Abstract
Background & Aims
Children with Cushing syndrome present with growth delay and excess adiposity that tends to be generalized rather than centripetal. There are no prospective studies of this phenotype as it evolves before and after treatment in children. The aims of this study were to evaluate children prior to and one-year after surgical cure compared to controls and to determine fasting insulin levels and their possible association with waist circumference and waist-height ratio, pre- and post-cure of Cushing syndrome.
Methods
30 children with Cushing syndrome were evaluated prior to and one-year post-treatment and compared to 14 age and body mass index-matched controls.
Results
Only triceps skin fold z- score showed a significant difference between patients with active Cushing syndrome and controls. A positive correlation between fasting insulin levels and waist circumference z- score was found for children with Cushing syndrome; this association persisted one-year following cure.
Conclusions
Unlike adults affected with Cushing syndrome, upper arm muscle area of children with Cushing syndrome did not differ from obese children without Cushing syndrome. The persistence of a positive correlation between waist circumference and fasting insulin despite remission of Cushing syndrome suggests that children with a history of Cushing syndrome may have an increased risk for adverse long-term effects of increased abdominal fat mass.
Clinical Trial numbers: NCT00001595, NCT00001452, NCT00005927
Keywords: Cushing, pediatric, obesity, insulin resistance, waist circumference, anthropometrics
Introduction
Children with Cushing syndrome (CS) typically present with growth delay and excess adiposity, which tends to be generalized rather than centripetal. Studies of adults with CS report a phenotype of higher abdominal and truncal fat percentages (as measured by dual-energy X-ray absorptiometry), intra-abdominal fat deposit (as measured by computed tomography) and reduced lean mass percentage compared with body mass index (BMI)- and age-comparable controls1, 2. In addition, studies with adult patients report that CS is associated with increased intra-abdominal fat stores compared to subcutaneous fat 3–5. However the effect of CS on the phenotype of children and adolescents remains largely unknown.
Glucocorticoid effects on glucose metabolism include impaired peripheral glucose uptake and hepatic insulin resistance. An increase in visceral fat is associated with insulin resistance and cardiovascular morbidity. We recently reported what appears to be an ‘East Asian’ phenotype of patients with CS, which is characterized by lower BMI and less significant abdominal obesity. In addition, compared to age-matched Caucasian patients with CS, East Asian CS patients had lower morning cortisol levels with no difference in urine free cortisol excretion (UFC)6. No prospective studies have been performed with a large group of children to determine the effect of CS on body habitus. One study reported an increase in visceral and subcutaneous fat that persisted after cure in an adolescent girl with CS 7. A prospective study of 14 children and adolescents with successfully treated (surgical) CS, reported significant alterations in body composition resulting in small but significant decrease in bone mass and an increase in visceral adiposity, suggesting that these patients are at increased risk of the metabolic syndrome 8.
Since both elevated cortisol and insulin contribute to visceral adiposity, the aim of this study was to evaluate the CS-related phenotype (i.e. anthropometric measurements) and the relationship between body fat distribution and fasting insulin levels in children with CS prior to and one-year after surgical cure compared to controls. The second aim of this study was to determine whether fasting insulin was associated with waist circumference and waist-height ratio, pre- and post-cure of CS. Our hypothesis was that children with CS may have increased insulin levels compared to age and BMI-z-score- matched controls, and that insulin levels in patients with CS may be positively associated with waist circumference even after cure of their disease.
Subjects and Methods
Thirty children (16 females, mean age 12 ± 2.8 years, range 8 to 18 years) with CS were evaluated prior to and one-year post surgical treatment: twenty-six children were diagnosed with pituitary tumors (Cushing disease- CD) and underwent transsphenoidal surgery (TSS), 4 were diagnosed with adrenal tumor(s) and underwent adrenalectomy (3 bilateral, 1 unilateral). Fourteen patients (9 females, 13.3 ± 3.6 years) referred for evaluation of CS, who had biochemical data not consistent with a diagnosis of CS, were included as controls. All children were enrolled in protocols (95CH0059, 97CH0076, and 00CH0160) approved by the institutional review board of the Eunice Kenney Shriver National Institute of Child Health and Human Development for evaluation and treatment of suspected CS (Clinical Trial numbers: NCT00001595, NCT00001452, NCT00005927). The cause of CS was confirmed by histology.
Endocrine and anthropometric assessments were done prior to and one-year post-TSS or adrenalectomy for patients in the CS treatment group. Evaluation and confirmation of CS was performed as described by Batista et al. 2007 9. Only CS patients with biochemical evidence consistent with cure were included in the final analysis. CS patients who did not complete baseline and one-year follow-up anthropometric measurements were not included; the design of the study was such that each patient served as his/her own control. Blood samples for glucose, insulin, and lipid panel were collected after an overnight fast.
A total of 64 children were referred for evaluation of CS from June 2004 to July 2007. Of the 64 children, 47 were confirmed to have CS and underwent surgery; of those 47 patients, 30 children (16 females, 11.8 ± 2.8 years) completed pre- and post- anthropometric measurements and had biochemical data consistent with cure of CS, and were included in the study. Seventeen patients with confirmed CS who either did not return for one-year follow-up (10), or due to scheduling limitations did not complete 1-year follow-up anthropometric measurements (5); or had biochemical data not consistent with a cure of CS (2), were not included in this study. Fourteen patients (9 females, 13.5 ± 3.4 years) referred for evaluation of their weight gain with biochemical data not consistent with a diagnosis of CS, were included as controls. Two patients referred for evaluation of CS that was not confirmed by biochemical testing, were diagnosed with an underlying genetic condition; they were excluded from the analysis. One patient did not complete anthropometric measurements and was not included in control group.
Height was measured to the nearest 0.1cm and weight to the nearest 0.1 kg. BMI (kilogram per meter squared) and BMI –for –age z scores were calculated from the CDC growth charts. The average of at least 3 measurements was recorded. Anthropometric measurements were obtained by one dietitian (JG) in triplicate to the nearest 0.1cm for circumference, and to the nearest 0.5mm for skin folds (using Lange calipers for skinfolds); the average of the measurements was used for analysis. Mid-upper arm circumference (MAC) and triceps skinfold (TS) were measured at the midpoint between the acromion and olecranon processes as described by Lohman et al10. TS and MAC were used to calculate upper arm muscle areas (AMA) and upper arm fat area (AFA), using the following equations: AMA= [MAC- (TS x π)]2 / (4 x π); AFA= [MAC2 / (4 x π)] – AMA11. The subscapular skinfold (SS) site was measured just inferior to the inferior angle of the scapula and was measured at a ~45 degree infero-lateral angle10. Waist circumference was measured immediately superior to the iliac crest. Hip circumference was measured at the level of maximum extension of the buttocks with subjects wearing only underwear 12.
Statistical analysis
Descriptive statistics were calculated for continuous variables. Only patients who had anthropometric measurements both pre- and post-operatively and had biochemical evidence of cure of CS were included in the analysis. Anthropometric measurement z scores (height, BMI, waist circumference, hip circumference, skin folds (subscapular and triceps), upper- arm fat and muscle area, and mid-arm circumference) were determined using anthropometric data for US children11, 13. Anthropometric and laboratory data are reported as means, standard deviations (SD), and/or z scores. Paired Student’s t-test, or its non-parametric parallel (Wilcoxon signed-rank test) were used to compare continuous data. Pearson correlation coefficients were used for correlation analysis. Data were analyzed using SPSS system software. Statistical significance was accepted for p values equal to or below 0.05.
Results
Clinical evaluations pre- and post-operatively
At baseline, CS was confirmed by biochemical testing, as described by Batista et al. 2007 9, including the confirmation of elevated UFC (385 ± 487 μg/24hr), and lack of diurnal variation in serum cortisol levels (midnight cortisol 16.6 ± 8.2 μg/dL; 8AM cortisol 20.4 ± 8.8 μg/dL). The estimated duration of CS based on clinical symptoms and/or decreased growth velocity was 2.4 ± 1 years. Patients in the control group had normal UFC (21 ± 23 μg/24hr); normal diurnal serum cortisol levels (midnight cortisol 1.4 ± 0.5 μg/dL; 8AM cortisol 10.1 ± 4.7 μg/dL). There was no significant difference between the CS and the control group of patients for age (12.8 ± 2.8 vs. 13.5 ± 3.4 yrs; p. > 0.05) or BMI standard deviation unit (SDU) (2.2 ± 0.5 vs. 1.9 ± 0.6; p> 0.05) at baseline evaluation. Physical examination revealed significant differences between children with CS and controls for the following: diastolic hypertension (30 vs. 7.6%), hirsutism (73.3 vs 28.5%), facial plethora (73.3 vs 7%), and easy bruising (16.6 vs.7%); however prevalence of systolic hypertension (26.6 vs 23%), acne (70 vs 50%), striae (66.6 vs. 50%) were not different between the two groups (Table 1).
Table 1.
Clinical features for patients with CS and controls
| Cushings | Controls | |
|---|---|---|
| Systolic hypertension | 26.6% | 23 % |
| Diastolic hypertension | 30% | 7.6% |
| Striae | 66.7% | 50% |
| Acne | 70% | 50% |
| Hirsutism | 73.3% | 28.5% |
| Facial plethora | 73.3% | 7% |
| Easy bruising | 16.6% | 7% |
At one-year follow-up of TSS or adrenalectomy, biochemical and clinical evidence cure was shown by: normal UFC (11.4 ± 11 μg/24hr), weight loss (BMI z score 2.2 ± 0.5 vs. 1.05 ± 1.1; p< 0.05), and improved linear growth (HT z score −1.1 ± 1.2 vs. −0.5 ± 1.1; p<0.05). Average interval for post-treatment evaluation was 12.5 ± 1.7 months. Eighteen of the patients who had TSS had full recovery of their adrenal function (serum cortisol response of >18 μg to ACTH) and one patient had panhypopituitarism and remained on full replacement. The patient who had unilateral adrenalectomy did not have full recovery of the other adrenal gland’s function and remained on glucocorticoid replacement, and the three patients who had bilateral adrenalectomy remained on both glucocorticoid and mineralocorticoid replacement.
Insulin, glucose, and lipids
Children with active CS had elevated fasting insulin (28 ± 11 μu/mL), LDL (130 ± 46 mg/dL), and HOMA index (7.5 ± 5), and borderline elevations of glucose (96 ± 27 mg/dL), total cholesterol (192 ± 46 mg/dL), and triglycerides (112 ± 72 mg/dL). Two patients were diagnosed with type II diabetes mellitus (T2DM) and started on insulin injections prior to surgical treatment. One- year post-surgical cure, there was a significant improvement in fasting insulin (9.5 ± 7.4 μu/mL; p<0.001), glucose (86.7 ±11 mg/dL; p<0.02), and lipid profile (total cholesterol: 139 ± 29 mg/dL (p<0001); and LDL: 86 ± 26 mg/dL (p<0.001), and HOMA index (2 ± 1.5; p<0.007) (Table 2). The two CS patients, who were diagnosed with T2DM, had normal fasting glucose off medication, at one-year follow-up.
Table 2.
Metabolic characteristics for patients with CS pre- and post treatment and in controls
| CS | 1yr after cure | Controls | |
|---|---|---|---|
| Fasting insulin (μu/mL) | 28 (± 11) | 9.5 (±7.4) | 25.1 (± 16.6) |
| Fasting glucose (mg/dL) | 103 (± 32) | 86.7 (± 11) | 97 (± 11.7) |
| Total cholesterol (mg/dL) | 192 (± 46) | 139 (± 29) | 161.5 (± 35.2) |
| HDL (mg/dL) | 54 (± 14) | 47 (±11) | 39.6 (± 8.7) |
| LDL (mg/dL) | 130 (± 46) | 86 (±25) | 120.3 (±40) |
| Triglycerides (mg/dL) | 112 (± 72) | 93.6 (± 78) | 174.6 (± 142) |
| HOMA index | 7.5 (±5) | 2 (± 1.5) | 5.9 (±4) |
Similar to the patients with active CS, children in the control group had elevated fasting insulin (25.1 ± 16.6 μu/mL), triglycerides (174.6 ± 142 mg/dL), and HOMA index (5.9 ± 4) and borderline elevation of glucose (97 ± 11.7 mg/dL) and LDL (120.3 ± 40 mg/dL). However, children in the control group had normal cholesterol (161.5 ± 35.2 mg/dL), as defined by the American Heart Association 14. (Table 2).
Anthropometric measures
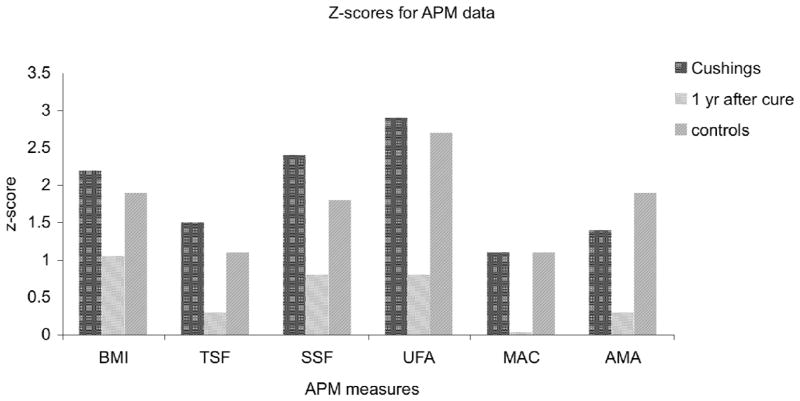
At baseline evaluation there were no significant differences between z scores of patients with active CS and controls for: BMI, hip or waist circumference (HC, WC), upper arm muscle area (AMA), upper arm fat area (AFA), waist-height ratio, subscapular skin fold (SS), mid-arm circumference (MAC); only triceps skin fold (TS) z score differed between CS and controls (1.5 ± 0.7 vs. 1.1 ± 1.2; p< 0.001). As expected, there was a significant difference in height z score between CS and control patients at baseline (p<0.002), height z score improved and at one-year follow-up there was no difference height z scores of the CS and control group (Table 3). One year following cure of CS, there was a significant decrease in z scores for: triceps skin fold (1.5 ± 0.7 vs. 0.3 ± 1; p<0.001), AFA (2.9 ±1.5 vs. 0.8 ± 1.6; p<0.001), MAC (1.1 ± 0.6 vs. −0.03 ± 1; p<0.001), AMA (1.4 ± 1.3 vs. 0.3 ± 1; p <0.001), waist circumference (WC)(1.6 ± 0.9 vs. 0.5± 0.8; p< 0.0001), and hip circumference (HC) (1.1 ± 0.8 vs. 0.2 ± 0.6; p<0.001), and waist-height ratio (0.7 ± 0.09 vs. 0.5 ± 0.14; p<0.001; (Figure 1)).
Table 3.
Anthropometric measurements
| Z-scores | Cushings | 1yr after cure | Controls |
|---|---|---|---|
| BMI | 2.2 ± 0.5 | 1.05 ± 1.1 | 1.9 ± 0.6 |
| Height | −1.1 ± 1.2 | −0.5 ± 1.1 | 0.2 ± 1.3 |
| Waist-Ht | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| Waist circumference | 1.6 ± 0.9 | 0.5 ± 0.8 | 1.2 ± 0.9 |
| Hip circ | 1.1 ± 0.8 | 0.2 ± 0.6 | 1.1 ± 1.1 |
| Triceps skin fold | 1.5 ± 0.7 | 0.3 ± 1 | 1.1 ± 1.2 |
| Subscapular skin fold | 2.4 ± 1.2 | 0.8 ± 1.3 | 1.8 ± 1.3 |
| Upper arm fat area | 2.9 ± 1.5 | 0.8 ± 1.6 | 2.7 ± 1.2 |
| Mid—arm circumference | 1.1 ± 0.6 | −0.03 ± 1 | 1.1 ± 1.2 |
| AMA (upper arm muscle area) | 1.4 ± 1.3 | 0.3 ± 1 | 1.9 ± 1.2 |
Figure 1.
z scores of anthropometric measures of body composition for patients with CS pre- and post-surgical treatment, and in controls. There was a significant decrease in body mass index (BMI), triceps skin fold (TSF), subscapular skin fold (SSF), upper arm fat area (UFA), mid-arm circumference (MAC), and upper arm muscle area AMA).
For patients with active CS, there was a positive correlation between fasting insulin levels and WC z score and waist-height ratio (r=0.8, p<0.01 for both). Even in patients, who were cured of their disease, one-year following cure there was a positive correlation for z WC and fasting insulin (r= 0.46, p<0.05). For controls, there was a modest positive correlation between fasting insulin and WC z score and waist-height ratio (r= 0.35), which, however, was not statistically significant.
Discussion
We conclude that, in children, one year after cure of CS a positive correlation between waist circumference and fasting insulin persists. This suggests that children with a history of CS may be at increased risk of a phenotype consistent with metabolic syndrome despite the lack of hypercortisolemia. These findings are consistent with other studies which report that abdominal fat percentage was significantly higher in adult patients with CS compared to age- and gender-matched controls, suggesting the crucial role of glucocorticoids in the pathogenesis of central obesity 15, 16. There is limited data regarding how well pediatric metabolic syndrome predicts adult disease due to a shortage of studies that track changes from childhood to adulthood. We recently reported the long-term instability of metabolic syndrome phenotype in children (6–12yo; mean BMI z score (1.14 =/− 1.15); 62% pre-pubertal); however, high waist circumference was the component of metabolic risks that was most prevalent at baseline and follow-up17. This is consistent with results of studies that note waist circumference is an independent predictor of insulin resistance, lipid levels, and blood pressure in children (non-CS) 18–20,
Studies of adults with CS reported a clinical phenotype of reduced lean tissue in the limbs and increased percentage of fat in the trunk compared to weight-matched controls 1, 3, 16, 21–24. Unlike adults affected with CS, upper arm muscle area of children with CS did not differ, and upper arm fat (TS) was greater than obese children without CS 24. These results are consistent with prior smaller studies, which reported a tendency towards generalized versus centripetal obesity in children compared to adults with CS. In this cohort, clinical features of children with CS differed from controls in terms of the prevalence of hypertension, hirsutism, facial plethora, and easy bruising, again, consistent with findings of prior studies 7, 9, 25, 26.
Recent studies of children with obesity show that waist-height ratio is strongly associated with adverse levels of risk factors for metabolic syndrome 27–29. Waist-height ratio is a simple anthropometric measurement that provides a strong indicator for risk associated with adult obesity. Results of the Bogalusa Heart Study by Freedman et al. 2009 30 indicated that in overweight children (BMI 85 – 94th percentile for age) waist-height ratio had the strongest association for levels of risk (odds ratio >2), and had statistically significant associations with triglycerides, insulin levels, cholesterol, and systolic blood pressure. In our study, both BMI SDU and waist-height ratio improved one-year after surgery: BMI SDU decreased to the 85– 87.4th percentile and waist-height ratio remained in the 92.5– 94.9th percentile as per Freedman et al. 2009 30. Although patients were eucorticolemic at one-year post-surgery, z-score waist circumference remained significantly correlated with fasting insulin, suggesting that these patients remain at increased risk for adverse long-term effects of increased abdominal fat mass. This is consistent with other reports suggesting that exposure to excess glucocorticoid during childhood may negatively affect the fat mass trajectory and increase the risk for an abnormal visceral adiposity in adulthood 7, 8.
The finding of persistent abdominal obesity in children after cure of CS and concern for adverse long-term effects of increased abdominal fat mass highlights the need for long-term clinical monitoring and treatment of the components of metabolic syndrome. Early identification of children who are at risk for developing long-term morbidity related to CS (i.e. cardiovascular disease, obesity, diabetes, metabolic syndrome) is important. The International Diabetes Foundation recently published a definition of “at-risk group and of metabolic syndrome in children and adolescents” which included: obesity >90th percentile as assessed by waist circumference, hyperlipidemia, hypertension, and or impaired glucose tolerance 31. Patients who exhibit risk factors for metabolic syndrome, such as BMI >95%, abnormal waist circumference z score, presence of glucose or lipid abnormalities, and/or family history (cardiovascular disease, diabetes, obesity, dyslipidemia, or metabolic syndrome), should receive ongoing counseling on diet and lifestyle changes aimed at decreasing obesity, increasing physical activity, and addressing each element of the metabolic syndrome that is present. Diet counseling should facilitate the transition to a balanced diet that is rich in fiber, fruits, and vegetables and low in simple sugars and saturated and trans fats in the setting of an energy balance that promotes both optimal growth and a reduction of BMI to below the 85th percentile on the CDC growth curves via age-specific goals. Additionally, emphasis must be placed on reducing sedentary leisure time activities and engaging the family to promote increased daily physical activity. Specific diet and lifestyle goals for various risk factors of metabolic syndrome and age ranges are outlined by the American Heart Association32.
Limitations of this study are the small number of controls and the reliance on anthropometric measurements alone for assessment of peripheral and visceral fat distribution. Anthropometric measurement is a widely available tool in the clinical setting that provides a method to assess patients without the radiation exposure and expense entailed with dexa scans that could not be clinically justified for young children and for the purposes of a research study.
In conclusion, pediatric patients with CS may continue to be at risk for adverse long-term effects of increased abdominal fat mass despite successful surgical treatment of their condition. Appropriate dietary and activity counseling should be incorporated into ongoing patient follow-up.
Acknowledgments
Statement of Authorship
MK designed and carried out the study, data analysis, and drafted the manuscript. JG participated in the design of the study, collection of data, and draft and editing of the manuscript. NG assisted with data collection, data analysis and editing of the manuscript; CAS was the principal investigator of all clinical trials used for this study, contributed to data analysis and the writing of the manuscript, and critical reviewed and edited all revisions. All authors read and approved the final manuscript.
This work was supported by NICHD, NIH intramural project Z01-HD-000642-04 to Dr. C.A. Stratakis.
We thank Donna Peterson, Sean Ivusic, and Kesa Koresko for their help with the clinical database.
Footnotes
Initial data presented as abstract at The Endocrine Society’s 88th Annual Meeting, 2006, Boston, MA
Institutional approval
All children were enrolled in protocols (95CH0059, 97CH0076, and 00CH0160) approved by the institutional review board of the Eunice Kenney Shriver National Institute of Child Health and Human Development.
Conflict of interest statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E. Body composition and metabolic features in women with adrenal incidentaloma or Cushing’s syndrome. J Clin Endocrinol Metab. 2001;86:5301–6. doi: 10.1210/jcem.86.11.8059. [DOI] [PubMed] [Google Scholar]
- 2.Schafroth U, Godang K, Ueland T, Berg JP, Bollerslev J. Leptin levels in relation to body composition and insulin concentration in patients with endogenous Cushing’s syndrome compared to controls matched for body mass index. J Endocrinol Invest. 2000;23:349–55. doi: 10.1007/BF03343737. [DOI] [PubMed] [Google Scholar]
- 3.Rebuffe-Scrive M, Krotkiewski M, Elfverson J, Bjorntorp P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67:1122–8. doi: 10.1210/jcem-67-6-1122. [DOI] [PubMed] [Google Scholar]
- 4.Mayo-Smith W, Rosenthal DI, Goodsitt MM, Klibanski A. Intravertebral fat measurement with quantitative CT in patients with Cushing disease and anorexia nervosa. Radiology. 1989;170:835–8. doi: 10.1148/radiology.170.3.2916039. [DOI] [PubMed] [Google Scholar]
- 5.Rebuffe-Scrive M. Steroid hormones and distribution of adipose tissue. Acta Med Scand Suppl. 1988;723:143–6. doi: 10.1111/j.0954-6820.1987.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao HP, Iglesias ML, Keil MF, Boikos S, Robinson-White A, Stratakis CA. Differences in cortisol levels and body mass index between East Asians and Caucasians with Cushing’s syndrome: an ‘East Asian’ phenotype for Cushing syndrome. Clin Endocrinol (Oxf) 2007;66:753–5. doi: 10.1111/j.1365-2265.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- 7.Abad V, Chrousos GP, Reynolds JC, et al. Glucocorticoid excess during adolescence leads to a major persistent deficit in bone mass and an increase in central body fat. J Bone Miner Res. 2001;16:1879–85. doi: 10.1359/jbmr.2001.16.10.1879. [DOI] [PubMed] [Google Scholar]
- 8.Leong GM, Abad V, Charmandari E, et al. Effects of child- and adolescent-onset endogenous Cushing syndrome on bone mass, body composition, and growth: a 7-year prospective study into young adulthood. J Bone Miner Res. 2007;22:110–8. doi: 10.1359/jbmr.061010. [DOI] [PubMed] [Google Scholar]
- 9.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–86. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 10.Lohman TRA, Martorel R. Anthropometric Standardization Manual. Champaign, IL: Human KInetics; 1988. [Google Scholar]
- 11.Frisancho . Anthropometric Standards for the Assessment of Growth and Nutritional Status. Ann Arbour, MI: The University of Michigan Press; 1990. [Google Scholar]
- 12.Lohman T, Roche A, Martorel R. Anthropometric Standardization Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 13.CDC. Epi Info. 2000. [Google Scholar]
- 14.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142:368–72. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 15.Kamel EG, McNeill G, Han TS, et al. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord. 1999;23:686–92. doi: 10.1038/sj.ijo.0800904. [DOI] [PubMed] [Google Scholar]
- 16.Kemink SA, Frijns JT, Hermus AR, Pieters GF, Smals AG, van Marken Lichtenbelt WD. Body composition determined by six different methods in women bilaterally adrenalectomized for treatment of Cushing’s disease. J Clin Endocrinol Metab. 1999;84:3991–9. doi: 10.1210/jcem.84.11.6143. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson JK, Yanoff LB, Easter BD, et al. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94:4828–34. doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr. 2006;149:809–16. doi: 10.1016/j.jpeds.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148:188–94. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72:490–5. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 21.Burt MG, Gibney J, Ho KK. Characterization of the metabolic phenotypes of Cushing’s syndrome and growth hormone deficiency: a study of body composition and energy metabolism. Clin Endocrinol (Oxf) 2006;64:436–43. doi: 10.1111/j.1365-2265.2006.02488.x. [DOI] [PubMed] [Google Scholar]
- 22.Lonn L, Kvist H, Ernest I, Sjostrom L. Changes in body composition and adipose tissue distribution after treatment of women with Cushing’s syndrome. Metabolism. 1994;43:1517–22. doi: 10.1016/0026-0495(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 23.Mayo-Smith W, Hayes CW, Biller BM, Klibanski A, Rosenthal H, Rosenthal DI. Body fat distribution measured with CT: correlations in healthy subjects, patients with anorexia nervosa, and patients with Cushing syndrome. Radiology. 1989;170:515–8. doi: 10.1148/radiology.170.2.2911678. [DOI] [PubMed] [Google Scholar]
- 24.Pirlich M, Biering H, Gerl H, et al. Loss of body cell mass in Cushing’s syndrome: effect of treatment. J Clin Endocrinol Metab. 2002;87:1078–84. doi: 10.1210/jcem.87.3.8321. [DOI] [PubMed] [Google Scholar]
- 25.Lodish MB, Sinaii N, Patronas N, et al. Blood pressure in pediatric patients with Cushing syndrome. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magiakou MA, Mastorakos G, Oldfield EH, et al. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994;331:629–36. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 27.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutr Clin Pract. 2008;23:397–404. doi: 10.1177/0884533608321700. [DOI] [PubMed] [Google Scholar]
- 29.Manios Y, Moschonis G, Kourlaba G, et al. Prevalence and independent predictors of insulin resistance in children from Crete, Greece: the Children Study. Diabet Med. 2008;25:65–72. doi: 10.1111/j.1464-5491.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. Risk factors and adult body mass index among overweight children: the Bogalusa Heart Study. Pediatrics. 2009;123:750–7. doi: 10.1542/peds.2008-1284. [DOI] [PubMed] [Google Scholar]
- 31.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 32.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]