Abstract
Tuberculosis (TB) remains a major health threat, killing near to 2 million individuals around this globe, annually. The sole vaccine developed almost a century ago, provides limited protection only during childhood. After decades without the introduction of new antibiotics, several candidates are currently undergoing clinical investigation. Curing TB requires prolonged combination chemotherapy with several drugs. Moreover, monitoring the success of therapy is questionable due to the lack of reliable biomarkers. To substantially improve the situation, a detailed understanding of the crosstalk between human host and the pathogen Mycobacterium tuberculosis (Mtb) is vital. Principally, Mtb’s enormous success is based on three capacities: First, reprogramming of macrophages after primary infection/phagocytosis in order to prevent its own destruction; second, initiating the formation of well-organized granulomas, comprising different immune cells to create a confined environment for the host–pathogen standoff; third, the capability to shut down its own central metabolism, terminate replication and thereby transit into a stage of dormancy rendering itself extremely resistant to host defense and drug treatment. Here we review the molecular mechanisms underlying these processes, draw conclusions in a working model of mycobacterial dormancy and highlight gaps in our understanding to be addressed in future research.
Keywords: granuloma, persistence, host immune response, latency
Introduction
The etiology of tuberculosis (TB) – one of the most devastating diseases of humankind – was first elucidated by Robert Koch (1843–1910) in 1882 (Kaufmann and Winau, 2005; Koch, 1882; Koch, 1932). Koch developed a specific staining method based on methylene blue combined with brown counterstaining of host tissues with vesuvin for the causative agent Mycobacterium tuberculosis (Mtb) which allowed visualization of bacteria not only in cultures, but also in tissues (Box 1). Precisely 130 years down the road his diagnostic method is still in use virtually unchanged. Potent antibiotics have been discovered and public health systems improved significantly in many parts of the world since Koch’s times. Nevertheless, Mtb remains as deadly as it was, claiming nearly 2 million lives annually and exploiting an estimated 2 billion as reservoir of latently Mtb-infected (LTBI) individuals (Dye et al., 1999; Yew and Leung, 2008). These figures were calculated when our globe hosted about 6 billion people. In 2011 when we reached the 7 billion mark, about 2.3 billion LTBI individuals appear more likely. Currently, up to 9 million new cases of TB arise each year, more than ever before (Dye and Williams, 2010). Most cases are not due to new infections but through reactivation of dormant Mtb residing in LTBI hosts (Figure 1).
Box 1. Profile of Mycobacterium tuberculosis (Mtb).
The bacillus causing TB in humans belongs to the genus Mycobacterium that includes several other obligate human pathogens, most importantly M. leprae (leprosy), M. africanum (TB-like symptoms, lower pathogenicity), and M. bovis (primarily TB in cattle). The family of pathogenic mycobacteria arose from soil-dwelling ancestors. They most likely became pathogens to animal and human hosts during domestication of animals about 10,000 years ago (Smith et al., 2009). At 37°C and under optimal availability of oxygen and nutrients a single Mtb organism has a generation time of 18–24 h and forms a white to light yellow colony on agar within 3–4 weeks. The aerobic-to-facultative anaerobe, Gram-positive pathogen is surrounded by an impermeable and thick cell wall/capsule that is made of peptidoglycans, polysaccharides, unusual glycolipids and lipids mainly consisting of long-chain fatty acids, such as mycolic acid. Unlike many other bacteria, Mtb does not form spores but has the capacity to become dormant – a non-replicating state characterized by low metabolic activity and phenotypic drug resistance. Note that phenotypic drug resistance is related to a specific physiologic state and independent from genetic mutations. Mtb is typically visualized by Ziehl-Neelsen (acid-fast) staining and appears as a rod-shaped red bacillus. The GC-rich (65.6%) 4.4-Mbp genome of Mtb is one of the biggest among the bacteria and encodes about 4,000 predicted proteins [http://genolist.pasteur.fr/TubercuList/, (Cole et al., 1998); integrated platform for TB research: http://www.tbdb.org/, (Reddy et al., 2009)]. M. bovis BCG (attenuated form of M. bovis) and M. smegmatis are non-pathogenic and therefore common surrogates for Mtb in research. The former is used as vaccine in children with partial success. Mtb is typically diagnosed by microscopy in sputum of active TB patients. A regime of several drugs is available to effectively cure the disease by 6–9 months of combination therapy. Incomplete treatment or non-compliance of patients often leads to drug-resistant Mtb, which is conferred by genetic mutations.
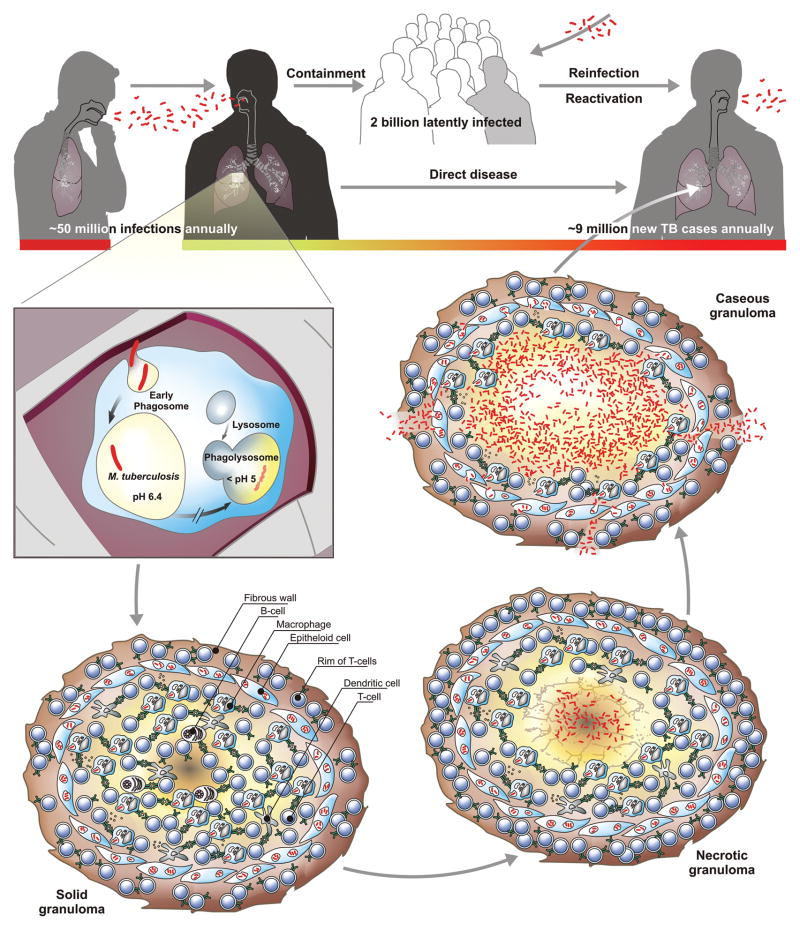
Figure 1. Transmission and pathology of tuberculosis (TB).
Transmission of TB between individuals occurs via aerosols of infectious bacilli. An estimated 50 million infections per year maintain a pool of ~2 billion latently infected individuals. In a few cases, infection directly transforms to active TB. Together with reactivation and reinfection this gives rise to approximately 9 million new TB cases annually. Upon inhalation of such droplets the pathogen reaches lung airways and is phagocytosed by alveolar macrophages. The infected host cell induces a localized proinflamatory response that attracts mononuclear cells and T lymphocytes to build up a granuloma, the hallmark tissue reaction of TB. Healthy individuals can control the pathogen at this stage but remain latently infected and thus at risk of reactivation lifelong. Granuloma maturation (solid, necrotic, caseous) occurs at different velocities and typically culminates in coexistence of all lesion forms during active TB. The caseating granuloma loses solidity due to decay of its center into a structureless accumulation of host cell debris, the caseum. Mycobacterium tuberculosis (Mtb) grows to high numbers, is released into airways and coughed out as contagious aerosol.
Primary infection can: (1) progress towards the active disease; (2) be contained as latent infection; (3) be eradicated by the host’s immune system. Less than 10% of infected individuals develop active TB during their lifetime. It is impossible to predict who can contain latent infection throughout lifetime and remain healthy, and who will develop active TB at some point. However, the risk of active disease is increased in immunocompromising situations such as during anti-tumor necrosis factor (TNF) therapy of patients with chronic inflammatory diseases, by diabetes/obesity or by co-infection with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) (Barry, III et al., 2009). Other risk factors include alcoholism and poor nutrition. Multiple factors are likely involved in defining overall risk of TB and the genetic make-up of both host and pathogen play a decisive role. Thus, biomarkers that would allow prognosis of TB reactivation in healthy individuals with LTBI would be of tremendous value.
Today, intervention measures for TB control are available. We have at hand numerous drugs to cure the disease, diagnostics to identify patients and a vaccine to prevent severe forms of childhood TB. Yet, these measures are insufficient for the following reasons:
Diagnosis of TB in low-income countries is correct in only an estimated half of all TB cases.
Treatment of TB requires 3–4 drugs given for 6 months or longer. Frequently, compliance is poor and premature termination of drug therapy often results in emergence of resistant strains. Today, an estimated 50 million individuals harbor multidrug-resistant (MDR)-Mtb of whom 500,000 fall ill annually. Even worse, strains of virtually untreatable extensively drug-resistant (XDR) Mtb are on the rise and XDR-TB has already been notified in 58 countries. Even totally drug-resistant (TDR)-TB has been described (Velayati et al., 2009).
The current vaccine, M. bovis Bacillus Calmette-Guérin (BCG), protects against severe forms of childhood TB, but fails to protect against adult pulmonary TB, which has become the most prevalent form of the disease today. Hence, BCG does not impact transmission of Mtb.
Many definitions in infectious disease research are difficult to apply in TB. Needless to say, the etiologic agent Mtb is a true pathogen and not an opportunistic microbe, even though active disease only develops in the minority of infections. Virulence describes the capability of the pathogen to cause disease in quantitative terms. High virulence, therefore, is often related to marked severity of disease and vice versa. Yet the decisive survival factor of Mtb is its capacity to persist in the host for long periods of time, both during non-contagious LTBI and contagious active TB before it is spread. To further complicate the situation, pathogenicity of TB is largely influenced by the host immune response. Hence, a discussion of the disease without regard for the host would remain incomplete. Although, our review of the molecular mechanisms of TB is oriented toward the pathogen’s perspective, we will consider host influences where appropriate.
Infection of the alveolar macrophage
Macrophages operate as prime defense cells against microbial intruders (Deretic et al., 2009; Liu and Modlin, 2008; Nathan and Shiloh, 2000). These microbes are ingested by phagocytosis, a process consisting of membrane invaginations finally culminating in phagosome formation (Aderem and Underhill, 1999). This organelle is part of the intracellular trafficking and transport system and the site to which the entire arsenal of host defense is targeted (Gruenberg and Stenmark, 2004; Rothman and Wieland, 1996; Schekman, 1994). Microbes captured in the phagosome experience increasing acidification, reactive oxygen and nitrogen species (ROS and RNS), hydrolytic enzymes and cationic antimicrobial peptides. Acidic pH inside the maturing phagosome activates enzymes that degrade bacterial lipids and proteins (Flannagan et al., 2009a; Huynh and Grinstein, 2007). Simultaneously microbial metabolism is suppressed by such conditions. ROS and RNS generated by the phagosomal enzymes NADPH phagocyte oxidase and inducible nitric oxide synthase (iNOS), damage captured microbes by modification of their DNA, lipids, thiols, tyrosine side chains and active centers of metal-dependent proteins (Fang, 2004). Further damage of ingested pathogens is incurred by cationic antimicrobial peptides via permeabilization of their cell membrane (Flannagan et al., 2009a; Purdy and Russell, 2007). It must be kept in mind that responses in mice, one of the most common model organisms, might be different from humans with respect to iNOS, ROS and RNS. The final steps of bacterial destruction and clearance require phagolysosome fusion. All of the described destruction pathways are influenced by the host’s immune status. Macrophage activation via cytokines, notably, interferon-gamma (IFN-γ), for instance, allows these host cells to control their intracellular predators (Cooper et al., 1993; North and Jung, 2004).
Phagosomal content can then be further processed towards the antigen presentation pathway (Pieters, 1997; Wolf and Ploegh, 1995). Components of Mtb, notably secreted proteins, are processed by macrophages and dendritic cells (DCs). Resulting peptides are loaded on gene products of the major histocompatability complex (MHC) and in this way T cells are instructed to allow for an appropriate adaptive immune response (Amigorena et al., 1994; Tulp et al., 1994; West et al., 1994). Thus, T lymphocytes are critical for control of Mtb during latent infection. Failure of T cells to maintain protective immunity promotes reactivation of TB.
Humans become infected with Mtb by inhaling minute aerosol droplets carrying a small number of bacteria (Kaufmann, 2001) (Figure 1). At the site of infection, the lung, Mtb bacilli are phagocytosed by alveolar macrophages. These cells are programmed to combat microbial intruders and to ultimately destroy them. However, Mtb manages to escape eradication by macrophages and survives within these cells (Armstrong and Hart, 1975; Kaufmann, 2001; Russell, 2001). The unique composition of the mycobacterial cell wall and envelope likely enables the tubercle bacillus to enter macrophages by employing multiple receptors such as Fc-, complement- or mannose-receptors and the DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) (Brennan and Nikaido, 1995; Cambi et al., 2005; Ernst, 1998; Greenberg, 1999). Some receptors allow silent entry (CR), others induce defense mechanisms (FcR). Once inside the resting macrophage, Mtb impairs phagosome maturation (Axelrod et al., 2008; Katti et al., 2008; Robinson et al., 2007; Russell et al., 2009; Vergne et al., 2003; Vergne et al., 2005; Walburger et al., 2004). Principally phagosome maturation is a highly complex process in which the phagosome harboring a particle other than Mtb constantly interacts with the recycling endosome, secretory organelles, multivesicular bodies, and the endoplasmic reticulum (Desjardins, 2009). Following the oxidative burst the phagosome gets acidified, a process lasting between 10 and 20 minutes. Acidification is mediated by proton pumps that reduce the neutral pH to an acidic pH of ca. 5.0 (Yates et al., 2005). Mtb arrests this process and therefore also down-stream events (Sturgill-Koszycki et al., 1994). In contrast to Mtb-containing phagosomes, those harboring inert particles go on to fuse with the lysosome within an hour to 1.5 hours. Acidification of the organelle is arrested by Mtb at pH 6.4, which is near the neutral pH and is significantly higher than in the terminal phagolysosome at pH 4.5–5.0 (MacMicking et al., 2003; Sturgill-Koszycki et al., 1996; Yates et al., 2005). IFN-γ activation of macrophages promotes delivery of Mtb into the mature phagolysosome, as shown by colocalisation with green fluorescent bacilli, and probably remains viable in this extremely hostile environment (Via et al., 1998). Some Mtb mutants that fail to prevent phagosome maturation do survive in the resting macrophage, while others are attenuated (MacGurn and Cox, 2007; Pethe et al., 2004). An alternative strategy of Mtb has been established more recently: its egress from the phagosome into the cytosol of macrophages (Behar et al., 2010; van der Wel et al., 2007). Additional host defense mechanisms include apoptosis and autophagy (Behar et al., 2010; Deretic, 2010; Levine et al., 2011). Apoptosis is a highly regulated process mediated by host mechanisms which likely contributes to host protection. In contrast, necrosis is driven by exogenous insults which might benefit the pathogen rather than the host. Studies on experimental TB in mice have provided evidence of such an association (Pan et al., 2005). Virulent Mtb inhibit apoptosis by a number of antiapoptotic genes and more recent evidence suggests a role of prostaglandin E2 in this mechanism (Behar et al., 2010). Autophagy is an essential mechanism for host cell integrity that can also serve as defense mechanism against bacterial pathogens (Deretic, 2010; Levine et al., 2011). In TB contribution of autophagy to protection has been described (Alonso et al., 2007). In sum, the intracellular survival stratagem of tubercle bacilli not only comprises active manipulation of host defense mechanisms to neutralize and counteract a highly aggressive armamentarium of activated macrophages but also robust resistance against assault.
Adaptation of Mtb to the intracellular environment of macrophages
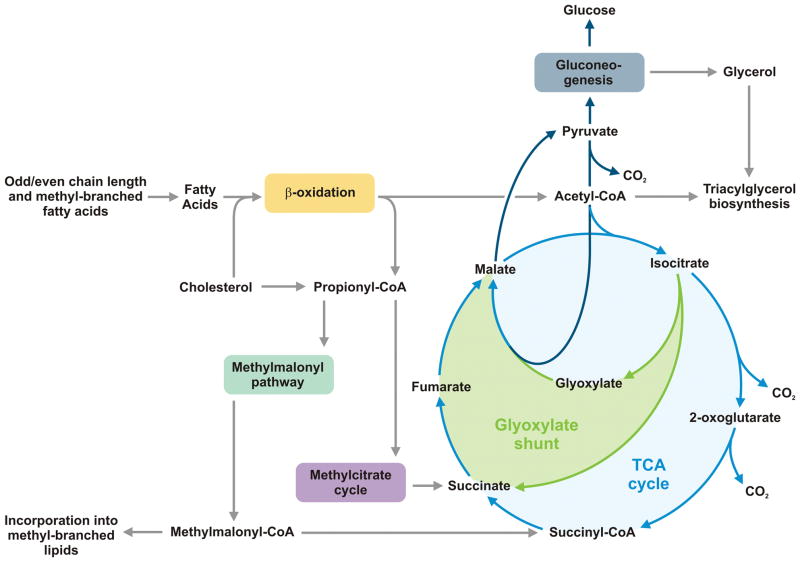
Mtb is shielded from the environment by a robust cell wall (Box 1). Upon phagocytosis by host cells, Mtb experiences drastic environmental changes and therefore has to realign its metabolism to assure survival. Genome-wide microarray techniques to study Mtb’s transcriptional response to this transition have provided deeper insights into the nature of the phagosomal environment, which was suggested to be nitrosative, oxidative, low in oxygen tension and limited in nutrients (Schnappinger et al., 2003). Additionally, the pathogen upregulated genes involved in lipid metabolism confirming previous evidence that lipids are critical for virulence of Mtb (Brzostek et al., 2007; Chang et al., 2009; McKinney et al., 2000; Movahedzadeh et al., 2004; Nesbitt et al., 2010). Isocitrate lyase (Icl) was identified as gate enzyme of the glyoxylate shunt, a short-cut of the tricarboxylic acid (TCA) cycle, bypassing steps of carbon loss by CO2 formation. The glyoxylate cycle is mobilized in Mtb growing on fatty acids as exclusive carbon source, and also during chronic infection in mice suggesting that lipids are accessible as nutrients in vivo (McKinney et al., 2000). Metabolic pathways that are relevant during infection are shown in Figure 2. Although the exact nature of carbon sources utilized during infection remains elusive, Mtb has been shown to metabolize host-derived cholesterol (Pandey and Sassetti, 2008). Disruption of mce4 encoding a cholesterol transporter, results in failure of Mtb to maintain chronic infection in mice while retaining full virulence during the acute phase comparable to observations made for an Icl-deficient mutant (McKinney et al., 2000; Mohn et al., 2008; Pandey and Sassetti, 2008). Recent data demonstrate that Mtb residing in phagosomes of macrophages utilizes triacylglycerol from the host cell to be stored in the form of intracellular lipid droplets (Daniel et al., 2011). Catabolism of cholesterol, odd-chain fatty acids, methyl-branched fatty acids and amino acids funnels into propionyl-CoA, a C3 intermediate, which is toxic in excess (Savvi et al., 2008; Yang et al., 2009). However, propionyl-CoA toxicity can be avoided by condensing the C3 body with oxaloacetate to form succinate and pyruvate by the 2-methylcitrate cycle (Figure 2). Intriguingly, both mycobacterial enzymes Icl-1 and Icl-2, can act as 2-methylcitrate lyase (Munoz-Elias et al., 2006). Thus, mycobacterial Icl plays a critical dual role during infection: (1) to bypass carbon loss by using the glyoxylate shunt under limited nutrient availability, and (2) to prevent excessive accumulation of toxic propionyl-CoA. Recent studies performed with steady state chemostat cultures have demonstrated the importance of Icl in slow-growing Mtb metabolizing glycerol as main carbon source. Under such conditions, anapleuric reactions prevailed while the pathogen was also capable of carbon dioxide fixation as demonstrated by isotope flux (Beste et al., 2011). Propionyl-CoA metabolization can also be performed by the methylmalonyl-CoA pathway ending up in methylmalonyl-CoA, which can either be converted into succinyl-CoA by a vitamin B12-dependent mutase or directly incorporated into methyl-branched fatty acids (Savvi et al., 2008) (Figure 2). These fatty acids are found among the large family of unique mycobacterial lipids which build up the pathogen’s cell envelope (Brennan and Nikaido, 1995; Jackson et al., 2007). Typically, loss of cell wall components leads to decreased virulence (Glickman et al., 2000; Makinoshima and Glickman, 2005). In conclusion, propionyl-CoA detoxification is extremely critical for Mtb in vivo. The asymmetric cleavage of isocitrate by Icl produces glyoxylate, which is converted into malate and succinate. The TCA cycle intermediate malate can be used to generate pyruvate and further, to replenish the pool of glycolytic intermediates by gluconeogenesis (Figure 2). Such intermediates are required to produce the essential building blocks of proteins, DNA, and the cell wall. Gluconeogenesis is critical throughout TB infection in mice and thus, might be relevant in dormancy (Marrero et al., 2010).
Figure 2. Metabolic pathways of Mtb important during infection.
Growing evidence suggests that pathogenic mycobacteria rely on lipids in vivo. Degradation of fatty acids by β-oxidation leads to acetyl-CoA (C2) and for uneven chain length or methyl-branched fatty acids additionally to propionyl-CoA (C3). The pathogen can directly metabolize C2 units via the tricarboxcylic acid (TCA) cycle while excessive accumulation of toxic propionyl-CoA is prevented by two metabolic routes: (1) the methylcitrate cycle and (2) the methylmalonyl pathway. The products of both pathways can enter the TCA cycle, either directly (succinate) or after conversion to succinyl-CoA (methylmalonyl-CoA). Moreover, methylmalonyl-CoA is a building block of methyl-branched lipids. Mycobacterial isocitrate lyase (Icl) plays a key role in the methylcitrate cycle and the glyoxylate shunt. The intermediate glyoxylate can be used terminally to generate pyruvate (via malate), from which glycolytic substrates can be replenished by gluconeogenesis. C3 bodies of the glycolysis/gluconeogenesis and acetyl-CoA are required for biosynthesis of triacylglycerol, a lipid relevant during dormancy.
Mtb tolerates low pH (i.e., 4.5–5.4) inside the phagolysosome of INF-γ-activated macrophages (Schaible et al., 1998; Via et al., 1998). The lack of complete acidification in Mtb-infected resting macrophages is likely–at least in part–caused by the exclusion of the phagosomal proton ATPase and by secretion of mycobacterial urease, an enzyme producing neutralizing ammonia from urea (Grode et al., 2005; Reyrat et al., 1995; Sturgill-Koszycki et al., 1996). Yet, Mtb is exposed to acidic conditions in vivo as evidenced by the fact that the first-line TB drug pyrazinamide kills the pathogen only at low pH in vitro (Zhang and Mitchison, 2003). In further support of this notion acid-sensitive Mtb mutants are attenuated in mice, and transcriptional analysis of the bacillus residing inside activated macrophages revealed upregulation of pH-responsive genes (Buchmeier et al., 2000; Raynaud et al., 2002; Rohde et al., 2007; Vandal et al., 2008). Interestingly, most acid-sensitive Mtb mutants show defects in genes associated with cell wall biogenesis (Vandal et al., 2009). This unique lipid-rich permeability barrier has been suggested to provide effective protection to protons more than 100 years ago (Metchnikoff, 1905). It is therefore not surprising that acid-sensitive mutants also confer hypersensitivity to detergents or lipophilic antibiotics due to increased cell wall permeability (Vandal et al., 2008). Although, a pH-sensing adenylate cyclase (Rv1264) has been described in Mtb, it turned out to be non-essential in vivo (Dittrich et al., 2006; Tews et al., 2005). More recently the membrane-associated serine protease Rv3671c has been characterized. Even though the precise functions of the protein remain elusive the authors elegantly showed that an Rv3671c mutant failed to maintain a neutral intrabacterial pH in acidic culture medium as well as in the late (acidic) phagolysosome of activated macrophages (Vandal et al., 2008). Moreover, the mutant was attenuated in mice suggesting that acid resistance is critical for virulence of the tubercle bacillus. A recent study demonstrated that aprABC, a locus unique to pathogenic mycobacteria, is involved in adaptation of Mtb to the phagosomal low pH environment. Disruption of aprABC conferred a defect in intracellular growth of the pathogen and influenced lipid abundance of intracellular stores and cell wall (Abramovitch et al., 2011). In sum, Mtb is resistant to even elevated acidic stress in the late phagolysosome compartment of macrophages at conditions that are lethal for many other microbial pathogens (Flannagan et al., 2009a; Huynh and Grinstein, 2007).
The defense repertoire any bacterial intruder experiences in the phagosome of an activated macrophage is not limited to low pH but also includes ROS and RNS. The enzyme phagocyte oxidase (NOX2) transfers electrons from cytosolic NADPH to phagosomal oxygen to form superoxide anions. These highly reactive anions dismutate into hydrogen peroxide and finally produce toxic hydroxyl radicals, which are members of the ROS family (Bedard and Krause, 2007). Accordingly, this reaction is often referred to as ‘oxidative burst’. In monocytes and neutrophils chlorination further adds to the toxicity of ROS (Bedard and Krause, 2007). RNS are produced by iNOS, an enzyme that generates nitrate and nitrite. The latter intermediate reacts at low pH to nitrous acid that forms nitric oxide and nitrogen dioxide, two highly reactive radicals (Nathan and Shiloh, 2000). Finally, the two molecules, nitric oxide and superoxide, form the toxic peroxynitrite (Beckman et al., 1990; Bogdan, 2001; Nathan and Ehrt, 2004). To avert toxicity caused by ROS and NOS, Mtb follows a dual strategy of detoxification and damage repair.
The mycobacterial enzyme catalase peroxidase encoded by katG converts hydrogen peroxide into water and oxygen. Accordingly, a katG loss-of-function Mtb mutant showed hypersensitivity to hydrogen peroxide in vitro (Ng et al., 2004). In NOX2-deficient mice the mutant was fully virulent while a unique phenotype of transient attenuation was reported in iNOS−/− mice: initial growth was followed by rapid decline and a lag phase of about 6–8 weeks characterized by stable bacterial burden in lungs, after which replicative activity was resumed. However, the molecular mechanisms of the observed phenomena remain unclear. Several other mycobacterial genes have been implicated in ROS and RNS detoxification [in-depth reviewed by (Ehrt and Schnappinger, 2009)]:
sodA and sodC, both encoding a superoxide dismutase (Dussurget et al., 2001; Edwards et al., 2001; Piddington et al., 2001; Sassetti and Rubin, 2003).
secA2, part of an accessory export system (Braunstein et al., 2003; Kurtz et al., 2006; Rigel and Braunstein, 2008).
mshA, required for mycothiol biosynthesis (Vilcheze et al., 2008).
cysH, involved in sulfur assimilation (Senaratne et al., 2006).
ahpC, ahpD, dalT, lpd, encoding the multifunctional NADH-dependent peroxidase and peroxynitrite reductase (Bryk et al., 2008; Shi and Ehrt, 2006).
Although the underlying molecular mechanisms require further elucidation, it is clear that Mtb employs a large variety of counterstrategies to detoxify ROS and RNS.
The second principal strategy to oppose effects of highly reactive intermediates includes repair of the damage caused, or degradation of affected biomolecules and their rapid replacement via de novo synthesis. Proteasomes are multi-protein complexes that degrade peptides by multiple proteolyic activities (Etlinger and Goldberg, 1977; Lowe et al., 1995). In Mtb, two putative accessory factors PafA and Mpa are involved in coupling the prokaryotic ubiquitin-like protein Pup to peptides that are destined for turnover and recognition of Pup-tagged proteins, respectively (Pearce et al., 2008; Striebel et al., 2009; Sutter et al., 2009). Mpa is an ATPase likely involved in unfolding and delivery of proteins into the proteolytic core of proteasomes (Wang et al., 2009). Mutations in the respective genes mpa and pafA increase the sensitivity of the tubercle bacillus to RNS (Darwin, 2009). Disruption of the mpa gene reduced virulence of Mtb in wild type mice, which was less profound in iNOS−/− mice. Conditional silencing of the essential genes prcB and prcA which encode proteolytic core proteins of the proteasome not only conferred sensitivity to RNS but also impaired survival of Mtb during chronic infection in mice (Gandotra et al., 2007). In sum, proteasome-mediated protein turnover becomes critical during RNS-related stress. Two different but not mutually exclusive scenarios or combinations, could explain these result: (1) degradation of a transcriptional repressor that controls expression of proteins required for synthesis of antioxidants; (2) removal of irreversibly damaged proteins with toxic potential (Darwin, 2009).
More recently, transcriptional profiling of Mtb and human macrophages during infection provided evidence for heavy metal poisoning (Botella et al., 2011; Tailleux et al., 2008). In particular, ctpC encoding a putative zinc efflux pump in mycobacteria was strongly induced upon infection suggesting exposure of the bacillus to zinc ions inside the phagosomal compartment. Indeed disruption of the ctpC gene rendered Mtb hypersensitive to zinc. While infection progresses zinc ions are quickly released from stores inside host cells and translocated to the phagosome. Thus, zinc poisoning is likely exploited by macrophages to destroy Mtb. Indeed, zinc is known to be of particular importance for the immune system playing multiple roles for instance in defense and signaling (Haase and Rink, 2009; Rink and Gabriel, 2000). Although not yet directly shown, accumulating evidence suggests that Mtb counteracts the stressor zinc by expression of an appropriate efflux pump (Botella et al., 2011).
Inside the mature phagolysosome, Mtb experiences the permeabilizing properties of cationic antimicrobial peptides (CAMPs) (Flannagan et al., 2009b; Purdy and Russell, 2007). The positively charged CAMPs – cathelicidin, hepcidin and ubiquitin-related peptides – gain their bactericidal activity by disrupting the negatively charged bacterial cell wall (Alonso et al., 2007; Liu et al., 2007; Sow et al., 2007). Microbes lower their affinity to CAMPs by reduction of their negative surface charge (Peschel and Sahl, 2006). The Mtb plasma membrane contains a positively charged lipid consisting of phosphatidyl glycerol linked to lysine moieties. Its generation requires the lysine transferase LysX (Maloney et al., 2009). Sensitivity to positively charged antibiotics and a specific CAMP of neutrophils was increased in an Mtb lysX loss-of-function mutant. Virulence of this mutant was lowered in mice and guinea pigs demonstrating a critical role of LysX in vivo (Maloney et al., 2009). The unique impermeable cell envelope of Mtb represents a physical barrier for CAMPs. Expression of the major porin of M. smegmatis, MspA, in Mtb, which does not encode an ortholog, increases membrane permeability (Mailaender et al., 2004). Concomitantly such strains become more sensitive to ubiquitin-derived CAMPs (Purdy et al., 2009). Probably the most obvious strategy of pathogenic mycobacteria to protect themselves from phagosomal assault is to translocate from the hostile organelle to the cytosol of host cells. Such behavior has been suggested for Mtb and M. leprae (van der Wel et al., 2007). If true this could have deep implications, not only for the fate of bacilli in host cells, but also for granuloma formation. Although, the host macrophage has developed a remarkable arsenal of bactericidal mechanisms, during millennia of co-evolution, Mtb has learned to survive in this hostile intracellular environment. As a result the co-evolutionary tie was shifted to the tissue level, namely, the formation of a remarkable self-organizing capsular structure for bacterial containment, the granuloma (Reece and Kaufmann, 2011).
Existence of Mtb inside granulomas
Infected macrophages migrate and thereby transport Mtb from the airways into pulmonary tissue sites. There, an inflammatory focus is formed comprising of infected macrophages and freshly immigrant monocytes (Figure 1). The primary lesion matures into a granuloma, the hallmark of TB, while some infected cells disseminate to seed secondary lesions in the lung. In the majority of individuals the pathogen is controlled at this stage by the immune system and does not spread further: LTBI is established. The solid granuloma is not only the site of Mtb containment during latency but also the source of tissue damage at the early stage of disease (Reece and Kaufmann, 2011). In other words, it is the histological correlate of both protection and pathology. In humans, the granuloma shows high plasticity and three major types can be distinguished. These are (1) solid granulomas which contain Mtb; (2) necrotic granulomas typical for early stages of active TB; (3) caseous granulomas during end-stage or severe TB. These different stages are not distinct entities but form a continuum.
Solid granulomas prevail during LTBI. These highly structured tissue reactions are comprised of mononuclear phagocytes of different developmental stages, DCs, as well as T and B lymphocytes. Although T lymphocytes are the critical mediators of protection in TB, B lymphocytes are also abundant. Often, lymphocytes form an outer ring whereas in the central parts mononuclear phagocytes, fibroblasts and DCs predominate. The solid granuloma is typically encircled by a fibrotic wall that separates it from surrounding tissue. The burden of Mtb inside solid granulomas is low. Most likely these bacilli are in a stage of dormancy, characterized by low metabolic activity with non- to low-replicating persistence. Often, such bacteria are difficult to grow under normal culture conditions and therefore have been termed viable but not culturable (VBNC).
The necrotic granuloma remains well structured, but the center becomes increasingly necrotic, i.e., composed of solid cell detritus which is often hypoxic. Later Mtb organisms can be resuscitated: they start replicating and become metabolically active. In caseous granlomas the center becomes liquefied leading to cavity formation. The structure of these granulomas wanes. Evidence has been presented for a harmful role for polymorphic neutrophilic granuloyctes (Lowe et al., 2011). In cavitary lesions, high oxygen content is reestablished. Moreover, the caseous material provides a fertile source of nutrients promoting growth of the pathogen up to some trillion organisms. Finally, Mtb finds access to blood capillaries and the alveolar space paving the way not only for dissemination to other organs but also for transmission to other individuals.
During active TB, different stages of granulomas coexist and provide a multitude of diverse microenvironments to which the pathogen has to adapt. Hence, the bacillus is found in different areas of the granulomas, intracellularly within the rim of host mononuclear phagocytes, some DCs and perhaps fibroblasts as well as extracellularly in the caseous center consisting of host cell debris. It has been proposed that unfavorable conditions inside the granuloma, such as nutrient limitation and low oxygen tension, trigger the metabolic downshift of subpopulations of Mtb to dormancy. Since most TB drugs target functions essential for growth they fail to eradicate non-replicating bacilli. This could explain the prolonged treatment time required to cure disease. In vitro models of dormancy have been developed to study non-replicating persistence of Mtb. As early as 1933, Loebel and colleagues observed that carbon starvation terminates growth of the tubercle bacillus and causes a drastic drop in respiration indicating a low metabolic rate (Loebel et al., 1933). More recent work confirmed that nutrient-starved non-replicating bacilli undergo a global downregulation of metabolic genes including those involved in respiration (Betts et al., 2002). Such bacilli are extremely tolerant to TB drugs (Xie et al., 2005). However, nutrient-starved Mtb remains sensitive to inhibition of NADH dehydrogenase 2, a single protein enzyme that can serve as alternate entry point of electrons into the electron transport chain but lacks proton translocation capacity (Gengenbacher et al., 2010; Teh et al., 2007; Xie et al., 2005; Yano et al., 2006). Other characteristics of the pathogen analyzed in this in vitro dormancy model include significantly reduced intracellular ATP levels and very low but continuous respiration. The glyoxylate shunt enzyme Icl is essential for survival of nutrient-starved non-replicating Mtb (Gengenbacher et al., 2010). Promotion of the glyoxylate cycle under limited nutrient access is in the best interest of the bacillus, since no carbon is lost by CO2-formation in contrast to the citrate cycle. Icl is required for maintenance of a chronic infection in the mouse model of TB suggesting a relevant role of nutrient limitation in vivo (McKinney et al., 2000).
The influence of oxygen shortage on Mtb has been extensively studied. In 1996 Wayne and coworkers introduced an Mtb-in vitro dormancy model based on gradual oxygen depletion (Wayne and Hayes, 1996). The pathogen passes through two phases of declining metabolic activity to dormancy and phenotypic drug resistance. More detailed physiological characterization revealed reduction of intracellular ATP in hypoxic non-replicating bacilli and sensitivity of the pathogen to further depletion, observations which later on were also made for nutrient starved non-replicating Mtb (Gengenbacher et al., 2010; Rao et al., 2008). Even though both models generate quiescent organisms by contrary conditions – carbon depletion in an oxygen-rich environment versus oxygen starvation in a nutrient-rich medium – physiological overlaps identified could qualify as core features of dormancy. In line with general upregulation of lipid metabolism genes during oxygen starvation of mycobacteria, hypoxic non-replicating BCG accumulates neutral lipid triacylglycerol to form visible intracellular droplets. Importantly, such lipid stores are required for regrowth of the hypoxic non-replicating organism in nutrient-rich medium (Low et al., 2009). Accumulation of triacylglycerol droplets might therefore be important during dormancy and could be useful for identification of dormant mycobacteria.
On the genetic level the adaption to changes in oxygen availability is mediated by the DosS/DosT-DosR regulatory complex that controls roughly 50 genes (Boon and Dick, 2002; Park et al., 2003). In other words, the DosR regulon governs metabolic shift of Mtb from aerobic to anaerobic functioning, ensures survival of the bacillus during hypoxia-induced in vitro dormancy and controls reversal to replication upon re-exposure to oxygen (Leistikow et al., 2010; Rustad et al., 2009). Furthermore, dosR responds to nitric oxide and carbon monoxide (Kumar et al., 2007). Yet, disruption of the dosR gene only slightly affects survival of the pathogen in different animal models such as mouse, guinea pig or rabbit; the molecular basis of this finding remains unclear (Converse et al., 2009). The role of hypoxia in vivo was impressively analyzed by Via and colleagues who introduced the hypoxia-activated compound pimonidazole to different experimental animal species and directly measured oxygen tension in granulomas of guinea pigs, rabbits and non-human primates (Via et al., 2008). This study revealed that low oxygen pressure could restrict growth of aerobic to microaerophilic Mtb in the hypoxic core of necrotic and solid granulomas. Note that TB histopathology of non-human primates most closely resembles active TB in humans (Leong et al., 2011). The characteristic continuum of granulomatous lesions in human TB is rarely reflected in small animal models. Guinea pigs show lesions of different types. Since they are extraordinarily susceptible to Mtb, caseous lesions predominate. Mice, the most widely used experimental animals for research on immunology and infection, develop non-hypoxic ill-structured lesions and distinct stages are not observed. To capitalize on the wealth of information available from the mouse model, mice which develop human-like TB pathology would be of great value. Recently, a mouse model that mimics the different stages of granulomas similar to human TB has been introduced. The iNOS-deficient mouse mutant, infected with Mtb developed well-structured solid granulomas, which controlled the pathogen at low to intermediate load. Neutralization of IFN-γ led to granuloma necrosis with hypoxic areas, followed by massive caseation. In this model, cathepsin G (CatG) was identified as critical effector molecule of both protection and pathology. CatG activity in turn was controlled by serpin b3 and fine-tuning of this control mechanism seems to decide whether pathology or protection prevails (Reece et al., 2010). Production of RNS by the iNOS system represents a vital antimicrobial defense mechanism but due to its strict oxygen-dependence it is likely insufficiently active in hypoxic areas of granulomas. In another murine model of TB the sst1 locus of mice has been demonstrated to prevent formation of necrotic lesions. The intracellular pathogen resistance 1-protein encoded within this locus possesses the ability to direct infected macrophages to undergo apoptosis rather than necrosis (Pan et al., 2005). Such necrotic lesions have recently been shown to be hypoxic (Harper et al., 2012). Whether ‘human-like’ mouse models have potential for broad application in TB research has yet to be determined. The widespread assumption that chronic TB infection is caused by a rather static equilibrium of slow or non-replicating bacilli has recently been questioned. Authors engineered Mtb to harbor an instable plasmid that was lost during cell division. This replication clock used to study TB infection in mice provided evidence for active replication of Mtb, not only during the acute stage, but also throughout the chronic phase of infection (Gill et al., 2009).
In vitro models aim at reproducing impacts of the host environment on Mtb. Thus the pathogen has been debarred from iron or phosphate and exposed to low concentrations of nitric oxide to mimic either the phagosome of host macrophages or the necrotic center of granulomas (Fisher et al., 2002; Ohno et al., 2003; Rifat et al., 2009). Other studies in M. bovis BCG combined different stressors in one model system to better represent the microenvironment of mycobacteria in vivo (Bryk et al., 2008). Most recently, drug-tolerant persister bacilli have been isolated from in vitro cultures of Mtb by D-cycloserine treatment. A few persisters were found during lag and early exponential phase while they made up to 1% in late exponential and stationary phase. The global transcriptome of such persisters was then profiled and compared to the transcriptomes obtained from various in vitro dormancy models. Authors identified a set of five genes upregulated in all models that probably represents a core dormancy response (Keren et al., 2011). These were acr2, encoding a heat shock protein, the transcriptional regulator gene Rv1152, pdhA, the gene of a putative pyruvate dehydrogenase subunit, a hypothetical protein encoded by Rv2517c and lat encoding an L-lysine-epsilon-aminotransferase. To date, an unusually high number of 65 toxin-antitoxin (TA) loci were identified in the genome of Mtb. A TA module produces a toxin which is detoxified by its respective antitoxin. In E. coli, a number of TA modules are relevant in dormancy (Keren et al., 2004; Vazquez-Laslop et al., 2006). Interestingly, in the recent transcriptome study of Keren et al., 10 TA loci were overexpressed in Mtb persisters suggesting their importance in survival without replication. Altogether, in vitro dormancy models of Mtb suggest that stress-related genes and alternative pathways are upregulated, while genes of central metabolic routes including glycolysis, TCA cycle, energy production and respiration are downregulated. The specific roles of distinct genes such as TA modules remain to be elucidated.
Reactivation and resuscitation
In the human host, Mtb persisting in a dormant stage causes LTBI without clinical disease. While Mtb is well equipped for persistence in the host, the term ‘persister’ is used for those Mtb organisms that are phenotypically resistant to drugs although they are in fact genetically susceptible to these antibiotics. The reason for this is probably transformation of bacteria into a non-replicating stage with low-to-absent metabolic activity – the precise conditions of dormancy. Principally, this feature underlies the so-called Cornell model of Mtb persistence in mice. In this model, animals infected with Mtb are treated with the drugs pyrazinamide and isoniazid to reduce bacterial load to a level, which is non-detectable by culture. At first sight, drug treatment achieves sterile eradication of the pathogen, but after termination of drug treatment, some bacilli recover and grow to high abundance causing reactivation of TB (McCune et al., 1966). Prolonged culture of stationary phase Mtb can generate bacteria that fail to grow to visible colonies on agar. Regrowth of these VBNC organisms was only supported in spent media taken from exponentially growing Mtb (Shleeva et al., 2002). VBNC bacilli in sputum of TB patients could lead to false-negative results of diagnostics, since those organisms are ‘invisible’ in standard cultures (Mukamolova et al., 2010). A very recent report showed that M. smegmatis (Box 1) generates cell-to-cell heterogeneity by asymmetric growth in combination with time-controlled cell division. Most importantly, distinct subpopulations showed different susceptibility to antibiotics (Aldridge et al., 2011).
Accumulating evidence suggests that regrowth of dormant Mtb is initiated by resuscitation, which is the reestablishment of metabolic and replicative activity. Resuscitation has been studied at the molecular level in Micrococcus luteus where a so-called resuscitation-promoting factor (Rpf) has been shown to induce resuscitation. Rpf orthologs of Mtb possess a conserved domain with putative lysozyme activity and therefore might cleave the peptidoglycan network that makes up the cell wall (Cohen-Gonsaud et al., 2005). Similarly, germination of spores of Bacillus anthracis begins with hydrolysis of the cell wall (Giebel et al., 2009). Recent studies in Bacillus subtilis have revealed a signaling cascade that is initiated by peptidoglycan degradation products (Shah et al., 2008). Altogether, regrowth could be initiated through a cell wall hydrolysis step but further steps involved are thus far unknown. The genome of Mtb comprises five rpf genes and it has been claimed that such genes can facilitate recovery of Mtb in sputum of patients with active TB or freeze-dried M. bovis BCG (Mukamolova et al., 2010; Wu et al., 2008). Since Mtb possesses several rpf genes, redundancy likely exists. As a result only multi-deletion mutants, not single knockouts of rpf genes, show impaired resuscitation in vitro and attenuation in mice (Kana et al., 2008). In the mouse chronic progression of disease characterized by high bacterial burden is observed while reactivation in humans develops from minute bacterial load of LTBI. Hence, mice are probably not a suitable model to study reactivation of TB.
Evidence for coexistence of different Mtb stages in infected individuals is increasing. Thus, probably only a few dormant bacilli coexist in face of a large number of metabolically active replicating organisms during active TB. The reciprocal is also true: during latent infection, in addition to non-replicating metabolically inactive (i.e., dormant) Mtb, some actively replicating Mtb are present. In other words, the equilibrium of dormant/replicating Mtb represents the distinguishing factor between LTBI and active TB.
Isoniazid only targets replicating Mtb and yet has been used widely and successfully for chemoprophylaxis of LTBI where there is considered to be elevated risk, suggesting that during several months treatment time, bacteria transform into an isoniazid-susceptible stage (Fox et al., 1999). A more recent hypothesis describing the latent infection as dynamic process of constant reinfection could explain the efficacy of isoniazid therapy during latency (Cardona, 2009). Although formal proof is lacking, the clinical observation is taken as evidence for sporadic emergence of some replicating Mtb during LTBI. Reciprocally, addition of Rpf significantly increases recovery of Mtb in sputum from active TB patients providing circumstantial evidence that these bacteria are resuscitated from dormancy (Mukamolova et al., 2010). It has been argued that the long treatment time of 6 or more months to cure TB depends, at least in part, on the coexistence of both replicating and non-replicating Mtb since current drugs preferentially, if not exclusively, target metabolically active replicating Mtb. On the one hand, replicating bacilli are the main culprits causing active disease; on the other hand they are the target of current chemotherapy. In contrast, the dormant pathogen is likely a ‘bystander’ in disease but mainly contributes to phenotypic drug resistance. Hence the dormant pathogen serves as reservoir of renascent active Mtb to sustain pathology and disease. It is therefore assumed, that treatment with available drugs has to eradicate replicating tubercle bacilli in several waves as they are resuscitated from dormancy.
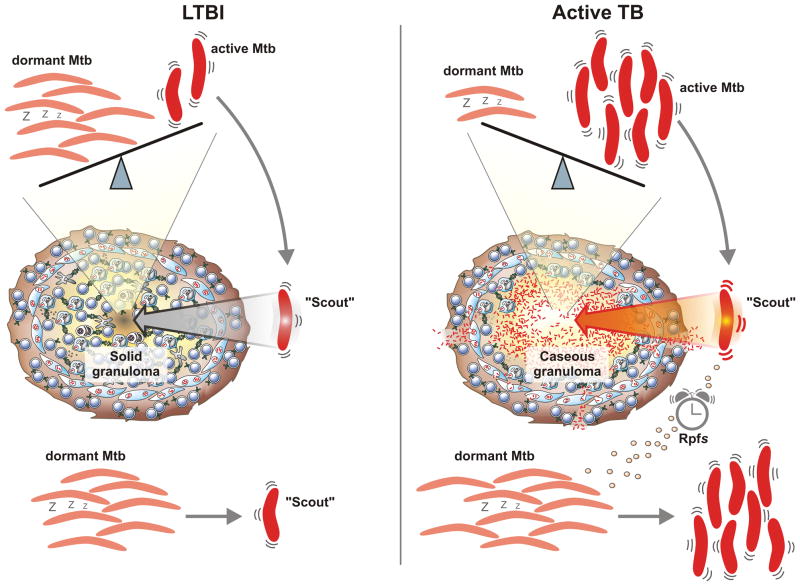
Currently, the scenario of equilibrium between dormant and replicating Mtb during the course of infection from LTBI to active TB remains speculative. In one attractive model, a few dormant Mtb resuscitate either stochastically or through signals such as those emitted by Rpf (Figure 3). These few Mtb organisms have been termed ‘scouts’ since they sense the environment for its appeal to awake and replicate. Under adverse conditions, the scouts die and the bulk of organisms remain dormant. In an appealing environment, scouts send activation signals to the dormant majority of bacteria which then resuscitate (Chao and Rubin, 2010; Epstein, 2009). The biochemical nature of these signals could involve Rpfs but has to be further investigated. In an environment attractive for growth the bacterial population starts replicating, causes pathology and leads to reactivation of TB. A small population will stay in or fall into dormancy thus showing tolerance to drug treatment (Lewis, 2010). Active disease caused by reinfection could involve similar factors since Mtb newly entering the host is actively growing and hence can provide wake-up signals to dormant bacilli in an LTBI host. Deletion of Rv2623, a gene of unknown function under the control of the dosR regulon, for instance, confers hypervirulence to the tubercle bacillus in mice and guinea pigs while its overexpression leads to growth delay in vitro (Drumm et al., 2009). Thus, Rv2623 could be involved in restricting replication. Reciprocally, regrowth of dormant bacilli could be promoted by the downregulation of Rv2623. The dosR regulon provides an instructive example of how environmental changes can trigger a complex adaptation program in Mtb. It is well understood how DosR responds to decreasing oxygen tension (Park et al., 2003). But how a change in oxygen availability is sensed on a molecular level and ultimately translated into an impulse for the dos regulatory system is not yet understood. Simply turning off the ‘dos program’ will most likely not be sufficient to allow for resuscitation. Although, molecular mechanisms of resuscitation are largely unknown, evidence for several aspects is unfolding.
Figure 3. Dynamic model of latent tuberculosis infection (LTBI) and active tuberculosis.
In this model, LTBI is characterized by predominance of dormant bacilli and only very few active scouts capable of sensing the environment for growth attractiveness, which is low inside the solid granuloma. Some Mtb wake-up stochastically to maintain a small pool of scouts (left panel). Once the environment provides more favorable conditions, for example, in a caseating granuloma, scouts resuscitate dormant bacilli to become active probably by secretion of resuscitation promoting factors (Rpfs) (right panel). A few organisms remain dormant and therefore phenotypically drug-resistant, explaining the long treatment time required to cure active TB.
Concluding remarks
The last decade has witnessed a remarkable increase in our knowledge on Mtb, not only on the bacillus itself but also on the communication with its host. We have learned that Mtb has invented complex mechanisms to survive in the intracellular environment, that it counteracts or evades the numerous defense mechanisms of macrophages, and that the crosstalk between pathogen and host immune system is focused on granulomas, which serve as both, habitat and containment for Mtb. However, many interesting observations remain fragmented in our current view of Mtb. Remarkable efforts are ongoing to integrate the relatively new science of systems biology into mycobacterial research (http://www.systemtb.org/ and http://www.broadinstitute.org/annotation/tbsysbio/home.html). Ideally this will tie up loose ends and improve our understanding, allowing for a broad network-oriented view of Mtb and its host.
After decades of dormancy, TB drug discovery has reawakened. Although the boost expected to come from the whole genome sequence of Mtb and application of high-throughput screening to target-based approaches did not pay off so far, currently nine TB drug candidates are undergoing clinical investigation – more than ever before (Cole et al., 1998; Ma et al., 2010; Payne et al., 2007). Most of these compounds belong to existing classes of antibiotics and thus are derivates of the respective parental molecule: oxazolidinones target protein synthesis; fluoroquinolones are well-known DNA gyrase inhibitors; 1,2-ethylene diamines interfere with cell wall biosynthesis pathways; nitroimidazoles cause multiple damage to Mtb by RNS. The target of sudoterb has yet to be identified. The ATP synthase inhibitor TMC207 is a diarylquinoline and represents a relatively new antimycobacterial class [current TB drug discovery reviewed by (Koul et al., 2011)]. Which new entity will complete the stony road to drug application is yet to be seen. Applying our increasing knowledge of host–pathogen interactions to TB drug discovery will most likely result in more new candidate compounds (Nathan et al., 2008).
Most impressive are the dynamics and the plasticity of the infection process, which unfolds as a continuum in which different populations of Mtb, as well as different pathologic forms of granulomas, coexist. As a corollary, different stages of infection are characterized by different ratios in abundance of pathogen populations rather than distinct periods of life cycle. Accordingly, analysis of single cells ‘frozen’ in a distinct stage, rather than of populations will be required in the future.
Development of next-generation vaccines will benefit from our deeper insights into the adaption of Mtb to different host environments, as well. Currently, over a dozen vaccine candidates are at different stages of clinical trial development (Kaufmann, 2011a; Kaufmann et al., 2010). All these candidates are pre-exposure vaccines, which do not prevent or eradicate infection with Mtb but rather aim at precluding emergence of active TB. Accordingly, these vaccine candidates stimulate an immune response that targets the pathogen promptly after infection, presumably by means of antigens expressed by the metabolically active, replicating pathogen. However, during LTBI Mtb changes its genetic program and antigens expressed by the dormant pathogen prevail. To sustain efficacious control of dormant Mtb, the vaccine-induced immune response needs to target antigens expressed by dormant bacilli, so-called latency antigens (Kaufmann, 2010). This stratagem becomes even more valid for post-exposure vaccines which are administered during LTBI – more than 2 billion individuals with elevated risk of developing TB. A first example of a post-exposure vaccine comprising an antigen selectively expressed during nutrient starvation in addition to canonical pre-exposure vaccine antigens has been described recently (Aagaard et al., 2011; Kaufmann, 2011B). This vaccine was highly successful in containing Mtb in the mouse model. Hence, better understanding of the tubercle bacillus’ life cycle will facilitate rational design of next generation vaccine candidates.
Acknowledgments
We would like to thank Olivier Neyrolles and colleagues for providing most recent experimental data prior to publication. We are grateful for outstanding editorial support of M.L. Grossmann and excellent graphic design of D. Schad. This work received financial support from the European 7th Framework Program SYSTEMTB (HEALTH-2009-2.1.2-1-241587) and the National Institutes of Health (SysBio NIAID-DMID-08-22).
Definition of terms (to be displayed on side margins)
- active TB
characterized by the presence of clinical symptoms caused by a high bacterial burden in the lung and sometimes in other organs; patients spread the disease
- latent TB infection (LTBI)
asymptomatic infection with a low number of Mtb in the absence of clinical signs; patients are not capable of spreading the disease
- reactivation of TB
transition from latency to the full blown active disease
- relapse of TB
re-development of active disease after incomplete or wrong treatment with antibiotics; single- or multiple-drug resistance is often observed
- active Mtb
metabolically active replicating bacilli; susceptible to drug-inhibiting processes essential for growth (i.e. DNA replication, RNA synthesis, cell wall biogenesis)
- dormant Mtb
non-replicating bacilli maintaining full viability at a very low metabolic rate; organisms show minor susceptibility or phenotypic drug resistance to antibiotics targeting functions required for growth
- resuscitation of Mtb
transition of the pathogen from dormancy to growth
- drug-resistance
inheritable resistance to a drug conferred by genetic mutation
- phenotypic drug-resistance
non-inheritable resistance to a drug conferred by a specific metabolic state (usually dormancy)
- viable but not culturable (VBNC)
refers to a state of viable Mtb that is not capable of colonizing on nutrient-rich solid media without resuscitation
Reference List
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection pre- and post-exposure. Nat Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- Abramovitch RB, Rohde KH, Hsu FF, Russell DG. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol Microbiol. 2011;80:678–694. doi: 10.1111/j.1365-2958.2011.07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. Asymmetry and Aging of Mycobacterial Cells Lead to Variable Growth and Antibiotic Susceptibility. Science. 2011 doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod S, Oschkinat H, Enders J, Schlegel B, Brinkmann V, Kaufmann SH, Haas A, Schaible UE. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008;10:1530–1545. doi: 10.1111/j.1462-5822.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- Barry CE, III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste DJ, Bonde B, Hawkins N, Ward JL, Beale MH, Noack S, Noh K, Kruger NJ, Ratcliffe RG, McFadden J. (1)(3)C metabolic flux analysis identifies an unusual route for pyruvate dissimilation in mycobacteria which requires isocitrate lyase and carbon dioxide fixation. PLoS Pathog. 2011;7:e1002091. doi: 10.1371/journal.ppat.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Boon C, Dick T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol. 2002;184:6760–6767. doi: 10.1128/JB.184.24.6760-6767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de CC, Neyrolles O. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Bryk R, Gold B, Venugopal A, Singh J, Samy R, Pupek K, Cao H, Popescu C, Gurney M, Hotha S, Cherian J, Rhee K, Ly L, Converse PJ, Ehrt S, Vandal O, Jiang X, Schneider J, Lin G, Nathan C. Selective killing of nonreplicating mycobacteria. Cell Host Microbe. 2008;3:137–145. doi: 10.1016/j.chom.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzostek A, Dziadek B, Rumijowska-Galewicz A, Pawelczyk J, Dziadek J. Cholesterol oxidase is required for virulence of Mycobacterium tuberculosis. FEMS Microbiol Lett. 2007;275:106–112. doi: 10.1111/j.1574-6968.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier N, Blanc-Potard A, Ehrt S, Piddington D, Riley L, Groisman EA. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35:1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- Cardona PJ. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection. 2009;37:80–86. doi: 10.1007/s15010-008-8087-y. [DOI] [PubMed] [Google Scholar]
- Chang JC, Miner MD, Pandey AK, Gill WP, Harik NS, Sassetti CM, Sherman DR. igr Genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol. 2009;191:5232–5239. doi: 10.1128/JB.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MC, Rubin EJ. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol. 2010;64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- Cohen-Gonsaud M, Barthe P, Bagneris C, Henderson B, Ward J, Roumestand C, Keep NH. The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes. Nat Struct Mol Biol. 2005;12:270–273. doi: 10.1038/nsmb905. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence (vol 393, pg 537, 1998) Nature. 1998;396:190–198. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Converse PJ, Karakousis PC, Klinkenberg LG, Kesavan AK, Ly LH, Allen SS, Grosset JH, Jain SK, Lamichhane G, Manabe YC, McMurray DN, Nuermberger EL, Bishai WR. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun. 2009;77:1230–1237. doi: 10.1128/IAI.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin KH. Prokaryotic ubiquitin-like protein (Pup), proteasomes and pathogenesis. Nat Rev Microbiol. 2009;7:485–491. doi: 10.1038/nrmicro2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V. Autophagy in infection. Curr Opin Cell Biol. 2010;22:252–262. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Delgado M, Vergne I, Master S, De HS, Ponpuak M, Singh S. Autophagy in immunity against Mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol. 2009;335:169–188. doi: 10.1007/978-3-642-00302-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M. The good fat: a link between lipid bodies and antigen cross-presentation. Immunity. 2009;31:176–178. doi: 10.1016/j.immuni.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Dittrich D, Keller C, Ehlers S, Schultz JE, Sander P. Characterization of a Mycobacterium tuberculosis mutant deficient in pH-sensing adenylate cyclase Rv1264. Int J Med Microbiol. 2006;296:563–566. doi: 10.1016/j.ijmm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Drumm JE, Mi K, Bilder P, Sun M, Lim J, Bielefeldt-Ohmann H, Basaraba R, So M, Zhu G, Tufariello JM, Izzo AA, Orme IM, Almo SC, Leyh TS, Chan J. Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-Binding: requirement for establishing chronic persistent infection. PLoS Pathog. 2009;5:e1000460. doi: 10.1371/journal.ppat.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussurget O, Stewart G, Neyrolles O, Pescher P, Young D, Marchal G. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect Immun. 2001;69:529–533. doi: 10.1128/IAI.69.1.529-533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, Lakey DL, Bochan MR, Kernodle DS. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164:2213–2219. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SS. Microbial awakenings. Nature. 2009;457:1083. doi: 10.1038/4571083a. [DOI] [PubMed] [Google Scholar]
- Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger JD, Goldberg AL. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977;74:54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Fisher MA, Plikaytis BB, Shinnick TM. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol. 2002;184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009a;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009b;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–S279. [PubMed] [Google Scholar]
- Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med. 2007;13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M, Rao SP, Pethe K, Dick T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology. 2010;156:81–87. doi: 10.1099/mic.0.033084-0. [DOI] [PubMed] [Google Scholar]
- Giebel JD, Carr KA, Anderson EC, Hanna PC. The germination-specific lytic enzymes SleB, CwlJ1, and CwlJ2 each contribute to Bacillus anthracis spore germination and virulence. J Bacteriol. 2009;191:5569–5576. doi: 10.1128/JB.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman MS, Cox JS, Jacobs WR. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell. 2000;5:717–727. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- Greenberg S. Fc receptor-mediated phagocytosis. In: Gordon S, editor. Phagocytosis and the host. Stamford, Connecticut: JAI Press; 1999. pp. 149–191. [Google Scholar]
- Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, Bancroft GJ, Reyrat JM, van Soolingen D, Raupach B, Kaufmann SHE. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Harper J, Skerry C, Davis SL, Tasneen R, Weir M, Kramnik I, Bishai WR, Pomper MG, Nuermberger EL, Jain SK. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis. 2012;205:595–602. doi: 10.1093/infdis/jir786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh KK, Grinstein S. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol Mol Biol Rev. 2007;71:452–462. doi: 10.1128/MMBR.00003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Stadthagen G, Gicquel B. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis (Edinb ) 2007;87:78–86. doi: 10.1016/j.tube.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kana BD, Gordhan BG, Downing KJ, Sung N, Vostroktunova G, Machowski EE, Tsenova L, Young M, Kaprelyants A, Kaplan G, Mizrahi V. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol Microbiol. 2008;67:672–684. doi: 10.1111/j.1365-2958.2007.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti MK, Dai G, Armitige LY, Rivera MC, Daniel S, Singh CR, Lindsey DR, Dhandayuthapani S, Hunter RL, Jagannath C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010;33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis. 2011a;11:633–640. doi: 10.1016/S1473-3099(11)70146-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Tuberculosis vaccines-a new kid on the block. Nat Med. 2011b;17:159–160. doi: 10.1038/nm0211-159. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Hussey G, Lambert PH. New vaccines for tuberculosis. The Lancet. 2010;375:85–94. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Winau F. From bacteriology to immunology: the dualism of specificity. Nat Immunol. 2005;6:1063–1066. doi: 10.1038/ni1105-1063. [DOI] [PubMed] [Google Scholar]
- Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. MBio. 2011:2. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol. 2004;186:8172–8180. doi: 10.1128/JB.186.24.8172-8180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. Die Aetiologie der Tuberculose (Nach einem in der physiologischen Gesellschaft zu Berlin am 24. März gehaltenem Vortrage) Berliner klin Wochenschr. 1882;19:221–230. [PubMed] [Google Scholar]
- Koch R. In: The aetiology of tuberculosis. Pinner B, Pinner M, translators. New York City: National Tuberculosis Association; 1932. [Google Scholar]
- Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- Kumar A, Toledo JC, Patel RP, Lancaster JR, Jr, Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc Natl Acad Sci U S A. 2007;104:11568–11573. doi: 10.1073/pnas.0705054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S, McKinnon KP, Runge MS, Ting JP, Braunstein M. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect Immun. 2006;74:6855–6864. doi: 10.1128/IAI.01022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol. 2010;192:1662–1670. doi: 10.1128/JB.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong FJ, Dartois V, Dick T, editors. A color atlas of comparative pathology of pulmonary tuberculosis. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Loebel RO, Shorr E, Richardson HB. The influence of adverse conditions upon the respiratory metabolism and growth of human tubercle bacilli. J Bacteriol. 1933;26:167–200. doi: 10.1128/jb.26.2.167-200.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KL, Rao PS, Shui G, Bendt AK, Pethe K, Dick T, Wenk MR. Triacylglycerol utilization is required for regrowth of in vitro hypoxic nonreplicating Mycobacterium bovis bacillus Calmette-Guerin. J Bacteriol. 2009;191:5037–5043. doi: 10.1128/JB.00530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2011 doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Lowe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995;268:533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. The Lancet. 2010;375:75–84. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- MacGurn JA, Cox JS. A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect Immun. 2007;75:2668–2678. doi: 10.1128/IAI.01872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology. 2004;150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- Makinoshima H, Glickman MS. Regulation of Mycobacterium tuberculosis cell envelope composition and virulence by intramembrane proteolysis. Nature. 2005;436:406–409. doi: 10.1038/nature03713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney E, Stankowska D, Zhang J, Fol M, Cheng QJ, Lun S, Bishai WR, Rajagopalan M, Chatterjee D, Madiraju MV. The two-domain LysX protein of Mycobacterium tuberculosis is required for production of lysinylated phosphatidylglycerol and resistance to cationic antimicrobial peptides. PLoS Pathog. 2009;5:e1000534. doi: 10.1371/journal.ppat.1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci U S A. 2010;107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune RM, Feldmann FM, Lambert HP, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney JD, zu Bentrup KH, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR, Russell DG. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- Mohn WW, van der Geize R, Stewart GR, Okamoto S, Liu J, Dijkhuizen L, Eltis LD. The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem. 2008;283:35368–35374. doi: 10.1074/jbc.M805496200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedzadeh F, Smith DA, Norman RA, Dinadayala P, Murray-Rust J, Russell DG, Kendall SL, Rison SC, McAlister MS, Bancroft GJ, McDonald NQ, Daffe M, Av-Gay Y, Stoker NG. The Mycobacterium tuberculosis ino1 gene is essential for growth and virulence. Mol Microbiol. 2004;51:1003–1014. doi: 10.1046/j.1365-2958.2003.03900.x. [DOI] [PubMed] [Google Scholar]
- Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. Resuscitation-promoting factors reveal an occult population of tubercle Bacilli in Sputum. Am J Respir Crit Care Med. 2010;181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Elias EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- Nathan C, Gold B, Lin G, Stegman M, de Carvalho LP, Vandal O, Venugopal A, Bryk R. A philosophy of anti-infectives as a guide in the search for new drugs for tuberculosis. Tuberculosis (Edinb ) 2008;88(Suppl 1):S25–S33. doi: 10.1016/S1472-9792(08)70034-9. [DOI] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan CF, Ehrt S. Nitric oxide in tuberculosis. In: Rom W, Garay S, editors. Tuberculosis. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 215–235. [Google Scholar]
- Nesbitt NM, Yang X, Fontan P, Kolesnikova I, Smith I, Sampson NS, Dubnau E. A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect Immun. 2010;78:275–282. doi: 10.1128/IAI.00893-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52:1291–1302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr, Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5:637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science. 2008;322:1104–1107. doi: 10.1126/science.1163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci U S A. 2004;101:13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun. 2001;69:4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters J. MHC class II restricted antigen presentation. Curr Opin Immunol. 1997;9:89–96. doi: 10.1016/s0952-7915(97)80164-1. [DOI] [PubMed] [Google Scholar]
- Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy GE, Russell DG. Lysosomal ubiquitin and the demise of Mycobacterium tuberculosis. Cell Microbiol. 2007;9:2768–2774. doi: 10.1111/j.1462-5822.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud C, Papavinasasundaram KG, Speight RA, Springer B, Sander P, Bottger EC, Colston MJ, Draper P. The functions of OmpATb, a pore-forming protein of Mycobacterium tuberculosis. Mol Microbiol. 2002;46:191–201. doi: 10.1046/j.1365-2958.2002.03152.x. [DOI] [PubMed] [Google Scholar]
- Reddy TB, Riley R, Wymore F, Montgomery P, DeCaprio D, Engels R, Gellesch M, Hubble J, Jen D, Jin H, Koehrsen M, Larson L, Mao M, Nitzberg M, Sisk P, Stolte C, Weiner B, White J, Zachariah ZK, Sherlock G, Galagan JE, Ball CA, Schoolnik GK. TB database: an integrated platform for tuberculosis research. Nucleic Acids Res. 2009;37:D499–D508. doi: 10.1093/nar/gkn652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece ST, Kaufmann SHE. Floating between the poles of pathology and protection: Can we pin down the granuloma in tuberculosis? Curr Opin Microbiol. 2011 doi: 10.1016/j.mib.2011.10.006. In press. [DOI] [PubMed] [Google Scholar]
- Reece ST, Loddenkemper C, Askew DJ, Zedler U, Schommer-Leitner S, Stein M, Mir FA, Dorhoi A, Mollenkopf HJ, Silverman GA, Kaufmann SH. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120:3365–3376. doi: 10.1172/JCI42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyrat JM, Berthet FX, Gicquel B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guerin. Proc Natl Acad Sci U S A. 1995;92:8768–8772. doi: 10.1073/pnas.92.19.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifat D, Bishai WR, Karakousis PC. Phosphate depletion: a novel trigger for Mycobacterium tuberculosis persistence. J Infect Dis. 2009;200:1126–1135. doi: 10.1086/605700. [DOI] [PubMed] [Google Scholar]
- Rigel NW, Braunstein M. A new twist on an old pathway--accessory Sec [corrected] systems. Mol Microbiol. 2008;69:291–302. doi: 10.1111/j.1365-2958.2008.06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59:541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- Robinson N, Wolke M, Ernestus K, Plum G. A mycobacterial gene involved in synthesis of an outer cell envelope lipid is a key factor in prevention of phagosome maturation. Infect Immun. 2007;75:581–591. doi: 10.1128/IAI.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe. 2007;2:352–364. doi: 10.1016/j.chom.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad TR, Sherrid AM, Minch KJ, Sherman DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol. 2009;11:1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, Dawes SS. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190:3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- Schekman RW. Regulation of membrane traffic in the secretory pathway. Harvey Lect. 1994;90:41–57. [PubMed] [Google Scholar]
- Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratne RH, De Silva AD, Williams SJ, Mougous JD, Reader JR, Zhang T, Chan S, Sidders B, Lee DH, Chan J, Bertozzi CR, Riley LW. 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol. 2006;59:1744–1753. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- Shah IM, Laaberki MH, Popham DL, Dworkin J. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell. 2008;135:486–496. doi: 10.1016/j.cell.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Ehrt S. Dihydrolipoamide acyltransferase is critical for Mycobacterium tuberculosis pathogenesis. Infect Immun. 2006;74:56–63. doi: 10.1128/IAI.74.1.56-63.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shleeva MO, Bagramyan K, Telkov MV, Mukamolova GV, Young M, Kell DB, Kaprelyants AS. Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology. 2002;148:1581–1591. doi: 10.1099/00221287-148-5-1581. [DOI] [PubMed] [Google Scholar]
- Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7:537–544. doi: 10.1038/nrmicro2165. [DOI] [PubMed] [Google Scholar]
- Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J Leukoc Biol. 2007;82:934–945. doi: 10.1189/jlb.0407216. [DOI] [PubMed] [Google Scholar]
- Striebel F, Imkamp F, Sutter M, Steiner M, Mamedov A, Weber-Ban E. Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nat Struct Mol Biol. 2009;16:647–651. doi: 10.1038/nsmb.1597. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Sutter M, Striebel F, Damberger FF, Allain FH, Weber-Ban E. A distinct structural region of the prokaryotic ubiquitin-like protein (Pup) is recognized by the N-terminal domain of the proteasomal ATPase Mpa. FEBS Lett. 2009;583:3151–3157. doi: 10.1016/j.febslet.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Tailleux L, Waddell SJ, Pelizzola M, Mortellaro A, Withers M, Tanne A, Castagnoli PR, Gicquel B, Stoker NG, Butcher PD, Foti M, Neyrolles O. Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS ONE. 2008;3:e1403. doi: 10.1371/journal.pone.0001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh JS, Yano T, Rubin H. Type II NADH: menaquinone oxidoreductase of Mycobacterium tuberculosis. Infect Disord Drug Targets. 2007;7:169–181. doi: 10.2174/187152607781001781. [DOI] [PubMed] [Google Scholar]
- Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308:1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]
- Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D, Fluitsma D, van ZM, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol. 2009;191:625–631. doi: 10.1128/JB.00932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Laslop N, Lee H, Neyfakh AA. Increased persistence in Escherichia coli caused by controlled expression of toxins or other unrelated proteins. J Bacteriol. 2006;188:3494–3497. doi: 10.1128/JB.188.10.3494-3497.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest. 2009;136:420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/Calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198:653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111 ( Pt 7):897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcheze C, Av-Gay Y, Attarian R, Liu Z, Hazbon MH, Colangeli R, Chen B, Liu W, Alland D, Sacchettini JC, Jacobs WR., Jr Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol Microbiol. 2008;69:1316–1329. doi: 10.1111/j.1365-2958.2008.06365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburger A, Koul A, Ferrari G, Nguyen L, Prescianotto-Baschong C, Huygen K, Klebl B, Thompson C, Bacher G, Pieters J. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- Wang T, Li H, Lin G, Tang C, Li D, Nathan C, Darwin KH, Li H. Structural insights on the Mycobacterium tuberculosis proteasomal ATPase Mpa. Structure. 2009;17:1377–1385. doi: 10.1016/j.str.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang Y, Han Y, Zhang J, Liang Y, Li H, Li B, Wang L. Effect of recombinant Rv1009 protein on promoting the growth of Mycobacterium tuberculosis. J Appl Microbiol. 2008;105:1121–1127. doi: 10.1111/j.1365-2672.2008.03850.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Siddiqi N, Rubin EJ. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2005;49:4778–4780. doi: 10.1128/AAC.49.11.4778-4780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Nesbitt NM, Dubnau E, Smith I, Sampson NS. Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis. Biochemistry. 2009;48:3819–3821. doi: 10.1021/bi9005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Li LS, Weinstein E, Teh JS, Rubin H. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2) J Biol Chem. 2006;281:11456–11463. doi: 10.1074/jbc.M508844200. [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6:413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- Yew WW, Leung CC. Update in tuberculosis 2007. Am J Respir Crit Care Med. 2008;177:479–485. doi: 10.1164/rccm.200710-1561UP. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]