Abstract
OBJECTIVES
To determine if aggressive treatment initiated early in the course of rheumatoid factor positive or negative polyarticular juvenile idiopathic arthritis (poly-JIA) can induce clinical inactive disease (CID) within 6 months.
METHODS
Between May 2007 and October 2010 a multi-center, prospective, double blind, randomized, placebo controlled trial of two aggressive treatments was conducted in 85 children aged 2 to 16 years with polyarticular JIA of less than 12 months duration. Patients received either methotrexate 0.5 mg/kg/wk SQ (40 mg max), etanercept 0.8 mg/kg/wk (50 mg max), prednisolone 0.5 mg/kg/d (60 mg max) tapered to 0 by 17 weeks (Arm 1), or methotrexate (same dose as Arm 1), etanercept placebo, and prednisolone placebo (Arm 2). The primary outcome was CID at 6 months. An exploratory phase determined the rate of clinical remission on medication (6 months of continuous CID) at 12 months.
RESULTS
By 6 months, 17 of 42 (40%) of patients in Arm 1 and 10 of 43 (23%) in Arm 2 had achieved CID (X2 = 2.91; p = 0.088). After 12 months, 9 patients in Arm 1 and 3 in Arm 2 achieved clinical remission on medication (p = 0.0534). There were no significant inter-arm differences in adverse events.
CONCLUSIONS
Although this study did not meet its primary endpoint, early aggressive therapy in this cohort of children with recent onset polyarticular JIA resulted in substantial proportions of patients in both arms achieving CID by 6 months and clinical remission on medication within 12 months of treatment.
Keywords: Juvenile idiopathic arthritis, early aggressive therapy, clinical inactive disease, randomized controlled trial
Juvenile idiopathic arthritis (JIA) encompasses a group of diseases of unknown etiology, defined by the International League of Associations for Rheumatology as having in common arthritis in one or more joints that persists for at least 6 weeks and beginning before 16 years of age with other conditions excluded (1). With a prevalence of approximately one per thousand children in the US, JIA is the most common pediatric rheumatic illness and cause of acquired childhood disability (2, 3). During the last 20 years the advent of a host of immune response modifiers (biologics) that directly inhibit the action of pro-inflammatory mediators has revolutionized the treatment and expected outcome of JIA (4-7) such that extended periods of clinically quiescent disease may now be induced. Newly published guidelines from the American College of Rheumatology (ACR) provide some guidance for the initiation and safety monitoring of drugs commonly used in JIA, including biologics (8). However, it remains unclear as to exactly when in the course of the disease and in what combination these treatments should be started to produce optimal outcomes. At present, it cannot be predicted with confidence which children with JIA have a less favorable outcome, although some risk factors have been identified (8-10). The polyarticular (both rheumatoid factor positive and negative) categories comprise approximately 30% of all patients with JIA, and the majority of these children remain on combinations of multiple medications for many years (11, 12); disease free periods off medication greater than 1 year are uncommon (13).
Investigations in adult rheumatoid arthritis (RA) have demonstrated improved outcomes, including less radiographic progression of joint damage, when aggressive treatment is started early in the disease course (14-17). Thus, many rheumatologists now believe there is a “window of opportunity” early in the disease during which aggressive therapy has a profound long term effect (17-19).
To date, there have not been any double-blind, randomized placebo controlled trials of biological agents in children with recent onset JIA in which the primary endpoint is clinical inactive disease (CID) (20). The trial described here was designed to determine if two aggressive treatment regimens started early in the course of polyarticular JIA results in CID within 6 months of initiation. An exploratory phase investigated the potential of the treatments to induce clinical remission on medication (CRM: 6 continuous months of CID while on treatment) within 12 months of initiation.
PATIENTS AND METHODS
Patients
Patients aged 2 to 17 years were recruited from 15 sites in the United States. All patients had a diagnosis of active JIA polyarthritis (rheumatoid factor positive or negative) less than 12 months in duration (1), however patients without psoriasis but a first degree relative with psoriasis were allowed to enroll. None had received previous biological therapy. The only disease modifying anti-rheumatic drug allowed was methotrexate (MTX) at a dose of less than or equal to 0.5 mg/kg/wk (40 mg max) started no longer than 6 weeks prior to enrollment. Eligible patients were permitted to have received up to 2 intra-articular corticosteroid injections before or up to 2 weeks after baseline; and oral prednisolone for up to 4 weeks, but must have been off corticosteroids for at least 1 week prior to enrollment. Patients with past or current JIA-associated uveitis were excluded. Female patients who had reached puberty were tested for pregnancy throughout the study.
Study design and treatments
This multi-centered, randomized, double-blind, placebo-controlled study compared the efficacy of two aggressive treatment arms to induce CID within 6 months of therapy initiation and CRM by 12 months. The study consisted of a 6 month pivotal phase, and an exploratory phase that lasted up to 12 months after enrollment (Figure 1).
Figure 1.
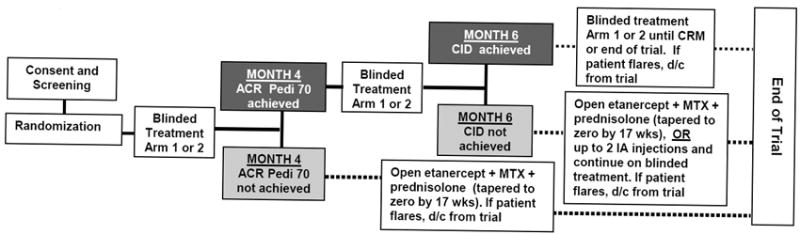
Treatment Arm 1: MTX 0.5mg/kg/wk subcutaneously (max 40 mg/wk) + daily prednisolone 0.5mg/kg/d (max 60 mg) with forced taper to zero by 17 wks + etanercept 0.8mg/kg/wk subcutaneously (max 50mg).
Treatment Arm 2: MTX as in Arm 1 + placebo prednisolone tapered to zero + placebo etanercept injected weekly.
Dark shaded boxes indicate the study flow for the patient if the end-points were achieved; Lighter shaded boxes indicate the study flow for the patient if the endpoints were not achieved. Solid lines represent the pivotal phase of the study. Dotted lines represent the exploratory phase of the study. Maximum total study duration = 12 months.
CID = Clinical Inactive Disease CRM= Clinical Remission on Medications
Pivotal Phase
A non-stratified blocked randomization scheme (block size = 10 in a 1:1 ratio across all centers) was used to assign patients to one of two treatment arms. Subject allocation to study arms was done at the baseline visit by the electronic data capture system (EDC) after the screening visit and inclusion/exclusion case report forms were completed. Pharmacists at each institution were given password protected access to the EDC which permitted them to determine which Arm the subject had been assigned.
Pivotal Phase Medications
Treatment Arm 1 medications were open label subcutaneously administered MTX at a dose of 0.5 mg/kg/wk (maximum of 40 mg/wk), blinded etanercept (ETN) 0.8 mg/kg/wk (maximum of 50 mg) administered subcutaneously, and blinded oral prednisolone daily at 0.5 mg/kg/d (maximum 60 mg/d) tapered to zero over the first 4 months of therapy. Treatment Arm 2 medications included open label methotrexate as in Arm 1, blinded placebo ETN administered subcutaneously every week and daily blinded placebo oral prednisolone tapered to zero over 4 months.
Patients in each Arm took 1 mg/d of folic acid and were allowed use of a single non-steroidal anti-inflammatory drug as concomitant therapy during the study period. Up to 2 intra-articular corticosteroid injections within 2 weeks after the baseline visit were allowed. The use of other anti-inflammatory or anti-rheumatic therapies was not allowed during the 12 month study period.
Exploratory Phase
The exploratory phase lasted up to 12 months after enrollment. Patients who failed to meet endpoints in the pivotal phase were given open label Treatment Arm 1 medications in the exploratory phase. Up to 2 intra-articular corticosteroid injections were allowed in the exploratory phase.
Study procedures
Study visits occurred at screening, baseline, 1, 2, 4, 5, 6, 7, 8, 10 and 12 months. After the baseline visit, the following assessments were done at every visit: joint examination by a trained certified blinded joint assessor (whose only contact with the subject was to conduct the joint assessment) to determine the number of joints with active arthritis and number of joints with limited range of motion; physician’s global assessment of disease activity (0-10 Likert-like scale) assessed by the treating pediatric rheumatologist; parents’ global assessment of subject’s overall well-being (0-10 Likert-like scale); Child Health Assessment Questionnaire; vital signs; review of concomitant medications; review of medication diary card for medication compliance. Laboratory tests to assess medication safety and disease activity included complete blood count, erythrocyte sedimentation rate (ESR), chemistry panel, urinalysis, and pregnancy test for girls who had reached puberty. Adverse events of Grade III or higher were recorded using the National Cancer Institute’s Common Toxicity Criteria Version 3 (21). All infections requiring systemic therapy were documented and followed until resolution.
Endpoints
The primary efficacy endpoint in the pivotal phase was the attainment of CID at 6 months. CID is defined as (1) no joints with active arthritis, (2) no fever, rash, serositis, splenomegaly or generalized lymphadenopathy attributable to JIA, (3) no active uveitis, (4) ESR in the normal range in the laboratory where tested, and (5) a physician’s global assessment of disease activity score of 0 (20). A secondary endpoint in the pivotal phase was achievement of at least an ACR Pediatric 70 level of response (22) at the 4 month visit. The ACR Pediatric 70 is defined as greater than or equal to 70% improvement from baseline in a minimum of 3 of the 6 ACR Pediatric Core Set of variables (physician’s global assessment of disease activity, parent’s assessment of overall well-being, the number of joints with active arthritis, the number of joints with limited range of motion, the Childhood Health Assessment Questionnaire, and the ESR), with no more than one of the remaining variables worsening by greater than 30% from baseline (22). Patients who failed to achieve an ACR Pediatric 70 were considered treatment failures in the pivotal phase and were treated with open label Arm 1 medications and placed in the exploratory phase of the trial.
The endpoint in the exploratory phase was the attainment of CRM, defined as 6 continuous months of CID while on medication (20). Patients who achieved CID at any time point and subsequently experienced a disease flare were discontinued from study participation. Flare was defined as worsening of 3 or more of any of the 6ACR core set variables by the following amounts from the previous visit: physician’s global assessment of disease activity by at least 2 units on a 0-10 Likert scale; parent/subject assessment of overall well-being by a least 2 units on a 0-10 Likert scale; increase of at least 2 joints with active arthritis; increase of at least 2 joints with limitation of motion; increase of a minimum of +0.125 on the CHAQ; increase of the ESR from normal to abnormal.
Safety was assessed in all patients who received at least 1 dose of study medication.
This study was approved by the NIH-NIAMS, an independent Data and Safety Monitoring Board, and the Institutional Review Boards of all participating sites, and was conducted in compliance with the Helsinki Declaration. Patients of sufficient age provided assent and parents/legal guardians provided written informed consent. Study participation occurred from May 2007 to October 2010.
Statistical considerations
Based on preliminary data from both adult and pediatric studies we estimated that 60% of patients in Arm 1 and 20% of those in Arm 2 would attain CID at the 6 month visit in the pivotal phase. Using an alpha error level of 0.05 and a beta error level of 0.1, NQuery Advisor Version 5.0 yielded a necessary evaluable sample size of 36 patients per arm, or 72 in total. Based upon previous trials, we estimated a non-evaluable rate of 15%, thus yielding a needed enrollment of 86 patients.
Baseline demographic and disease characteristics and comparison of differences in frequencies of patients who attained the primary endpoint were compared between treatment groups by the Wilcoxon rank test for continuous numeric or ordinal level data, or the likelihood ratio chi square or Fisher’s Exact test for nominal data. Logistic regression was used to identify independent variables that were predictive of the outcome in the pivotal phase. The intent-to-treat last-observation-carried forward approach was used for missing data or for patients who withdrew from the study.
RESULTS
Baseline characteristics
A total of 92 patients were assessed for eligibility and 87 patients were randomized, 2 of whom were found to be ineligible shortly after enrollment and were excluded from study participation: one prior to receiving study medication and the other after a single dose of medication. The baseline demographic and disease characteristics of the 85 patients who received medication are summarized in Table 1. Forty-two patients were randomized to Arm 1 and 43 to Arm 2. Sixty-three females and 22 males had a mean age of 10.5 years and mean disease duration of 5.1 months at the baseline visit. The groups were well balanced except for the mean number of joints with active arthritis and the ESR. Although the mean ESR was statistically higher in Arm 2, there was no difference in the proportion of patients with an elevated ESR between the 2 treatment arms.
Table 1.
Baseline Demographic and Disease Characteristics of the Patient Sample
| Characteristic | Arm 1 MTX + ETN + Pred (N = 42) |
Arm 2 MTX only (N = 43) |
P value* |
|---|---|---|---|
| Sex | |||
| Female – no. (%) | 29 (69.0%) | 34 (79.1%) | NS |
| Male – no. (%) | 13 (31.0%) | 9 (20.9%) | 0.3 |
| Ethnic origin | |||
| White – no. (%) | 35 (83.3%) | 38 (88.4%) | NS |
| Black – no. (%) | 4 (9.5%) | 1 (2.3%) | 0.16 |
| Other – no. (%) | 3 (7.1%) | 4 (9.3%) | NS |
| Mean (SD) age at baseline – years | 9.9 (4.6) | 11.1 (4.1) | 0.22 |
| Mean (SD) duration of Symptoms – months | 4.9 (0.5) | 5.2 (0.6) | NS |
| Mean (SD) number of active joints | 18.3 (11.0) | 25.5 (14.4) | 0.018 |
| Mean (SD) number of joints with limited motion | 13.6 (11.8) | 16.3 (13.2) | NS |
| Mean (SD) CHAQ disability index | 1.1 (0.8) | 1.3 (0.7) | NS |
| Mean (SD) parent assessment of well-being | 5.6 (2.1) | 5.2 (2.8) | NS |
| Mean (SD) physician assessment of disease activity | 7.0 (1.8) | 7.1 (1.9) | NS |
| Mean (SD) erythrocyte sedimentation rate mm/hr | 29.0 (21) | 44.6 (30) | 0.017 |
| Elevated erythrocyte sedimentation rate – no. (%) | 20 (47.6%) | 27 (62.8%) | 0.16 |
| Positive for rheumatoid factor – no. (%) | 14 (33.3%) | 17 (39.5%) | 0.24 |
| Positive for anti-nuclear antibodies – no. (%) | 33 (79.5%) | 25 (59.5%)** | 0.10 |
| Positive for anti-CCP – no. (%) | 14 (35%)*** | 16 (39%)*** | NS |
| Previous treatment with methotrexate – no. (%) | 6 (14.2%) | 4 (9.3%) | 0.48 |
| Previous treatment with Prednisolone – no. (%) | 2 (4.7%) | 2 (4.6%) | NS |
Based on either Chi square, Fisher’s Exact test, or Wilcoxon Rank test. NS indicates P > 0.5
One missing value
Two missing values
Outcome
Figure 2 is a flow diagram of patient progress through the phases of the trial. By the 4 month visit, 2 patients in Arm 1 were discontinued due to adverse events and the remaining 40 were evaluated. Of these 40, 30 achieved an ACR Pediatric 70 and continued in the double-blind phase, while 10 failed to meet this level of response and were placed on open label medication. Of the 43 patients in Arm 2, 3 discontinued due to inadequate response to therapy prior to the 4 month visit. Of the 40 evaluated in Arm 2, 19 achieved an ACR Pediatric 70, and 21 failed to achieve this level of improvement and were placed on open label medications. At the 6 month visit (end of pivotal phase of the trial), an additional 2 patients in Arm 1 had discontinued, and 28 were evaluated. Of these 28, 17 achieved CID and 11 did not and were entered into the open-label exploratory phase. Among the 19 in Arm 2 who remained in the double-blind after the 4 month visit, 10 achieved CID (1 of whom was discontinued at the 6 month visit due to fear of needles), 9 did not and subsequently entered the open label exploratory phase. All 17 in Arm 1 who had achieved CID at 6 months remained in the double-blind until the 12 month visit and all continued to be in a state of CID, 9 of who attained CRM. Of the 9 in Arm 2 who continued in the double-blind after 6 months, 1 discontinued prior to the 12 month visit due to disease flare. The remaining 8 were evaluated and 7 were found to be in CID, 3 of whom attained CRM. Figure 2 provides additional details regarding the outcome of those who entered the open-label exploratory phase.
FIGURE 2.
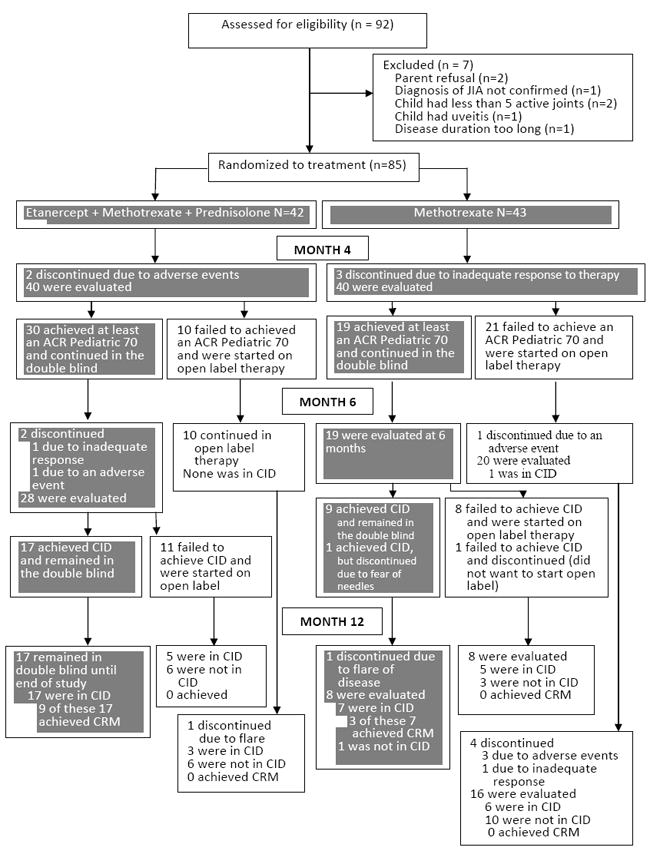
Flow diagram of subject progress through the trial. Dark shaded boxes indicate assessments done under double-blind conditions.
Table 2 summarizes outcome data for all patients. A statistically higher proportion of patients in Arm 1 attained at least an ACR Pediatric 70 at the 4 month visit and remained in the double blind phase. There was no significant difference between Arm 1 and Arm 2 in the proportion of patients who achieved CID by the 6 month visit. Of those that achieved an ACR Pediatric 70 at 4 months, 73% in Arm 1 and 58% in Arm 2 achieved CID between 7 and 12 months. Of those NOT achieving an ACR Pediatric 70 at 4 months, 50% achieved CID between 7 and 12 months, but it lasted only a mean of 1 month. The proportion of patients in each arm who attained CRM by 12 months approached significance, but the numbers were small (p = 0.053).
Table 2.
Summary of outcomes by treatment arm based on the last-observation-carried-forward of the intent to treat principle. (Numbers in cells represent subject numbers and (%). The primary outcome for the trial was clinical inactive disease [CID] at month 6. Clinical remission on medication [CRM] was the primary outcome for the exploratory phase.)
| Month 4 data | |||
|---|---|---|---|
| Arm 1 | Arm 2 | ||
| ACR Pedi 70 = yes | 30 (71%) | 19 (44%) | Chi square = 6.46; p = 0.011 |
| ACR Pedi 70 = no | 12 (28%) | 24 (55%) | |
| Totals | 42 | 43 | |
| Month 6 data | |||
| Arm 1 | Arm 2 | ||
| CID = yes | 17 (40%) | 10 (23%) | Chi square = 2.91; p = 0.088 |
| CID = no | 25 (60%) | 33 (77%) | |
| Totals | 42 | 43 | |
| Month 12/End of Study data | |||
| Arm 1 | Arm 2 | ||
| CRM = yes | 9 (21%) | 3 (7%) | Fisher’s Exact probability = 0.053 |
| CRM = no | 33 (79%) | 40 (93%) | |
| Totals | 42 | 43 | |
Figure 3 displays the proportions of patients in each arm who achieved ACR Pediatric 30, 50, 70 and 90 levels of improvement and CID at each study visit during the 6 month pivotal phase, and then at the 12 month/end of study visit. Arm 1 showed a consistently higher proportion of patients who improved as compared to Arm 2 across all visits for all levels of improvement except CID at the 2 month visit. There were a total of 440 visits during which assessment for CID was conducted (this excludes the baseline visit) under double blind conditions; 246 in Arm 1 and 194 in Arm 2. Ninety-four (38%) of the assessments in Arm 1 showed the subject to be in CID compared to 44 (22%) in Arm 2. Sixteen of the 27 patients that achieved CID at 6 months but did not maintain CID during the next 6 months (60%), 3 due to flare of disease (Arm 2) and 13 due to elevation of ESR, or increase of the physician’s global assessment to 1 (on a 0 to 10 point scale) or the development of an active joint by blinded joint assessment (9 Arm 1, 4 Arm 2).
FIGURE 3.

Proportions of patients who had ACR pediatric 70 response rates (Figure 3A) and Clinical Inactive Disease (Figure 3B) at 1, 2, 4, 5, 6, and 12 months. Open = MTX, etanercept and prednisolone; Arm 1 = MTX, etanercept and prednisolone; Arm 2= MTX and placebo etanercept and placebo prednisolone.
With the exception of parent assessment of overall well-being in Arm 2, all 6 of the core set variables used to determine the level of ACR improvement showed statistically significant improvements from baseline as early as 4 months after initiation of treatment (data not shown).
Because two potential predictors of outcome were statistically higher at the baseline visit in Arm 2 compared to Arm 1, logistic regression was used to determine if either the number of joints with active arthritis or the ESR influenced the likelihood of CID at the 6 month visit. The resulting odds ratios were well below statistical significance (p = 0.745 for the ESR, and 0.378 for the active joint count.) Specifically, the odds ratios were 0.997 (95% CI 0.979, 1.016) for the ESR, and 0.982 (95% CI 0.944, 1.022) for the active joint count. We also used logistic regression to determine if the anti-nuclear antibody, rheumatoid factor, anti-cyclic citrullinated peptide status or disease duration at the baseline visit predicted for the primary outcome. Only disease duration was statistically predictive; the shorter the disease duration at baseline, the more likely CID would be achieved at 6 months (odds ratio per month = 1.324, p <0.011). Thus, for each month sooner after disease onset aggressive treatment was started, the subject was 1.324 times more likely to achieve CID at 6 months. Rheumatoid factor positivity had no influence on the primary outcome when the two Arms were combined in the regression analysis. Among the 31 who were rheumatoid factor positive, 9 (29%) achieved CID at 6 months, compared to 19 of 54 (35%) who were rheumatoid factor negative (p = 0.56).
There was no difference between treatment arms in the number of patients that received intra-articular corticosteroid injections within 2 weeks of baseline or in the exploratory phase.
A total of 8 patients, 2 from Arm 1 and 6 from Arm 2 discontinued the study due to insufficient therapeutic effect or a flare of disease after CID had been achieved.
Safety
Serious and Unexpected Adverse Events
Three patients experienced serious adverse events. Two events occurred during open label dosing: pneumonia, which resulted in the subject being withdrawn from the study, and a single psychotic event that resolved with taper of prednisone and the patient continued in the study. A septic hip joint was identified at the last study visit in a subject 2 months after discontinuing open label therapy. The hip had not undergone intra-articular steroid injection and no organism was isolated. All 3 SAEs resolved without sequelae and none was unexpected.
Grade 3 or higher adverse events
In addition to the SAEs mentioned above, there were a total of 5 grade 3 (severe) events: 2 patients in Arm 1 (1 elevation of transaminases and 1 peritonsillar abscess), none in Arm 2, and 3 in patients on open label medication (1 low white blood cell count, 1 elevation of transaminases, and 1 adjustment reaction). Only the two patients with transaminase elevation were withdrawn from the study.
Four additional patients with less than Grade 3 AEs were withdrawn from the study: 1 due to worsening of a pre-existing, recurrent herpes simplex, 1 pneumonia, and 2 due to persistent elevations in transaminases, as per protocol. The recurrent herpes simplex infection returned to its pre-study status, while the pneumonia and transaminase elevations resolved completely.
The rates of infections that required systemic therapy were low and did not differ significantly based upon what agent/s the subject was taking when the infection occurred. Eighteen infections occurred during 247 months of MTX monotherapy (0.87/year); 16 occurred during 297 months of MTX and ETN therapy (0.65/year), and 17 occurred during 360 months of MTX-ETN-prednisolone therapy (0.57/year).
DISCUSSION
Irreparable damage to the joints and surrounding tissue is known to occur early in the course of JIA in perhaps as many as 60% of patients with polyarticular JIA (23-25). The rate of radiographic wrist destruction in these children progresses over time during which the disease remains active, but is most pronounced during the first year following onset of symptoms (24). Furthermore, clinically important osteoporosis can occur with some patients failing to recover normal bone mineralization, even if arthritis resolves, increasing bone fracture risk (26-28). These well-known findings underscore the urgency of bringing new onset disease under control as rapidly as possible. This study is the first double blind randomized placebo controlled trial to use aggressive treatment regimens as the initial therapy in polyarticular JIA with CID as soon as 6 months as the primary outcome. Both treatment arms consisted of aggressive treatment: higher dose subcutaneous methotrexate in both arms with the addition of etanercept as a first line agent plus 4 months of tapered prednisolone in the more aggressive arm. While there was a trend toward a higher rate of induction of CID in the more aggressive treatment arm, (etanercept, methotrexate, and prednisolone) the difference was not statistically significant. Despite this lack of statistical significance, results presented here have a high degree of clinical relevance for several reasons. Both treatment arms demonstrated dramatic effectiveness in these severe categories of childhood arthritis. Overall, CID was induced in 32% of patients by 6 months and in 66% by 12 months.
An important finding of this trial is the clinically relevant impact of timing of therapy on the achievement of CID. The chance of achieving CID increased by 1.324 for each month earlier a patient was treated. By comparison, the average time to achievement of CID in the published literature is 13 months (11, 13). Achievement of CID is undoubtedly a high bar to attain. Although a third of patients achieved CID by 6 months in this study, this state was not continuously maintained in 60% of patients. This loss of CID was usually due to minimal elevations in the ESR, an increase of the physician’s global assessment to 1 on a 10 point scale, or the development of an active joint by blinded joint assessment.
Comparison of results from this study to those from most other trials of biologics in JIA is difficult for a number of reasons. Patients in other studies were not treated early in disease, had failed prior MTX therapy, or were retrospective reports of uncontrolled, non-randomized treatment. A recently reported trial of early aggressive therapy in 60 children with polyarticular JIA and who were disease modifying anti-rheumatic drug naïve found similar results supportive of those reported here. The ACUTE–JIA study (Aggressive Combination Drug Therapy in Very Early Polyarticular Juvenile Idiopathic Arthritis) was a 54 week, multicenter, open-label randomized trial that compared 3 treatments - MTX, MTX with infliximab, and MTX with sulfasalazine and hydroxychloroquine (29). Patients had a mean disease duration of 1.9 months, a physician’s global assessment of 5.5 (0-10 VAS), 37% were positive for anti-nuclear antibody and 2% were rheumatoid factor positive. At 6 months, a modified CID was achieved by 60% in the MTX-infliximab arm, 30% in the combination arm and 5% in the MTX only arm. Although the ACUTE study patient sample, concomitant medications, and protocol design were different from those of TREAT, the results provide additional evidence of the benefit of early aggressive therapy.
In the TREAT trial, achievement of ACR Pediatric 70 improvement by 4 months was an important predictor of CID at 6 months. Fifty percent of those who did not reach an ACR Pediatric 70 at 4 months and were placed on open label medication did achieve CID between 7 and 12 months. However, it was short lived, lasting a median of only 1 month. This may suggest that the durability of CID is greatest when achieved early in the treatment course.
There are several reasons that may explain why the difference in proportion of patients attaining CID between the two treatment arms was not statistically significant. The initial assumption that 60% of patients in the more aggressive treatment arm would achieve the primary endpoint of CID at 6 months proved too optimistic. This may be due to the high disease activity and proportion of rheumatoid factor positivity of the cohort of patients enrolled in this trial.
MTX administered subcutaneously at a dose of 0.5 mg/kg/week was slightly more effective than had been anticipated (23% observed versus 20% expected). The excellent response to subcutaneous MTX suggests a new standard dose and route for maximal effectiveness for use of MTX in treating polyarticular JIA.
A crucial observation is that there were few serious adverse events, and that there were no clinically important differences in Grade 3 or higher AEs or infections requiring systemic treatment between the treatment arms. While this study has limited power to detect rare side effects, these treatments appear to have acceptable short term safety profiles. Studies with larger subject-years of follow up, such as a disease specific, consolidated registry, will provide more valid assessments of safety for these treatment approaches.
This trial has several shortcomings that may have influenced results. The study was limited to only 2 of the 7 categories of JIA; rheumatoid factor positive and negative polyarticular JIA. Validity of extrapolation of results presented here to other JIA categories is unknown. Uveitis was an exclusion criterion for this trial, thus precluding conclusions regarding the therapeutic utility of the treatment regimens studied here on JIA-associated eye disease. While enrolled patients were referred to as newly diagnosed, to make recruitment feasible we permitted patients who had symptoms up to 12 months to be eligible and to have received small doses of MTX and prednisolone prior to enrollment. The finding that patients with shorter duration of disease at enrollment were more likely to attain CID at 6 months is undoubtedly one of the more clinically important findings of this trial. This suggests, however, that had enrollment been limited to those with disease of shorter duration, results may have been different. The ACR recently adopted Provisional Criteria for CID in Select Categories of JIA (30). The newly adopted criteria contain a slight modification to those used in this study: the ESR may be elevated if not attributable to JIA and the duration of morning stiffness cannot exceed 15 minutes. Neither the cause for elevation of the ESR nor the duration of morning stiffness were collected in this trial and, while the impact on our findings is likely negligible, we are unable to state this with certainty. While MTX monotherapy given in the dose of 0.5 mg/kg/week SQ may no longer be considered aggressive therapy by some pediatric rheumatologists, it is still an aggressive approach for many. This dose and route are not standard of care for children with poly JIA. Rather than start at a low dose, or start orally and increase and/or switch to SQ dosing, we chose to use the maximally effective dose and route of MTX at the start of treatment.
This study represents the largest, most homogeneous cohort of children with new onset polyarticular JIA treated in a blinded, placebo controlled trial using a standardized protocol. The substantial proportion of patients who achieved CID after only 6 months of aggressive early treatment, and CRM by 12 months may serve as a template for new standards of care for polyarticular JIA. Follow up of this cohort is underway to determine the durable, long-term benefits of this treatment approach.
Acknowledgments
The investigators wish to thank all of the subjects and their families for their dedicated participation in this study and the following core study research team members: Morty Cohen, Kim Gama, Audrey Hendrickson, Susan Jacob, Elena Mano, Anne Murphy, and Nora Singer; and the following site study team members: Stacy Ardoin, Aimee Baker, Shawna Baker, Imelda Balboni, Lilliana Barillas-Arias, Tara Barker, Samantha Bell, Joseph Benavides, Heather Benham, John Bohnsack, Bonny Bowen, Ann Clark, Bronte Clifford, Irene Borras Coughlin, Sonya Crook, Fatma Dedeoglu, Lauren Dickey, Anne Eberhard, Helen Emery, Maria Espinosa, Alisa Gotte, Kimberly Fluker, Jennifer Frankovich, Tracy Fuelling, Robert Fuhlbrigge, Sharon Goodwin, Tom Griffin, Alexei Grom, Kathleen Haines, Heather Hanson, Theresa Harris, Marla Hashiguchi, Kristen Hayward, Michael Henrickson, Shirley Henry, Aimee Hersch, Sarah Holland, Joyce Hsu, Jennifer Huggins, Patricia Irigoyen, Jim Jarvis, Kathleen Kenney-Riley, Susan Kim, Patricia Lee, Suzanne Li, Bernadette McNally, Jackie Morrill, Su-Ellen Mortland, Tzielan Lee, Katherine Madson, Esi Morgan-Dewitt, Daphne Nayar, Marilynn Orlando, Julisa Patel, Karin Peterson, Egla Rabinovich, Jennifer Rammel, Kathy Redmond, Mary Ellen Riordan, Jenny Rossette, , Kandice Roush, Ann Rutherford, Christy Sandborg, Robert Sheets, Abi Siva, Erin Smith, Mary Beth Son, Charles Spencer, Ann Stevens, Janalee Taylor, Tracy Ting, Jennifer Turner, Katherine Tuthill, Heather Van Mater, Emily vonScheven, Jennifer Weiss, Jolene Wesley, Katharine Willcoxon, Janet Wootton, and Justine Zasa. The authors would also like to thank TARGET HEALTH INC for providing and partially subsidizing the electronic data capture system and electronic data base.
SUPPORT: This work was supported by NIH Grants: 1R21AR48355-1, 1RO1 AR049762-01A2, 3R01AR049762-04S1, P60 AR047784-07, P30 AR47636, the Howe Endowment for Juvenile Rheumatoid Arthritis Research, Friends of CARRA, and the Arthritis Foundation. This publication was supported in part by the CTSA Grants: UL1RR025014, UL1RR025764, CO6RR11234, UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
INDUSTRIAL SUPPORT: AMGEN provided etanercept and etanercept placebo and medication transport supplies.
References
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 2.Gortmaker S. Chronic childhood disorders: prevalence and impact. Ped Clin North Am. 1984;31:3–18. doi: 10.1016/s0031-3955(16)34532-1. [DOI] [PubMed] [Google Scholar]
- 3.Singsen BH. Rheumatic disease of childhood. Rheum Dis Clin North Am. 1990;16(3):581–99. [PubMed] [Google Scholar]
- 4.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 5.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810–20. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 6.Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58(5):1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 7.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–91. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 8.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63(4):465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena N, Aggarwal A, Misra R. Elevated concentrations of monocyte derived cytokines in synovial fluid of children with enthesitis related arthritis and polyarticular types of juvenile idiopathic arthritis. J Rheumatol. 2005;32(7):1349–53. [PubMed] [Google Scholar]
- 10.Black AP, Bhayani H, Ryder CA, Pugh MT, Gardner-Medwin JM, Southwood TR. An association between the acute phase response and patterns of antigen induced T cell proliferation in juvenile idiopathic arthritis. Arthritis Res Ther. 2003;5(5):R277–84. doi: 10.1186/ar791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers HM, Brinkman DM, Kamphuis SS, van Suijlekom-Smit LW, van Rossum MA, Hoppenreijs EP, et al. Clinical course and prognostic value of disease activity in the first two years in different subtypes of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2010;62(2):204–12. doi: 10.1002/acr.20069. [DOI] [PubMed] [Google Scholar]
- 12.Shenoi S, Wallace CA. Remission in juvenile idiopathic arthritis: current facts. Curr Rheumatol Rep. 2010;12(2):80–6. doi: 10.1007/s11926-010-0085-2. [DOI] [PubMed] [Google Scholar]
- 13.Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(11):3554–62. doi: 10.1002/art.21389. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43(1):22–9. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Moreland LW, Bridges SL., Jr Early rheumatoid arthritis: a medical emergency? Am J Med. 2001;111(6):498–500. doi: 10.1016/s0002-9343(01)00973-1. [DOI] [PubMed] [Google Scholar]
- 16.Mottonen T, Hannonen P, Korpela M, Nissila M, Kautiainen H, Ilonen J, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46(4):894–8. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 17.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 18.O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46(2):283–5. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 19.Quinn MA, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol. 2003;21(5 Suppl 31):S154–7. [PubMed] [Google Scholar]
- 20.Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4. [PubMed] [Google Scholar]
- 21.Program CTE. Common Terminology Criteria for Adverse Events, Version 3. DCTD NCI NIH DHHS. 2003 [Google Scholar]
- 22.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Bowyer SL, Roettcher PA, Higgins GC, Adams B, Myers LK, Wallace C, et al. Health status of patients with juvenile rheumatoid arthritis at 1 and 5 years after diagnosis. J Rheumatol. 2003;30(2):394–400. [PubMed] [Google Scholar]
- 24.Magni-Manzoni S, Rossi F, Pistorio A, Temporini F, Viola S, Beluffi G, et al. Prognostic factors for radiographic progression, radiographic damage, and disability in juvenile idiopathic arthritis. Arthritis Rheum. 2003;48(12):3509–17. doi: 10.1002/art.11337. [DOI] [PubMed] [Google Scholar]
- 25.Mason T, Reed AM, Nelson AM, Thomas KB. Radiographic progression in children with polyarticular juvenile rheumatoid arthritis: a pilot study. Ann Rheum Dis. 2005;64(3):491–3. doi: 10.1136/ard.2003.017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zak M, Hassager C, Lovell DJ, Nielsen S, Henderson CJ, Pedersen FK. Assessment of bone mineral density in adults with a history of juvenile chronic arthritis: a cross-sectional long-term followup study. Arthritis Rheum. 1999;42(4):790–8. doi: 10.1002/1529-0131(199904)42:4<790::AID-ANR24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Duffy CM. Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: clinical science for the pediatrician. Ped Clin North Am. 2005;52:359–72. doi: 10.1016/j.pcl.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Malattia C, Damasio MB, Pistorio A, Ioseliani M, Vilca I, Valle M, et al. Development and preliminary validation of a paediatric-targeted MRI scoring system for the assessment of disease activity and damage in juvenile idiopathic arthritis. Ann Rheum Dis. 2011;70(3):440–6. doi: 10.1136/ard.2009.126862. [DOI] [PubMed] [Google Scholar]
- 29.Tynjala P, Vahasalo P, Tarkiainen M, Kroger L, Aalto K, Malin M, et al. Aggressive Combination Drug Therapy in Very Early Polyarticular Juvenile Idiopathic Arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70:1605–12. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 30.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. for the Childhood Arthritis and Rheumatology Research Alliance, the PRCSG, and the Pediatric Rheumatology International Trials Organization. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 2011;63:929–936. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]


