen LM, Takahashi K, Johnson RT, Vaisberg A, Moench TR, Wesselingh SL, Griffin DE. Brain endothelial cell infection in children with acute fatal measles. J Clin Invest. 1995;96:2478–2481. doi: 10.1172/JCI118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Munoz R, Celma ML. Measles virus from a long-term persistently infected human T lymphoblastoid cell line, in contrast to the cytocidal parental virus, establishes an immediate persistence in the original cell line. J Gen Virol. 1992;73:2195–2202. doi: 10.1099/0022-1317-73-9-2195. [DOI] [PubMed] [Google Scholar]
- Finke D, Brinckmann UG, ter Meulen V, Liebert UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman D, Wolfson M, Bazarski E, Segal S, Rager-Zisman B. The effects of measles virus persistent infection on AP-1 transcription factor binding in neuroblastoma cells. FEBS Letters. 1997;410:191–194. doi: 10.1016/s0014-5793(97)00586-3. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Magoffin RL, Lennette EH, Herndon RM. Additional evidence of the relation between subacute inclusion-body encephalitis and measles virus. Lancet. 1967;2:129–131. doi: 10.1016/s0140-6736(67)92965-0. [DOI] [PubMed] [Google Scholar]
- Friedrichs WE, Reddy SV, Bruder JM, Cundy T, Cornish J, Singer FR, Roodman GD. Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget’s disease. J Bone Miner Res. 2002;17:145–151. doi: 10.1359/jbmr.2002.17.1.145. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Oldstone MBA. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979;279:529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Furuse Y, Suzuki A, Oshitani H. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virol J. 2010;7:52. doi: 10.1186/1743-422X-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne M. Sex differences in measles mortality: a world review. Int J Epidemiol. 1994;23:632–642. doi: 10.1093/ije/23.3.632. [DOI] [PubMed] [Google Scholar]
- Good RA, Zak SJ. Disturbances in gammaglobulin synthesis as “experiments of nature”. Pediatrics. 1956;18:109–149. [PubMed] [Google Scholar]
- Grais RF, Dubray C, Seal Gerstl. Unacceptably high mortality related to measles epidemics in Niger, Nigeria, and Chad. PLoS Med. 2007;4:0122–0129. doi: 10.1371/journal.pmed.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE, Ward BJ, Jauregui E, Johnson RT, Vaisberg A. Natural killer cell activity during measles. Clin Exp Immunol. 1990;81:218–224. doi: 10.1111/j.1365-2249.1990.tb03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT, Stowring L, Ventura P, Burks J, Ebers G, Tourtellotte W, Warren K. Detection by hybridization of viral infection of the human central nervous system. Ann N Y Acad Sci. 1984;436:103–108. doi: 10.1111/j.1749-6632.1984.tb14780.x. [DOI] [PubMed] [Google Scholar]
- Haase AT, Ventura P, Gibbs CJ, Jr, Tourtellotte WW. Measles virus nucleotide sequences: Detection by hybridization in situ. Science. 1981;212:672–675. doi: 10.1126/science.7221554. [DOI] [PubMed] [Google Scholar]
- Hall WW, Lamb RA, Choppin PW. Measles and subacute sclerosing panencephalitis virus proteins: Lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci USA. 1979;76:2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey NA, Modlin JF, Jabbour JT, Dubey L, Eddins DL, Ludwig DD. Risk factors in subacute sclerosing panencephalitis: A case-control study. Am J Epidemiol. 1980;111:415–424. doi: 10.1093/oxfordjournals.aje.a112916. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T, Kajikawa M, Maita N, Takeda M, Kuroki K, Sasaki K, Kohda D, Yanagi Y, Maenaka K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci USA. 2007;104:19535–19540. doi: 10.1073/pnas.0707830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi T, Ose T, Kubota M, Maita N, Kamishikiryo J, Maenaka K, Yanagi Y. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat Struct Mol Biol. 2011;18:135–141. doi: 10.1038/nsmb.1969. [DOI] [PubMed] [Google Scholar]
- He B, Paterson RG, Stock N, Durbin JE, Durbin RK, Goodbourn S, Randall RE, Lamb RA. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology. 2002;303:15–32. doi: 10.1006/viro.2002.1738. [DOI] [PubMed] [Google Scholar]
- Helin E, Vainionpaa R, Hyypia T, Julkunen I, Matikainen S. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology. 2001;290:1–10. doi: 10.1006/viro.2001.1174. [DOI] [PubMed] [Google Scholar]
- Herndon RM, Rubinstein LJ. Light and electron microscopy observations on the development of viral particles in the inclusions of Dawson’s encephalitis (subacute sclerosing panencephalitis) Neurology. 1968;18:8–20. doi: 10.1212/wnl.18.1_part_2.008. [DOI] [PubMed] [Google Scholar]
- Horzinek MC. Rinderpest: the second viral disease eradicated. Vet Microbiol. 2011;149:295–297. doi: 10.1016/j.vetmic.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Indoh T, Yokota S, Okabayashi T, Yokosawa N, Fujii N. Suppression of NF-kappaB and AP-1 activation in monocytic cells persistently infected with measles virus. Virology. 2007;361:294–303. doi: 10.1016/j.virol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Jabbour JT, Duenas DA, Sever JL, Krebs HM, Horta-Barbosa L. Epidemiology of subacute sclerosing panencephalitis: A report of the SSPE registry. J Am Med Assoc. 1972;220:959–962. [PubMed] [Google Scholar]
- Jaye A, Magnusen AF, Sadiq AD, Corrrah T, Whittle HC. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J Clin Invest. 1998;102:1969–1977. doi: 10.1172/JCI3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Beard S, Hunjan R, Brown DW, Miller E. Characterization of measles virus strains causing SSPE: a study of 11 cases. J Neurovirol. 2002;8:335–344. doi: 10.1080/13550280290100752. [DOI] [PubMed] [Google Scholar]
- Kaiserlian D, Dubois B. Dendritic cells and viral immunity: friends or foes? Semin Immunol. 2001;13:303–310. doi: 10.1006/smim.2001.0326. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Hirano A, Wong TC. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating production of alpha/beta interferon. J Virol. 2000;74:1252–1257. doi: 10.1128/jvi.74.3.1252-1257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Hotta H, Nishimura A, Tatsuno Y, Homma M. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol. 1995;76:3201–3204. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kohso K, Nishimura A, Tatsuno Y, Homma M, Hotta H. Detection of measles virus mRNA from autopsied human tissues. J Clin Microbiol. 1998;36:299–301. doi: 10.1128/jcm.36.1.299-301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H, Miyajima T, Mori T, Yuan L, Ogihara M, Kinoue K, Takekuma K, Hoshika A. A case of intractable epilepsy positive for the detection of measles virus genome in the cerebrospinal fluid and peripheral mononuclear cells using reverse transcriptase-polymerase chain reaction. Brain Dev. 1996;18:220–223. doi: 10.1016/0387-7604(95)00154-9. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Kirk JL, Zhou AL, McQuaid S, Cosby SL, Allen IV. Cerebral endothelial cell infection by measles virus in subacute sclerosing panencephalitis: ultrastructural and in situ hybridization evidence. Neuropathol Appl Neurobiol. 1991;17:289–297. doi: 10.1111/j.1365-2990.1991.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Koschel K, Brinckmann U, Hoyningen-Huene VK. Measles virus antisense sequences specifically cure cells persistently infected with measles virus. Virology. 1995;207:168–178. doi: 10.1006/viro.1995.1063. [DOI] [PubMed] [Google Scholar]
- Kouomou DW, Wild TF. Adaptation of wild-type measles virus to tissue culture. J Virol. 2002;76:1505–1509. doi: 10.1128/JVI.76.3.1505-1509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne M, Brown DW, Jin L. Genetic variability of measles virus in acute and persistent infections. Infect Genet Evol. 2006;6:269–276. doi: 10.1016/j.meegid.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Patterson CE, Gales TL, D’Orazio JL, Vaughn MM, Rall GF. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J Virol. 2000;74:1908–1918. doi: 10.1128/jvi.74.4.1908-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon K, de Vries RD, Mesman AW, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011;7:e1001263. doi: 10.1371/journal.ppat.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi R, Hyypia T, Vainionpaa R. Effect of interferon-alpha on measles virus replication in human peripheral blood mononuclear cells. APMIS. 1992;100:125–131. doi: 10.1111/j.1699-0463.1992.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert UG, Baczko K, Budka H, ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986;67:2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Liebert UG, Schneider-Schaulies S, Baczko K, ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990;64:706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston P, Briedis DJ. Measles virus V protein binds zinc. Virology. 1994;198:399–404. doi: 10.1006/viro.1994.1050. [DOI] [PubMed] [Google Scholar]
- Ludlow M, Allen I, Schneider-Schaulies J. Systemic spread of measles virus: overcoming the epithelial and endothelial barriers. Thromb Haemost. 2009;102:1050–1056. doi: 10.1160/TH09-03-0202. [DOI] [PubMed] [Google Scholar]
- Makhortova NR, Askovich P, Patterson CE, Gechman LA, Gerard NP, Rall GF. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology. 2007;362:235–244. doi: 10.1016/j.virol.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse N, Ainouze M, Neel B, Wild TF, Buckland R, Langedijk JP. Measles virus (MV) hemagglutinin: evidence that attachment sites for MV receptors SLAM and CD46 overlap on the globular head. J Virol. 2004;78:9051–9063. doi: 10.1128/JVI.78.17.9051-9063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid S, Cosby SL. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab Invest. 2002;82:403–409. doi: 10.1038/labinvest.3780434. [DOI] [PubMed] [Google Scholar]
- McQuaid S, Cosby SL, Koffi K, Honde M, Kirk J, Lucas SB. Distribution of measles virus in the central nervous system of HIV-seropositive children. Acta Neuropathol. 1998;96:637–642. doi: 10.1007/s004010050945. [DOI] [PubMed] [Google Scholar]
- Menna HH, Collins AR, Flanagan TD. Characterization of an in vitro persistent-state measles virus infection: establishment and virological characterization of the BGM/MV cell line. Infect Immun. 1975;11:152–158. doi: 10.1128/iai.11.1.152-158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Farrington CP, Harbert K. The epidemiology of subacute sclerosing panencephalitis in England and Wales 1970–1989. Int J Epidemiol. 1992;21:998–1006. doi: 10.1093/ije/21.5.998. [DOI] [PubMed] [Google Scholar]
- Miller CA, Carrigan DR. Reversible repression and activation of measles virus infection in neuronal cells. Proc Natl Acad Sci USA. 1982;79:1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin JF, Jabbour JT, Witte JJ, Halsey NA. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing encephalitis. Pediatrics. 1977;59:505–512. [PubMed] [Google Scholar]
- Moench TR, Griffin DE, Obriecht CR, Vaisberg AJ, Johnson RT. Acute measles in patients with and without neurological involvement: Distribution of measles virus antigen and RNA. J Infect Dis. 1988;158:433–442. doi: 10.1093/infdis/158.2.433. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Jaye A, Callan MFC, Magnusen AF, McMichael AJ, Whittle HC. Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. J Virol. 1999;73:67–71. doi: 10.1128/jvi.73.1.67-71.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WJ. Measles still has a devastating impact in unvaccinated populations. PLoS Med. 2007;4:e24. doi: 10.1371/journal.pmed.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WJ, Fisher C, Scott S, Monze M, Ryon JJ, Quinn TC, Griffin DE, Cutts FT. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin Infect Dis. 2008;46:523–527. doi: 10.1086/526525. [DOI] [PubMed] [Google Scholar]
- Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WJ, Monze M, Ryon JJ, Quinn TC, Griffin DE, Cutts F. Prospective study of measles in hospitalized, human immunodeficiency virus (HIV)-infected and HIV-uninfected children in Zambia. Clin Infect Dis. 2002;35:189–196. doi: 10.1086/341248. [DOI] [PubMed] [Google Scholar]
- Moss WJ, Ryon JJ, Monze M, Griffin DE. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J Infect Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- Muhlebach MD, Mateo M, Sinn PL, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat M, Bang H, Wohlfahrt J, Glismann S, Molbak K. Measles in Europe: an epidemiological assessment. Lancet. 2009;373:383–389. doi: 10.1016/S0140-6736(08)61849-8. [DOI] [PubMed] [Google Scholar]
- Myou S, Fujimura M, Yasui M, Ueno T, Matsuda T. Bronchoalveolar lavage cell analysis in measles viral pneumonia. Eur Resp J. 1993;6:1437–1442. [PubMed] [Google Scholar]
- Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, Strebel P, Cairns L. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin Infect Dis. 2006;42:322–328. doi: 10.1086/499240. [DOI] [PubMed] [Google Scholar]
- Naniche D, Varior-Krishnan G, Cervoni F, Wild F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D, Yeh A, Eto DS, Manchester M, Friedman R, Oldstone BA. Evasion of host defenses by measles virus: Wild-type measles virus infection interferes with induction of alpha/beta interferon production. J Virol. 2000;74:7478–7484. doi: 10.1128/jvi.74.16.7478-7484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer HP, Gantumur T, Neubert WJ, Arnold W. Measles virus and otosclerosis. Adv Otorhinolaryngol. 2007;65:86–92. doi: 10.1159/000098676. [DOI] [PubMed] [Google Scholar]
- Noyce RS, Bondre DG, Lin L-T, Sisson G, Tsao MS, Richardson CD, Ha MN. Tumor cell marker PVRL4 (Nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obojes K, Andres O, Kim KS, Daubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. 2005;79:7768–7776. doi: 10.1128/JVI.79.12.7768-7776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Seki F, Ono N, Yanagi Y. Histidine at position 61 and its adjacent amino acid residues are critical for the ability of SLAM (CD150) to act as a cellular receptor for measles virus. J Gen Virol. 2003;84:2381–2388. doi: 10.1099/vir.0.19248-0. [DOI] [PubMed] [Google Scholar]
- Oldstone MBA, Dales S, Tishon A, Lewicki H, Martin L. A role for dual viral hits in causation of subacute sclerosing panencephalitis. J Exp Med. 2005;202:1185–1190. doi: 10.1084/jem.20051376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Homann D, Lewicki H, Stevenson D. One, two, or three step: measles virus receptor dance. Virology. 2002;299:162–163. doi: 10.1006/viro.2002.1507. [DOI] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa HY. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001;75:4399–4401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CH, Valsamakis A, Colella T, Nair N, Adams RJ, Polack FP, Greer CE, Perri S, Polo JM, Griffin DE. Inaugural Article: Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc Natl Acad Sci USA. 2005;102:11581–11588. doi: 10.1073/pnas.0504592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Cornu TI, Redwine J, Dales S, Lewicki HA, Holz A, Thomas D, Billeter M, Oldstone MBA. Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease. Virology. 2001;291:215–225. doi: 10.1006/viro.2001.1182. [DOI] [PubMed] [Google Scholar]
- Permar SR, Klumpp SA, Mansfield KG, et al. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J Infect Dis. 2004;190:998–1005. doi: 10.1086/422846. [DOI] [PubMed] [Google Scholar]
- Permar SR, Klumpp SA, Mansfield KG, et al. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol. 2003;77:4396–4400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183:532–538. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- Pfaller CK, Conzelmann KK. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J Virol. 2008;82:12365–12373. doi: 10.1128/JVI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza JA, Nuovo GJ. Histologic and molecular correlates of fatal measles infection in children. Diagn Mol Pathol. 2005;14:97–102. doi: 10.1097/01.pas.0000149877.70494.cf. [DOI] [PubMed] [Google Scholar]
- Polack FP, Auwaerter PG, Lee S-H, Nousari HC, Valsamakis A, Leiferman KM, Diwan A, Adams RJ, Griffin DE. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat Med. 1999;5:629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- Pueschel K, Tietz A, Carsillo M, Steward M, Niewiesk S. Measles virus-specific CD4 T-cell activity does not correlate with protection against lung infection or viral clearance. J Virol. 2007;81:8571–8578. doi: 10.1128/JVI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B, Egan JE, Kress Y, Bloom BR. Isolation of cold-sensitive mutants of measles virus from persistently infected murine neuroblastoma cells. J Virol. 1984;51:845–855. doi: 10.1128/jvi.51.3.845-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Horvath CM. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J Virol. 2010;84:11152–11163. doi: 10.1128/JVI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Parisien JP, Horvath CM. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J Virol. 2008;82:8330–8338. doi: 10.1128/JVI.00831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammohan KW, McFarland HF, McFarlin DE. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981;290:588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Rammohan KW, McFarland HF, McFarlin DE. Subacute sclerosing panencephalitis after passive immunization and natural measles infection: role of antibody in persistence of measles virus. Neurology. 1982;32:390–394. doi: 10.1212/wnl.32.4.390. [DOI] [PubMed] [Google Scholar]
- Richard JL, Masserey Spicher V. Large measles epidemic in Switzerland from 2006 to 2009: consequences for the elimination of measles in Europe. Euro Surveill. 2009;14:1–9. [PubMed] [Google Scholar]
- Riddell MA, Moss WJ, Hauer D, Monze M, Griffin D. Slow clearance of measles virus RNA after acute infection. J Clin Virol. 2007;39:312–317. doi: 10.1016/j.jcv.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Rima BK, Davidson WB, Martin SJ. The role of defective interfering particles in persistent infection of Vero cells by measles virus. J Gen Virol. 1977;35:89–97. doi: 10.1099/0022-1317-35-1-89. [DOI] [PubMed] [Google Scholar]
- Rima BK, Duprex WP. Molecular mechanisms of measles virus persistence. Virus Res. 2005;111:132–147. doi: 10.1016/j.virusres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Rima BK, Earle JAP, Yeo RP, Herlihy L, Baczko K, ter Meulen V, Carabana J, Caballero M, Celma ML, Fernandez-Munoz Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76:1173–1180. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- Robinzon S, Dafa-Berger A, Dyer MD, Paeper B, Proll SC, Teal TH, Rom S, Fishman D, Rager-Zisman B, Katze MG. Impaired cholesterol biosynthesis in a neuronal cell line persistently infected with measles virus. J Virol. 2009;83:5495–5504. doi: 10.1128/JVI.01880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos RP, Graves MC, Wollmann RL, Chilcote RR, Nixon J. Immunologic and virologic studies of measles inclusion body encephalitis in an immunosuppressed host: The relationship to subacute sclerosing panencephalitis. Neurology. 1981;31:1263–1270. doi: 10.1212/wnl.31.10.1263. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966;92:1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha V, John TJ, Mukundan P, Gnanamuthu C, Prabhakar S, Arjundas G, Sayeed ZA, Kumaresan G, Srinivas K. High incidence of subacute sclerosing panencephalitis in south India. Epidemiol Infect. 1990;104:151–156. doi: 10.1017/s0950268800054637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Bjorling E, Stehle T, Casasnovas JM. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J Biol Chem. 2002;277:32294–32301. doi: 10.1074/jbc.M202973200. [DOI] [PubMed] [Google Scholar]
- Santiago C, Celma ML, Stehle T, Casasnovas JM. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat Struct Mol Biol. 2010;17:124–129. doi: 10.1038/nsmb.1726. [DOI] [PubMed] [Google Scholar]
- Sato H, Honma R, Yoneda M, Miura R, Tsukiyama-Kohara K, Ikeda F, Seki T, Watanabe S, Kai C. Measles virus induces cell-type specific changes in gene expression. Virology. 2008;375:321–330. doi: 10.1016/j.virol.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Sawaishi Y, Yano T, Wantanabe Y, Takada G. Migratory basal ganglia lesions in subacute sclerosing panencephalitis (SSPE): clinical implications of axonal spread. J Neurol Sci. 1999;168:137–140. doi: 10.1016/s0022-510x(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Spielhofer P, Cattaneo R, Baczko K, ter Meulen V, Billeter MA. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992;188:910–915. doi: 10.1016/0042-6822(92)90552-z. [DOI] [PubMed] [Google Scholar]
- Schneider U, von Messling V, Devaux P, Cattaneo R. Efficiency of measles virus entry and dissemination through different receptors. J Virol. 2002;76:7460–7467. doi: 10.1128/JVI.76.15.7460-7467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Bieback K, Avota E, Klagge I, ter Meulen V. Regulation of gene expression in lymphocytes and antigen-presenting cells by measles virus: consequences for immunomodulation. J Mol Med. 2002;80:73–85. doi: 10.1007/s00109-001-0299-x. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Kreth HW, Hofmann G, Billeter M, ter Meulen V. Expression of measles virus RNA in peripheral blood mononuclear cells of patients with measles, SSPE, and autoimmune diseases. Virology. 1991;182:703–711. doi: 10.1016/0042-6822(91)90611-e. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Schneider-Schaulies J, Schuster A, Bayer M, Pavlovic J, ter Meulen V. Cell type-specific MxA-mediated inhibition of measles virus transcription in human brain cells. J Virol. 1994;68:6910–6917. doi: 10.1128/jvi.68.11.6910-6917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr J-J, Schneider-Schaulies S, Simon-Jodicke A, Pavlovic J, Horisberger MA, ter Meulen V. MxA-dependent inhibition of measles virus glycoprotein synthesis in a stably transfected human monocytic cell line. J Virol. 1993;67:4760–4768. doi: 10.1128/jvi.67.8.4760-4768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann KM, Pfaller CK, Conzelmann KK. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J Virol. 2011;85:3162–3171. doi: 10.1128/JVI.02342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks GD, Lee S-H, Howard A, Brunden KR. Extreme mortality after first introduction of measles virus to the Polynesian Island of Rotuma, 1911. Am J Epidemiol. 2011;173:1211–1222. doi: 10.1093/aje/kwq504. [DOI] [PubMed] [Google Scholar]
- Sheppard RD, Raine CS, Bornstein MB, Udem SA. Rapid degradation restricts measles virus matrix protein expression in a subacute sclerosing panencephalitis cell line. Proc Natl Acad Sci USA. 1986;83:7913–7917. doi: 10.1073/pnas.83.20.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai M, Ayata M, Ishida H, Matsunaga I, Katayama Y, Seya T, Tatsuo H, Yanagi Y, Ogura H. Receptor use by vesicular stomatitis virus pseudotypes with glycoproteins of defective variants of measles virus isolated from brains of patients with subacute sclerosing panencephalitis. J Gen Virol. 2003;84:2133–2143. doi: 10.1099/vir.0.19091-0. [DOI] [PubMed] [Google Scholar]
- Shirogane Y, Takeda M, Tahara M, Ikegame S, Nakamura T, Yanagi Y. Epithelial-mesenchymal transition abolishes the susceptibility of polarized epithelial cell lines to measles virus. J Biol Chem. 2010;285:20882–20890. doi: 10.1074/jbc.M110.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Williams G, Vongpunsawad S, Cattaneo R, McCray PB., Jr Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J Virol. 2002;76:2403–2409. doi: 10.1128/jvi.76.5.2403-2409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson JR, Siddell SG, ter Meulen V. Persistent and lytic infections with SSPE virus: A comparison of the synthesis of virus-specific polypeptides. J Gen Virol. 1981;57:191–197. doi: 10.1099/0022-1317-57-1-191. [DOI] [PubMed] [Google Scholar]
- Stubblefield P, Sr, Widness M, Levine AD, Patterson CE. T cell-, interleukin-12-, and gamma interferon-driven viral clearance in measles virus-infected brain tissue. J Virol. 2011;85:3664–3676. doi: 10.1128/JVI.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Watari E, Shinya E, Shimizu T, Takahashi H. Suppression of virus replication via down-modulation of mitochondrial short chain enoyl-CoA hydratase in human glioblastoma cells. Antiviral Res. 2007;75:152–158. doi: 10.1016/j.antiviral.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Takaki H, Watanabe Y, Shingai M, Oshiumi H, Matsumoto M, Seya T. Strain-to-strain difference of V protein of measles virus affects MDA5-mediated IFN-beta-inducing potential. Mol Immunol. 2011;48:497–504. doi: 10.1016/j.molimm.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Takasu T, Mgone JM, Mgone CS, et al. A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidemiol Infect. 2003;131:887–898. doi: 10.1017/s0950268803008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Miyajima N, Nagata N, Takeda M, Tashiro M. Wild-type measles virus induces large syncytium formation in primary human small airway epithelial cells by a SLAM(CD150)-independent mechanism. Virus Res. 2003;94:11–16. doi: 10.1016/s0168-1702(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Takeda M, Miyajima N, Kobune F, Tanabayashi K, Tashiro M. Recombinant wild-type and Edmonston strain measles viruses bearing heterologous H proteins: role of H protein in cell fusion and host cell specificity. J Virol. 2002;76:4891–4900. doi: 10.1128/JVI.76.10.4891-4900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro VG, Perez HH, Griffin DE. Prospective study of the magnitude and duration of changes in tuberculin reactivity during complicated and uncomplicated measles. Ped Infect Dis J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Kurita-Taniguchi M, Takeuchi K, Takeda M, Ayata M, Ogura H, Matsumoto M, Seya T. Mechanism of up-regulation of human toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem Biophys Res Commun. 2003;311:39–48. doi: 10.1016/j.bbrc.2003.09.159. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–898. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Tenoever BR, Servant MJ, Grandvaux N, Lin R, Hiscott J. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J Virol. 2002;76:3659–3669. doi: 10.1128/JVI.76.8.3659-3669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A, Lewicki H, Andaya A, McGavern D, Martin L, Oldstone MB. CD4 T cell control primary measles virus infection of the CNS: regulation is dependent on combined activity with either CD8 T cells or with B cells: CD4, CD8 or B cells alone are ineffective. Virology. 2006;347:234–245. doi: 10.1016/j.virol.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Van Binnendijk RS, van den HS, van den KH, Kohl RH, Woonink F, Berbers GA, Conyn-van Spaendonck MA, Kimman TG. Evaluation of serological and virological tests in the diagnosis of clinical and subclinical measles virus infections during an outbreak of measles in The Netherlands. J Infect Dis. 2003;188:898–903. doi: 10.1086/377103. [DOI] [PubMed] [Google Scholar]
- Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM or CD46-induced fusion and their localization on a new hemmagglutinin structural model. J Virol. 2004;78:302–313. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield AJ, Pittilo RM, Sim R, Cosby SL, Stephenson JR, Dhillon AP, Pounder RE. Evidence of persistent measles virus infection in Crohn’s disease. J Med Virol. 1993;39:345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- Ward BJ, Johnson RT, Vaisberg A, Jauregui E, Griffin DE. Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol. 1991;61:236–248. doi: 10.1016/s0090-1229(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Ward SV, George CX, Welch MJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci USA. 2011;108:331–336. doi: 10.1073/pnas.1017241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear DJ, Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971;107:1593–1598. [PubMed] [Google Scholar]
- Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol. 2009;38:192–205. doi: 10.1093/ije/dyn224. [DOI] [PubMed] [Google Scholar]
- Wong TC, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991;65:2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Ono N, Tatsuo H, Hashimoto K, Minagawa H. Measles virus receptor SLAM (CD150) Virology. 2002;299:155–161. doi: 10.1006/viro.2002.1471. [DOI] [PubMed] [Google Scholar]
- Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- Young KK, Heineke BE, Wechsler SL. M protein instability and lack of H protein processing associated with nonproductive persistent infection of HeLa cells by measles virus. Virology. 1985;143:536–545. doi: 10.1016/0042-6822(85)90392-7. [DOI] [PubMed] [Google Scholar]
- Yount JS, Gitlin L, Moran TM, Lopez CB. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai Virus defective interfering particles. J Immunol. 2008;180:4910–4918. doi: 10.4049/jimmunol.180.7.4910. [DOI] [PubMed] [Google Scholar]
- Yu XL, Cheng YM, Shi BS, et al. Measles virus infection in adults induces production of IL-10 and is associated with increased CD4+ CD25+ regulatory T cells. J Immunol. 2008;181:7356–7366. doi: 10.4049/jimmunol.181.10.7356. [DOI] [PubMed] [Google Scholar]
- Zilliox MJ, Moss WJ, Griffin DE. Gene expression changes in peripheral blood mononuclear cells during measles virus infection. Clin Vaccine Immunol. 2007;14:918–923. doi: 10.1128/CVI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]