Abstract
Background
At low and clinically-relevant doses, psychostimulants enhance cognitive and behavioral function dependent on the prefrontal cortex (PFC) and extended frontostriatal circuitry. These actions are observed in individuals with attention deficit hyperactivity disorder (ADHD) as well as in normal human and animal subjects. Despite the widespread use of these drugs, the sites of action involved in their cognition-enhancing and therapeutic effects are poorly understood. Indirect and/or correlative evidence suggests the cognition-enhancing/therapeutic effects of psychostimulants may involve actions directly within the PFC or extended frontostriatal circuitry. The current studies examined the degree to which methylphenidate (MPH; Ritalin®) acts within distinct frontostriatal subfields to improve PFC-dependent cognition as measured in a delayed-response test of spatial working memory.
Methods
Working memory performance was assessed following microinfusion of vehicle or varying doses of MPH (0.03-8.0 μg/500 nl) directly into the dorsomedial PFC (dorsal prelimbic and dorsal anterior cingulate cortex), the ventromedial PFC (infralimbic) and the dorsomedial striatum of rats (n=69).
Results
MPH infusion into the dorsomedial PFC, but not ventromedial PFC, elicited an inverted-U shaped facilitation of PFC-dependent cognition as measured in this task. The magnitude of this improvement was comparable to that seen with systemic administration. Additional studies demonstrated that although the dorsomedial striatum is necessary for accurate performance in this task, MPH infusion into this region did not affect working memory performance.
Conclusions
These observations provide the first definitive evidence that the PFC is a site of action in the cognition-enhancing and presumably therapeutic actions of low-dose psychostimulants.
Keywords: ADHD, Psychostimulants, Ritalin, Cognition, Striatum, Executive Function
Millions of prescriptions are written annually for psychostimulants, particularly methylphenidate (MPH; Ritalin) and amphetamine, to treat the cognitive and behavioral symptoms of attention deficit hyperactivity disorder (ADHD) (1). Extensive research demonstrates that low doses of these drugs improve cognitive and behavioral processes dependent on the prefrontal cortex (PFC)/frontal cortex in ADHD patients. These observations are consistent with structural and functional imaging evidence implicating dysregulation of the PFC/frontal cortex and extended frontostriatal circuitry in this disorder (2–5). Importantly, the cognition enhancing actions of psychostimulants are not unique to ADHD, as similar effects occur in normal humans and animals when administered at low and clinically-relevant doses (6–9). Indeed, this is evident through the increasing use of these drugs in the general population as cognitive enhancers (10). Despite their widespread use, the neural circuitry responsible for the therapeutic and cognition-enhancing actions of low-dose psychostimulants is surprisingly poorly understood.
A number of observations suggest that the cognition-enhancing/therapeutic actions of these drugs may stem from direct action within the PFC (2; 11). For example, in animals, clinically-relevant and cognition-enhancing doses of psychostimulants elevate extracellular catecholamine levels and increase responsiveness to afferent signals preferentially within the PFC (8; 12). Moreover, structural and functional imaging studies demonstrate psychostimulants reverse ADHD-associated hypofrontality (2; 13–15). However, interpretation of these observations is confounded both by their correlational nature and the fact that systemic administration of psychostimulants, as used in all of these studies, can influence PFC neuronal activity indirectly via actions in regions that project to the PFC. Combined, these observations provide intriguing, though indirect, support that the cognition-enhancing/therapeutic effects of low-dose psychostimulants involve drug action directly within the prefrontal/frontal cortex.
An additional or alternative site of action in the cognition-enhancing/therapeutic actions of psychostimulants is the striatum, a region also implicated in the neuropathology of ADHD (16). For example, a variety of observations indicate the striatum is anatomically and functionally connected with the PFC and plays a prominent role in cognitive/behavioral processes historically viewed as ‘PFC-dependent’ (17; 18). Additionally, studies in both humans and animals demonstrate that clinically-relevant doses of psychostimulants impact dopamine (DA) signaling within striatal regions (19; 20), albeit to a lesser degree than seen in the PFC (8).
To test whether the cognition-enhancing effects of psychostimulants involve direct action within the PFC and/or striatum, the current studies examined the effects of microinfusion of MPH into select PFC and striatal subfields on performance in a PFC-dependent delayed-alternation test of working memory (21). Importantly, the pharmacology of performance in this test closely aligns with the pharmacology of ADHD (8; 9; 22), in contrast with other tests of PFC-dependent cognition (23). This close alignment may reflect the fact that performance in these tasks is simultaneously dependent on a variety of cognitive and behavioral processes known to be affected in ADHD, including attention, working memory, and planning.
The medial PFC (mPFC) of rats is functionally and anatomically heterogeneous, with the dorsomedial PFC (dmPFC), encompassing the dorsal anterior cingulate (dAcg) and dorsal prelimbic subregions, implicated in higher cognitive function (21; 24). In contrast, the ventromedial PFC (vmPFC) comprised of the infralimbic and ventral prelimbic subregions, is strongly associated with autonomic, visceromotor, and affective processes (3; 24; 25). Consistent with this, the current studies demonstrate that MPH infusion into the dmPFC, but not vmPFC, improve working memory performance comparable to that seen with systemic administration.
The dorsomedial striatum (dmSTR) receives direct projections from the dmPFC. Moreover, pharmacological and lesion studies demonstrate that the dmSTR participates in higher cognitive functions typically associated with the dmPFC (26; 27). Therefore, additional studies examined the degree to which MPH acts within the dmSTR to improve working memory performance. We first identified a region of the dmSTR that receives prominent and direct projections from the dmPFC. Subsequent studies demonstrated that reversible inactivation of the dmSTR impairs performance comparable to that seen with PFC inactivation, indicating the dmSTR is necessary for performance of this task. Nonetheless, MPH infusion into this region had no effect on PFC-dependent cognition as measured in this task.
Combined, these studies provide the first demonstration that the cognition-enhancing actions of psychostimulants believed to underlie the therapeutic effects in treatment of ADHD involve direct action within the PFC.
Methods and Materials
Animals
Male Sprague-Dawley rats (260-280 grams; Charles River, Wilmington, MA) were pair-housed in clear polycarbonate cages on a 13-11 hour light/dark cycle (lights on 06:00). Animals were fed ad libitum for the first 7 days and subsequently restricted to 15-17 grams of food per day following training/testing. Training/testing was conducted between 09:00 and 16:00 hours (typically 6 days/week). Rats were weighed twice weekly to confirm animals did not lose weight and were assigned a single experimenter who handled them extensively prior to behavioral testing. All facilities and procedures were in accordance with the guidelines regarding animal use and care put forth by the National Institutes of Health of the United States and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Surgery
Following training in the T-maze (see below), rats were anesthetized with isoflurane and placed in a standard stereotaxic device with the skull flat. Indwelling stainless steel cannulae (25 ga.) were implanted bilaterally over either the dAcg/prelimbic PFC (A+3.0; L±0.8; V−0.2 mm measured from dura; Fig. 1A,C), infralimbic PFC (same coordinates as dAcg, with longer needle; Fig. 2A,B), or dmSTR (A+0.45; L±2.0; V−3.2 mm; Fig. 3B) and secured to the skull with stainless steel screws and dental acrylic (Plastics One, Roanoke,VA). Stainless steel stylets prevented occlusion of the cannulae, and were replaced as needed to maintain patency.
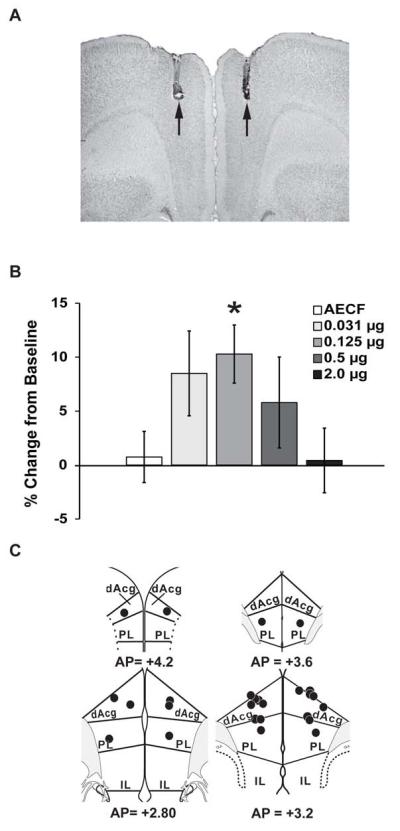
Figure 1.
(A) Representative photomicrograph depicting an infusion site into dorsomedial prefrontal cortex (dmPFC). Note minimal damage to dorsal areas. (B) Infusion of MPH into the dmPFC improved working memory performance in an inverted-U dose-dependent manner, with 0.125 μg/hemisphere producing a maximal improvement. (C) Schematic diagram indicating all 0.125 μg infusion sites into the dmPFC. dAcg, dorsal anterior cingulate; PL, prelimbic; IL, infralimbic. Numbers represent AP level (52). *P < 0.05 relative to vehicle treatment.
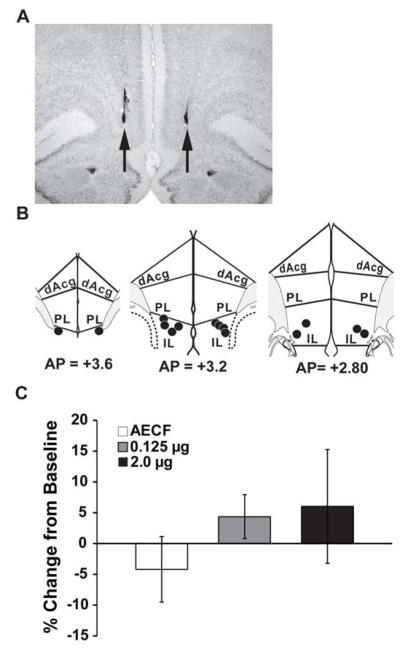
Figure 2.
(A) Representative photomicrograph indicating site of MPH infusion into the infralimbic subregion of the mPFC. (B) Schematic of 0.125μg MPH infralimbic infusion sites. (C) Infusion of MPH into the IL PFC had no significant effect on working memory performance measured as the percent change from baseline (mean ± SEM). mPFC, medial prefrontal cortex; dAcg, dorsal anterior cingulate; PL, prelimbic; IL, infralimbic. Numbers represent AP level (52).
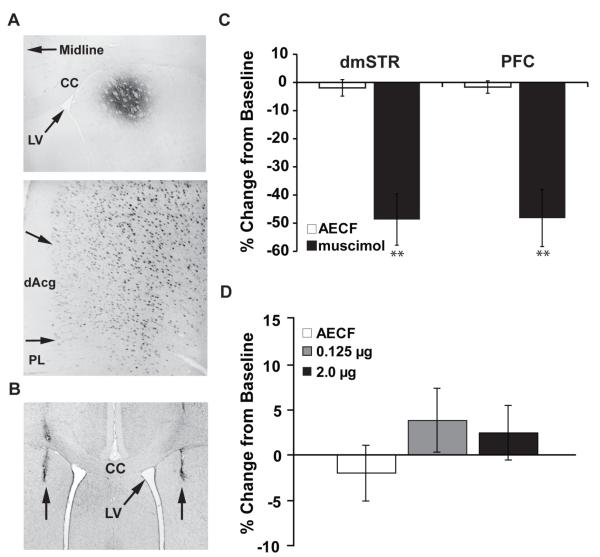
Figure 3.
(A) Fluorogold retrograde tracer infused into dmSTR (top panel) results in strong neuronal labeling of dorsomedial PFC (Top Panel, 40x; Bottom Panel, 200x; A+2.7). (B) Representative photomicrograph depicting muscimol/MPH dmSTR infusion sites. (C) Intra-dmSTR muscimol significant impaired working memory performance as measured by the percent change from baseline (mean ± SEM). The magnitude of this impairment is comparable to that seen with PFC inactivation, indicating a critical role of the dmSTR in working memory performance. (D) Intra-dmSTR infusion of MPH had no significant effect on working memory performance as measured by the percent change from baseline (mean ± SEM). dmSTR, dorsomedial striatum; dAcg, dorsal anterior cingulate; PL, prelimbic; IL, infralimbic. **P < 0.01 relative to vehicle (AECF) treatment.
Great care was taken to maintain the structural integrity of the mPFC in these experiments. Given the anatomical and behavioral evidence implicating the dorsal mPFC (dAcg, dorsal prelimbic PFC) in higher cognitive/behavioral processes (21; 24), cannulae were only lowered 200 μm below dura to avoid cannula-related damage to this region.
Behavioral Training and Testing
Training and testing were similar to that described previously (8). Black plastic sheeting surrounded the maze to obscure any external spatial cues. Animals were trained to enter the maze arm not chosen on the previous trial to gain food reward (1 chocolate chip/trial; 20 trials per session, 1 session per day). Between trials, rats were placed in the start box located at base of the T and prevented from exiting by a removable acrylic glass gate during the delay interval. Following surgery, rats resumed testing in the T-maze until performance reached pre-surgery levels. Delay intervals were used that resulted in 65%-80% accurate performance. Stable performance was defined as 2 consecutive days of 65-80% accuracy in which performance did not differ by more than 10%. Accuracy in performance improves gradually with testing at a given delay, thus it was necessary to extend the delay interval over several weeks of testing. For these studies, delay intervals ranged between 3 and 80 seconds (mean = 21 seconds). Performance at the same delay was further tested on the first two days following infusion to confirm stable performance. No consistent effects of any of the treatments were observed in the days following treatment. Thus, when a difference between the pre-infusion average and post-infusion average was greater than 10%, the animal was considered unstable and that treatment data point excluded.
All treatments were separated by at least two days. Before receiving a drug treatment, rats were given two mock infusions, consisting of an initial needle insertion followed by a vehicle infusion 48 hours later. This permitted animals to acclimate to the mild restraint associated with the infusions, as well as minimize detrimental behavioral effects of tissue damage related to needle insertion.
Drug Infusion
Methylphenidate HCl (Sigma, St. Louis, MO; (0.035, 0.125, 0.5, 2.0, and 8.0 μg/500 nl)) and the GABAA agonist muscimol (Sigma, St. Louis, MO; 75 ng/500 nl) were dissolved in AECF (147 mmol/L NaCl, 1.3 mmol/L CaCl2, 0.9 mmol/L MgCl2, 2.5 mmol/L KCl; pH=7.4). 0.5 μl infusions of drug or AECF were made bilaterally through 33 gauge needles that projected below the guide cannulae by 1.6 mm for dAcg, 2.5 mm for prelimbic (See Fig. 1C), 4 mm for infralimbic (see Fig. 2B), and 3.5 mm for dmSTR infusions (See Fig. 3B). Infusions were performed using a microprocessor pump (Harvard Apparatus, South Natick, MA), set at a rate of 250 nl/minute for 2 minutes. Needles remained in tissue for 2 minutes following infusions after which the stylets were replaced. Rats were placed in their home cage for an additional 15 minutes before testing.
Histological Analyses of Drug Infusion Sites and Data Selection
At the end of testing, rats were deeply anesthetized with isoflurane and transcardially perfused with 10% w/v formaldehyde. Brains were stored in formaldehyde for a minimum of 24 hours prior to sectioning. Placement of injectors was verified in 40 μm-thick coronal sections stained with Neutral Red dye. Data from a given experiment were included only when histological analyses verified accurate placement of injectors and minimal tissue damage.
Striatal Retrograde Tracer Infusion
In a limited number of animals (n=4), the retrograde tracer Fluorgold (FG; Fluorochrome, Denver, CO) was infused into the dmSTR (see Surgery above) or dorsolateral STR (+0.45A, ±3.8L, −3.5V), using glass pipettes, as described previously (28). For these infusions, single-barrel glass micropipettes (15-25 μm diameter; Friedrich and Dimmock Inc. Millville, NJ) were filled with 2.0 % FG solution (dissolved in saline) as previously described. Once the infusion pipette was in position, FG was iontophoresed (5.0 μA, 15-minutes, 5-second pulses, 50% duty cycle) with the pipette remaining in place for an additional 10-minutes. Animals were sacrificed 7 days following FG infusions. FG was visualized using immunohistochemical procedures as described previously (28). Briefly, animals were perfused with 4% paraformaldehyde, 40 μm sections were collected through the PFC and later incubated for 48 hours at 4° with rabbit anti-FG antibody (1:2,000; Chemicon International, Temecula, CA; cat# AB153) diluted in 0.01 M PBS-TX. After incubation, tissue was rinsed with 0.01 M PBS-TX, and incubated with donkey-anti-rabbit antibody (1:500; Jackson ImmunoResearch, West Grove, PA) for 90-minutes. Tissue was then rinsed with 0.01 M PBS-TX, exposed to rabbit PAP (1:500; Dako Corporation, Carpinteria, CA) for 90-minutes and rinsed with 0.01 M PBS-TX. Sections were reacted with diaminobenzidene (DAB; Vector Laboratories) to yield a brown precipitate.
Statistical Analyses
Given the number of infusions/animal was limited to four, it was not possible that every animal receive every dose of MPH. Thus, the effect of intra-PFC infusions on performance (change in % accuracy compared to baseline) was statistically analyzed using a between-subjects 1-way ANOVA. Post-hoc analyses were conducted comparing each dose to vehicle using one-way Dunnett’s t-tests. In addition, given systemically-administered psychostimulants improve working memory performance following a non-monotonic dose-response curve (inverted-U) a planned comparison tested the fit of data to a quadratic trend. For muscimol studies and 8.0 μg MPH vs. AECF, independent T-tests were used for analysis.
RESULTS
Cognitive Effects of Intra-PFC MPH Infusion
Given the likely involvement of rat dAcg in working memory performance (21), we avoided placement of guide cannulae directly into this dorsal-most region of the rat mPFC and utilized small gauge (33ga) infusion needles. As shown in Figure 1, this approach resulted in minimal damage to the mPFC. Vehicle infusions had minimal, non-significant effects on working memory performance (Figure 1; 0.75% ± 2.37% change from baseline, n=13). In contrast, direct infusion of MPH (500 nl) into the dmPFC resulted in an inverted-U-shaped dose-dependent improvement in working memory (Figure 1; F(1,49)=8.36, p<0.01 for quadratic fit of data). MPH maximally improved accuracy by 10.3% ± 2.7% at the 0.125 μg/hemisphere dose (p=0.04; n=14). Both a four-fold lower dose (0.031 μg/hemisphere, n=7) and a four-fold higher dose (0.5 μg/hemisphere, n=10) of MPH produced a non-significant trend for improvement (8.5% ± 3.9%, p=0.17; 5.8% ± 4.2%, p=0.33, respectively), while the highest dose (2.0 μg/hemisphere, n=10) had no distinguishable effects on performance relative to vehicle treatment (0.42% ± 3.0%, p=0.84).
When administered systemically, MPH impairs working memory performance at a dose 4-fold higher than one that elicits maximal cognition enhancement. Thus, it was surprising that when infused into the dmPFC at concentrations that were 4-fold higher (0.5 μg/hemisphere) and 16-fold higher (2.0 μg/hemisphere) MPH lacked cognition-impairing actions. Therefore, in a limited subset of subjects (n=6) we examined the effects of a 64-fold higher dose (8.0 μg/hemisphere) on delayed response performance. Performance of animals treated with this highest dose did not differ significantly from vehicle treatment (5.7% ± 4.8%, t(17)=1.05, p=0.16).
To assess whether the cognition-enhancing actions of MPH are limited to the dmPFC, additional studies examined the working memory effects of MPH when infused into the infralimbic subfield of the vmPFC. In contrast to that seen with more dorsally placed infusions, infusion of MPH into the vmPFC did not substantially alter performance at any dose examined (See Figure 2; vehicle (n=4) = −4.2 ± 5.3% change from baseline; 0.125 μg (n=7) = 4.4 ± 3.6%, p=0.46; 2.0 μg (n=4) = 6.0 ± 9.2%, p=0.43).
Cognitive Effects of Intra-Striatal MPH Infusion
Additional studies examined the involvement of the dmSTR in working memory performance. We initially identified a region of the dmSTR that receives prominent projections from the dmPFC using iontophoretic infusions of the retrograde tracer, Fluorogold (Fluorochrome, Denver, CO). As shown in Figure 3A, and consistent with prior observations (3), placement of this tracer into the dmSTR resulted in robust labeling of neurons in the dmPFC but not the vmPFC. In contrast, retrograde tracer placement within the dorsolateral striatum resulted in minimal retrograde labeling within the dmPFC and more robust labeling within the vmPFC (data not shown). Subsequent intra-tissue infusions targeted the region of the dmSTR identified in these retrograde tracing studies that receives a prominent projection from the dmPFC.
Although the dmSTR is implicated in higher cognitive function, the degree to which this region contributes to performance in tests of working memory is unknown. Therefore, we next assessed the degree to which temporary inactivation of the dmSTR affects working memory performance using intra-dmSTR infusions of the GABA agonist, muscimol (75 ng/hemisphere). Temporary inactivation of the dmSTR profoundly impaired performance in this test (Figure 3C). Indeed, as shown in Figure 3C, the magnitude of this impairment was comparable to that seen with mPFC inactivation (dmSTR, vehicle (n=13) = −1.9 ± 2.9% change from baseline; muscimol (n=8) = −48.7 ± 9.1%, t(19)=5.87, p<0.001: PFC, vehicle (n=17) = −1.66% ± 2.21%; muscimol (n=6) = −48.2% ± 10.1%, t(21)=6.78, p< 0.001).
Finally, additional studies examined whether MPH acts within the dmSTR to improve working memory performance (Figure 3D). For these studies, vehicle (n=13), 0.125 μg MPH (n=6) and 2.0 μg MPH (n=4) were infused into the dmSTR prior to testing. In contrast to that seen in the dmPFC, neither dose of MPH infused into the dmSTR infusion affected working memory performance (vehicle; −1.94% ± 2.92% change from baseline; 0.125 = 3.83% ± 3.46%, p=0.20; 2.0 = 2.47% ± 2.98%, p=0.35).
DISCUSSION
Despite the widespread use of psychostimulants as cognitive enhancers, surprisingly little is known about the neural circuitry involved in their cognitive/therapeutic actions. Although systemic administration of low-dose psychostimulants improves frontostriatal function, whether this reflects direct or indirect actions in the PFC or striatum is unclear. The current studies provide unambiguous evidence that psychostimulant action within the PFC is sufficient to promote higher cognitive function as assessed in a delayed-response test of working memory. Moreover, the magnitude of the cognition-enhancing effect of intra-PFC MPH was virtually identical to that seen with systemic administration of clinically-relevant doses of this drug (8; 12; 29). In contrast, while our results demonstrate the dmSTR is necessary for performance in this test of working memory, MPH infusion into the dmSTR failed to affect performance. These observations indicate a prominent role of select PFC/frontal cortex subfields in the cognition-enhancing and therapeutic actions of psychostimulants and other drugs used in the treatment of ADHD. Additionally, these results are consistent with previous research indicating a prominent role of the PFC/frontal cortex in the pathophysiology of ADHD.
Site of Action within the mPFC
The rat PFC is heterogeneous, with differing subfields associated with distinct cognitive, behavioral, affective, and physiological functions (21). It has been posited that there exists a dorsal/ventral divide within the rat mPFC, with the dorsal aspects consisting of the dAcg and prelimbic subfields linked to ‘cognitive’ functions, while the ventrally-situated IL subregion is anatomically and functionally associated with processing of visceral, autonomic and affective information (3; 25; 30). The current results are consistent with this proposed functional subdivision of the mPFC: MPH improved working memory performance when infused into the dorsal, but not ventral, mPFC. Additionally, the ability of MPH to improve working memory performance when infused into the dmPFC is consistent with prior lesion studies that implicate this region in egocentric-based motor memory and the temporal sequencing of behavior (21). The current studies utilized an infusion volume that provides for consistent behavioral effects and is typical of studies that examine the cognitive/behavioral effects of catecholamine-related drugs in rats. However, this volume likely precludes making strong conclusions regarding the degree to which an infusion selectively influences the dAcg vs. dorsal prelimbic subfields of the mPFC. Future mapping studies using smaller infusion volume are needed to address this issue.
These observations are also consistent with functional imaging studies that indicate a dysregulation of the dorsolateral PFC and anterior cingulate in ADHD (4; 31; 32). Limited evidence suggests the rat dmPFC is functionally homologous to both of these regions in the human/primate (33), Thus, the current results cannot necessarily be extrapolated to identify the degree to which the cognition-enhancing/therapeutic actions of psychostimulants in humans involve actions within the dorsolateral PFC vs. anterior cingulate. Further studies in non-human primates will need to determine the relative role of the anterior cingulate and PFC in the cognition-enhancing actions of psychostimulants.
A variety of cognitive processes affected in ADHD are dependent on the PFC, including working memory, sustained attention, impulsivity, and planning (34). The current studies utilized a well-characterized task of PFC-dependent function that requires a variety of cognitive and motivational processes, including working memory, attention, motivation and response outcome evaluation (8; 12; 29). Given the pharmacology of performance in this task is closely aligned with the pharmacology of ADHD, our current results likely extend to the therapeutic actions of psychostimulants (7; 8). This close alignment between the pharmacology of working memory performance and ADHD contrasts with that seen in other tests used to assess PFC-dependent function in animals, including sustained attention and attentional set-shifting (23). Nonetheless, it will be of interest for future studies to examine the actions of intra-PFC infusions of MPH in additional tests of cognitive processes known to be affected in ADHD.
Both the magnitude and general inverted-U shaped dose-dependent actions of intra-PFC MPH-induced improvement in working memory performance are comparable to that seen with systemic administration of clinically-relevant doses of MPH (8; 12; 29). However, while systemically administered MPH impairs working memory performance when administered at a dose 4-fold higher than an optimally-improving dose (12), intra-PFC infusion of MPH at doses 16-fold (2 μg/hemisphere) and 64-fold (8 μg/hemisphere) greater than the maximally-facilitating dose (0.125 μg/hemisphere) failed to hinder performance. These observations indicate that although psychostimulants act within the dmPFC to facilitate PFC-dependent cognition, the impairment of PFC-dependent function associated with higher doses of these drugs likely involves either actions outside the dmPFC or vmPFC or requires concurrent activity in these medial PFC subfields and regions outside the PFC. The hippocampus, medial-dorsal thalamus, and striatum are all implicated in higher cognitive processing (30), including working memory (35; 37). Thus, these regions would be of particular interest in future studies exploring the neurocircuitry underlying the cognition-impairing actions of higher doses of psychostimulants.
Potential Receptor Mechanisms
Catecholamines act within the PFC to facilitate performance in tests of working memory in an inverted-U shaped manner (36; 38). In the case of DA, this involves inverted-U shaped modulatory actions of DA D1 receptors (39) For norepinephrine (NE), activation of postsynaptic α2-receptors promotes, while activation of α1-receptors impairs, working memory performance (40; 41). α2-receptors possess a higher affinity for NE than α1-receptors (42). Thus, it has been proposed that under conditions of moderate rates of NE release PFC α2-receptor activation predominates, facilitating PFC-dependent function, whereas at higher rates of NE release (e.g. stress) α1-receptors are engaged, impairing PFC-dependent function (42)
Consistent with these behavioral observations, electrophysiological evidence suggests optimal catecholamine modulation is needed for proper PFC neuronal signal processing. For example, in monkeys, PFC α2-and D1 receptors act in concert to produce optimal spatial tuning of delay-related neurons (39; 43). In contrast, stimulation of PFC α1 receptors reduces neuronal responsiveness and decreases spatial tuning (44). Combined, these observations suggest that there is an ideal level of both NE and DA signaling within the PFC that supports optimal signal processing abilities of PFC neurons and PFC-dependent behavior.
Microdialysis studies demonstrate that low and clinically relevant doses of psychostimulants preferentially elevate extracellular NE and DA within the PFC (8). When combined with the current results, this information indicates that cognition-enhancing actions of low-dose psychostimulants likely involve α2 and/or D1 receptor activation within the PFC. Consistent with this, the cognition-enhancing effects of systemic MPH are prevented by pretreatment with either an α2 or D1 receptor antagonist (29).
Striatal Involvement in ‘PFC-Dependent’ Function
As reviewed above, evidence suggests that the striatum may be critically involved in both the pathophysiology of ADHD and the therapeutic/cognition-enhancing actions of psychostimulants. Of particular relevance to this discussion, frontostriatal projections are topographically organized, with the dmSTR of the rat receiving direct projections from the dmPFC (27). Additionally, lesion and pharmacological studies suggest the dmSTR acts in concert with the dmPFC to support flexible, goal-directed behavior (18; 26). Thus, the current studies examined the degree to which the dmSTR is involved in working memory performance. We observed that inactivation of the dmSTR profoundly impaired performance of spatial delayed alternation, providing the first demonstration that the dmSTR is critically involved in performance of this task. Nonetheless, infusion of MPH into the dmSTR had no noticeable effect on performance in this task, indicating that this region does not play a prominent role in the cognition-enhancing or impairing actions of psychostimulants.
The ventromedial striatum (vmSTR), particularly the nucleus accumbens, has been implicated in ADHD as well as the regulation of impulsivity (45–48). Functional imaging studies indicate that MPH-induced improvement in certain cognitive/behavioral tasks is associated with alterations in vmSTR activity (15; 49). Additionally, limited observations also indicate the vmSTR is involved in the performance of delayed response tasks (50; 51). Indeed, MPH-induced changes in DA receptor occupancy in the ventral striatum predict the magnitude of improvement in a spatial working memory task (19). However, it should be noted that although it is not possible to image DA receptor/transporter occupancy in the PFC with current methodology, available evidence indicates there is likely a similar or stronger association between MPH-induced changes in DA/NE receptor occupancy within the PFC and changes in cognition (8). Collectively, these observations indicate that vmSTR function may contribute to the cognitive/therapeutic effects low-dose psychostimulants. However, whether these effects involve direct or indirect actions of psychostimulants on the vmSTR remains to be determined. Future studies will address whether the cognition-enhancing effects of MPH involve actions within the vmSTR.
Conclusion
These results provide the first direct evidence that psychostimulants act within the PFC, but not the dmSTR, to improve PFC-dependent higher cognitive function, an action closely associated with the effective treatment of ADHD. The ability of intra-PFC psychostimulants to improve cognitive function was regionally selective, with infusions into the dorsal, but not the ventral, medial PFC improving PFC-dependent cognition. While the receptor mechanisms that support the cognition-enhancing actions of psychostimulants within the PFC remain to be definitively determined, previous research indicates a likely role of NE α2 and/or DA D1 receptors. The fact that the PFC is a site of action in the cognition-enhancing actions of psychostimulants is consistent with the posited role of the PFC in the etiology of ADHD and suggests that selective targeting of the PFC may be of particular benefit in the treatment of ADHD and other conditions associated with PFC dysfunction.
Acknowledgements
This work was supported by PHS grants, MH081843, DA000389, and MH08138, the Wisconsin Institutes of Discovery and the University of Wisconsin Graduate School.
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Greenhill LL. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; New York: 2001. Clinical effects of stimulant medication in ADHD; pp. 31–71. [Google Scholar]
- 2.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 4.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 5.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta MA, Sahakian BJ, Robbins TW. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; New York: 2001. Comparative psycholpharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals; pp. 303–331. [Google Scholar]
- 7.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Gamo NJ, Wang M, Arnsten AFT. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J. Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 12.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci U.S.A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 21.Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- 22.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e101–111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, et al. Differential Sensitivity to Psychostimulants Across Prefrontal Cognitive Tasks: Differential Involvement of Noradrenergic α(1)- and α(2)-Receptors. Biol. Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.07.022. epublished ahead of print, doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Re. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 26.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann NY Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 27.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.España RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 29.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 33.Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- 34.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J. Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 35.Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann NY Acad Sci. 1999;877:711–716. doi: 10.1111/j.1749-6632.1999.tb09308.x. [DOI] [PubMed] [Google Scholar]
- 36.Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G-W, Cai J-X. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 40.Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 41.Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 42.Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 45.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang G-J, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang G-J, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating Dopamine Reward Pathway in ADHD. J Am Med Assoc. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 49.Dodds CM, Muller U, Clark L, van LA, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mair RG, Koch JK, Newman JB, Howard JR, Burk JA. A double dissociation within striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J Neurosci. 2002;22:6756–6765. doi: 10.1523/JNEUROSCI.22-15-06756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–468. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]