Abstract
Oral complications associated with immunodeficiency virus (HIV) with antiretroviral drugs are becoming a mounting concern in HIV patients. Protease inhibitors have been shown to change the proliferation and differentiation state of oral tissues but the effect of nucleoside inhibitors is currently unknown. This study examines the effect of Zidovudine, also known as AZT, on the growth and differentiation of gingival epithelium.
Methods
Gingival keratinocytes Organotypic (raft) cultures were established. Raft cultures were treated with a range of Zidovudine concentrations. Hematoxylin and eosin staining was performed to examine the effect of Zidovudine on gingival epithelium growth and stratification. Raft cultures were immunohistochemically analyzed to determine the effect of this drug on the expression of key differentiation and proliferation markers including cytokeratins and PCNA.
Results
Zidovudine dramatically changed the proliferation and differentiation state of gingival tissues both when it was present throughout the growth period of the tissue and when it was added to established tissue at day 8. Zidovudine treatment increased the expression of cytokeratin 10, PCNA and cyclin A. Conversely, cytokeratins 5, involucrin and cytokeratin 6 expression was decreased. The tissue exhibited characteristics of increased proliferation in the suprabasal layers as well as an increased fragility and an inability to heal itself.
Conclusions
Zidovudine treatments, even when applied in low concentrations for short periods of time, deregulated the cell cycle/proliferation and differentiation pathways resulting in abnormal epithelial repair and proliferation. Our system could be developed as a potential model for studying HIV/ highly active antiretroviral therapy affects in vitro.
Keywords: antiretroviral therapy, cytokeratins, HIV infections, zidovudine, oral complications, organotypic culture
Introduction
An estimated 33.4 million patients are infected with human immunodeficiency virus (HIV) world-wide (1). The advent of antiviral drugs has greatly decreased the mortality from this virus and improved life expectancy of HIV patients. Highly active antiretroviral therapy (HAART) the combination of reverse transcriptase inhibitors and protease inhibitors is able to greatly reduce the HIV-viral load of patients and help restore their immune function. However, the continuous drug regimen and the patients’ ability to live longer with a suppressed immune system have led to complications. Oral complications are very common in HIV-positive patients. The incidence of the oral complications oral candidiasis and oral hairy leukoplakia has been shown to drop significantly in HAART patients (2–4). Other oral complications that are common in HIV-positive patients, such as, Kaposi’s sarcoma and oral apthous ulceration have shown to be unaffected by HAART (2, 3, 5). Long-term use of HAART has been associated with the increase of many complications including: oral warts (2, 5), erythema multiforme (6, 7), xerostomia (6, 7), toxic epidermal necrolysis, lichenoid reactions (7, 8), exfoliative cheilitis (6), oral ulceration and paraesthesia (6, 9). The adverse oral complications greatly affect the quality of life in HAART patients thus leading to noncompliance with drug regimens. This in turn results in interrupted and subfavorable levels of exposure to the drugs. Non-adherence to a strict drug regimen could eventually lead to drug resistance and damage all future therapy (10).
Zidovudine is also known as Azidothymidine (AZT) or 3’-azido-3’-deoxythymidine. Nucleoside analogs, like Zidovudine, were first approved by the U.S. Food and Drug Administration for use against HIV-AIDS in 1987 (11). This drug belongs to the nucleoside reverse transcriptase inhibitors (NRTIs) class and has become an essential part of HAART. Zidovudine has a two-pronged antiviral effect. It disrupts the virus both by incorporating itself into viral DNA and by inhibiting the viral reverse transcriptase (11). Zidovudine also exhibit some affinity for cellular polymerases (12, 13). It has been reported that the toxic sequelae of NRTI exposures are correlated with the kinetics of AZT incorporation into mitochondrial DNA (mtDNA) by the mitochondrial DNA polymerase (14).
Epithelial tissues, both cutaneous and mucosal provide protection from the environment to the underlying tissues. It is particularly important in the oral cavity, where masticatory functions increase damage, that epithelial lining be intact or injuries be quickly repaired, in order to prevent microorganism and toxic material from entering the underlying tissues. Epithelial cells undergo a complicated, well-defined program of differentiation that allows the expression of structural proteins designed to preserve the integrity and function of these tissues (15). Damage cannot completely be avoided in an environment such as the oral cavity and epithelial turnover rates in the oral cavity are second only to that of the small intestine (16). Typically, this would allow for a rapid wound healing response of compromised tissue. It is possible that changes in the turnover rate and wound healing abilities of oral epithelial in response to HAART may affect the occurrence of oral disease.
The epithelium is predominantly comprised of cytokeratins. The expression of dytokeratins depends on the type of tissue, its proliferations and differentiation state and pathologic conditions (17, 18). In short, examining a tissue’s cytokeratin profile allows for a snapshot into the proliferation and differentiation state of that tissue.
Zidovudine’s affect on oral epithelium is currently unknown. In the present study, the organotypic (raft) tissue culture model system derived from primary gingival cells is used, to examine, for the first time, the effect of Zidovudine on gingival epithelium growth, and the expression patterns of differentiation and proliferation markers.
Materials and Methods
Primary gingival keratinocytes and organotypic raft cultures
Primary gingival keratinocytes were isolated from a mixed pool of tissues obtained from patients undergoing dental surgery in accordance with Penn State University College of Medicine Institutional review Board (IRB# 25284) procedures. The tissue was washed three times in PBS containing 50 µg/mL gentamycin sulfate (Gibco BRL, Bethesda, MD, USA) and 1X nystatin (Sigma Chemical Co., St. Louis, MO, USA.) The connective tissue and dermis were removed, leaving the epithelium. The epithelial tissue was then minced with a scalpel and trypsinized in a sterile glass universal with a stir bar containing 25 mL of 0.05% Trypsin-EDTA (Gibco BRL, Bethesda, MD, USA). The sample was stirred on a magnetic stirrer at 37°C and incubated for 45 minutes. The supernatant was removed, neutralized with 25 mL of E media plus 5% fetal bovine serum (FBS) (19) and cells were pelleted by centrifugation. The supernatant was removed and the cell pellet was resuspended in 10 mL of 154 media (Cascade Biologics, Inc., Portland, OR, USA) and then added to a 100 cm tissue culture plate. The procedure was repeated an additional two times. When cultures became of 80% confluent, they were passaged at a ratio 1:6. When the passaged cells reached 80% confluence they were used for growing raft cultures.
Raft cultures were grown as previously described (19, 20). Briefly, mouse fibroblast 3T3 J2 cells were trypsinized and resuspended to a concentration of 2.5×105 cells/mL in 1% reconstitution buffer, 10% 10X DMEM (Life Technologies, Gaithersburg, MD, USA), 2.4µl of 10 M NaOH and 80% collagen (Dickinson, Franklin Lakes, NJ, USA) on ice. The combination was then aliquoted into 6-well plates, each well containing 2.5 mL of the mixture. The plates were incubated at 37°C for 2 hours to allow the collagen matrices to solidify. Two mL of E-media was then added to each well so that the matrix could equilibrate. Human gingival epithelial keratinocytes were trypsinized and resuspended in E-media plus 5ng/mL epidermal growth factor (EGF) at a concentration of 1×106 cells/mL and 1 mL of the suspension was added to the top of each collagen matrix. Epithelial cells were allowed to attach to the collagen for 2–4 hours before the media was removed and the matrices were lifted onto stainless steel grids. The grids rested at the air-liquid interface and the raft cultures were fed by diffusion from below with E-media supplemented with Zidovudine. Zidovudine capsules (Aurobindo Pharma, USA) were purchased from the pharmacy at The Milton S. Hershey Medical Center, Penn State University. The contents of either a 100 mg or 300 mg capsule was removed and resuspended in sterile PBS. Serial dilutions were made directly in E-media to reach the correct concentration. The Cmax of Zidovudine is 2 µg/mL (21–23). Two additional concentrations on either side of the Cmax were also used. In the first set of experiments, the rafts were treated with Zidovudine at concentrations of 0.5, 1, 2, 4, and 6 µg/mL from day 0. Control rafts were fed with E-media only. In the second set of experiments the same concentrations of Zidovudine were used but the rafts were treated on day 8. All rafts were fed every other day and harvested at the indicated time points (Figure 1).
Figure 1.
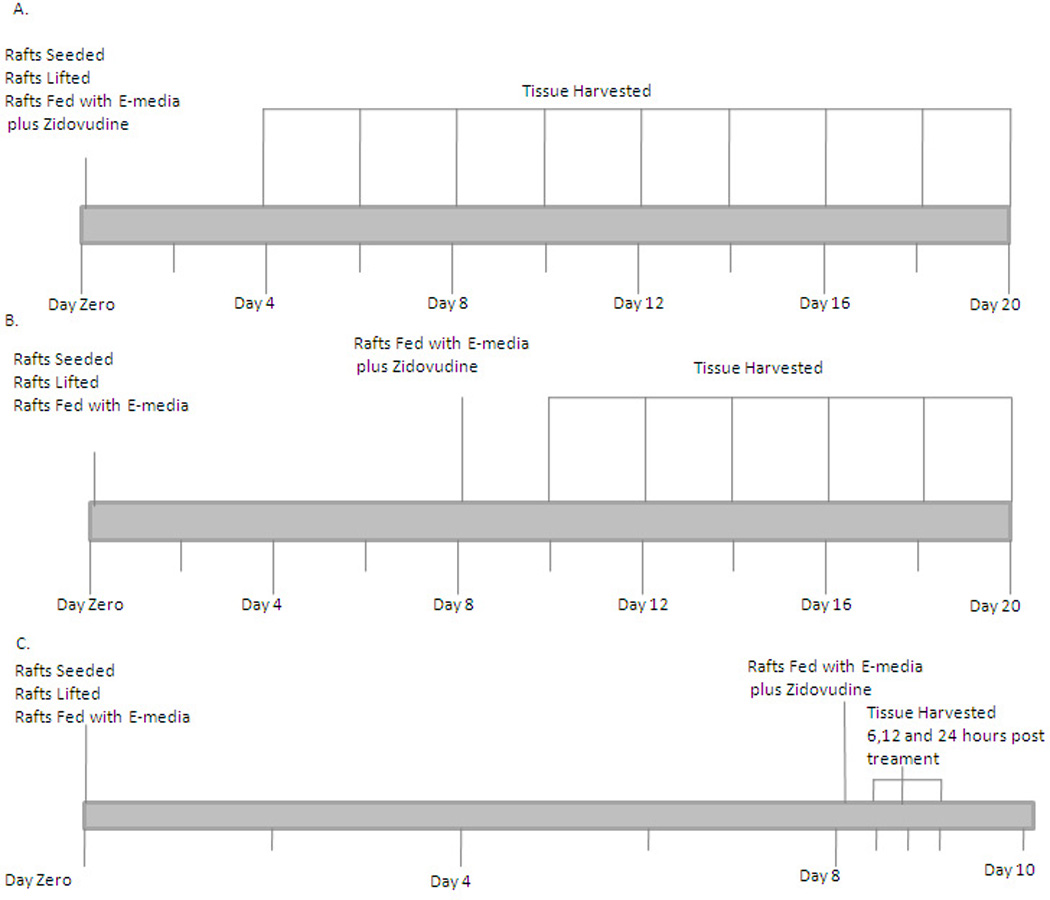
Time line for experiments.
Histochemical analyses
Raft cultures were fixed in 10% buffered formalin, and embedded in paraffin. Four micrometer sections were cut and stained with hematoxylin and eosin as described previously (19).
Immunostaining was performed with the Vectastain Elite ABC kit (Vector laboratories Burlingame, CA, USA) (19). Briefly, a vacuum oven was used to bake slides at 55°C for 1 hour. Tissue sections were then dehydrated in xylene and rehydrated in alcohol gradients. Endogenouse peroxide activity was neutralized by incubating the slides in a 3% H202 solution. Tissue sections were blocked for 1 h with 3% normal horse serum and 20% normal goat serum for primary mouse antibodies and rabbit antibodies, respectively. Next slides were incubated in primary antibodies for 1 hour. The following primary antibodies were used: mouse monoclonal keratin 5 (clone XM26; dilution 200 mg/mL), mouse monoclonal keratin 10 (clone DEK10; dilution 200 mg/mL), mouse monoclonal involucrin (200 mg/mL), mouse monoclonal keratin 6 (clone LHK6B; dilution10 ng/mL) (all from Lab Vision, Fremont, CA, USA), rabbit polyclonal proliferating cell nuclear antigen (PCNA) (clone FL-261; dilution 2 mg/mL) and rabbit polyclonal cyclin A (clone H-432; dilution 4 mg/mL) (both from Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA).
Results
Effect of Zidovudine on morphological differentiation and stratification of gingival tissues
Hematoxylin and eosin staining was performed to examine the effect of Zidovudine on gingival epithelial morphology and stratification in raft cultures. The raft culture system has been shown to accurately mimic the in vivo physiology of the gingival epidermis (24, 25). In the first set of experiments, we applied Zidovudine treatments every other day throughout the period of raft culture growth and differentiation for a total of 16 days. We treated the raft cultures with a range of Zidovudine concentrations, two on either side of the Cmax: 0.5, 1, 2 (Cmax), 4, and 6µg/ml. Control rafts were fed with E-media only (Figure 1).
The raft cultures treated with all concentrations of Zidovudine showed dramatic change in morphology and stratification. Even at four days there is obvious change in tissues treated from day zero. Keratin pearls become evident in treated tissues. Drug treatment also caused a change in differentiation. Normally, nuclei are only present in the basal layer of cells, as is the case with our untreated rafts. However in Zidovudine-treated rafts nuclei become visible throughout the layers of tissue. Additionally, in rafts allowed to grow for 10–16 days, there is a dramatic loss of vaculation of the upper tissue layers of all Zidovudine-treated raft cultures (Figure 2A).
Figure 2. Effect of Zidovudine on gingival epithelium morphology and stratification.
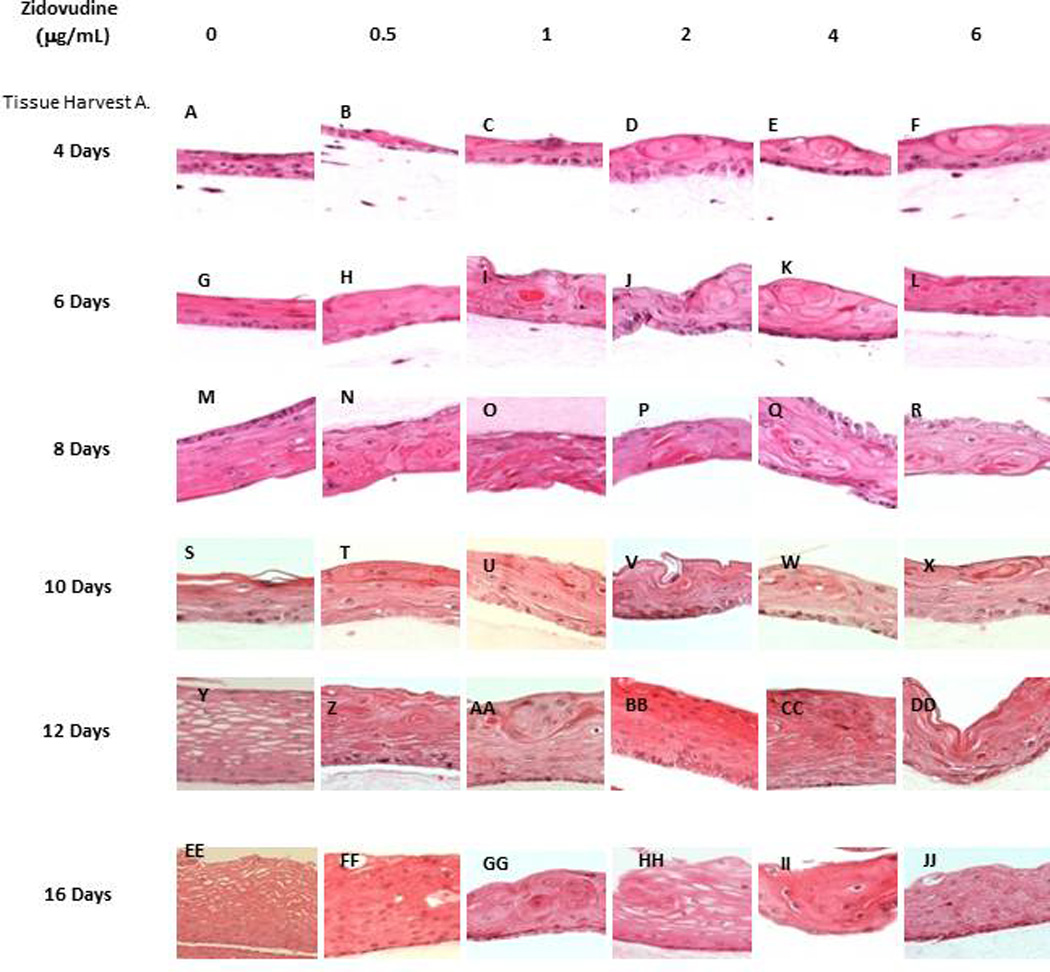
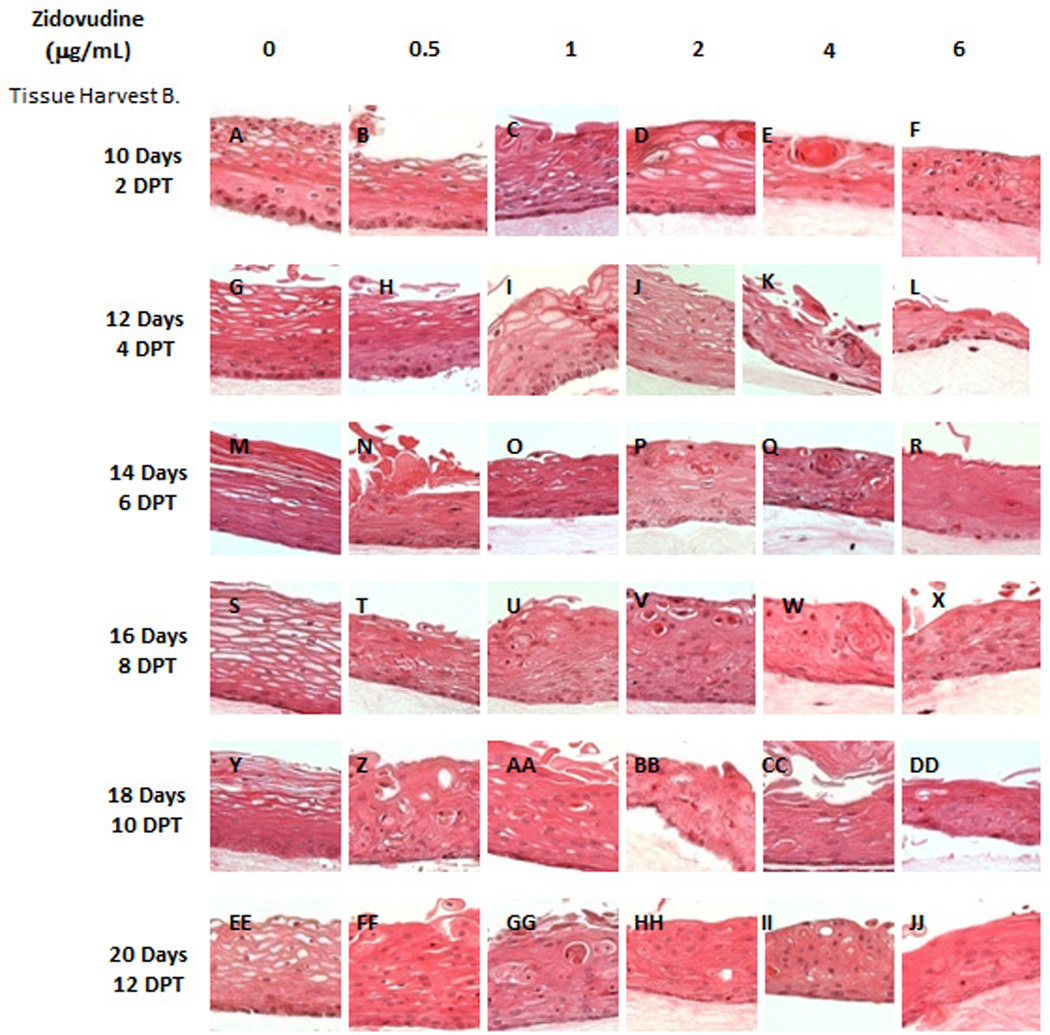
Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of Zidovudine, beginning either at day 0 (Tissue Harvest A) or at day 8 (Tissue Harvest B). (Panels A, G, M, S, Y and EE) untreated rafts; (Panels B, H, N, T, Z, and FF) rafts treated with 0.5 µg/ml Zidovudine; (Panels C, I, O, U, AA, and GG) rafts treated with 1 µg/ml Zidovudine; (Panels D, J, P, V, BB and HH) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, Q, W, CC, and II) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, R, X, DD, and JJ) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at different points and stained with hematoxylin and eosin. DPT denotes days post treatment (Tissue Harvest B). Images are at 20 X original magnification.
A second set of experiments were designed to examine the effect of Zidovudine on already established growing tissue, rafts were grown to day 8 in E-media alone (Figure 2B). At day 8, Zidovudine was added at the above concentrations and applied every other day until the tissue was harvested. This allowed us to examine the effect the Zidovudine had on already differentiated tissue and to compare the results to those seen in tissues treated with protease inhibitors (26, 27). The effect of Zidovudine on tissue first grown to day 8 was similar to that of Zidovudine added to tissue on day 0. Figure 2B demonstrates the effect of Zidovudine on day 8 gingival tissues as compared with non-treated rafts. The raft cultures treated with Zidovudine below the Cmax reflected the same morphology at 2 and 4 days post treatment, and were similar to untreated rafts (Figure 2B, Panel A–C). There was a change in morphology, including the presence of keratin pearls, a change in differentiation and a loss of vaculation as early as two days post treatment in these rafts at concentrations at or above Cmax (Figure 2B, Panels D–F). At 6 or more days post treatment these changes in morphologies were evident at all concentrations. These results indicated that Zidovudine treatments on day 8 tissue changed the gingival epithelium differentiation, morphology and stratification, perhaps affecting the integrity of the tissue even after the tissue had differentiated. Overall, the change in gingival tissue after Zidovudine treatment, whether it was begun at day zero or at day 8 was dramatic (Figure 2).
Zidovudine treatment changes the expression pattern of basal layer markers in gingival epithelium
Cytokeratins 5 and 14 are associated with the basal layer of terminally differentiated gingival keratinocytes (28). In order to assess the expression pattern of biochemical markers of differentiation in Zidovudine treated and untreated samples, rafts were harvested and paraffin embedded as described in Methods. Tissue sections of both treated and untreated rafts were analyzed by immunostaining with monoclonal antibodies.
Typically, cytokeratin 5 and its partner cytokeratin 14 are expressed in the basal layer of gingival stratified epithelium and have been used as proliferative cell markers (28–30). Although only expressed in the basal layer the cytokeratins are maintained in the upper layers of tissue. In this study, cytokeratin 5 was visualized in all layers of gingival tissue. If the rafts were treated with Zidovudine from day 0, tissue harvested after 4 and 6 days of continuous treatment did not differ from untreated tissues (Figure 3, Panels A–F and data not shown). However, Zidovudine treatments decreased and changed the expression pattern of cytokeratin 5 at all drug concentrations with the most prominent change with tissue harvested at day 10. (Figure 3 Panels G–L). Tissues that were grown to day eight and then drug-exposed were also affected even if they were only drug-exposed for 2 days (Figure 3, Panels M-X). Cytokeratin 14 showed similar staining patterns (data not shown). Cytokeratin 5 and its partner cytokeratin 14 form dimers that help give tissue its integrity. Without these cytokeratins present tissue becomes very fragile and the small injuries cause tissue to fall apart and blisters to form (28). Zidovudine treatments demonstrated the potential to compromise epithelium integrity during all stages of tissue development.
Figure 3. Expression pattern of cytokeratin 5 in untreated and Zidovudine treated gingival raft cultures.
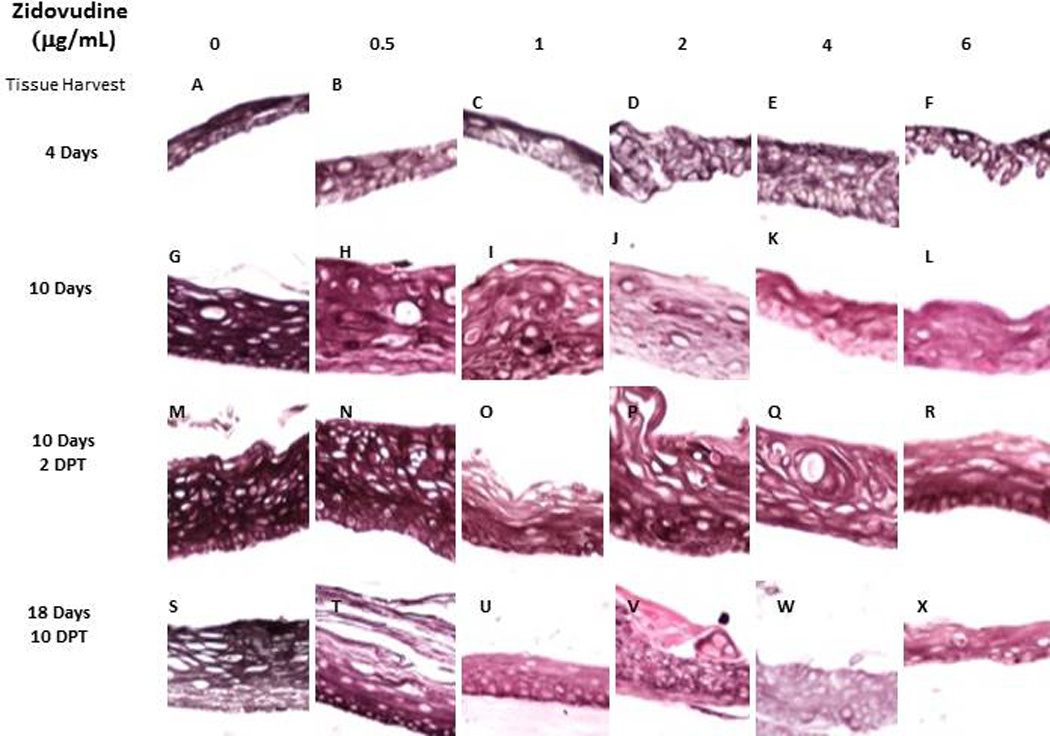
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at either day 0 or day 8.
(Panels A, G, M, and S) untreated rafts; (Panels B, H, N and T) rafts treated with 0.5 µg/ml Zidovudine; (Panels C, I, O, and U) rafts treated with 1 µg/ml Zidovudine; (Panels D, J, P, and V) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, Q, and W) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, R, and X) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at the indicated timepoints (DPT denotes days post treatment) and stained with anti-cytokeratin 5. Images are at 20 X original magnification.
Zidovudine treatment changes the expression pattern of differentiation markers in gingival epithelium
Involucrin is a protein present in keratinocytes of epidermis and other stratified squamous epithelia. Involucrin first appears in the cell cytosol, but ultimately becomes cross-linked to membrane proteins by transglutaminase thus helping in the formation of an insoluble envelope beneath the plasma membrane. Zidovudine decreased the expression pattern of involucrin at all drug concentrations and at all timepoints, whether added at day 0 or day 8 of tissue development (Figure 4).
Figure 4. Expression pattern of involucrin in untreated and Zidovudine treated gingival raft cultures.
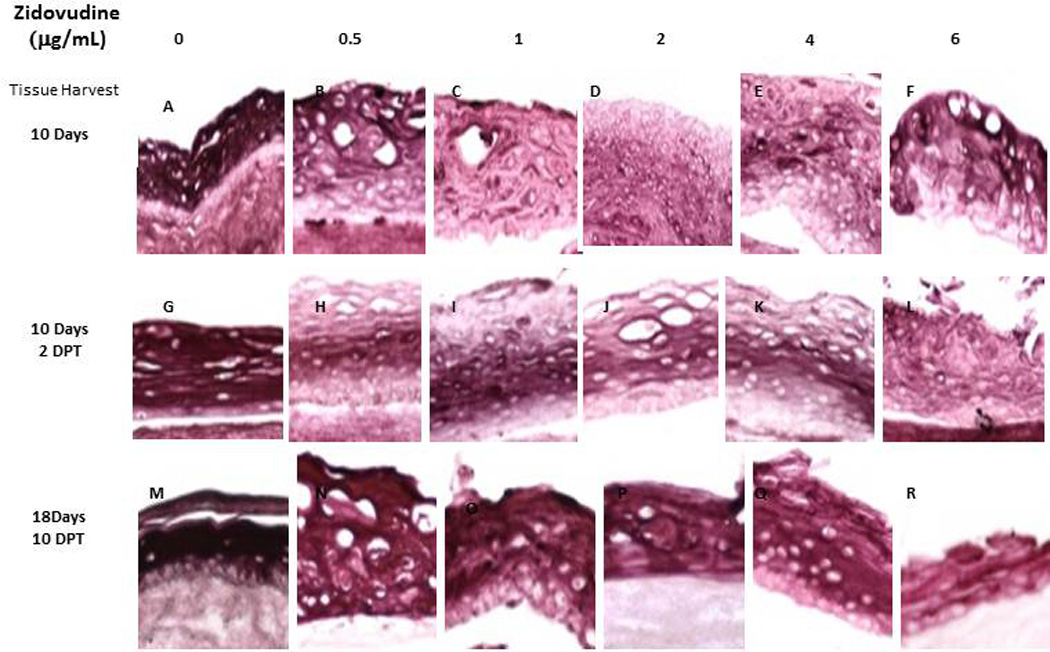
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at either day 0 or day 8.
(Panels A, G, and M) untreated rafts; (Panels B, H and N) rafts treated with 0.5 µg/ml Zidovudine; (Panels C, I and O) rafts treated with 1 µg/ml Zidovudine; (Panels D, J and P) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, and Q) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, and R) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at the indicated time points (DPT denotes days post treatment) and stained with anti-cytokeratin involucrin. Images are at 20 X original magnification.
Cytokeratin 10 expression is indicative of terminal differentiation of tissue and it was the second differentiation marker we studied. Cytokeratin 10 is usually expressed in low levels in the suprabasal layers of oral keratinocytes (29, 31). When the drug was added at day zero, Zidovudine treatments induced the expression of cytokeratin 10 at concentrations at Cmax or higher. The effect on this cytokeratin was weak and most apparent in tissue harvested at later days (Figure 5). When the drug was added at day eight of growth, the effect on tissue was similar to that seen in tissue treated from day zero. Expression of cytokeratin 10 was induced at concentration at or above Cmax. However, the effect on this cytokeratin under these conditions was minimal and again most apparent in tissue harvested at later days (Figure 5, Panels J–L; V–X). The keratin 10 expression may be an attempt by the tissue to protect itself from damage.
Figure 5. Expression pattern of cytokeratin 10 in untreated and Zidovudine treated gingival raft cultures.
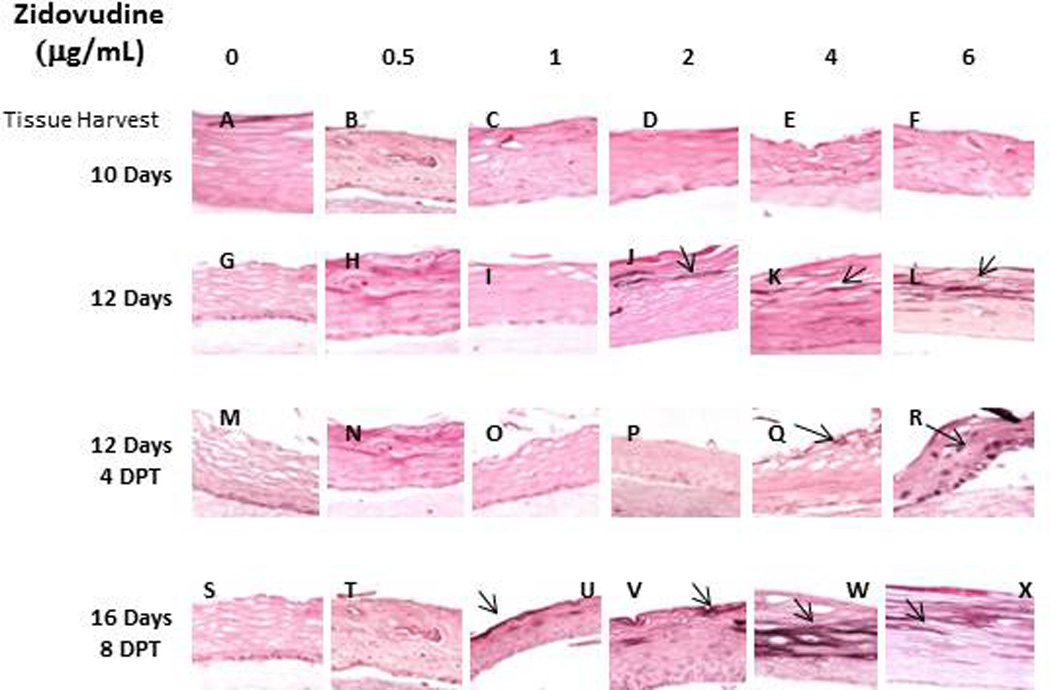
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at either day 0 or day 8.
((Panels A, G, M, and S) untreated rafts; (Panels B, H, N and T) rafts treated with 0.5 µg/ml Zidovudine; (Panels C, I, O, and U) rafts treated with 0.1 µg/ml Zidovudine; (Panels D, J, P, and V) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, Q, and W) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, R, and X) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at the indicated time points (DPT denotes days post treatment) and stained with anti-cytokeratin 5. Images are at 20 X original magnification. Rafts were harvested at the indicated time points (DPT denotes days post treatment) and stained with anti-cytokeratin 10. Images are at 20 X original magnification.
Effects of Zidovudine treatment on the expression of wound healing keratin 6
Cytokeratin 6 expression is related with the wound healing process and is expressed in the suprabasal layer. Epidermal injury results in induced cytokeratin 6 expression in keratinocytes undergoing activation at the wounded edge (32, 33). The raft culture is a wound healing environment and as such, cytokeratin 6 is typically expressed in raft tissues. In our study, cytokeratin 6 expression was dramatically reduced at all concentrations of Zidovudine when the drug was added at day zero (Figure 6, Panels A–L). There was a similar decrease in expression, at all Zidovudine concentrations, when the drug was added at day 8 (Figure 6, Panels M–X). A marked decrease in cytokeratin 6 was seen after just two days when the drug was added to tissue after 8 days of growth (data not shown). Such an immediate and strong decrease in expression of cytokeratin 6 at 2 and 4 days post treatment suggests an impaired wound healing response of the tissue.
Figure 6. Expression pattern of cytokeratin 6 in untreated and Zidovudine treated gingival raft cultures.
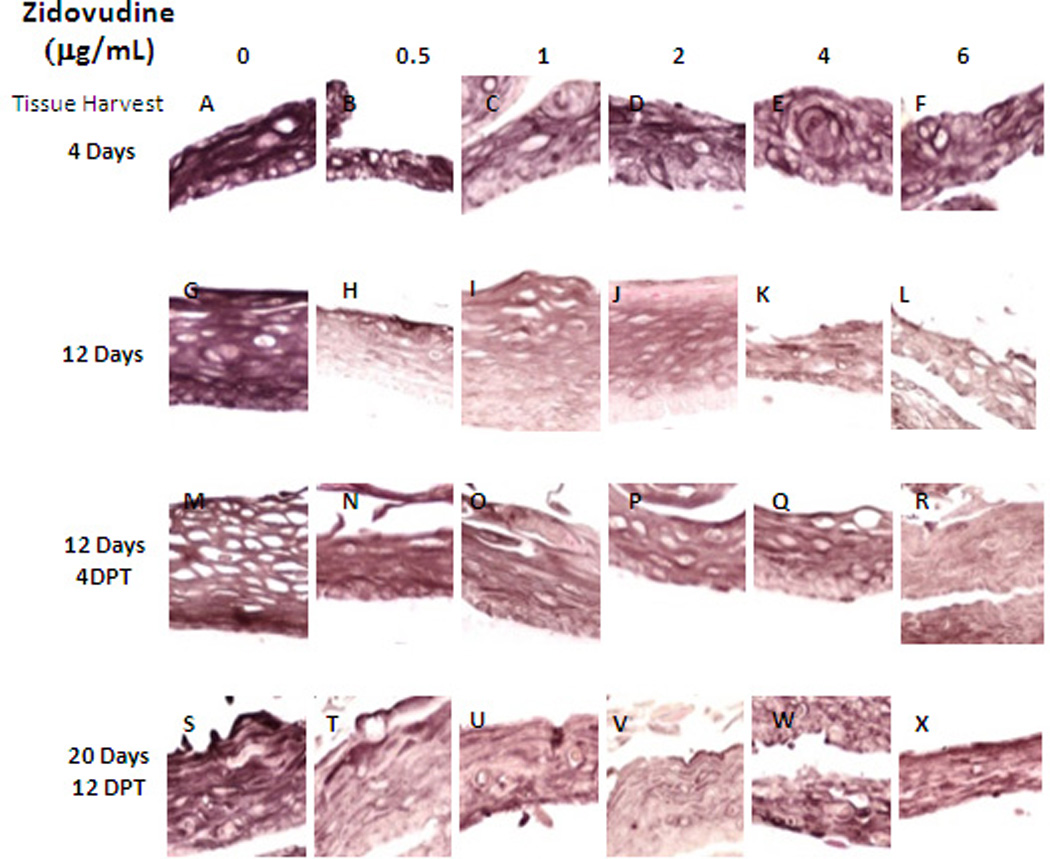
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at either day 0 or day 8.
(Panels A, G, M, and S) untreated rafts; (Panels B, H, N and T) rafts treated with 0.5 µg/ml Zidovudine; (Panels C, I, O, and U) rafts treated with 1 µg/ml Zidovudine; (Panels D, J, P, and V) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, Q, and W) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, R, and X) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at the indicated timepoints (DPT denotes days post treatment) and stained with anti-cytokeratin 6. Images are at 20 X original magnification.
Zidovudine treatments induce cell proliferation
Since Zidovudine treatments changed the expression patterns of proliferation markers cytokeratins 5 and 6, we then decided to evaluate the effect of Zidovudine on the expression of well characterized cell proliferation markers. PCNA is a nuclear protein associated with DNA polymerase delta which is present throughout the cell cycle in the nuclei of proliferating cells (34). Cyclin A, however, plays a role in proliferation by regulating entry into the DNA synthesis phase (S phase) of the cell cycle (35, 36). Immunohistochemical analysis of PCNA and cyclin A allow a spatial view of cell proliferation to be generated. Typically, cells only proliferate in the basal layer of tissue. In this study, the PCNA and cyclin A expression in untreated rafts was limited to the basal layer. This expression, particularly of PCNA was strong and even throughout the experiment in untreated tissues. The Zidovudine-treated rafts, however, both PCNA and cyclin A were strongly expressed in both the basal and differentiating layers of the tissue (Figure 7). When applied from day 0, Zidovudine causes an increase in the expression pattern of PCNA. Zidovudine treatment also changed where PCNA was being expressed in these tissues. Where PCNA expression in untreated tissues was confined to the basal layers treated tissues showed expression of PCNA in differentiating layers of the tissue (Figure 7A). When tissue is treated with Zidovudine beginning at day 8, an effect on PCNA expression is seen as early as two to four days post treatment (Figure 7B, Panels A–F and data not shown). There is an increase of expression of PCNA in differentiating layers of treated tissues.
Figure 7. Proliferating cell nuclear antigen expression pattern in untreated and Zidovudine treated gingival raft cultures.
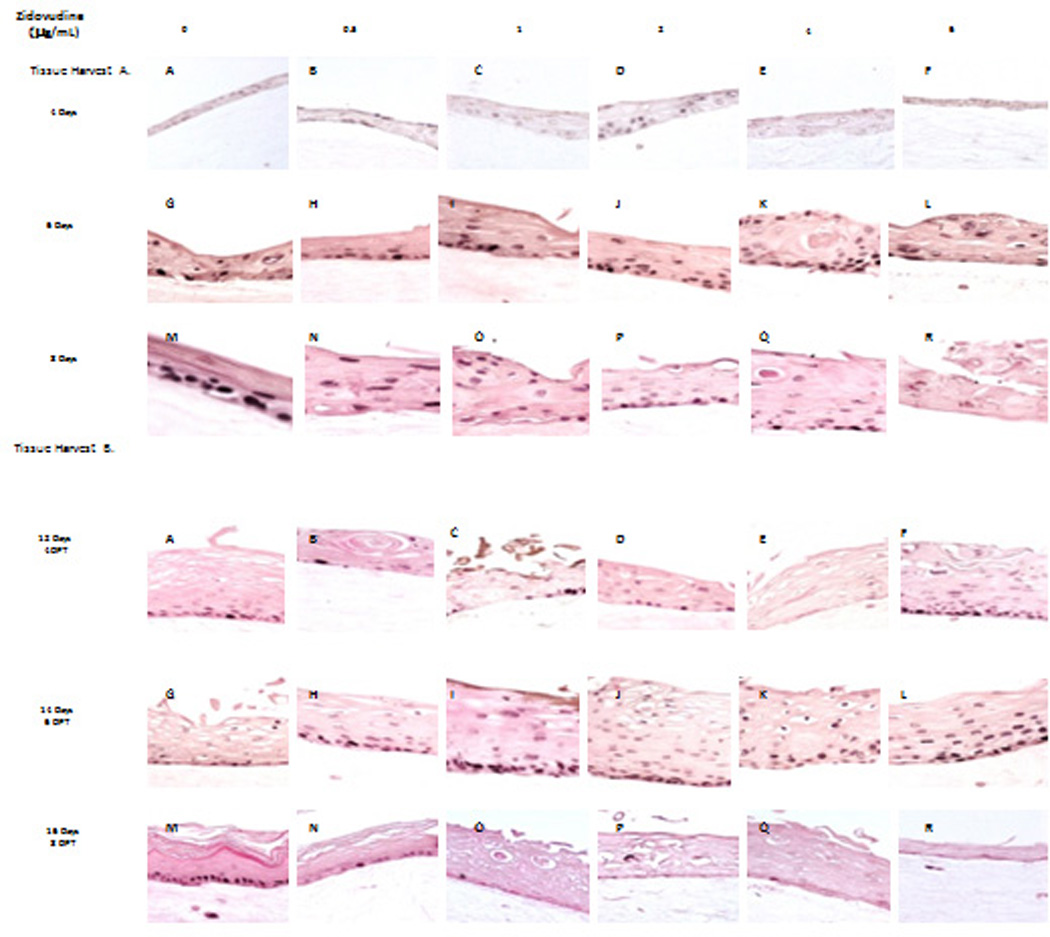
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at either day 0 (Tissue Harvest A) or day 8 (Tissue Harvest B). (Panels A, G, and M) untreated rafts; (Panels B, H and N) rafts treated with 0.05 µg/ml Zidovudine; (Panels C, I and O) rafts treated with 1 µg/ml Zidovudine; (Panels D, J and P) rafts treated with 2 µg/ml, the cMax of Zidovudine; (Panels E, K, and Q) rafts treated with 4 µg/ml Zidovudine; (Panels F, L, and R) rafts treated with 6 µg/ml Zidovudine. Rafts were harvested at the indicated timepoints (DPT denotes days post treatment) and stained with anti-cytokeratin pcna or cyclin A. Images are at 20 X original magnification.
The changed expression pattern of PCNA (Figure 7) and cyclin A (data not shown) could indicate the activation of the wound healing pathway against drug induced damage. However, it is more likely that the changed expression patterns of PCNA and cyclin A indicate that exposure to Zidovudine induces a loss of cell cycle control which could play a role in the generation of oral complications in HIV patients under treatment with this drug. Decreased cytokeratin 6 expression supports this possibility.
The Effect of 6, 12 and 24 hour Applications of Zidovudine on Raft Culture
The effects of Zidovudine were seen on established tissue as early as 48 hours after exposure to the drug. Therefore, we performed an experiment where 0.5, 1 and 2 µg/ml Zidovudine was added to the day 8 raft cultures for 6, 12 or 24 hours in order to examine the effects of short-term treatment on gingival tissue (Figure 1). The immunohistochemical staining was then performed as in the previous experiments.
Effects similar to those seen in previous experiments were seen at all concentrations and at all time points. Even as early as six hours and at the lowest concentration of Zidovudine, hematoxylin and eosin staining and immunohistochemistry reveal that the drug changes both the morphology and the differentiation and proliferation status of tissues.
Hematoxylin and eosin staining at the Cmax of Zidovudine show that keratin pearls become more visible in treated tissue. Nuclei become more evident in upper layers of the tissue and vaculation is reduced in tissues treated for 6, 12 and 24 hours (Figure 8, Panels A–D). Similar to tissue treated with Zidovudine for longer periods of time, tissue treated with drug for 6, 12 and 24 hours showed a decrease in cytokeration 5 and involucrin expression at all drug concentrations (Figure 8, Panels E–L and data not shown). When Zidovudine was added to tissues at the 6, 12 and 24 hour time points there was a marked increase in keratin 10 was seen in tissues at all concentrations of drug (Figure 8, Panels M–P and data not shown). This observation was different then that seen at longer time points. Tissues treated at day eight and harvest two and four day post treatment did not sustain this increase in cytokeratin expression. The wound healing cytokeratin 6 was decreased in tissues treated with Zidovudine for 6, 12 and 24 hours at all drug concentrations tested (Figure 8, Panels Q–T). Like tissues treated for longer periods of time, an increase in PCNA was seen in tissues after 6, 12 and 24 hours of Zidovudine exposure. PCNA expression also became evident in upper layers of tissue (Figure 8, Panels U–X and data not shown).
Figure 8. Effect of Zidovudine on gingival epithelium morphology, stratification and cytokeratin expression pattern in gingival raft cultures either untreated or treated with Zidovudine for 6, 12 or 24 hours.
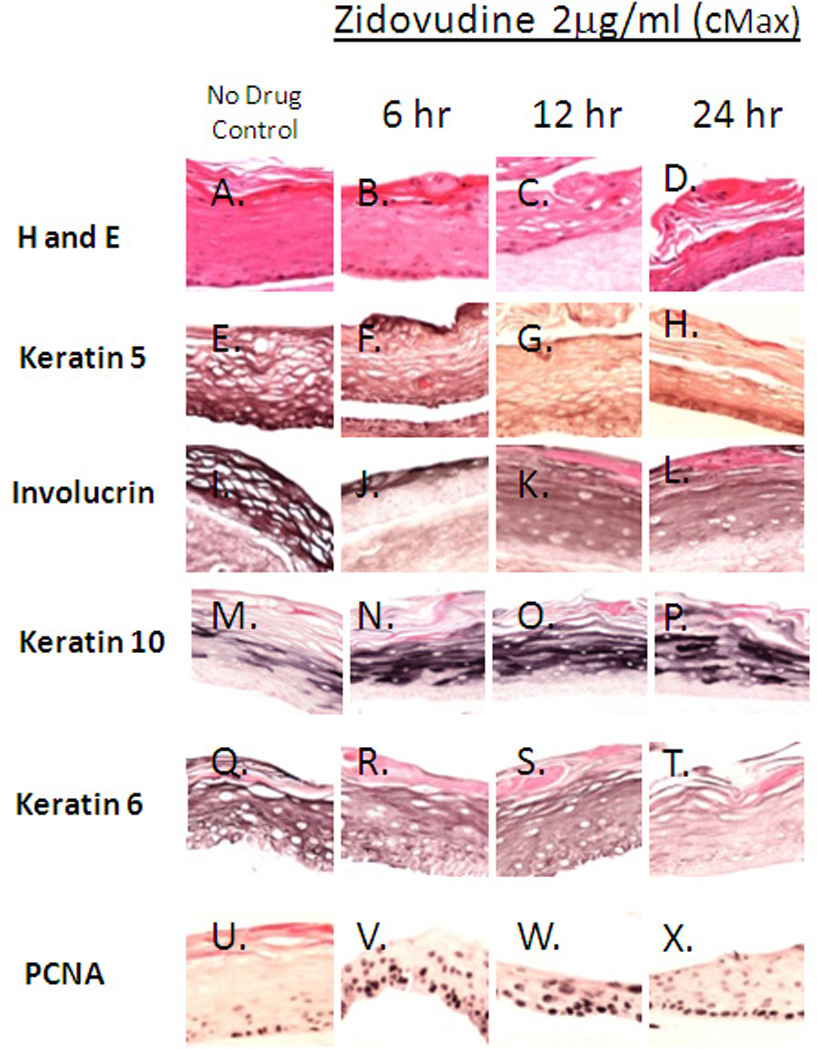
Primary gingival keratinocytes were grown in raft cultures and treated with different concentrations of Zidovudine at day 8 for either 6, 12 or 24 hours. All rafts were treated with 2 µg/ml, the cMax of Zidovudine.
Effects similar to those seen in previous experiments were seen at all concentrations and at all time points. The effects were strongest at the 2µg/ml concentration (Cmax)(Figure 6). These results suggest that Zidovudine is able to mediate its effects through quick acting pathways.
Discussion
HIV-positive patients taking highly active antiretroviral therapy (HAART) have reported many oral complications which have a major impact on their overall health and quality of life. Protease inhibitors have been shown to effect both the growth and differentiation of oral epithelium (26, 27), however, the effects of nucleoside reverse transcriptase inhibitors (NRTI) on the growth and differentiation of oral epithelium is currently unknown.
No data is available on the concentration of Zidovudine in the oral cavity. However, we expect that in the oral caity, the concentration of the drug should be at least close to or lower than its Cmax (2 µg/ml). This assumption and previous data from the effect of protease inhibitors on gingival tissues lead us to use the concentrations indicated.
The growth of gingival epithelium was inhibited when the drug, Zidovudine, an NRTI, was added at day 0 and was present throughout the growth period. In the present study, Zidovudine, even at lower concentrations (0.5 and 1 µg/ml), below the Cmax, affected the growth of gingival epithelium, disrupting its proliferation and stratification status. These results support previous information that indicated the use of antiretroviral drugs resulted in the development of oral complications, especially with long-term use (2, 3, 5, 7, 9). Our observations suggest the possibility that oral epithelium in HIV-positive patients exposed to HAART, including Zidovudine, experience drug-induced abnormalities in the tissue’s molecular and cellular biology which give rise to these oral complications.
Epithelial tissues express different pairs of cytokeratin proteins depending on epithelial cell type and stage of differentiation (17, 18). During the process of terminal differentiation, keratinocytes lose their ability to proliferate and migrate from the basal layer to the superficial layers while undergoing a co-ordinated series of morphological, biochemical and genetic changes. The terminal differentiated cell is a flattened dead cell that consists of a network of cytokeratin filaments surrounded by an insoluble envelope of heavily crosslinked protein(37). When cells make the commitment to terminally differentiate one of changes to occur is a switch in cytokeratin gene expression. Expression of cytokeratins 5 and 14 is shut off and that of cytokeratin 1 and 10 is turned on (38). Cytokeratin 10 is indicative of terminal differentiation and is expressed in the suprabasal layer of keratinized epithelia. It has also been reported that cytokeratin 10 protects epithelium from trauma and damage (31).
To examine the effect of Zidovudine on the proliferation and differentiation of oral keratinocytes, we treated raft cultures at day 0 and at day 8. Normally, gingival stratified epithelia express the cytokeratin pair of cytokeratins 5 and 14 only in the proliferative basal layer however, it is maintained in all layers of tissue (28, 30). In this study, cytokeratins 5 and 14 were detected in all layers of the tissue in untreated samples. Application of Zidovudine decreased the presence of cytokeratin 5 (Figure 3) and 14 (data not shown). The decrease in cytokeratin 5 and 14 was seen in all levels of the oral tissue. Cytokeratin 5 and its partner cytokeratin 14 form dimers that help give tissue its integrity. Without these cytokeratins present tissue becomes fragile and small injuries can cause tissue to fall apart and blisters to form. These cytokeratins have also been shown to be enhance in hyperproliferative situations such as wound healing (33, 39). These data suggest that Zidovudine treatment is impairing the ability of oral tissue to heal itself.
In this study, Zidovudine treatments induced the expression of cytokeratin 10 particularly at the 6, 12 and 24 hours time points. (Figure 3 and 6). Increased levels of cytokeratin 10 in drug-treated gingival epithelium may be an attempt by the tissue to protect itself against damage caused by the Zidovudine (31, 40, 41). Additionally, it has been shown that cytokeratin 10 is more strongly expressed in both oral lesions and hyperproliferative epidermis when compared to ordinary epidermis (42). Thus, the elevated levels of cytokeratin 10 may be linked to the proliferative effect of the Zidovudine on treated rafts. Additionally, the normal balance of cytokeratin proliferation and differentiation may be disrupted upon injury and under pathologic conditions (43–45).
Involucrin is expressed in response to the same pathway as cytokeratin 5. In addition to a change in cytokeratin expression, envelope formation is a hallmark of terminal differentiation. In order for the envelope to be formed correctly, the envelope precursors and transglutaminase, the enzyme activity responsible for the assembly of the envelope, must be expressed at the correct time and level during the differentiation process (37). Involucrin is a component of the cornified envelope. Involucrin is specifically expressed in the suprabasal layers of the epidermis (37). While in the spinous and granular layer, involucrin amasses as a non-crosslinked precursor. During the final stages of keratinocytes differentiation, involucrin becomes cross-linked to other proteins to form the cornified envelope (37). Involucrin expression, like that of cytokeratin 5, is regulated by the Sp1 protein (37), and in our study, involucrin, like cytokeratin 5, was decreased in response to Zidovudine treatment. A lack of involucrin available for cross-linking may explain the lack of a vaculated, cornified layer seen in Zidovudine treated tissues and may account for the fragility of oral tissues in HAART patients.
Induction of cytokeratin 6 expression in protease inhibitor-treated rafts (26, 27), as well as a slight increase in cytokeratin 10 expression in Zidovudine-treated tissues suggested the possibility that HAART drugs, including Zidovudine were causing damage to the gingival epithelium. To examine this possibility, we looked at expression patterns cytokeratin 6, a wound healing keratin, which is activated in response to injury in the suprabasal layer of stratified epithelium. In the current study, cytokeratin 6 expression was reduced significantly by all concentrations of Zidovudine at all time points examined, including 2 days post treatment, in treated rafts compared with untreated rafts where the cytokeratin was strongly expressed. Injury to stratified epithelia causes induction of cytokeratin 6 in the differentiating layers of epidermis (31–33, 45). The inability of oral tissue, which is constantly in a wounding environment, to repair itself through the cytokeratin 6 mechanism could explain some of the oral complications seen in HAART patients.
The results of hematoxylin and eosin staining suggested a change in the proliferation status of Zidovudine-treated rafts. Nuclei were present in all layers of the drug-treated tissue. Increased cell proliferation is a feature of many disorders such as wounds, ulcers and tumors, and the identification and use of reliable markers of proliferative activity is important in clinical practice (46, 47). Therefore, we examined the effect of Zidovudine on two well-known cell proliferation markers, PCNA and cyclin A. These nuclear proteins play important roles in DNA synthesis and cell cycle progression, allowing cell proliferation. Both PCNA and cyclin A are generally found in cell nuclei between the G1 and M phases of the cell cycle (36, 48). Under normal conditions of cell proliferation and tissue differentiation PCNA and cyclin A are expressed in only a few basal layer cells, thus their expression and allocation make them useful histochemical indicators of cellular proliferation and DNA synthesis (49). In contrast to the decreased expression of cytokeratin 6, an increased expression of PCNA and cyclin A in drug-treated rafts in this study. Not only was there increased PCNA in the basal layer of the drug-treated tissue, PCNA also became apparent in the suprabasal layers of the drug-treated tissue. This increased expression of PCNA could indicate the activation of wound healing pathways attempting to counteract drug induced tissue damage. Elevated levels of cytokeratin 10 in Zidovudine-treated rafts support this conclusion. However, it is more likely that exposure to the Zidovudine deregulated cell proliferation and differentiation pathways allowing abnormal proliferation independent of wound healing pathways. This argument is supported by the decrease in cytokeratin 6 in drug-treated tissues and the short-lived induction of cytokeratin 10. Overall, the Zidovudine-treated tissue is highly and abnormally proliferative and has impaired epithelial repair mechanisms thus making the tissue more vulnerable to the oral complications seen in HIV patients taking this drug. The increased levels of PCNA, cyclin A and cytokeratin 10 indicate that the tissue is in a hyperproliferative state that may make in more conducive to the viral cancers common in HIV-positive patients. Decreased levels of cytokeratin 5 and 6 and involucrin suggest that the tissue is fragile and unable to repair itself sufficiently, adding to an environment that is ripe for opportunistic infections and other complications.
In conclusion, we have observed that Zidovudine can inhibit and change the growth of gingival tissue both when the drug was added at day 0 or day 8 of raft growth. Zidovudine increased the expression of PCNA, cyclin A and cytokeratin 10. The expression of cytokeratin 5 and 6 and involucrin were decreased in Zidovudine-treated rafts. Together these results indicate that Zidovudine deregulated the growth, differentiation and proliferation profiles in human gingival raft tissue. These results are consistent with the finding of oral complications in patients undergoing long-term HAART. Additional studies will be needed to determine the exact mechanism through which Zidovudine is exerting its effect.
ACKNOWLEDGEMENTS
We thank Lynn Budgeon for technical assistance in preparing histological slides. This work was supported by NIDCR grant DE018305 to Craig Meyers.
Contributor Information
Danielle Mitchell, The Pennsylvania State University College of Medicine, Microbiology and Immunology.
Mohd Israr, The Pennsylvania State University College of Medicine, Microbiology and Immunology.
Samina Alam, The Pennsylvania State University College of Medicine, Microbiology and Immunology.
Joseph Kishel, The Pennsylvania State University College of Medicine, Pharmacology.
Donald Dinello, Oral Surgery Associates.
Craig Meyers, The Pennsylvania State University College of Medicine, Microbiology and Immunology.
References
- 1.Global summary of the AIDS epidemic. The Joint United Nations Programe on HIV AIDS. 2007 http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001 May 5;357(9266):1411–1412. doi: 10.1016/S0140-6736(00)04578-5. [DOI] [PubMed] [Google Scholar]
- 3.Greenspan JS, Greenspan D. The epidemiology of the oral lesions of HIV infection in the developed world. Oral Dis. 2002;8 Suppl 2:34–39. doi: 10.1034/j.1601-0825.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- 4.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Mar;89(3):299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson TA, Greenspan D, Greenspan JS. Oral lesions of HIV disease and HAART in industrialized countries. Adv Dent Res. 2006;19(1):57–62. doi: 10.1177/154407370601900112. [DOI] [PubMed] [Google Scholar]
- 6.Diz Dios P, Scully C. Adverse effects of antiretroviral therapy: focus on orofacial effects. Expert Opin Drug Saf. 2002 Nov;1(4):307–317. doi: 10.1517/14740338.1.4.307. [DOI] [PubMed] [Google Scholar]
- 7.Scully C, Diz Dios P. Orofacial effects of antiretroviral therapies. Oral Dis. 2001 Jul;7(4):205–210. [PubMed] [Google Scholar]
- 8.Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. Aids. 2001 Sep 28;15(14):1843–1848. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 9.Jordan RA. Implications of antiretroviral therapy in oral medicine--a review of literature. Schweiz Monatsschr Zahnmed. 2007;117(12):1210–1216. [PubMed] [Google Scholar]
- 10.Hawkins T HIV drug resistance and you. The limitations of drug resistance testing. Many factors influence the results. Posit Aware. 2006 Winter; Spec No:13-6. [PubMed] [Google Scholar]
- 11.Olivero OA. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen. 2007 Apr–May;48(3–4):215–223. doi: 10.1002/em.20195. [DOI] [PubMed] [Google Scholar]
- 12.Parker WB, White EL, Shaddix SC, Ross LJ, Buckheit RW, Jr, Germany JM, et al. Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases alpha, beta, and gamma by the 5'-triphosphates of carbovir, 3'-azido-3'-deoxythymidine, 2',3'-dideoxyguanosine and 3'-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs. J Biol Chem. 1991 Jan 25;266(3):1754–1762. [PubMed] [Google Scholar]
- 13.Lim SE, Copeland WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem. 2001 Jun 29;276(26):23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Hanes J, Johnson KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003 Dec 23;42(50):14711–14719. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11(4):383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 16.Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001 29;:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 17.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 18.Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, et al. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- 19.Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 20.Meyers C. Organotypic (raft) epithelial tissue culture system for the differentiation dependent replication of papillomavirus. Meth Cell Sci. 1996;18:1–10. [Google Scholar]
- 21.Cressey TR, Jourdain G, Rawangban B, Varadisai S, Kongpanichkul R, Sabsanong P, et al. Pharmacokinetics and virologic response of zidovudine/lopinavir/ritonavir initiated during the third trimester of pregnancy. Aids. Sep 10;24(14):2193–2200. doi: 10.1097/QAD.0b013e32833ce57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremieux AC, Katlama C, Gillotin C, Demarles D, Yuen GJ, Raffi F. A comparison of the steady-state pharmacokinetics and safety of abacavir, lamivudine, and zidovudine taken as a triple combination tablet and as abacavir plus a lamivudine-zidovudine double combination tablet by HIV-1-infected adults. Pharmacotherapy. 2001 Apr;21(4):424–430. doi: 10.1592/phco.21.5.424.34497. [DOI] [PubMed] [Google Scholar]
- 23.Unadkat JD, Collier AC, Crosby SS, Cummings D, Opheim KE, Corey L. Pharmacokinetics of oral zidovudine (azidothymidine) in patients with AIDS when administered with and without a high-fat meal. Aids. 1990 Mar;4(3):229–232. doi: 10.1097/00002030-199003000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Klausner M, Ayehunie S, Breyfogle BA, Wertz PW, Bacca L, Kubilus J. Organotypic human oral tissue models for toxicological studies. Toxicol In Vitro. 2007 Aug;21(5):938–949. doi: 10.1016/j.tiv.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Tomakidi P, Fusenig NE, Kohl A, Komposch G. Histomorphological and biochemical differentiation capacity in organotypic co-cultures of primary gingival cells. J Periodontal Res. 1997 May;32(4):388–400. doi: 10.1111/j.1600-0765.1997.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Israr M, Mitchell D, Alam S, Dinello D, Kishel JJ, Meyers C. Effect of the HIV protease inhibitor amprenavir on the growth and differentiation of primary gingival epithelium. Antivir Ther. 15(2):253–265. doi: 10.3851/IMP1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israr M, Mitchell D, Alam S, Dinello D, Kishel JJ, Meyers C. The HIV protease inhibitor lopinavir/ritonavir (Kaletra) alters the growth, differentiation and proliferation of primary gingival epithelium. HIV Med. Aug 15; doi: 10.1111/j.1468-1293.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie IC, Rittman G, Gao Z, Leigh I, Lane EB. Patterns of cytokeratin expression in human gingival epithelia. J Periodontal Res. 1991 Nov;26(6):468–478. doi: 10.1111/j.1600-0765.1991.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 29.Eichner R, Bonitz P, Sun TT. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984 Apr;98(4):1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990 Sep;97(Pt 1):39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Bonan PR, Kaminagakura E, Pires FR, Vargas PA, Almeida OP. Cytokeratin expression in initial oral mucositis of head and neck irradiated patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Feb;101(2):205–211. doi: 10.1016/j.tripleo.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996 Feb;132(3):381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desdouets C, Sobczak-Thepot J, Murphy M, Brechot C, Cyclin A. Cyclin A: function and expression during cell proliferation. Prog Cell Cycle Res. 1995;1:115–123. doi: 10.1007/978-1-4615-1809-9_9. [DOI] [PubMed] [Google Scholar]
- 36.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. Embo J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banks EB, Crish JF, Eckert RL. Transcription factor Sp1 activates involucrin promoter activity in non-epithelial cell types. Biochem J. 1999 Feb 1;337(Pt 3):507–512. [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 39.Bernerd F, Magnaldo T, Darmon M. Delayed onset of epidermal differentiation in psoriasis. J Invest Dermatol. 1992 Jun;98(6):902–910. doi: 10.1111/1523-1747.ep12460344. [DOI] [PubMed] [Google Scholar]
- 40.Bloor BK, Seddon SV, Morgan PR. Gene expression of differentiation-specific keratins (K4, K13, K1 and K10) in oral non-dysplastic keratoses and lichen planus. J Oral Pathol Med. 2000 Sep;29(8):376–384. doi: 10.1034/j.1600-0714.2000.290803.x. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos JN, de Sousa SO, Nunes FD, Sotto MN, de Araujo VC. Altered cytokeratin expression in actinic cheilitis. J Cutan Pathol. 2003 Apr;30(4):237–241. doi: 10.1046/j.0303-6987.2002.028.x. [DOI] [PubMed] [Google Scholar]
- 42.Bloor BK, Tidman N, Leigh IM, Odell E, Dogan B, Wollina U, et al. Expression of keratin K2e in cutaneous and oral lesions: association with keratinocyte activation, proliferation, and keratinization. Am J Pathol. 2003 Mar;162(3):963–975. doi: 10.1016/S0002-9440(10)63891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetzels RH, Kuijpers HJ, Lane EB, Leigh IM, Troyanovsky SM, Holland R, et al. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991 Mar;138(3):751–763. [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan PR, Su L. Intermediate filaments in oral neoplasia. I. Oral cancer and epithelial dysplasia. Eur J Cancer B Oral Oncol. 1994 May;30B(3):160–166. doi: 10.1016/0964-1955(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 45.Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005 Sep–Oct;13(5):468–479. doi: 10.1111/j.1067-1927.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 46.Thomson PJ, Goodson ML, Booth C, Cragg N, Hamadah O. Cyclin A activity predicts clinical outcome in oral precancer and cancer. Int J Oral Maxillofac Surg. 2006 Nov;35(11):1041–1046. doi: 10.1016/j.ijom.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Lundqvist K, Schmidtchen A. Cyclin A expression in chronic leg ulcers. Acta Derm Venereol. 2006;86(1):61–62. doi: 10.1080/00015550510044172. [DOI] [PubMed] [Google Scholar]
- 48.Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuber M. Characterization of a cell cycle-dependent nuclear autoantigen. Mol Biol Rep. 1996;23(3–4):197–203. doi: 10.1007/BF00351169. [DOI] [PubMed] [Google Scholar]


